Abstract
The etiology of diabetes and associated metabolic derailments is a complex process that relies on crosstalk between metabolically active tissues. Dysregulation of secreted factors and metabolites from islets, adipose tissue, liver, and skeletal muscle contributes to the overall progression of diabetes and metabolic syndrome. Extracellular vesicles (EVs) are circulating nanovesicles secreted by most cell types and are comprised of bioactive cargoes that are horizontally transferred to targeted cells/tissues. Accumulating evidence from the past decade implicates the role of EVs as mediators of islet cell dysfunction, inflammation, insulin resistance, and other metabolic consequences associated with diabetes. This review covers a broad spectrum of basic EV biology (i.e., biogenesis, secretion, and uptake), including a comprehensive investigation of the emerging role of EVs in β-cell autocrine/paracrine interactions and the multidirectional crosstalk in metabolically active tissues. Understanding the utility of this novel means of intercellular communication could impart insight into the development of new treatment regimens and biomarker detection to treat diabetes.
Diabetes mellitus is one of the largest global epidemics to plague developing nations. With a staggering statistic of 422 million adults with this disease in 2014, the World Health Organization reported a doubling of global diabetes prevalence since 1980 (8.5% of adults in 2014) (1). In the United States alone, the incidences of diabetes in the adult population (diagnosed and undiagnosed) are thought to rise to 21% in 2050 (2). It is imperative that a better understanding of the pathophysiology of diabetes is needed to design and implement novel therapeutics for the prevention and treatment of this disease.
Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are multifactorial diseases that manifest due to loss of either physical or functional β-cell mass through autoimmune destruction (type 1) or a progressive decline in insulin secretion (insulin deficiency) typically associated with other metabolic abnormalities (type 2) (3–5). A concerted effort has been made to define these metabolic alterations, now termed “metabolic syndrome” (MetS), which is mainly comprised of central obesity, insulin resistance, hypertension, and hyperlipidemia (3). To maintain metabolic homeostasis, crosstalk between metabolically active tissues through the secretion of hormones and metabolites is necessary. However, a derailment in normal secretion and production of these metabolic mediators has been shown to be attributed to the progression of diabetes and other related metabolic diseases (6). Intercellular communication via direct cell–cell contact or paracrine/endocrine effects of secreted factors are necessary for both the maintenance of cellular homeostasis as well as a contributing factor to various disease pathologies (6).
Extracellular vesicles (EVs) are considered another major mediator of cellular crosstalk owing to their inherent ability to transfer important bioactive cargoes to targeted cells/tissues. Once described as the “trashcans of the cell,” the relevance of EVs in the pathophysiology of various disease states has changed current research paradigms, particularly in the past decade. EVs are secreted by a variety of different cell types and can be isolated from multiple biological fluid sources such as blood, saliva, urine, and breast milk. EVs have been shown to elicit a variety of functional outcomes through their pleiotropic effects on various biological processes such as tumorigenesis, immune modulation, neurodegeneration, and even in the maintenance of cellular homeostasis (7).
EVs in a broad sense are categorized by three distinct subpopulations of nanovesicles: exosomes (30 to 150 nm), microvesicles (100 to 1000 nm), and apoptotic bodies (1000 to 5000 nm). The distinction between the three classes lies not only in their size, but also in their content, mode of generation, and mechanism of release (8, 9) (Table 1). The bioactive cargoes within EVs consist of a heterogeneous subset of DNAs, RNAs (mRNAs and small regulatory RNAs), proteins, and lipids that are often reflective of the tissue of origin from which the EVs are formed (10). Whereas apoptotic bodies are known to bleb off of the plasma membrane of apoptotic cells (11), microvesicles and exosomes are released endogenously by healthy cells through vesicle secretion off the plasma membrane (12). In this review, the fundamentals of EV biogenesis and secretion with a focus on a comprehensive overview of existing literature on the role of EVs in the pathogenesis of T2DM and associated metabolic syndrome are introduced. With this insight, the current challenges of EV research and future perspectives in this field are highlighted. Understanding the relevancy of EVs in the progression of T2DM and related comorbidities could afford novel disease-related biomarkers and/or nano-based therapies for targeted cell deliveries.
Table 1.
Characteristics of EVs
| Characteristic | Exosomes | Microvesicles | Apoptotic Bodies |
|---|---|---|---|
| Size, nm | 30–150 | 100–1000 | 1000–5000 |
| Site of origin | MVBs | Plasma membrane | Plasma membrane |
| Mechanism of release | MVB fusion to plasma membrane and exocytosis | Plasma membrane budding | Plasma membrane budding (apoptotic cells) |
| Composition | Genomic DNA (double and single stranded), mitochondrial DNA, mRNA, small RNA (miRNA, tRNA, rRNA, long and short noncoding RNA), protein, lipids | mRNA, small RNA, proteins, lipids | Nuclear fractions, cell organelles |
| Common markers | ESCRT proteins (ALIX, TSG101), tetraspanins (CD63, CD81, CD9), flotillin-1 | CD40 ligand, selectins, integrins | Annexin V, phosphatidylserine |
EV Biogenesis and Secretion
The mechanism of EV formation and release differs among the three subtypes, as apoptotic bodies are known to bleb off the plasma membrane and microvesicles are known to form through mechanisms involving the redistribution of membrane lipids and contractility of actin-myosin–based machinery (13, 14). Alternatively, exosomes, which are derived from the endosomal pathway, are formed in intraluminal vesicles (ILVs) within multivesicular bodies (MVBs). This process requires the reorganization of the endosome membrane, making it highly enriched with tetraspanins, particularly CD9 and CD63 (15), and the recruitment of the endosomal sorting complex required for transport (ESCRT) machinery (16–19). The recruitment of ESCRT 0, I, and II is critical for intraluminal membrane budding through binding of ESCRT I to ubiquitinated proteins, which then activates ESCRT II on the endosomes. Programmed cell death 6 interacting protein (PDCD6IP or ALIX) interacts with tumor susceptibility gene 101 (TSG101) to recruit ESCRT III, which is essential for the final steps of exosomal release (20). Additionally, ESCRT-independent pathways have been described and may function in concert with the “classical” ESCRT-dependent pathway described above. This additional mechanism of exosomal formation requires ceramide-rich ILVs, which can induce endosome membrane curvature and eventual secretion of exosomes (21). Following the formation of exosomes in the ILVs, the next process requires the fusion of the MVB to the plasma membrane and the subsequent release of exosomes into the extracellular space. This process requires the aid of specific Rab proteins necessary for the docking and fusion of the MVB to the plasma membrane. In particular, Rab27a, Rab27b, and Rab11 have all been shown to have differing roles in the exosomal pathway; however, collectively they were found to be necessary for proper exosomal secretion (22, 23). Conversely, other literature suggests that exosomal release can occur independently of the Rab GTPases (24, 25). Therefore, further investigation into the molecular mechanisms behind the process of exosome release is needed.
EV Content
The heterogeneous cellular cargoes within EVs differ based on the parental cell it was secreted from, the mode of biogenesis, and/or various pathological states. In a general sense, EVs harbor a diverse cellular milieu consisting of single- and double-stranded DNAs, mitochondrial DNA, mRNAs, and small RNAs (e.g., miRNAs, tRNAs, rRNAs, long noncoding RNAs), various proteins, and lipids. Public databases such as ExoCarta and Vesiclepedia contain comprehensive lipidomic, transcriptomic, and proteomic analyses of EVs from a variety of different cell types (26, 27). Although comprehensive studies on the proteome of EVs show differing protein content due to the cell type and inconsistencies in isolation procedures, there remains a consistent set of proteins found in EVs that are related to biogenesis. The most common proteins found in EVs (specifically exosomes) are the tetraspanins (CD63, CD9, and CD81) and ESCRT pathway components TSG101 and ALIX. These common vesicle-specific proteins are often used as identification markers for EVs and to assess the purity of EV preparations. Additionally, EVs have been shown to be enriched with other protein markers such as major histocompatibility complex class I and II molecules, cytoskeletal proteins, integrins, and heat shock proteins (10).
The identification of diverse genetic material contained within EVs has been extensively reported in recent years owing to substantial technical advances in the detection of small RNAs. The advancement of high-throughput next generation sequencing has allowed the detection of mRNAs, miRNAs, tRNAs, rRNAs, long and short noncoding RNAs, and more (28, 29). Although intact mRNAs are found in EVs, most RNAs tend to be small fragments of ∼200 nucleotides (miRNA size or smaller) (30). There are over 1000 different miRNAs that have been collectively reported in EVs by hundreds of independent studies documented by databases such as Vesiclepedia (26, 31). To avoid degradation, miRNAs have been shown to bind either with RNA-binding proteins such as Argonaute 2 (Ago2) (32) and lipoproteins. EV miRNAs have been shown to be bioactive through translational inhibition of the mRNA of target cells but may also function through mechanisms independent of this process (30, 33). Several studies have reported that the RNA content of EVs is different than the content of the parental cells from which they were derived (31, 34, 35). However, the diversity of RNA content among differing EV populations still remains unclear. The utility in understanding the distribution and selective loading of miRNAs into EVs in a given population could have the potential to change current paradigms for treatment strategies and biomarker discoveries for a myriad of diseases.
The presence of both single- and double-stranded DNA as well as mitochondrial DNA in EVs has been demonstrated in several reports (36–38). As described with RNAs, these DNA cargoes are also biologically active and are present in different cell types. Perhaps one of the most striking aspects of DNA in EVs is that the DNA can often reflect the mutational status of the parent cell. For example, exosomes from pancreatic cancer (PC) cell lines and serum were shown to harbor double-stranded genomic DNA that contained mutations for KRAS and p53 (38). In another study, the full mitochondrial genome was found in cancer-associated fibroblast–derived EVs and EVs from patients with hormonal therapy–resistant breast cancer (37). The authors proposed that the horizontal transfer of EV mitochondrial DNA could act as an oncogenic signal to promote the survival of these cancer cells (37).
Mechanisms of EV Uptake
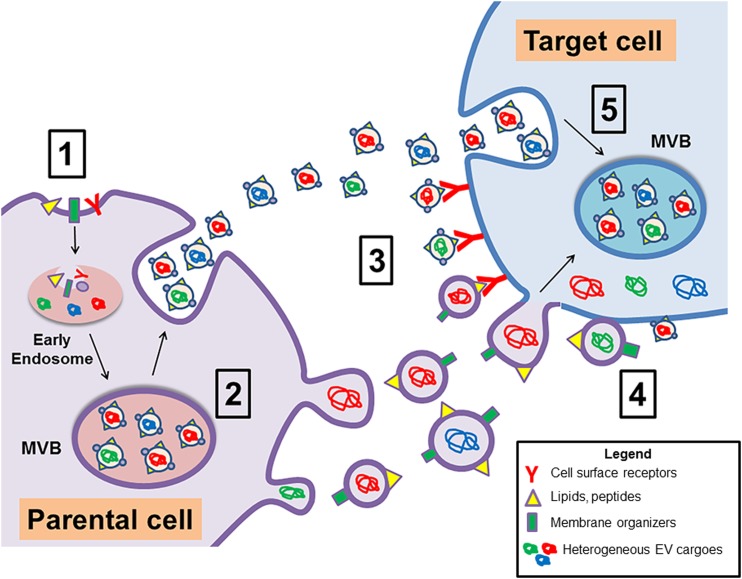
Once EVs are formed and secreted from the parental cell they can interact with a target cell in several proposed manners. The mechanisms of EV cargo delivery incorporate the endocytic pathway and include clathrin-mediated endocytosis, caveolin-dependent endocytosis, macropinocytosis, and phagocytosis (39). The primary mode of EV internalization is currently up for debate, as several factors can potentially influence this process. The cell type, expression of target cell surface proteins, EV surface ligands, and physiologic condition of the cell all contribute to the overall mechanism of EV internalization (Fig. 1). As an example of the importance of EV-specific ligands in direct communication with target cells, one study showed that dendritic cell–derived exosomes carried major histocompatibility complex II, and these exosomes could directly target interacting T cells (40). Direct fusion of EVs to the plasma membrane is an additional mode of internalization, but this is less common than endocytic pathway–related mechanisms (39). Once endocytosis occurs, EVs must escape the endogenous lysosomal degradative pathway through direct fusion to the early endosome and subsequent reformation and secretion via the MVB membrane fusion pathway (20). The complexity of EV target cell interactions still remains despite efforts by several groups to understand this process. In vitro and in vivo applications have exploited the manipulation of EVs to allow for tracking into target cells. For example, one group demonstrated that the fusion of EGFP to a palmitoylation sequence localizes to the plasma membrane and consequently into EVs (41). EV uptake and cargo delivery was visualized both in vitro in cancer cells and in vivo in mice using this reporter system with high-resolution microscopy to track vesicle formation (41). Several additional elegant approaches have been demonstrated in the tracking of vesicle content delivery to recipient cells (42, 43); however, detailed mechanisms of this process still remain, with a complete understanding of the overall impact of disease pathologies influencing this process.
Figure 1.
Mechanisms of EV release and cargo transfer to target cells. The schematic depicts the formation and mechanisms of secretion of exosomes and microvesicles (apoptotic bodies are not shown). (1) Exosomes are formed through the inward reverse budding of the limiting membrane and subsequent fusion to the early endosome. The early endosome fuses to the MVB, which is the site for newly formed exosomes. (2) The MVB will fuse to the plasma membrane and release the exosomes to target a recipient cell, whereas microvesicles will bleb off of the plasma membrane. (3) Once secreted into systemic circulation or interstitial fluid, EVs can interact with a target cell through multiple mechanisms. EV surface ligands can directly interact with cell surface membrane receptors to activate cell signaling pathways; (4) direct fusion of EVs with the plasma membrane to release cargoes into the cytosol; (5) or through mechanisms of endocytosis such as clathrin-mediated endocytosis, caveolin-dependent endocytosis, macropinocytosis, and phagocytosis.
Implications of EVs in Diabetes and Associated MetS
Diabetes is a multifactorial disease consisting of several metabolic disorders characterized by defective insulin release and glucose homeostasis. Complications arising from this disease have vast consequences in the form of heart disease, stroke, diabetic retinopathy, kidney disease, and macrovascular issues. The need for early detection biomarkers and novel therapeutic strategies is critical for the prevention and treatment of the disease. EVs have recently emerged as cellular conduits due to their distinct cargo selection in normal physiological conditions and in a variety of disease pathologies. Several studies have reported increases in EV production and secretion in individuals with T2DM and/or associated MetS (44–48). In a recent study, EV secretion was assessed in a large cross-sectional and longitudinal cohort consisting of prediabetic, diabetic, and euglycemic controls. The results showed that diabetic patients secreted more EVs and that insulin resistance was a potential cause for this increase (44). The observation of increased EV production suggests, in part, the relevancy of EVs in the pathophysiology of diabetes and associated MetS. In the next sections, additional contributions of EV work that further solidify their role in disease pathology are highlighted.
Paracrine Effects of EVs on Islets
Pancreatic islets are spherical micro-organs consisting of five hormone-secreting cells. The most highly abundant of the endocrine cells are the insulin-secreting β-cells, then the glucagon-secreting α-cells, and lastly the somatostatin-secreting δ-cells. Two additional islet cell types exist: the pancreatic polypeptide cells and ghrelin-secreting ε-cells. Collectively, these cell types sense nutrients (i.e., glucose) and respond accordingly by secreting their respective hormones into systemic circulation (49). Dysfunctional insulin and glucagon secretion coupled with peripheral insulin resistance are hallmark features of T2DM. One of the mechanisms that regulates proper insulin secretion is through paracrine/autocrine interactions within the cells of the islet (50). Paracrine interactions are necessary for each cell type of the islet to recognize whether normal cellular homeostasis is perturbed in neighboring cells. These interactions entail an exchange of intraislet signaling neurotransmitters, hormones/peptides, and most recently the secretion and uptake of EVs. The emerging role of EVs as intraislet cellular conduits has been reported by several groups. In one study, EVs secreted from human islets were primarily smaller vesicles, and the content of these EVs was reflective of islet cells. Most EVs characterized from human islets were found to be of β-cell origin, as proteins such as insulin, C-peptide, and GLP1R were present, whereas α-cell and endothelial cell (EC) markers such as endothelial nitric oxide synthase and glucagon were less expressed. Additionally, these smaller vesicles were shown to contain distinct miRNAs that are involved in insulin secretion, angiogenesis, and normal β-cell function (51). Paracrine interactions between EVs from other tissue types and β-cells have also been reported. PC is often associated with the development of new-onset diabetes. This type of diabetes is distinct in that it is likely formed through secretion of cancer-specific mediators that can alter normal glucose homeostasis. In this study, the authors found that PC EVs contain the polypeptide adrenomedullin and that the delivery of adrenomedullin to β-cells reduced insulin secretion. This bioactive transfer of adrenomedullin to β-cells was shown to increase endoplasmic reticulum stress and reactive oxygen species production and to induce a failure of the unfolded protein response (52).
Amyloid deposits are comprised of a significant amount of islet amyloid polypeptide (IAPP), which is found in the islets of T2DM patients. Intracellular IAPP accumulation in β-cells promotes endoplasmic reticulum and oxidative stress, thus impairing β-cell function and survival (53). In a recent report, it was found that EVs derived from normal healthy islets have the ability to reduce amyloid formation in a concentration-dependent manner; however, this effect was not found in the presence of T2DM islet or serum EVs (54). The ability of normal healthy islet EVs to suppress IAPP formation was corroborated by lipid composition analysis of healthy vs T2DM EVs. These results showed increased lipid membrane components involved in lipid raft formation and membrane fluidity in healthy islet EVs, which are known to inhibit IAPP formation (54). Further investigation is warranted to decipher the molecular mechanism and key protein/lipid modulators of this process.
The destruction of β-cells in both T1DM and T2DM is associated with immune cell infiltration and proinflammatory cytokine production (55–59). IL-1β, TNFα, and interferon γ are known inducers of apoptosis in β-cells (57). Interestingly, in situ perfusion of the rat pancreas with IL-1β created membrane blebbing, suggesting the role of proinflammatory cytokines in the formation and release of EVs (60). Proteomic analysis of EVs released by the β-cell line NHI 6F Tu28 in the presence and absence of cytokines showed alterations in protein content between groups, particularly an upregulation of TNFα signaling molecules. This body of work suggests the potential role of EVs in the inflammatory response (61). In a recent article by Guay et al. (62), autocrine signaling was demonstrated between EVs released from β-cells containing miRNAs, which were shown to be transferred to other nearby β-cells. Additionally, cytokine exposure of Min6B1 cells altered not only the release of miRNAs but had an impact on overall survival of recipient β-cells. Although several studies have provided evidence of the apoptotic effects of high-dose cytokines on cells, low-dose cytokine treatment has been shown to impart beneficial effects on the β-cell, potentially mediated through EVs. In one study, the authors demonstrated that low-dose cytokine treatment of INS-1 cells protected the cells from apoptosis through EVs containing neutral ceramidase, a key regulator of cytokine-induced effects on apoptotic signaling. Whereas high-dose cytokine stimulation inhibited neutral ceramidase release (63).
Emerging evidence has implicated EVs in the immunoregulatory response of the islet, particularly in the pathogenesis of T1DM. EVs isolated from Min6 cells were found to stimulate T cell proliferation in addition to inducing proinflammatory cytokine production in nonobese diabetic mice (64). Additionally, immunostimulated EVs isolated from islet-derived mesenchymal stem cells can activate autoreactive B and T cells in nonobese diabetic mice. The addition of these EVs promoted the expansion of autoreactive T cells subsequently leading to T-cell–mediated islet dysfunction (65). In another study, β-cell autoantigens GAD65, IA-2, and proinsulin/insulin were found in EVs isolated from both rat and human islets and were readily taken up by dendritic cells. Proinflammatory cytokines induced endoplasmic reticulum stress, which increased EV secretion by β-cells and subsequent packaging of immunostimulatory chaperones, thereby enhancing the ability of EVs to stimulate antigen-presenting cells (66).
EVs in Adipose Tissue
One of the main drivers of T2DM is obesity-associated insulin resistance in tissues such as the liver, skeletal muscle, and white adipose tissue (AT) coupled with insufficient insulin secretion and subsequent β-cell dysfunction. The balance between energy intake and expenditure is perturbed in obesity, leading to an increase in fat accumulation in multiple organs. The discovery of secretory adipokines from AT as a consequence of excess adipocyte accumulation has shed light on a more impactful role of cellular secreted factors in metabolic dysfunction. Several studies have identified EVs in obesity; however, in-depth functional studies and a link to disease pathology are needed. The amount of EVs in circulation from individuals with obesity, MetS, and diabetes has been extensively investigated (45–48). Studies done using a rat model of obesity (high-fat diet fed) showed significantly elevated EV counts compared with chow-fed rats. Elevated EVs were shown to originate from several cell types such as leukocytes, ECs, and platelets (67). Obese individuals tend to show an increase in circulating plasma EVs compared with healthy, lean controls, which was also found to be independent of MetS (48). Alternatively, weight loss decreased the overall amount of EVs produced in obese patients who underwent sleeve gastrectomy (68).
In addition to studying the production of EVs from AT, substantial strides have been made toward identifying the content of AT-derived EVs. In one study, EVs were extensively studied both in vitro using differentiated human adipocytes and ex vivo from human AT explants. Analyses of EV content from both of these sources revealed a distinct adipocyte-specific protein signature in addition to increased expression of immunomodulatory proteins such as MIF, TNFα, MCSF, and RBP-4 (69). Similarly, human AT EVs derived from omental AT compared with subcutaneous AT (SAT) showed an increase in immune factors MCP-1, IL-6, and MIF in hepatocytes, which may contribute to overall systemic insulin resistance (70). miRNA profiling of EVs from human visceral AT (VAT) and SAT from lean vs obese adolescents showed downregulation of miRNAs associated with TGF-β and Wnt signaling pathways in the obese VAT EVs only (71). Interestingly, weight loss from gastric bypass surgery was found to alter circulating AT EV miRNA profiles, correlating with improvements in both insulin resistance and glucose homeostasis in these patients (72). Proteomic profiling of AT EVs from obese diabetic and obese nondiabetic rats revealed 128 upregulated and 72 downregulated proteins found in the obese diabetic rat EVs (73). Collectively, these studies provide evidence to support the role of EVs in obesity-related diseases.
This notion is also supported by functional studies of AT EVs in insulin resistance and the inflammatory response. AT crosstalk with macrophages has been shown to be mediated by AT-derived EVs. SAT and VAT EVs were found to induce monocyte-to-macrophage differentiation with similar proinflammatory and anti-inflammatory secretory profiles of AT macrophages (ATMs). Additionally, insulin signaling was perturbed in human adipocytes in the presence of cell culture supernatants of macrophages that were prestimulated with AT EVs (69). In another study, EVs from ob/ob mice (obEVs) activated macrophages through TLR4 signaling mediated by RBP4 expression in these EVs. Proinflammatory cytokine stimulation was evident in macrophages cultured with obEVs, thereby allowing for macrophage infiltration into AT and the liver to induce insulin resistance. This effect was mitigated in TLR4 knockout mice injected with obEVs (74). In a recent report, the authors found that EVs secreted from ATMs from obese mice harbored miRNAs that could promote insulin resistance and glucose intolerance in insulin-targeting cells. Interestingly, treatment using ATM EVs from lean mice improved both glucose tolerance and insulin sensitivity in obese mice. These effects were shown to be mediated by obese ATM EVs containing the miRNA miR-155, which was shown to inhibit both insulin signaling and glucose tolerance via PPARγ suppression (75).
Understanding the global ramifications of AT-derived EVs on other tissues may impart clues to the overall pathophysiology of MetS. To understand the pathogenesis of nonalcoholic fatty liver disease (NAFLD) in obese patients, VAT EVs were shown to integrate into HepG2 and hepatic stellate cell lines and alter extracellular matrix regulatory molecules TIMP-1 and integrin αvβ5 while downregulating MMP-7 and PAI-1. These results suggest that increased expression of TIMP-1 and a decrease in MMP-7 could possibly promote extracellular matrix production, leading to a profibrotic state in liver cells mediated by TGF-β pathway activation. Further evidence is needed to solidify the role of VAT EVs in the pathogenesis of NAFLD (76). In a recent report by Crewe et al. (77), EVs isolated from white AT were shown to contain caveolin 1, which was found to be trafficked to adipocytes from caveolin 1–containing endothelial-derived EVs. Subsequently, AT was shown to secrete EVs back to ECs. This exchange of cellular cargo was found to be dependent on the nutrient state of the environment, as fasting/refeeding stimulated EV secretion from ECs, whereas mouse models of diet-induced obesity did not show this response in isolated ECs (77).
EVs in Skeletal Muscle
Skeletal muscle insulin resistance is thought to be one of the initiating factors contributing to the progression of T2DM preceding both hyperglycemia and β-cell dysfunction (78). Skeletal muscle has been proposed to be a secretory organ in which muscle-specific cytokines and peptides termed “myokines” are released in a hormone-like manner to affect distal organs, or through autocrine/paracrine interactions within muscle (79–83). Although there is emerging evidence on skeletal muscle crosstalk between various tissues, the field remains relatively unknown; however, recent work implicates EVs in this process. In one study, mice on a high-palmitate (HP) diet showed a significant increase in EV secretion from skeletal muscle compared with controls. These EVs were able to induce proliferation and alter genes in the cell cycle and in muscle differentiation (84).
Owing to the critical role that skeletal muscle plays in normal metabolic function and energy homeostasis, understanding the role of skeletal muscle EV crosstalk with other metabolically active tissues could reveal novel mechanisms of disease pathology. In the context of diabetes, it was found through in vivo and in vitro studies that skeletal muscle EVs could be readily taken up by pancreatic β-cells (85). To test whether insulin resistance could affect the function of skeletal muscle EVs on the β-cell, mice were fed either a chow or HP diet for 16 weeks. The quadriceps were excised and EVs were isolated from both sets of conditions. Culturing of HP EVs on MIN6 cells increased 460 mRNA transcripts associated with membrane receptors, various transcription factors, and the immune response. Specifically, miRNA profiling of HP EVs showed that insulin resistance in skeletal muscle was associated with the increased release of the miRNA miR-16 as compared with chow diet–fed EVs (85). miR-16 has been shown to regulate the cell proliferation gene Ptch1, a receptor found in the sonic hedgehog pathway and a known regulator of insulin transcription and secretion (85). Taken together, these studies suggest the importance of skeletal muscle EV crosstalk in the progression of diabetes, although further exploration is needed to fully understand the mechanisms that govern this process.
EVs in the Liver
The global surge in diabetes and obesity prevalence has been paralleled by a rise in related metabolic complications. NAFLD and its association with diabetes is a reciprocal relationship in which both serve as mediators of progression for the other. Diabetes has been shown to promote inflammation and liver fibrosis, thus promoting the acceleration of NAFLD to nonalcoholic steatohepatitis (NASH) (86–88). Almost all cell types in the liver (e.g., hepatocytes, cholangiocytes, hepatic stellate cells) secrete EVs and are a target of systemic EVs from other tissues (89). Similar to AT and skeletal muscle EVs, liver EVs are responsive to metabolic stressors such as lipotoxicity (90–92). In one study, culturing hepatocytes in excess free fatty acids stimulated the release of EVs that could promote angiogenesis, which was Vanin-1 (VNN1)–dependent. RNA interference against VNN1 or genetic ablation of caspase-3 prevented angiogenesis formation in the liver and reduced the proangiogenic effects of liver EVs (90).
Similarly, primary hepatocytes and Huh7 cells released significantly more EVs in the presence of palmitate or lysophosphatidylcholine compared with control (untreated) cells. EVs from primary hepatocytes of a diet-induced model of NASH induced an inflammatory phenotype in bone marrow–derived macrophages in vitro as noted by an increase in proinflammatory cytokine production of IL-1β and IL-6. This effect was shown to be mediated by DR5 signaling, as NASH-induced mice treated with the Rho-associated, coiled-coil containing protein kinase 1 (ROCK1) inhibitor fasudil showed a reduction in serum EVs correlating to a decrease in liver fibrosis and hepatic inflammation (91). Additionally, palmitic acid treatment in hepatocytes not only increased the production of EVs as previously reported, but the miRNA profiles of the EVs changed and favored a more than fivefold increase in NAFLD- and NASH-related miRNAs (miR-24, miR-19b, miR-34a, miR-122, and miR-192) (92). Moreover, the addition of palmitic acid–treated hepatocyte EVs on the human hepatic stellate cell line LX-2 increased the expression of profibrotic genes in comparison with control hepatocyte EVs (92). In total, the work presented provides evidence for the role of hepatic EVs in liver disorders; however, further investigation into the mechanisms of liver EV communication with other metabolically active tissues is needed.
Future Perspectives and Conclusions
The knowledge base in the field of EV biology has exponentially increased during the past two decades as a growing understanding of the technical challenges in the field coupled with improvements in EV isolation, characterization, and functional assessment are evident. Although considerable efforts have been made in understanding the basics of EV biology (i.e., formation, uptake, and secretion), there remains a large question of their relevance in the pathophysiology of disease. As outlined in this review, preliminary contributions made to the EV biology field in the context of T2DM and MetS are highlighted. These bodies of work impart insight into a novel mechanism of intracellular communication and metabolic tissue crosstalk, which could aid in the development of treatment regimens for diabetes. EVs are well suited as delivery carriers due to their ease of uptake, low rejection rate, stability in circulation, and their ability to be re-engineered to carry selected cargoes (e.g., therapeutic drugs, proteins, nucleic acids) (93). Additionally, it has been suggested that cell type–specific EVs derived from stem cells can improve diabetes outcomes (94–96). Although EVs hold much promise in way of therapy, there remains a gap in knowledge in the overall importance of EVs in β-cell dysfunction and metabolic tissue crosstalk in disease progression. Identification of the mechanisms that govern these processes through EV-based means will ultimately give rise to valuable insight into preventative and therapeutic regimes to combat T2DM and the associated MetS.
Acknowledgments
The author thanks Dr. Aleksey Matveyenko for assistance in reviewing this manuscript.
Financial Support: This work was supported by National Institutes of Health Grant T32-HL 105355.
Disclosure Summary: The author has nothing to disclose.
Glossary
Abbreviations:
- AT
adipose tissue
- ATM
adipose tissue macrophage
- EC
endothelial cell
- ESCRT
endosomal sorting complex required for transport
- EV
extracellular vesicle
- HP
high-palmitate
- IAPP
islet amyloid polypeptide
- ILV
intraluminal vesicle
- MetS
metabolic syndrome
- MVB
multivesicular body
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- obEV
extracellular vesicle from ob/ob mice
- PC
pancreatic cancer
- SAT
subcutaneous adipose tissue
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TSG101
tumor susceptibility gene 101
- VAT
visceral adipose tissue
References
- 1. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12(10):616–622. [DOI] [PubMed] [Google Scholar]
- 2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group . The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. [DOI] [PubMed] [Google Scholar]
- 4. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 5. Matveyenko AV, Butler PC. Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(Suppl 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3–18. [DOI] [PubMed] [Google Scholar]
- 7. Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27(1):31–39. [DOI] [PubMed] [Google Scholar]
- 8. Meckes DG Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85(24):12844–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. [DOI] [PubMed] [Google Scholar]
- 11. Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104(9):2761–2766. [DOI] [PubMed] [Google Scholar]
- 12. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 13. Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528–537. [DOI] [PubMed] [Google Scholar]
- 14. Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315(9):1584–1592. [DOI] [PubMed] [Google Scholar]
- 16. Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. [DOI] [PubMed] [Google Scholar]
- 17. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adell MA, Vogel GF, Pakdel M, Müller M, Lindner H, Hess MW, Teis D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J Cell Biol. 2014;205(1):33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morvan J, Rinaldi B, Friant S. Pkh1/2-dependent phosphorylation of Vps27 regulates ESCRT-I recruitment to endosomes. Mol Biol Cell. 2012;23(20):4054–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. [DOI] [PubMed] [Google Scholar]
- 22. Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. [DOI] [PubMed] [Google Scholar]
- 23. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. [DOI] [PubMed] [Google Scholar]
- 24. Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila-Flores A, Merida I, Izquierdo M. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011;18(7):1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fader CM, Sánchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793(12):1901–1916. [DOI] [PubMed] [Google Scholar]
- 26. Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a Web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nolte-’t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, ’t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crescitelli R, Lässer C, Szabó TG, Kittel A, Eldh M, Dianzani I, Buzás EI, Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2(1):20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct. 2013;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 32. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108(12):5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–E2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafé M, Cricca M, Gasparre G, Lyden D, Bromberg J. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer [published correction appears in Proc Natl Acad Sci USA. 2017;114(47):E10255] Proc Natl Acad Sci USA. 2017;114(43):E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. [DOI] [PubMed] [Google Scholar]
- 41. Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6(1):7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Höglinger G, Sültmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation [published correction appears in PLoS Biol. 2018;16(3):e1002623] PLoS Biol. 2014;12(6):e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, Wurdinger T, Pegtel DM, van Rheenen J. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, Egan J, Becker KG, Mattson MP, Ejiogu N, Evans MK. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67(11):2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(12):E2271–E2276. [DOI] [PubMed] [Google Scholar]
- 46. Santovito D, De Nardis V, Marcantonio P, Mandolini C, Paganelli C, Vitale E, Buttitta F, Bucci M, Mezzetti A, Consoli A, Cipollone F. Plasma exosome microRNA profiling unravels a new potential modulator of adiponectin pathway in diabetes: effect of glycemic control. J Clin Endocrinol Metab. 2014;99(9):E1681–E1685. [DOI] [PubMed] [Google Scholar]
- 47. Eguchi A, Lazic M, Armando AM, Phillips SA, Katebian R, Maraka S, Quehenberger O, Sears DD, Feldstein AE. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94(11):1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L, Alessi MC, Msika S, de Prost D. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring). 2013;21(11):2236–2243. [DOI] [PubMed] [Google Scholar]
- 49. Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes Metab. 2017;19(Suppl 1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rutter GA, Hodson DJ. Minireview: intraislet regulation of insulin secretion in humans. Mol Endocrinol. 2013;27(12):1984–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Figliolini F, Cantaluppi V, De Lena M, Beltramo S, Romagnoli R, Salizzoni M, Melzi R, Nano R, Piemonti L, Tetta C, Biancone L, Camussi G. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS One. 2014;9(7):e102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Javeed N, Sagar G, Dutta SK, Smyrk TC, Lau JS, Bhattacharya S, Truty M, Petersen GM, Kaufman RJ, Chari ST, Mukhopadhyay D. Pancreatic cancer-derived exosomes cause paraneoplastic β-cell dysfunction. Clin Cancer Res. 2015;21(7):1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795–826. [DOI] [PubMed] [Google Scholar]
- 54. Ribeiro D, Horvath I, Heath N, Hicks R, Forslöw A, Wittung-Stafshede P. Extracellular vesicles from human pancreatic islets suppress human islet amyloid polypeptide amyloid formation. Proc Natl Acad Sci USA. 2017;114(42):11127–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–S113. [DOI] [PubMed] [Google Scholar]
- 56. Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183(7):4432–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eizirik DL, Mandrup-Poulsen T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44(12):2115–2133. [DOI] [PubMed] [Google Scholar]
- 58. Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55(8):1139–1149. [DOI] [PubMed] [Google Scholar]
- 59. Rabinovitch A, Suarez-Pinzon WL. Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Rev Endocr Metab Disord. 2003;4(3):291–299. [DOI] [PubMed] [Google Scholar]
- 60. Wogensen LD, Kolb-Bachofen V, Christensen P, Dinarello CA, Mandrup-Poulsen T, Martin S, Nerup J. Functional and morphological effects of interleukin-1 beta on the perfused rat pancreas. Diabetologia. 1990;33(1):15–23. [DOI] [PubMed] [Google Scholar]
- 61. Palmisano G, Jensen SS, Le Bihan MC, Lainé J, McGuire JN, Pociot F, Larsen MR. Characterization of membrane-shed microvesicles from cytokine-stimulated β-cells using proteomics strategies. Mol Cell Proteomics. 2012;11(8):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guay C, Menoud V, Rome S, Regazzi R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal. 2015;13(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu Q, Kang J, Miao H, Feng Y, Xiao L, Hu Z, Liao DF, Huang Y, Jin J, He S. Low-dose cytokine-induced neutral ceramidase secretion from INS-1 cells via exosomes and its anti-apoptotic effect. FEBS J. 2014;281(12):2861–2870. [DOI] [PubMed] [Google Scholar]
- 64. Sheng H, Hassanali S, Nugent C, Wen L, Hamilton-Williams E, Dias P, Dai YD. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J Immunol. 2011;187(4):1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rahman MJ, Regn D, Bashratyan R, Dai YD. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes. 2014;63(3):1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, Piemonti L, Hirosue S, Swartz MA, De Palma M, Hubbell JA, Baekkeskov S. Primary human and rat β-cells release the intracellular autoantigens GAD65, IA-2, and proinsulin in exosomes together with cytokine-induced enhancers of immunity. Diabetes. 2017;66(2):460–473. [DOI] [PubMed] [Google Scholar]
- 67. Heinrich LF, Andersen DK, Cleasby ME, Lawson C. Long-term high fat feeding of rats results in increased numbers of circulating microvesicles with pro-inflammatory effects on endothelial cells. Br J Nutr. 2015;113(11):1704–1711. [DOI] [PubMed] [Google Scholar]
- 68. Campello E, Zabeo E, Radu CM, Spiezia L, Foletto M, Prevedello L, Gavasso S, Bulato C, Vettor R, Simioni P. Dynamics of circulating microparticles in obesity after weight loss. Intern Emerg Med. 2016;11(5):695–702. [DOI] [PubMed] [Google Scholar]
- 69. Kranendonk ME, Visseren FL, van Balkom BW, Nolte-’t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22(5):1296–1308. [DOI] [PubMed] [Google Scholar]
- 70. Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-’t Hoen EN, de Jager W, Wauben MH, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22(10):2216–2223. [DOI] [PubMed] [Google Scholar]
- 71. Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, Gordish-Dressman H, Koeck E, Sevilla S, Wiles AA, Freishtat RJ. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh JH, Wang J, Dohm GL, Pories WJ, Mietus-Snyder M, Freishtat RJ. Circulating adipocyte-derived exosomal microRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee JE, Moon PG, Lee IK, Baek MC. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka Long-Evans Tokushima fatty (OLETF) rats. Protein J. 2015;34(3):220–235. [DOI] [PubMed] [Google Scholar]
- 74. Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, Shah SV, Sun D, Michalek S, Grizzle WE, Garvey T, Mobley J, Zhang HG. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58(11):2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384.e12. [DOI] [PubMed] [Google Scholar]
- 76. Koeck ES, Iordanskaia T, Sevilla S, Ferrante SC, Hubal MJ, Freishtat RJ, Nadler EP. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res. 2014;192(2):268–275. [DOI] [PubMed] [Google Scholar]
- 77. Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R, Scherer PE. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175(3):695–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. [DOI] [PubMed] [Google Scholar]
- 80. Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007;103(3):1093–1098. [DOI] [PubMed] [Google Scholar]
- 82. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. [DOI] [PubMed] [Google Scholar]
- 83. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. [DOI] [PubMed] [Google Scholar]
- 84. Aswad H, Forterre A, Wiklander OP, Vial G, Danty-Berger E, Jalabert A, Lamazière A, Meugnier E, Pesenti S, Ott C, Chikh K, El-Andaloussi S, Vidal H, Lefai E, Rieusset J, Rome S. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57(10):2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H, Forterre A, Meugnier E, Pesenti S, Regazzi R, Danty-Berger E, Ducreux S, Vidal H, El-Andaloussi S, Rieusset J, Rome S. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049–1058. [DOI] [PubMed] [Google Scholar]
- 86. Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34(1):84–129. [DOI] [PubMed] [Google Scholar]
- 87. Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hui E, Xu A, Bo Yang H, Lam KS. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: role of adipokines. J Diabetes Investig. 2013;4(5):413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Deng F, Magee N, Zhang Y. Decoding the role of extracellular vesicles in liver diseases. Liver Res. 2017;1(3):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, Lazic M, Thapaliya S, Parola M, Patel HH, Feldstein AE. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6(296):ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150(4):956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, Je J, Suh SJ, Jung YK, Kim JH, Seo YS, Yim HJ, Jeong WI, Yeon JE, Um SH, Byun KS. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7(1):3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. [DOI] [PubMed] [Google Scholar]
- 94. Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. [DOI] [PubMed] [Google Scholar]
- 95. Favaro E, Carpanetto A, Caorsi C, Giovarelli M, Angelini C, Cavallo-Perin P, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325–333. [DOI] [PubMed] [Google Scholar]
- 96. Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, Bruno S, Amoroso A, Giovarelli M, Porta M, Perin PC, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014;57(8):1664–1673. [DOI] [PubMed] [Google Scholar]