Abstract
Purpose
To investigate the association between serum 25-hydroxyvitamin D (25[OH]D) concentration and refractive error in a community-based cohort of adults aged 46 to 69 years.
Methods
Residents of the City of Busselton in Western Australia born between 1946 and 1964 were invited to participate. Participants underwent cycloplegic autorefraction and completed questionnaires on education, occupational sun exposure, and physical activity. Blood samples were collected and serum frozen at −80°C. Serum 25[OH]D concentration was measured by immunoassay. Data on 25[OH]D were deseasonalized and multivariate models built to analyze the association between 25[OH]D concentration and spherical equivalent and myopia, defined as spherical equivalent <−0.50 D.
Results
After exclusions, data were available for 4112 participants. Serum 25[OH]D concentration was not associated with spherical equivalent or myopia after adjustment for confounding factors (β = −0.01, 95% confidence interval [CI]: −0.03 to −0.008, P = 0.25, and odds ratio = 1.02, 95% CI: 0.99 to 1.05, P = 0.12, respectively). When participants were classified into 25[OH]D groups of lower (<50 nmol/L), medium (≥50 to <75 nmol/L), and upper (≥75 nmol/L), the upper group had slightly greater myopic refractive error than the medium group (P = 0.02) but not the lower group, after adjustment for confounders.
Conclusions
There was no substantial association between 25[OH]D levels and spherical equivalent or odds of myopia in this study. The association previously noted between low serum 25[OH]D level and myopia in younger Western Australians is not evident in later adulthood.
Translational Relevance
This study provides further evidence suggesting that vitamin D levels are unrelated to myopia risk in adults and thus not a suitable target for myopia intervention.
Keywords: myopia, refractive error, vitamin D, adult, 25-hydroxyvitamin D
Introduction
The rising prevalence of myopia is a major global public health issue.1,2 Myopia is associated with significant individual and societal costs through treatment and lost productivity.3 Additionally, myopia increases the risk of potentially blinding diseases such as glaucoma, retinal detachment, and myopic maculopathy.4–6
Increasing the amount of time children spend outdoors reduces the incidence of myopia.7–10 The mechanism underlying the link between time spent outdoors and myopia is unknown. One theory suggests that vitamin D may be a potential mediator of this relationship, and could therefore be a possible target for myopia interventions.11–13 Vitamin D3 is produced in the skin following exposure to ultraviolet B radiation and both vitamin D3 and vitamin D2 can be obtained from dietary sources. In Australian populations, vitamin D3 makes up the majority of an individual's total vitamin D.14,15 Dietary vitamin D3 intake is typically low in Australian adults and most vitamin D in Australians is therefore derived from sunlight.15,16 All forms of vitamin D are metabolized into the inactive 25-hydroxyvitamin D (25[OH]D) in the liver; assessing serum 25[OH]D concentration is the standard method for monitoring vitamin D status.
Several previous cross-sectional studies have found an inverse association between the serum concentration of 25[OH]D and measures of myopia in children and young adults.11,12,17,18 On the other hand, a small (n = 50) cross-sectional study of young adults in Northern Ireland recently found no significant difference in 25[OH]D3 concentration in those with and without myopia, possibly due to a lack of power.19 The only longitudinal study investigating this relationship found no association between myopia at 15 years of age and previous serum 25[OH]D3 or total serum 25[OH]D, after adjusting for reported time spent outdoors at age 9.14 Moreover, a recent Mendelian randomization study found that genetically low 25[OH]D was not associated with refractive error in a large sample of European and Asian adults.20 These latter studies suggest that 25[OH]D concentration may be just a marker of time spent outdoors and not directly involved in the onset of myopia. However, these studies have their own limitations, such as the inherent difficulty in accurately measuring time spent outdoors, possible residual confounding, and potential pleiotropy of the tested single nucleotide polymorphisms, which may have downstream effects on refractive error.14,20
Only one study, the European Eye Study, has investigated the association between serum 25[OH]D and myopia in older adults.21 In this cross-sectional study in European individuals over 65 years of age, serum 25[OH]D3 concentrations were lower in myopic adults compared to nonmyopic adults, but there was no independent association after adjustment for confounding factors. The authors did find that higher estimated personal ultraviolet B radiation, particularly during childhood and adolescence, was associated with a lower prevalence of myopia in adulthood.21 As myopia is typically incident in childhood and adolescence, it is likely that any association with adult 25[OH]D concentration arises as a result of a correlation between childhood and adult 25[OH]D levels. There is some evidence for tracking of 25[OH]D levels through adulthood and childhood/adolescence.22–25
A limitation of the European Eye Study is that only individuals with reduced visual acuity (<0.3 logMAR) underwent refraction, hence mild uncorrected or undercorrected myopia may have been missed. Additionally, the 25[OH]D3 levels in that study were relatively low, with a median 25[OH]D3 concentration of 47.2 nmol/L.21 It is therefore worthwhile re-examining the possible association in a climate with more sunlight, to test the possible benefits of higher 25(OH)D levels.
We aimed to further investigate the relationship between serum 25[OH]D concentration and spherical equivalent in middle-aged to older Australian adults residing in a sunny, inner-regional, coastal community.
Methods
This research was approved by the Human Research Ethics Committee of the University of Western Australia (RA/4/1/2203) and adhered to the tenets of the Declaration of Helsinki. Prior to participating, all subjects provided written informed consent after an explanation of the nature and possible consequences of the study.
Study Population
Data for the research were collected from the baseline visit of the Busselton Healthy Ageing Study (BHAS), which has been described previously.26 In brief, the BHAS is a cross-sectional study of adults born between 1946 and 1964 living in the city of Busselton in Western Australia. Busselton is a semi-rural, coastal city located at latitude of approximately 34°South and is classified as inner-regional according to the Accessibility/Remoteness Index of Australia. All noninstitutionalized residents listed on the 2010 Australian Electoral Roll, a compulsory requirement for Australian citizens aged 18 years and over, were eligible to participate. Data collection began in May 2010 and was completed in December 2015.
Questionnaires
All participants were asked to complete an extensive 40-page questionnaire on demographics, health, and lifestyle. Questions included level of highest education, longest and current occupation, ethnicity, time spent in physical activity (short form of the International Physical Activity Questionnaire),27 hours spent sitting per day, current medications including over-the-counter or prescribed supplements, and medical history.
An occupational sun exposure variable was created based on self-reported longest occupation by classifying occupations into categories according to the methods described by Carey et al.28 An additional “domestic duties” (n = 86) and a “not elsewhere classified” (NEC) category (n = 96) were created. The NEC group was excluded from any further analysis involving occupational sun exposure. The remaining 31 groups were further categorized into three occupational sun exposure groups—mostly indoors, mixed indoor/outdoor, and mostly outdoors—according to published data that reported the percentage of Australians exposed to solar ultraviolet radiation at work within occupation groups (% exposed by group: mostly indoors: 0% to 19.4%; mixed indoor/outdoor: 31.6% to 42.0%; mostly outdoors: 63.6% to 97.5%).20,29 The domestic duties category was classified as mostly indoors based on data from the Ausimmune Study30 in which 83% of those with a similar occupation classified their job as indoors (unpublished data, 2017).
Eye Examination
All willing participants underwent cycloplegic refraction using an automated refractor/keratometer (ARK-501a, Nidek, Osaka, Japan) 15 to 20 minutes after instillation of tropicamide 1% in both eyes. The autorefraction instrument was able to record only +/−10D of spherical or cylindrical refractive error; values exceeding this range were assigned a +10D if they were positive and −10D if they were negative.
Serum 25[OH]D Measurement
Participants had fasting blood samples collected via venepuncture on the day of examination. Samples were frozen at −80°C and analyzed in two batches, one in late 2014 and one in early 2016 using immunoassay (Abbott ARCHITECT platform, Abbott Laboratories, IL). The intrabatch coefficients of variation were 3.4% and 2.3% at mean 25[OH]D concentrations of 51 and 74 nmol/L, respectively, and the interbatch coefficients of variation ranged from 2.4% to 5.3%. A random subset of 117 BHAS serum samples from three strata of 25[OH]D concentration were re-measured using isotope dilution liquid chromatography/tandem mass spectrometry as described previously.31 The two methods showed very good correlation (R2 = 0.88), although there was a tendency for the immunoassay to overestimate 25[OH]D at high concentrations (for immunoassay 25[OH]D > 110 mol/L, mean bias = +30.41, n = 574; for 25[OH]D <=110 nmol/L, mean bias = +1.17, n = 4503).
Data Analysis
Only refraction data from the right eye were used in this analysis. Spherical equivalent, defined as sphere + ½ cylinder of the refraction, and the presence of myopia, defined as spherical equivalent <−0.50D, were the outcomes of this study. Serum 25[OH]D concentration was deseasonalized according to month of collection by fitting a sinusoidal model as previously described32; however, we fitted a separate model for each year of collection to allow for variation in seasonal 25[OH]D measurements. For further analyses, 25[OH]D was categorized into quintiles, and into groups of lower (<50 nmol/L), medium (≥50 to <75 nmol/L), and upper (≥75 nmol/L), which have been suggested as cut-off points for deficient, insufficient, and sufficient vitamin D levels, respectively.18,33
The “Did not go to school,” “Primary School,” and “Secondary School” groups were combined due to lack of numbers in the former two groups. A “Vitamin D supplement” variable was created by classifying individuals who reported taking a medication or supplement containing vitamin D, including cod liver oil, as “yes,” and all others as “no.”
Only participants with right eye cycloplegic autorefraction and serum 25[OH]D data were eligible for this study. Participants were excluded from the analysis if they identified as being of non-Caucasian ethnicity or self-reported a history of cataract diagnosis, keratoconus diagnosis, or laser refractive surgery.
Demographic data were compared in males and females, and in those with and without myopia, using the two-sided independent samples t-test for quantitative variables and the Pearson χ2 test for categorical variables. Linear and logistic regression models were constructed to test univariate associations of 25[OH]D variables (continuous and categorical) and potential confounders with spherical equivalent and myopia as the outcomes, respectively. A confounding variable was defined as a variable that had P < 0.1 on univariate linear regression testing with both spherical equivalent and deseasonalized 25[OH]D.
Finally, we constructed both multivariate linear and logistic regression models with spherical equivalent and odds of myopia as outcomes, respectively, and investigated their associations with deseasonalized 25[OH]D as a continuous or categorical variable. These models included all identified confounding factors as covariates: age, highest education, occupational sun exposure, hours spent sitting per day, and retirement status. We tested for nonlinear relationships between continuous 25[OH]D and the outcomes by using the fractional polynomial test to identify the optimal power transformation of 25[OH]D. We then added this term to the model and tested for significant improvement based on the change in deviance or residual sum of squares (RSS).
We conducted a sensitivity analysis to determine if differently categorizing 25[OH]D affected our findings by re-grouping deseasonalized 25[OH]D into <40, ≥40 to <60, ≥60 to <80, and ≥100 nmol/L and re-fitting the multivariate models for myopia and spherical equivalent.
All analyses were conducted using R statistical software version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria), and a statistically significant association was defined as P < 0.05.
Results
Participants
A total of 5107 individuals participated in the BHAS (82% of eligible baby boomers on the electoral roll were located and contacted, 76% of whom participated). Of these, 30 (0.59%) were excluded due to missing 25[OH]D data and 190 (3.72%) were excluded due to missing right eye autorefraction data. Of those remaining, 365 (7.2%), 2 (0.04%), and 1 (0.02%), were excluded for a reported diagnosis of cataract, keratoconus, and laser refractive surgery, respectively. A further 56 (1.1%) reported non-Caucasian ethnicity and were excluded, as were 351 (6.9%) participants who had noncycloplegic autorefraction only, leaving data on 4112 (80.52%) to be included in the analysis. After exclusions, only one individual had a right eye autorefraction sphere value −10D or less; this individual was assigned a right eye sphere value of −10D and included in the analysis.
The characteristics of the study participants are shown in Table 1. The mean age of participants in the study was 57.5 years (range: 45.5 to 69.8 years). Women were overrepresented in the study sample (women: n = 2197 [53.4%] versus male: n = 1915 [46.6%], test of equal proportions: P < 0.001) and women were slightly younger (mean difference: 0.40 years, 95% confidence interval [CI]: 0.05 to 0.75, P = 0.03), more likely to have had an indoor occupation (P < 0.001), more likely to take a vitamin D supplement (P < 0.001), more likely to be retired (P < 0.001), had a lower mean body mass index (BMI) (P < 0.001), spent less time sitting per day (P < 0.001), and had a lower mean 25[OH]D concentration (mean difference = 6.94 nmol/L, 95% CI: 5.45 to 8.44, P < 0.001) than men. The mean spherical equivalent of the sample was −0.19D (SD: 1.59, 95% CI: −0.24 to −0.14), and 1364 (33.2%) individuals were classified as myopic. There was no significant difference in mean spherical equivalent or proportion of myopic individuals between males and females (mean spherical equivalent difference: 0.04D, 95% CI: −0.14 to 0.05, P = 0.38; myopia: males n = 649 [33.9%], females n = 715 [32.6%], P = 0.38). We investigated whether significant anisometropia may have biased our results due to the use of right eye data only. Right and left eye spherical equivalents were highly correlated (r = 0.91, 95% CI: 0.91 to 0.92). Of the 427 individuals who had myopia in only one eye, 216 (50.58%) were myopic in the right eye. In those with unilateral myopia, there was no significant difference in 25[OH]D between those with myopia in the right eye and those with myopia in the left eye (mean difference = 3.64 nmol/L, 95% CI: −8.85 to 1.57, P = 0.17).
Table 1.
Characteristics of Individuals in the BHAS Who Have, and Have Not Been Categorized as Having Myopia
| Non-myopia |
Myopia |
Total |
|
| Age, mean (SD) | 57.7 (5.8) | 57.2 (5.6) | 57.5 (5.7) |
| Female, n (%) | 1482 (53.9) | 715 (52.4) | 2197 (53.4) |
| Highest education, n (%) | |||
| Secondary school or lower | 1443 (52.7) | 634 (46.7) | 2077 (50.7) |
| Other (e.g., TAFEa) | 818 (29.9) | 415 (30.6) | 1233 (30.1) |
| University | 477 (17.4) | 308 (22.7) | 785 (19.2) |
| Longest occupation, n (%) | |||
| Mostly indoor | 1741 (64.9) | 935 (70.5) | 2676 (66.7) |
| Mixed indoor/outdoor | 186 (6.9) | 92 (6.9) | 278 (6.9) |
| Mostly outdoor | 757 (28.2) | 300 (22.6) | 1057 (26.4) |
| Retired, n (%) | 191 (7.0) | 123 (9.0) | 314 (7.6) |
| BMI, n (%)b | |||
| Underweight | 10 (0.4) | 5 (0.4) | 15 (0.4) |
| Healthy weight | 744 (27.1) | 341 (25.0) | 1085 (26.4) |
| Overweight | 1180 (42.9) | 616 (45.2) | 1796 (43.7) |
| Obese | 814 (29.6) | 402 (29.5) | 1216 (29.6) |
| Minutes of physical activity per week, median (IQR) | 510 (210–1140) | 490 (200–1110) | 510 (210–1125) |
| Hours sitting per day, median (IQR) | 4.0 (2.0–6.0) | 4.0 (2.2–6.0) | 4.0 (2.0–6.0) |
| Taking vitamin D supplement, n (%) | 312 (11.4) | 158 (11.6) | 470 (11.4) |
| Deseasonalized 25[OH]D concentration (nmol/L), median (IQR) | 77.8 (64.7–93.2) | 79.8 (65.0–95.2) | 78.5 (64.7–93.8) |
| 25[OH]D status, n (%) | |||
| Lower (<50 nmol/L) | 191 (7.0) | 101 (7.4) | 292 (7.1) |
| Medium (≥50 to <75 nmol/L) | 1045 (38.0) | 473 (34.7) | 1518 (36.9) |
| Upper (≥75nmol/L) | 1512 (55.0) | 790 (57.9) | 2302 (56.0) |
| 25[OH]D Quintile, n (%) | |||
| 1 (range: 17.79–61.27) | 551 (20.1) | 272 (19.9) | 823 (20.0) |
| 2 (range: 61.28–72.56) | 567 (20.6) | 255 (18.7) | 822 (20.0) |
| 3 (range: 72.56–83.83) | 561 (20.4) | 261 (19.1) | 822 (20.0) |
| 4 (range: 83.83–98.35) | 547 (19.9) | 275 (20.2) | 822 (20.0) |
| 5 (range: 98.35–302.16) | 522 (19.0) | 301 (22.1) | 823 (20.0) |
IQR, interquartile range.
Vocational or technical tertiary education in Australia. TAFE, Technical and Further Education.
Categories according to the Australian Government Department of Health: underweight: <18.5 kg/m2; healthy weight: 18.5–24.9 kg/m2, 25–29.9 kg/m2; obese: ≥30 kg/m2.
Three individuals had very high 25[OH]D levels (>250 nmol/L), which exhibited high leverage in the models including 25[OH]D as a continuous variable. To determine the impact of these data on the results, the regression models were constructed with and without these three cases. There was little difference in the results before and after exclusion of these data (Table 2); therefore, they were included in the analysis.
Table 2.
The Association Between Continuous and Categorical Measures of Deseasonalized 25[OH]D and Myopia in Participants of the BHAS
| Spherical Equivalent |
Spherical Equivalenta |
|||
| Beta (95% CI) |
P Value |
Beta (95% CI) |
P Value |
|
| Deseasonalized 25[OH]D per 10 nmol/L change | −0.005 (−0.02 to 0.001) | 0.60 | −0.01 (−0.03 to 0.008) | 0.25 |
| Deseasonalized 25[OH]D per 10 nmol/L changeb | −0.003 (−0.02 to 0.02) | 0.76 | −0.009 (−0.03 to 0.01) | 0.39 |
| Deseasonalized 25[OH]D group | 0.05 | 0.02* | ||
| Lower | −0.18 (−0.32 to 0.02) | 0.07 | −0.17 (−0.37 to 0.03) | 0.10 |
| Medium | Reference | Reference | ||
| Upper | −0.11 (−0.22 to −0.01) | 0.03* | −0.14 (−0.25 to 0.04) | 0.007* |
| Deseasonalized 25[OH]D quintile | 0.29 | 0.14 | ||
| 1 (n = 823) | 0.05 (−0.10 to 0.20) | 0.51 | 0.10 (−0.05 to 0.26) | 0.20 |
| 2 (n = 822) | 0.15 (−0.003 to 0.30) | 0.05 | 0.18 (0.03 to 0.34) | 0.02* |
| 3 (n = 822) | 0.08 (−0.07 to 0.24) | 0.29 | 0.08 (−0.07 to 0.24) | 0.29 |
| 4 (n = 822) | 0.006 (−0.15 to 0.16) | 0.94 | 0.02 (−0.14 to 0.17) | 0.84 |
| 5 (n = 823) | Reference | Reference | ||
Ranges of the quintiles are as follows: quintile 1: 17.79 to 61.27 nmol/L; quintile 2: 61.28 to 72.56 nmol/L; quintile 3: 72.56 to 83.83 nmol/L; quintile 4: 83.83 to 98.35 nmol/L; quintile 5: 98.35 to 302.16 nmol/L. *P < 0.05.
Adjusted for age, occupational sun exposure, hours spent sitting per day, highest education, and retirement status.
Three outliers with deseasonalized 25[OH]D >250 nmol/L excluded from this analysis.
Table 2.
Extended
| Myopiaa |
||
| OR (95% CI) |
P Value |
|
| Deseasonalized 25[OH]D per 10 nmol/L change | 1.02 (0.99 to 1.05) | 0.12 |
| Deseasonalized 25[OH]D per 10 nmol/L changeb | 1.02 (0.99 to 1.05) | 0.20 |
| Deseasonalized 25[OH]D group | 0.06 | |
| Lower | 1.13 (0.86 to 1.48) | 0.37 |
| Medium | Reference | |
| Upper | 1.18 (1.03 to 1.37) | 0.02* |
| Deseasonalized 25[OH]D quintile | 0.09 | |
| 1 (n = 823) | 0.80 (0.65 to 0.99) | 0.04* |
| 2 (n = 822) | 0.75 (0.61 to 0.83) | 0.008* |
| 3 (n = 822) | 0.81 (0.65 to 0.99) | 0.04* |
| 4 (n = 822) | 0.85 (0.69 to 1.04) | 0.12 |
| 5 (n = 823) | Reference | |
Univariate Analyses Between 25[OH]D and Refractive Error
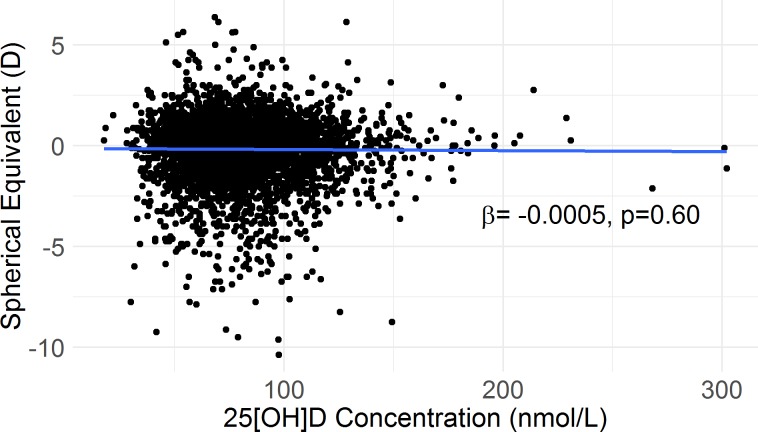
There was no association between deseasonalized 25[OH]D concentration and spherical equivalent (Fig. 1; Table 2) nor was there an association between deseasonalized 25[OH]D concentration and odds of myopia (OR = 1.00). Quintiles of 25[OH]D were not associated with either spherical equivalent (Table 2) or odds of myopia (P = 0.29 and P = 0.15, respectively). Serum 25[OH]D group (low/medium/upper) was associated with spherical equivalent (Table 2), but not with odds of myopia (P = 0.11).
Figure 1.
Scatterplot of spherical equivalent over 25[OH]D concentration before adjustment for confounders. β and P value are obtained from simple linear regression.
Identifying Confounders
Supplementary Table S1 shows the results of the analysis of potential confounders. Age, occupational sun exposure, and sitting (in hours per day) all met the criteria for a confounder and were included in the multivariate models. Being retired was associated with increased odds of myopia (odds ratio [OR] = 1.33, 95% CI: 1.05 to 1.68, P = 0.02) but not with spherical equivalent (β = −0.01, 95% CI: −0.28 to 0.08, P = 0.29). After adjustment for age, retirement status was associated with both spherical equivalent (β = −0.24, 95% CI: −0.43 to −0.05, P = 0.01) and odds of myopia (OR = 1.51, 95% CI: 1.17 to 1.93, P = 0.001) and was therefore included in the regression models.
We considered that the association between spherical equivalent and occupational sun exposure could be confounded by education, a well-known risk factor for myopia. After adjustment for age and highest education, the change in sum of squares induced by adding occupational sun exposure to the model reduced from 28.3 (P = 0.004) to 11.8 (P = 0.09). Thus, we included both education and occupational sun exposure in the multivariate analysis. There was no significant interaction between occupational sun exposure and education (P = 0.72).
There was substantial collinearity between sex and occupational sun exposure (90% of females were in the mostly indoor group), and both could not be included in the same model. Only occupational sun exposure met the definition of a confounder and so was not used in the multivariate models.
Multivariate Models
Table 2 shows the results of the multiple regression analyses. After adjustment for age, occupational sun exposure, highest education, sitting hours, and retirement status, there was no association between deseasonalized 25[OH]D concentration and spherical equivalent nor was deseasonalized 25[OH]D associated with odds of myopia.
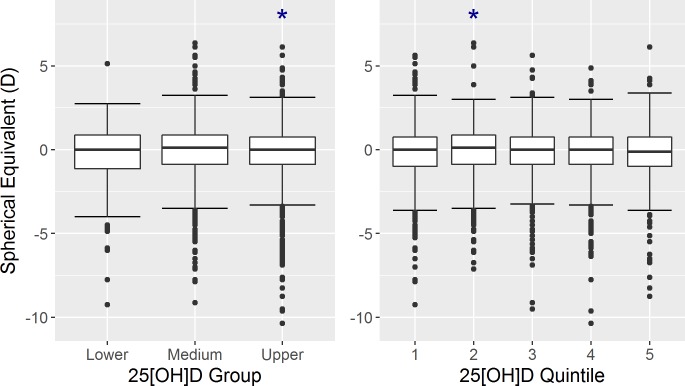
This association was also present when odds of myopia was the outcome. Overall, quintiles of 25[OH]D was not significantly associated with spherical equivalent or odds of myopia. However, when comparing quintiles against each other, the second quintile had a lower myopic spherical equivalent than the fifth quintile (Table 2; Fig. 2). Additionally, the odds of myopia were significantly lower in the first, second, and third quintiles compared to the fifth quintile. The significant associations between spherical equivalent and 25[OH]D group or 25[OH]D quintile were not altered significantly after the exclusion of those with high myopia <−6.0D (data not shown). We investigated curvilinearity in the model with continuous 25[OH]D through fractional polynomial modelling and identified that the addition of 25[OH]D raised to the power of −1 or −0.5 constructed the best curvilinear multiple linear regression models. The addition of either the −1 or −0.5 term actually worsened the model, although not significantly, and these were therefore not included (F-test: ΔRSS = 0.8, P = 0.58; and ΔRSS = 0.4, P = 0.70, respectively). In addition, inclusion of these terms did not improve the model when odds of myopia were considered as the outcome (Likelihood ratio test: ΔDeviance = 0.03, P = 0.86; and ΔDeviance = 0.01, P = 0.92, respectively).
Figure 2.
Boxplots of spherical equivalent in each of the three 25[OH]D groups (left) and in each of the 25[OH]D quintiles (right). Significant differences (P < 0.05) in mean spherical equivalent compared to the reference group (medium group in the left plot and quintile 5 in the right plot) are marked with an asterisk.
Sensitivity Analysis
Using alternate grouping methods for 25[OH]D made little difference to the overall results. Supplementary Table S2 shows the results from using 40, 60, 80, and 100 nmol/L as cutoff points. Similar to other results, individuals in the ≥40 to <60 nmol/L and the ≥60 to <80 nmol/L had significantly less myopia than those in the ≥100 nmol/L group.
Discussion
We found no association between deseasonalized serum 25[OH]D concentration and spherical equivalent or odds of myopia before or after adjustment for confounding factors. As shown in Figure 2, there was a modest inverse U-shaped pattern between the 25[OH]D groups, defined by commonly used cut-points, and spherical equivalent such that the upper group had a slightly more myopic mean refraction than the medium group. Quintiles of 25[OH]D displayed a similar relationship with spherical equivalent and odds of myopia; the second quintile had a more positive spherical equivalent than the fifth quintile and the first, second, and third quintiles had lower odds of myopia compared to the fifth quintile. However, the overall associations between both outcome variables and 25[OH]D quintiles were not statistically significant.
It is interesting to note that while the differences were small, the highest 25[OH]D quintile and the upper 25[OH]D group had the most myopia. This is the reverse of what is reported in the literature, including from studies in older European adults and young adult Western Australians.18,21 Given that there was no significant association between continuous deseasonalized 25[OH]D and myopia or spherical equivalent, and that any associations with categorical 25[OH]D as a predictor are weak and in an unexpected direction, we do not believe that our findings suggest that higher 25[OH]D in adulthood may increase the risk of myopia.
Compared to the study by Williams et al.21 in European adults, our sample had a much higher mean 25[OH]D concentration (81 vs. 47 nmol/L), and a much smaller proportion of participants with a 25[OH]D concentration <50 nmol/L (7% vs. ∼50%). The participants in this study also had higher mean 25[OH]D levels, with a smaller proportion of individuals with 25[OH]D <50 nmol/L than 20-year-old Western Australians (81 vs. 71 nmol/L and 7% vs. 15%, respectively).18 Recent evidence suggests that an increased risk of diseases such as osteoporosis and bowel cancer occurs only in association with very low levels of serum 25[OH]D (<25 to 30 nmol/L).34,35 Therefore, it is possible that we were unable to detect an association between low 25[OH]D and myopia because of the relatively few participants with concentrations well below 50 nmol/L, who may have been most at risk of myopia in our study. However, the European Eye Study and a study in young adults in Northern Ireland did have high proportions of vitamin D-deficient participants and also found no independent association between 25[OH]D and myopia.21 Equally, it is possible that we detected a previously unreported association between more myopic refractive error and very high 25[OH]D level because of the larger proportion of participants with high 25[OH]D concentration in this study.
Another potential explanation for the lack of association between low 25[OH]D and myopia in our study is that those who spent less time outdoors and were consequently at higher risk of myopia during the myopia development period, typically between 5 and 25 years of age, have since changed their sun exposure behaviors and therefore increased their 25[OH]D level. Indeed, an example of this behavior change can be found within our results; those who reported being retired were more likely to have had an indoor occupation previously, were more myopic, and had higher 25[OH]D concentrations than nonretirees. Additionally, we identified two variables that were associated with both increased 25[OH]D and less myopic refractive error on univariate analysis: higher occupational sun exposure (determined from self-reported longest occupation) and lower hours spent sitting per day. Both these relationships are in keeping with evidence pointing toward a beneficial effect of spending time outside or increased vitamin D for preventing myopia.
Our results replicate the findings of Williams et al.21 in a population with higher 25(OH)D levels and using cycloplegic autorefraction and show that the relationship between 25(OH)D concentration and myopia in younger life that had been previously identified,11,14,18 is not apparent in later adulthood. If there is a causal association between low 25[OH]D and increased risk of myopia, the lack of an association in this analysis of older adults may suggest that 25[OH]D levels in this older adult population do not reflect those of their young childhood, when risk factors for myopia may be most influential.
Limitations of our study include the use of self-reported ocular to exclude those with a history of cataract, laser refractive surgery, or keratoconus because self-reported data may be unreliable. Additionally, participants were not directly asked if they had a history of keratoconus or refractive surgery, and so we could exclude only those who volunteered the information under “other medical history.” We also did not directly collect data on participant's time spent outdoors, which could be confounding this analysis. We did, however, have information on hours spent sitting and occupational sun exposure, which may act as proxies for time spent outdoors. Selection or collider bias may have been introduced by excluding individuals with a history of cataract diagnosis.36 This exclusion is necessary due to inherent changes in refractive error after cataract extraction, but those with myopia are at higher risk of this procedure and there is some evidence that high 25[OH]D concentration lowers an individual's risk of cataract diagnosis.37,38 Therefore, exclusion of these individuals may have induced bias in the analysis and could mask or artificially induce an association between 25[OH]D and myopia. The strengths of our study are its large sample size, population-based method, the use of cycloplegic autorefraction to measure refractive error, and the consistent method of 25[OH]D measurement.
Our study indicates that 25[OH]D is not associated with refractive error in a cross-sectional study of older Australian adults and replicates previous results in European adults.21 These findings do not refute the possibility that 25[OH]D may be involved in the development of myopia in younger age groups. Therefore, further investigations into the relationship between 25[OH]D and myopia should be directed at these younger populations.
Supplementary Material
Acknowledgments
The authors sincerely thank the managers and research assistants of the Busselton Healthy Ageing Study for their work collecting the data for this study and for their ongoing support. The authors wish to thank Lyn Fritschi and Ellie Darcey for their expert advice on the classification of self-reported occupations and the creation of an occupational sun exposure variable. The authors also thank Kashif Syed for his valuable work on coding the occupational sun exposure variable.
The Busselton Healthy Ageing Study was funded by a grant from the Office of Science and Department of Health of the Government of Western Australia (#G02016BHS), the City of Busselton, a bequest of the late Janet Elder, and private donors. G.L. receives financial support through an Australian Government Research Training Program Scholarship. R.M.L. is supported by a National Health and Medical Research Senior Research Fellowship and S.Y. by a CJ Martin Biomedical Fellowship.
Gareth Lingham (ORCID: 0000-0002-8957-0733), Seyhan Yazar (ORCID: 0000-0003-0994-6196), Robyn M. Lucas (ORCID: 0000-0003-2736-3541), John P. Walsh (ORCID: 0000-0002-1766-2612), Kun Zhu (ORCID: 0000-0002-8723-7574), Michael Hunter (ORCID: 0000-0001-6704-4815), David A. Mackey (ORCID: 0000-0001-7914-4709)
Disclosure: G. Lingham, None; S. Yazar, None; R.M. Lucas, None; J.P. Walsh, None; K. Zhu, None; M. Hunter, None; E.M. Lim, None; B.R. Cooke, None; D.A. Mackey, None
References
- 1.Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015;122:1489–1497. doi: 10.1016/j.ophtha.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 3.Saw SM, Zheng Y, Chay J, et al. The economic cost of myopia in Singapore. Invest Ophthalmol Vis Sci. 2013;54:5711–5711. doi: 10.1167/iovs.13-12795. [DOI] [PubMed] [Google Scholar]
- 4.Marcus MW, de Vries MM, Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94:678–684. doi: 10.1136/bjo.2009.157727. [DOI] [PubMed] [Google Scholar]
- 6.Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109:704–711. doi: 10.1016/s0161-6420(01)01024-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125:1239–1250. doi: 10.1016/j.ophtha.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 9.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119:2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Tideman JW, Polling JR, Voortman T, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol. 2016;31:491–499. doi: 10.1007/s10654-016-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci. 2011;88:377–382. doi: 10.1097/OPX.0b013e31820b0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan CW, Qian DJ, Saw SM. Time outdoors, blood vitamin D status and myopia: a review. Photochem Photobiol Sci. 2017;16:426–432. doi: 10.1039/c6pp00292g. [DOI] [PubMed] [Google Scholar]
- 14.Guggenheim JA, Williams C, Northstone K, et al. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Invest Ophthalmol Vis Sci. 2014;55:8550–8558. doi: 10.1167/iovs.14-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdette CQ, Camara JE, Nalin F, et al. Establishing an accuracy basis for the vitamin D external quality assessment scheme (DEQAS) J AOAC Int. 2017;100:1277–1287. doi: 10.5740/jaoacint.17-0306. [DOI] [PubMed] [Google Scholar]
- 16.Nowson CA, Mergerison C. Vitamin D intake and vitamin D status in Australians. Med J Aust. 2002;177:149–152. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi JA, Han K, Park YM, La TY. Low serum 25-Hydroxyvitamin D Is associated with myopia in Korean adolescents. Invest Ophthalmol Vis Sci. 2014;55:2041–2047. doi: 10.1167/IOVS.13-12853. [DOI] [PubMed] [Google Scholar]
- 18.Yazar S, Hewitt AW, Black LJ, et al. Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci. 2014;55:4552–4559. doi: 10.1167/iovs.14-14589. [DOI] [PubMed] [Google Scholar]
- 19.Kearney S, O'Donoghue L, Pourshahidi LK, et al. Conjunctival ultraviolet autofluorescence area, but not intensity, is associated with myopia. Clin Exp Optom. 2019;102:43–50. doi: 10.1111/cxo.12825. [DOI] [PubMed] [Google Scholar]
- 20.Cuellar-Partida G, Williams KM, Yazar S, et al. Genetically low vitamin D concentrations and myopic refractive error: a Mendelian randomization study. Int J Epidemiol. 2017;46:1882–1890. doi: 10.1093/ije/dyx068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams KM, Bentham GG, Young IS, et al. Association between myopia, ultraviolet B radiation exposure, serum vitamin D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a multicountry European study. JAMA Ophthalmol. 2017;135:47–53. doi: 10.1001/jamaophthalmol.2016.4752. [DOI] [PubMed] [Google Scholar]
- 22.Poopedi MA, Norris SA, Micklesfield LK, Pettifor JM. Does vitamin D status track through adolescence? Am J Clin Nutr. 2015;102:1025–1029. doi: 10.3945/ajcn.115.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176:615–621. doi: 10.1093/aje/kws141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 25.Zhu K, Oddy WH, Holt P, et al. Tracking of vitamin D status from childhood to early adulthood and its association with peak bone mass. Am J Clin Nutr. 2017;106:276–283. doi: 10.3945/ajcn.116.150524. [DOI] [PubMed] [Google Scholar]
- 26.James A, Hunter M, Straker L, et al. Rationale, design and methods for a community-based study of clustering and cumulative effects of chronic disease processes and their effects on ageing: the Busselton Healthy Ageing Study. BMC Public Health. 2013;13:1–12. doi: 10.1186/1471-2458-13-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Carey RN, Driscoll TR, Peters S, et al. Estimated prevalence of exposure to occupational carcinogens in Australia (2011–2012) Occup Environ Med. 2014;71:55–62. doi: 10.1136/oemed-2013-101651. [DOI] [PubMed] [Google Scholar]
- 29.Carey RN, Glass DC, Peters S, et al. Occupational exposure to solar radiation in Australia: who is exposed and what protection do they use? Aust N Z J Public Health. 2014;38:54–59. doi: 10.1111/1753-6405.12174. [DOI] [PubMed] [Google Scholar]
- 30.Lucas R, Ponsonby AL, McMichael A, et al. Observational analytic studies in multiple sclerosis: controlling bias through study design and conduct. The Australian Multicentre Study of Environment and Immune Function. Mult Scler. 2007;13:827–839. doi: 10.1177/1352458507077174. [DOI] [PubMed] [Google Scholar]
- 31.Cooke DJ, Cooke BR, Bell DA, Vasikaran SD, Glendenning P. 25-Hydroxyvitamin D C3-epimer is universally present in neonatal Western Australian samples but is unlikely to contribute to diagnostic misclassification. Ann Clin Biochem. 2016;53:593–598. doi: 10.1177/0004563215625693. [DOI] [PubMed] [Google Scholar]
- 32.van der Mei IAF, Blizzard L, Ponsonby A-L, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15:1538–1544. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Vitamin D deficiency. N Eng J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 34.Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3:784–794. doi: 10.1039/c2fo10262e. [DOI] [PubMed] [Google Scholar]
- 35.Scragg R. Emerging evidence of thresholds for beneficial effects from vitamin D supplementation. Nutrients. 2018;10(5):561. doi: 10.3390/nu10050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39:417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanthan GL, Mitchell P, Rochtchina E, Cumming RG, Wang JJ. Myopia and the long-term incidence of cataract and cataract surgery: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2014;42:347–353. doi: 10.1111/ceo.12206. [DOI] [PubMed] [Google Scholar]
- 38.Park S, Choi NK. Serum 25-hydroxyvitamin D and Age-Related Cataract. Ophthalmic Epidemiol. 2017;24:281–286. doi: 10.1080/09286586.2017.1281427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.