Abstract
Progesterone receptors (PRs) are key modifiers of estrogen receptor (ER) target genes and drivers of luminal breast cancer progression. Total PR expression, rather than isoform-specific PR expression, is measured in breast tumors as an indicator of functional ER. We identified phenotypic differences between PR-A and PR-B in luminal breast cancer models with a focus on tumorsphere biology. Our findings indicated that PR-A is a dominant driver of cancer stem cell (CSC) expansion in T47D models, and PR-B is a potent driver of anchorage-independent proliferation. PR-A+ tumorspheres were enriched for aldehyde dehydrogenase (ALDH) activity, CD44+/CD24−, and CD49f+/CD24− cell populations relative to PR-B+ tumorspheres. Progestin promoted heightened expression of known CSC-associated target genes in PR-A+ but not PR-B+ cells cultured as tumorspheres. We report robust phosphorylation of PR-A relative to PR-B Ser294 and found that this residue is required for PR-A–induced expression of CSC-associated genes and CSC behavior. Cells expressing PR-A S294A exhibited impaired CSC phenotypes but heightened anchorage-independent cell proliferation. The PR target gene and coactivator, FOXO1, promoted PR phosphorylation and tumorsphere formation. The FOXO1 inhibitor (AS1842856) alone or combined with onapristone (PR antagonist), blunted phosphorylated PR, and tumorsphere formation in PR-A+ and PR-B+ T47D, MCF7, and BT474 models. Our data revealed unique isoform-specific functions of phosphorylated PRs as modulators of distinct and opposing pathways relevant to mechanisms of late recurrence. A clear understanding of PR isoforms, phosphorylation events, and the role of cofactors could lead to novel biomarkers of advanced tumor behavior and reveal new approaches to pharmacologically target CSCs in luminal breast cancer.
Luminal or estrogen receptor (ER)–positive breast cancer accounts for ~75% of cases. ER+ tumors typically express a wide range of ER-target gene products, such as progesterone receptor (PR)-A and PR-B isoforms, derived from the single PGR gene locus (1, 2). Most ER+/PR+ cases (luminal A type) are initially estrogen responsive and most effectively treated with endocrine therapies aimed at blocking ER action or estrogen synthesis. As ER+/PR+ tumors progress, they are likely to become hormone independent, yet usually retain steroid receptor expression. In addition, ≤40% of women with ER+ tumors will exhibit de novo resistance or will have ER-targeted therapies fail (acquired resistance), with eventual progression to metastatic disease (3–5). Historically, PR has been used as a biomarker of ER transcriptional activity that predicts for a high likelihood of an initial response to endocrine therapy. As the PR levels decrease (luminal B type), breast tumors are more likely to become endocrine resistant. However, an increasing body of evidence has supported the role of the PR as an important ER binding partner and dominant modifier of ER activity and target gene selection (6–8). Although the presence of progesterone (P4) can limit estrogen-induced proliferation, either hormone alone is mitogenic in normal and neoplastic breast epithelial cells (9, 10). PR is also an important mediator of breast cancer cell survival (11, 12). PR has been emerging as a context-dependent driver of luminal breast cancer phenotypes associated with tumor progression in vitro (13, 14) and in vivo (15, 16). However, progress in the development of highly selective anti-progestins for clinical use as PR-targeted therapies has been limited.
In breast tissues, P4 signaling is mediated by two coexpressed PR isoforms, full-length PR-B and N-terminal truncated PR-A (truncated of the first 164 amino acids found in PR-B, termed the B-upstream segment). Although PR-A and PR-B share structural and sequence identity downstream of the B-upstream segment, these isoforms regulate the same, as well as distinct, gene sets (17–19). Mouse knockout studies showed that PR-B is integral for normal mammary gland development, and PR-A knockout mice displayed disrupted uterine development and infertility (20, 21). Consistent with these findings, P4 and progestins (e.g., R5020) that act through PR-B are proliferative in the breast (22). Although mammary epithelial cells often coexpress PR-A and PR-B, the ratio of PR-A/PR-B changes significantly with the developmental state of the gland such that it peaks (∼1:1 ratio) at puberty and progressively decreases during adulthood, pregnancy, and postpartum (i.e., PR-B predominates) (23). Although the total PR levels, rather than the individual PR isoforms, are measured clinically, altered PR isoform expression has been implicated in the etiology of breast cancer and contributes to tumorigenesis (24). Immunohistochemical analysis of PR-A and PR-B expression in human breast tumors indicated PR-A predominance (i.e., PR-A > PR-B) in ductal carcinoma in situ and invasive breast lesions (25). Furthermore, high PR-A expression relative to PR-B predicted for relapse to tamoxifen but not to aromatase targeted therapies (26). Recent studies have further defined PR isoform-specific gene expression profiles and associations with advanced tumor characteristics in ER+/PR+ breast cancer models and tumors (18, 24). However, opposite conclusions were reached with regard to the behavior of ER+ tumors that are either PR-A–rich or PR-B–rich; genetic data acquired using PR-A+ or PR-B+ cell lines did not accurately predict tumor behavior, suggesting that additional factors contribute to the PR isoform-specific influence on breast cancer biology, especially in the context of the high tumor heterogeneity that typifies human breast cancers (24).
Context-dependent factors predicted to influence PR expression and isoform-specific actions include the presence of altered and oncogenic signaling pathways. PRs are heavily phosphorylated by mitogenic or stress-sensing protein kinases that are elevated and activated in breast cancer. Modified PRs act as sensors for altered or active signaling pathways that modulate PR transcriptional activity and alter PR target gene selection via phosphorylation events (13, 14, 27). We previously established that although the intrinsic transcriptional activity of PR-B is unchanged on luciferase reporter genes (28), endogenous PR-B target gene expression is exquisitely sensitive to Ser294 phosphorylation in response to activation of p42/p44 MAPKs or cyclin-dependent kinase 2 in breast cancer models (14, 29, 30). We demonstrated abundant phosphorylated Ser294 PR levels in a majority (54%) of luminal breast tumor samples (27). Furthermore, phosphorylated Ser294 PR-B enhanced the expression of unique gene sets associated with endocrine resistance (13) and embryonic and cancer stem cell (CSC) and cancer stem-like cell biology (14, 27). PR, but not ER, is required for expansion of normal mammary epithelial stem cells (31, 32). These studies have been extended to show that PR also regulates the breast CSC compartment (2, 33, 34). Breast CSCs represent a small minority of the total tumor cell population, which makes them inherently more difficult to specifically target using standard chemotherapy or endocrine therapy. The individual roles of PR-A and PR-B with regard to breast cancer stem-like cell biology are unknown. However, this knowledge is required to target PR isoform-specific actions as a complement to ER-based endocrine therapies aimed at blocking both ER-driven tumor cell proliferation and PR-driven expansion of the breast CSC compartment.
We sought to define PR isoform-specific regulation of cancer stem or stem-like cells in ER+/PR+ breast cancer models. Our findings have indicated that PR-A is a dominant driver of CSC expansion in breast cancer cells, and PR-B is a more potent driver of breast cancer cell proliferation. Additionally, we found that PR-A Ser294 phosphorylation is required for CSC behavior as measured in secondary tumorsphere assays and expression of well-characterized CSC markers. Mutation of PR-A Ser294 to Ala (S294A) blocked CSC expansion but promoted cell proliferation. Finally, we have demonstrated that the classic PR target gene, FOXO1, cooperates with both PR isoforms to both inhibit proliferation and promote CSC behavior in breast cancer cell models. Taken together, our data revealed unique functions of PR isoforms and PR target genes (FOXO1) as modulators of distinct and opposing pathways (i.e., proliferation vs stem-like expansion) in luminal breast cancer models. Our findings have provided information regarding the important clinical question of why patients with ER+/PR+ luminal breast cancer initially respond well to endocrine therapies but have a high risk of late recurrence.
Materials and Methods
General reagents
Estradiol (E2; Sigma-Aldrich), R5020 (Sigma-Aldrich), P4 (Sigma-Aldrich), mifepristone (RU486; Sigma-Aldrich), onapristone (Arno Therapeutics), and hydrocortisone (Sigma-Aldrich) stocks were prepared in ethanol (EtOH). Epidermal growth factor (EGF; Sigma-Aldrich) was prepared in 0.1% BSA containing 10 mM acetic acid. AS1842856 (EMD Millipore) was prepared in dimethyl sulfoxide.
Cell culture
MCF-7 cells were cultured in modified improved MEM (Life Technologies) containing 5% FBS (Thermo Scientific HyClone), 1% penicillin-streptomycin (Life Technologies), and 67.5 ng/mL of insulin (Life Technologies). BT-474 cells were cultured in RPMI 1640 (Corning) containing 10% FBS and 1% penicillin-streptomycin. T47D (ATCC) cells were cultured in RPMI 1640 containing 10% FBS, 1% penicillin-streptomycin, and 0.2 U/mL insulin. T47D-Y and T47D CO cells were cultured in MEM (Corning) containing 5% FBS, 1% penicillin-streptomycin, 1× MEM nonessential amino acids (Life Technologies), and 6 ng/mL of insulin. T47D-YA and T47D-YB cells (35) were cultured in the same media as described for the T47D-Y and CO cells and maintained with 0.2 mg/mL G418 sulfate (Corning). T47D cells containing an inducible PR expression system (12) were maintained in MEM containing 5% FBS, 1% penicillin-streptomycin, 0.2 mg/mL G418 sulfate, and 0.2 mg/mL hygromycin B (Calbiochem). PR expression was induced within 24 hours after the addition of AP21967 (1 nM; ClonTech). HeLa cells were cultured in MEM containing 5% FBS, 1% penicillin-streptomycin, 1× MEM nonessential amino acids, and 6 ng/mL insulin. For experiments with hormone treatment (e.g., R5020, P4, E2), the cells were hormone starved in phenol-free modified improved MEM containing 5% dextran-coated charcoal-stripped FBS (HyClone) for 16 hours before treatment. For transfection experiments, HeLa cells were transfected with the appropriate constructs [green fluorescent protein-tagged PR-A or PR-B, plasmid cytomegalovirus promoter DNA (pcDNA) wild-type (WT) FOXO1, short hairpin (sh)FOXO1] using FugeneHD (Promega).
Stable cell line generation
Stable T47D models were generated by transfecting T47D-Y cells with pSG5-neo vector (which also served as the vector control) containing WT PR-A or S294A PR-A using FuGENE HD (Roche). Stable pool populations were selected in and maintained as described with 0.2 mg/mL G418 sulfate. Stable shPR-expressing cell lines (clones TRCN0000003321 and 3324) and shFOXO1-expressing cell lines (clones TRCN0000039578 and 39579) were created by transducing T47D-YA and T47D-YB cells with pLKO.1 lentiviral vectors containing target gene shRNA sequences. Stable pooled populations were selected in and maintained as described with 0.2 mg/mL G418 sulfate and 0.5 µg/mL puromycin (MP Biomedicals). Stable FOXO1 models were generated by transducing T47D-YA or T47D-YB cells with retroviral pBabe puroL vector (vector control) containing WT FOXO1 or FOXO1-AAA (36). The cells were selected in and maintained as described with 0.5 µg/mL puromycin. Single cell cloning was used to generate clonal cell lines expressing empty vector (EV), WT FOXO1, or FOXO1-AAA.
Soft agar assays
Cells were seeded (4 × 104 cells per well) in 1× sterile low melt agarose (Life Technologies) containing 5% dextran-coated charcoal and the appropriate treatment [vehicle (EtOH), R5020, 10 nM; P4, 10 nM; or E2, 1 nM]. Soft agar assays were allowed to proceed for 21 days at 37°C. Afterward, the cell colonies were counted using ImageJ (National Institutes of Health; available at: http://rsbweb.nih.gov/ij/). Data are presented as the average ± SD of three independent measurements.
Cell lysate preparation
The cells were harvested in radioimmunoprecipitation assay-lite lysis buffer (150 mM NaCl, 6 mM Na2HPO4, 4 mM NaH2PO4, 2 mM EDTA, 100 mM NaF, 1% Triton-X 100, 1× complete mini protease inhibitors (Roche), and 1× PhosSTOP (Roche), supplemented with 1 mM PMSF, 5 mM NaF, 0.05 mM Na3VO4, 25 mM β-glycerophosphate, and 20 µg/mL aprotinin].
Immunoblotting
Immunoblotting was performed with the following antibodies: phosphorylated PR [Ser294 (custom), Thermo Fisher], PR (H190; Santa Cruz Biotechnology; RRID: AB_2164331) (37), PR (F-4; Santa Cruz Biotechnology; RRID: AB_2166687) (38), phosphorylated FOXO1 (Ser256; Cell Signaling; RRID: AB_329831) (39), FOXO1 (C29H4; Cell Signaling; RRID: AB_2106495) (40), phosphorylated Akt (Ser473; Cell Signaling; RRID: AB_329825) (41), Akt (Cell Signaling; RRID: AB_329827) (42), glyceraldehyde 3-phosphate dehydrogenase (0411; Santa Cruz Biotechnology; RRID: AB_627678) (43), goat anti-rabbit IgG-horseradish peroxidase (BioRad; RRID: AB_11125142) (44), and goat anti-mouse IgG-horseradish peroxidase (BioRad; RRID: AB_11125547) (45). Blots were developed with ECL using Super Signal West Pico PLUS Chemiluminescence Substrate (Pierce) and imaged by film.
Tumorsphere assays
Adherent cells were washed with PBS and dissociated enzymatically in 0.25% trypsin-EDTA (Invitrogen). The cells were sieved through a 40-µm sieve (BD Falcon). Single cells were plated in ultra-low attachment plates (Corning) and grown in a serum-free DMEM/F12 phenol-free medium (Corning) containing 1% methylcellulose (Sigma-Aldrich), 1% B27 proprietary supplement (Invitrogen), 1% penicillin-streptomycin, 5 μg/mL insulin, 20 ng/mL EGF, 1 ng/mL hydrocortisone, and 100 μM β-mercaptoethanol. To generate secondary tumorspheres, primary spheres were collected and dissociated enzymatically in 0.25% trypsin-EDTA. Single cells were plated as described in conditioned media, which consisted of a 1:1 mixture of DMEM/F12 media (as described) and media from cultured parental cells. The tumorspheres were allowed to grow for 10 to 12 days. The tumorspheres were analyzed by total number and scored by manual counting using a uniformly scaled grid. Data are presented as the average ± SD of three independent measurements.
Flow cytometry
An ALDEFLUOR assay kit (Stem Cell Technologies) was used in accordance with the manufacturer’s instructions to assay for aldehyde dehydrogenase (ALDH) activity. The cells were plated in ultra-low attachment plates and grown in DMEM/F12 media (as described) to generate tumorspheres. The tumorspheres were collected and dissociated using StemPro Accutase (Life Technologies) and resuspended (5 × 105) in ALDEFLUOR assay buffer (1 mL). ALDEFLUOR reagent was added to this cell suspension and mixed. Next, one half of the cell suspension was transferred to a separate tube containing N,N-diethylaminobenzaldehyde. The cells were incubated at 37°C for 45 minutes, washed, and subjected to flow cytometry using the BD LSRII H4760 flow cytometer (BD Biosciences). Sorting gates were established using ALDEFLUOR-stained cells treated with N,N-diethylaminobenzaldehyde.
For CD44/CD24 or CD49f detection, tumorspheres were collected and dissociated as described. The cells (5 × 105) were resuspended in fluorescence-activated cell sorting buffer (Dulbecco’s PBS containing 2% FBS) with APC-CD44 (1:20; BD Pharmingen), CD24 phycoerythrin (PE; 1:50; BD Pharmingen), or CD49f-PerCP-Cy (1:100; BD Pharmingen) conjugated antibodies and incubated at 4°C for 45 minutes. The cells were washed, resuspended in cold fluorescence-activated cell sorting buffer, and subjected to flow cytometry using the BD LSRII H4760 flow cytometer (BD Biosciences). The cells were gated in forward scatter area vs side scatter area, after which the forward scatter area vs forward scatter height was used to identify single cells. Single cells were plotted as CD24-PE vs CD44-APC or CD24-PE vs CD49f-PerCP-Cy to identify populations based on single stained control samples.
Real-time quantitative PCR
Total RNA was extracted from cell samples using TriPure Isolation Reagent (Roche) and isopropanol precipitation. For two-dimensional (2D; adherent) conditions, the cells were plated (in triplicate) into their respective growth media. For three-dimensional (3D; tumorsphere) conditions, the cells were plated in triplicate into tumorsphere media and ultra-low attachment plates. RNA (1000 ng) was reverse transcribed to cDNA in accordance with the manufacturer’s instructions using qScript cDNA SuperMix (Quanta BioSciences). Quantitative PCR (qPCR) was performed using Light Cycler FastStart DNA Master SYBR Green I (Roche) using a Light Cycler 480 II Real-Time PCR System (Roche). The qPCR cycling conditions were initial denaturation at 95°C (10 minutes), denaturing at 95°C (10 seconds), annealing at 60°C (10 seconds), and extension at 72°C (5 seconds) for 45 cycles. The target gene levels were normalized to standard housekeeper genes (TBP or 18s) and represent the average of three independent measurements (mean ± SD).
Chromatin immunoprecipitation assays
The cells were fixed, harvested, and lysed using the ChIP-IT Express Magnetic Chromatin Immunoprecipitation Kit (Active Motif). The samples were homogenized using a Bioruptor sonicator (Diagenode Inc.). Chromatin immunoprecipitation (ChIP) reactions (100 µL isolated chromatin) were incubated with 2 µg of PR-A/B antibody (H190; Santa Cruz Biotechnology) overnight at 4°C with rocking. Normal rabbit IgG (Santa Cruz Biotechnology) was used in control samples. The samples were washed, eluted, reverse cross-linked, and treated with proteinase K according to the manufacturer’s instructions. DNA was analyzed using real-time qPCR, as described. Data are presented as the average ± SD of three technical replicates.
CSC-associated gene expression analysis within The Cancer Genome Atlas breast cancer public database
FOXO1 and NOTCH2 gene expression data generated and reported by The Cancer Genome Atlas (TCGA) consortium (46, 47) were downloaded from the TCGA data portal in October 2018. The downloaded data were provided as mean-centered and included all breast cancer subtypes (i.e., not restricted to ER+). Upper and lower quartile mRNA expression was correlated with relapse-free survival (RFS) over 8 years using the Kaplan-Meier curve in RStudio (48) and examined for statistical significance using the log-rank test.
Supplemental data
Supplemental figures have been provided in an online repository, available at: https://doi.org/10.6084/m9.figshare.7469948 (49).
Statistical analysis
The data were tested for a normal distribution using the Shapiro-Wilks normality test and the homogeneity of variances using the Bartlett test. Once the data were determined to meet these two requirements, statistical analyses were performed using one-way or two-way ANOVA in conjunction with the Tukey multiple comparison test for mean values between more than two groups or the Student t test for mean values between two groups, with significance determined with 95% confidence.
Results
PR-A promotes ALDH+/CD44+/CD24− breast CSC expansion
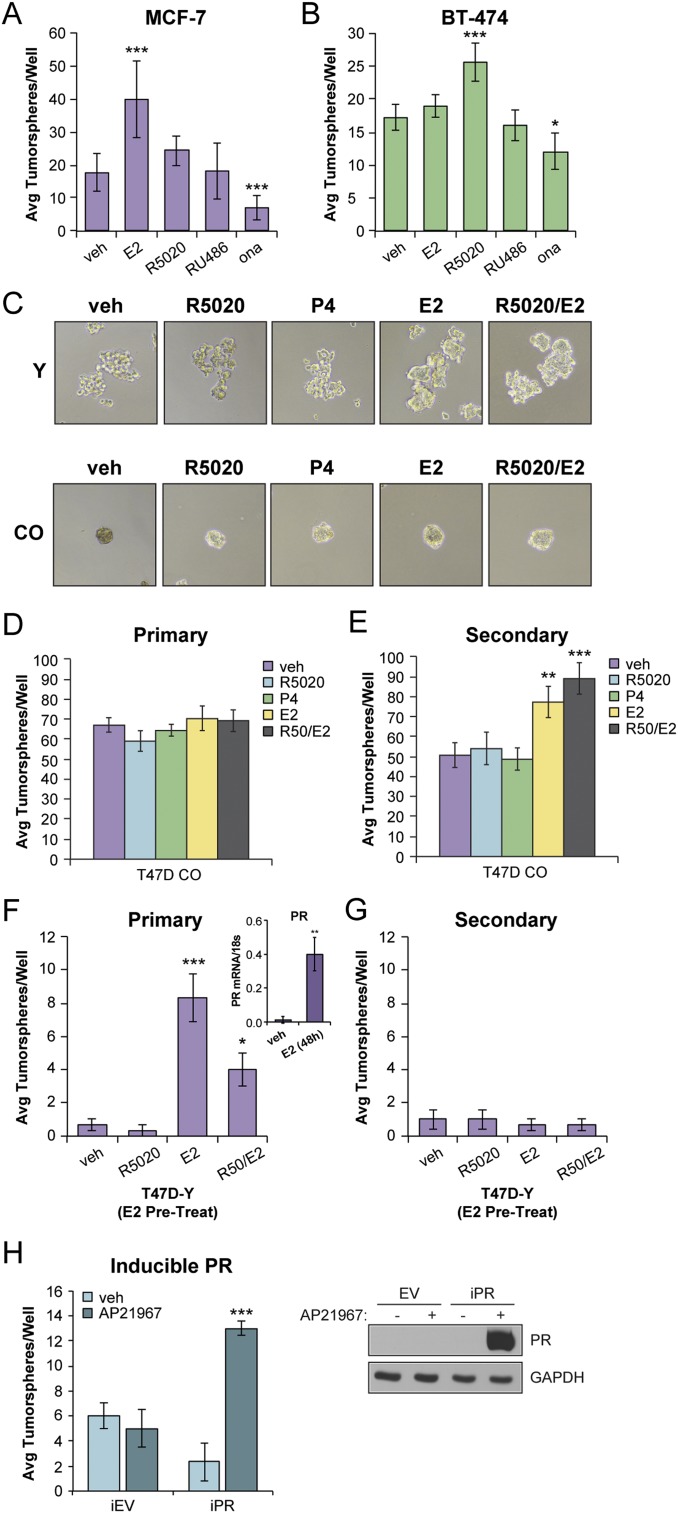
P4, but not E2, is a critical mediator of normal mammary gland stem cell self-renewal via induction of PR target genes that included paracrine signals (e.g.,WNT4, RANKL) (31, 50). Emerging data from human breast cancer cell lines and mouse models of breast cancer have also implicated PR-dependent signaling events in stem cell biology and early dissemination of circulating tumor cells (51–53). To elucidate the effect of P4 and PR in stem cell biology, we performed tumorsphere assays in luminal breast cancer cell lines (e.g., MCF-7 and BT-474; Fig. 1A and 1B). Tumorsphere assays assess the ability of a minority subpopulation of cancer stem or stem-like cells to expand in suspension culture (54). Equal numbers of cells were seeded into B27-supplemented tumorsphere media and treated with steroid hormones [i.e., E2, R5020 (synthetic progestin)] or PR antagonists (i.e., RU486, onapristone). As expected, both cell lines exhibited comparable levels of basal primary tumorsphere formation. E2 is typically required for PR expression in vitro; E2 treatment of ER+/PRlow MCF-7 cells induced an increase in the number of primary tumorspheres relative to steroid-free media; however, R5020 had no effect. In contrast, R5020, but not E2, increased the number of ER+/PR+ BT474 tumorspheres. The pan PR/glucocorticoid receptor antagonist mifepristone (RU486) had no effect on basal tumorsphere formation in either cell line. However, onapristone, which is PR-specific at low doses (14, 55), blocked basal tumorsphere formation in both MCF-7 and BT-474 cells. These results suggest that PR expression is important for tumorsphere formation.
Figure 1.
PR expression promotes tumorsphere formation in breast cancer cells. (A) Primary tumorsphere assays in MCF-7 cells. (B) Primary tumorsphere assays in BT-474 cells. Cells were treated with vehicle (veh; EtOH), R5020 (10 nM), E2 (1 nM), RU486 (100 nM), or onapristone (ona; 100 nM). (C) Tumorsphere assays in T47D-Y (PR-null) and T47D CO cells treated with vehicle (EtOH) or the indicated hormone treatment (R5020, 10 nM; P4, 10 nM; or E2, 1 nM). Representative images shown. (D) Primary and (E) secondary tumorspheres in T47D CO cells. (F) Primary and (G) secondary tumorsphere assays in T47D-Y cells. T47D-Y cells were pretreated with E2 (1 nM) for 48 hours, followed by tumorsphere assays with vehicle (EtOH) or the indicated hormone treatment. (F, Right Inset) mRNA levels of PR from primary tumorspheres in T47D-Y cells pretreated with E2. (H) Secondary tumorsphere assays in T47D-inducible EV control and PR-B cells. PR expression was induced within 24 hours after addition of AP21967 (1 nM); Right, Western blot. Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
To strengthen these results, we performed primary and secondary tumorsphere assays using ER+/PR+ T47Dco cells, a variant of T47D cells known to constitutively express ER and both PR isoforms (56). The power of this model is that E2, itself a potent breast mitogen, is not required to stimulate PR expression. We also examined tumorsphere formation in naturally occurring PR-null variant cells (T47D-Y) that were originally selected by seeding T47Dco cells at limiting dilution (35). PR-null T47D-Y cells formed flat sheet-like aggregates in both the presence and the absence of hormones, and T47Dco cells readily formed tumorspheres (Fig. 1C). The T47Dco cells formed abundant primary and secondary tumorspheres independently of the added steroid hormones (Fig. 1C–1E). However, as with unmodified MCF-7 cells, E2, but not P4 or R5020, stimulated increased formation of secondary T47Dco tumorspheres (Fig. 1E). We predicted this occurs via further induction of PR (57). Consistent with this finding, E2 pretreatment of T47D-Y cells stimulated weak induction of PR mRNA (Fig. 1F; Inset) and modest numbers of primary T47D-Y tumorspheres (Fig. 1F). However, the T47D-Y cells failed to sustain secondary tumorspheres, regardless of hormone pretreatment (Fig. 1G). Finally, we seeded T47D-Y cells harboring an inducible PR-B construct (12) into primary tumorsphere media and included vehicle (EtOH) or AP21967 to induce PR expression (i.e., in the absence of E2). After dissociation of primary tumorspheres, PR+ single cells formed secondary tumorspheres only in the presence of the AP21967 compound (Fig. 1H). These findings confirm that PR expression promotes secondary tumorsphere formation.
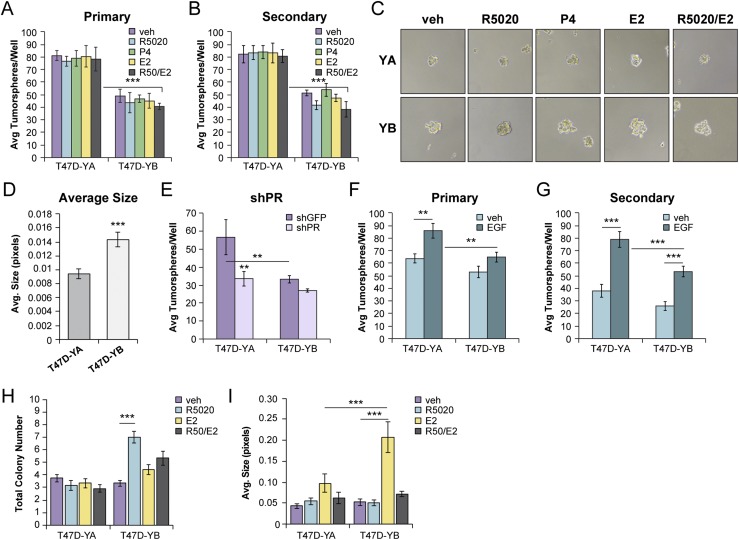
PR-B is required for mouse mammary gland development, suggesting a dominant role for PR-B in mammary gland stem cell biology. We have previously demonstrated a requirement for both EGF and PR-B Ser294 in secondary tumorsphere formation of T47D breast cancer cells (27). The significance of PR-A in promoting this biological feature remains unknown. To explore PR isoform-specific roles in CSC biology, we used PR-null T47D-Y cells engineered to stably re-express either PR-A (T47D-YA) or PR-B (T47D-YB) (35). PR-A–expressing cells formed increased tumorsphere numbers relative to cells expressing similar levels of PR-B (Fig. 2A and 2B). Hormone treatment (i.e., R5020, P4, or E2) had no substantial effect on tumorsphere number or size. However, PR-B+ tumorspheres were, on average, larger than the PR-A+ tumorspheres (Fig. 2C and 2D). Knockdown of PR in T47D-YA and T47D-YB cells decreased tumorsphere formation (Fig. 2E). Removal and add-back of EGF to tumorsphere media indicated that secondary tumorsphere formation was enhanced by growth factors (Fig. 2F and 2G). These data revealed PR isoform-specific functions in tumorsphere forming assays and confirmed that secondary tumorsphere formation is relatively insensitive to steroid hormones in T47D models but is promoted by growth factor signaling. Proliferative and stem cell programs are governed by separate and often opposing signaling pathways (58). Soft agar colony assays (i.e., anchorage independent growth) confirmed the hormone-induced proliferative response of T47D-YB cells relative to T47D-YA cells. R5020 (but not E2) increased the T47D-YB colony number (Fig. 2H), and E2 (but not R5020) increased the T47D-YB colony size (Fig. 2I). Combined treatment with both hormones attenuated both colony number and size induced by either hormone alone, indicative of reciprocal antagonistic ER/PR-B cross-talk, as reported by others (6, 8, 59). Parental T47D-Y and T47Dco cells remained insensitive to hormone treatment (49). These results suggest that proliferation in luminal breast cancer cells is mediated by PR-B but not PR-A.
Figure 2.
PR isoforms have distinct phenotypes in breast cancer cells. (A) Primary and (B) secondary tumorsphere assays in T47D-YA and T47D-YB cells. Cells were treated with vehicle (veh; EtOH), R5020 (10 nM), P4 (10 nM), or E2 (1 nM). (C) Representative images of T47D (YA and YB) primary tumorspheres. (D) Average size of T47D (YA and YB) primary tumorspheres. (E) Primary tumorsphere assays in T47D-YA and T47D-YB short hairpin green fluorescent protein (shGFP) control or short hairpin PR (shPR) knockdown cells. (F) Primary and (G) secondary tumorsphere assays in T47D-YA and T47D-YB cells. Cells treated with vehicle (water) or EGF (20 ng/mL). (H) Soft agar colony formation and (I) average colony size in T47D-YA and T47D-YB cells were assessed in the presence of vehicle (EtOH) or the indicated hormone treatments (R5020, 10 nM; P4, 10 nM; or E2, 1 nM). Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
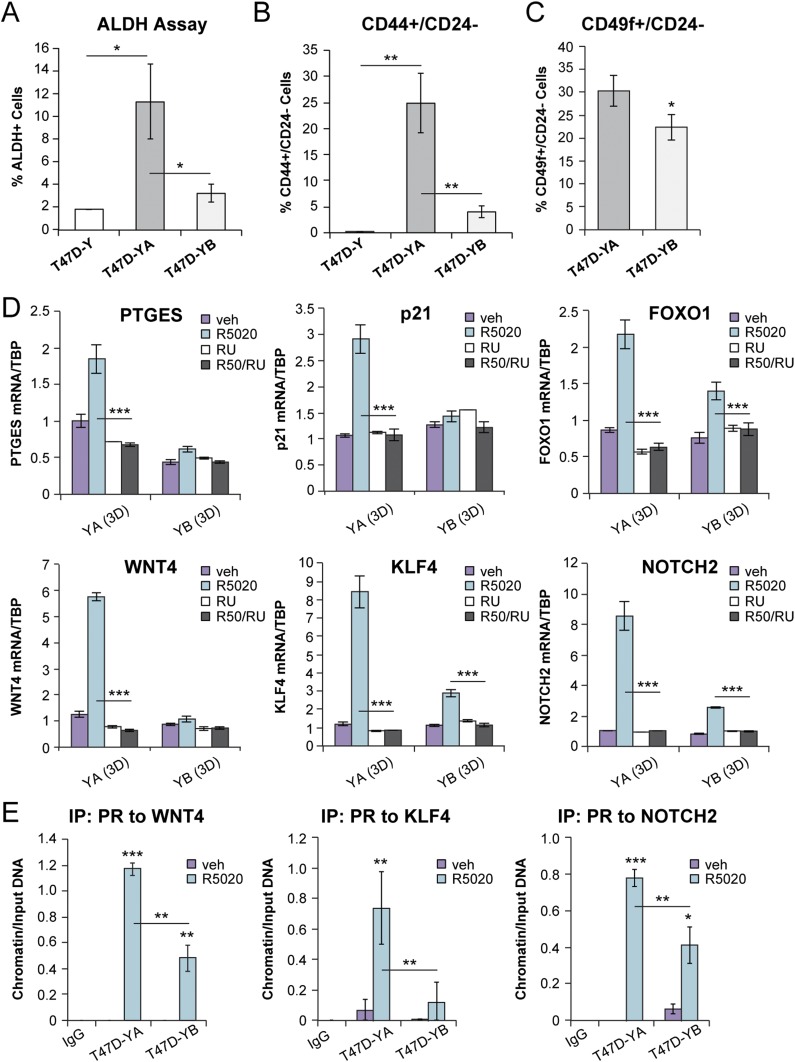
Enhanced ALDH activity is a hallmark of CSCs (60). ALDEFLUOR assays confirmed that T47D-YA tumorspheres were enriched for ALDH activity (11.3% ± 3.3%) relative to PR-null T47D-Y (1.8% ± 0.02%) or T47D-YB (3.2% ± 0.8%) tumorspheres (Fig. 3A) (49). Breast CSCs exhibit high expression of CD44+ and low expression of CD24− cell surface markers (61). Consistent with a breast CSC phenotype, T47D-YA tumorspheres exhibited increased CD44+/CD24− populations (24.9% ± 5.7%) relative to either PR-null T47D-Y (0.1% ± 0.02%) or T47D-YB tumorspheres (4.0% ± 1.1%; Fig. 3B) (49). Luminal breast cancer progenitor cells will exhibit a CD49f+/CD24+ profile (62), and basal breast CSC populations will be enriched for CD49f+/CD24− (63, 64). PR-A+ tumorspheres exhibited increased CD49f+/CD24− cell populations (30.3% ± 3.3%) relative to PR-B+ tumorspheres (22.4% ± 2.8%; Fig. 3C) (49). In contrast, PR-B+ tumorspheres shifted toward a luminal enriched (CD49f+/CD24+) population (49). These data suggest that PR-A induces expansion of basal-like breast CSCs, and PR-B induces luminal-enriched breast CSC populations. Collectively, these results have indicated that although PR-A and PR-B both drive breast cancer stem-like cell expansion as measured by tumorsphere assays and known CSC markers (e.g., ALDH, CD44/CD24/CD49f), they likely regulate distinct gene programs to promote divergent breast cancer stem-like lineages.
Figure 3.
PR-A expression enhanced breast CSC populations and target gene expression. (A) ALDH+ activity in T47D (Y, YA, YB) tumorspheres was assessed using flow cytometry. (B) CD44+/CD24− populations from T47D (Y, YA, YB) tumorspheres. (C) CD49f+/CD24− populations from T47D-YA and T47D-YB tumorspheres. (D) mRNA levels of select genes (e.g., PTGES, p21, FOXO1, WNT4, KLF4, NOTCH2) in T47D-YA and T47D-YB cells. Cells were cultured in tumorsphere conditions and treated with vehicle (veh), R5020 (10 nM), or RU486 (100 nM). (E) ChIP assays showing PR recruitment to a PRE-containing region of the WNT4, KLF4, or NOTCH2 promoter. T47D-YA and T47D-YB cells were stimulated with vehicle (EtOH) or R5020 (10 nM) for 1 hour. Fixed lysates were subjected to ChIP assays using specific antibodies targeting PR (or IgG control). Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Gene expression profiling studies performed in adherent (i.e., 2D) culture conditions have revealed that PR-A and PR-B regulate overlapping but distinct gene sets in breast and ovarian cancer models (18, 19). Given that CSCs typically represent a small minority of the total tumor cell population but are abundant in the circulation, we predict that tumorsphere (i.e. nonadherent or 3D) culture conditions enable the expression of gene sets not evident in 2D conditions. We, therefore, analyzed PR isoform-specific expression of CSC-associated genes [e.g.,FOXO1 (65), p21 (66), KLF4 (67), PTGES (68), WNT4 (69), and NOTCH2 (70)]. T47D-YA and T47D-YB cells were seeded in tumorsphere conditions containing vehicle (EtOH) or R5020 (10 nM). The tumorspheres were allowed to form (~7 days) before harvesting mRNA for real-time qPCR analyses. T47D-YA cells exhibited enhanced hormone-induced expression of numerous CSC-associated genes relative to T47D-YB cells (Fig. 3D). Progestin failed to regulate these PR target genes in T47D-Y (PR-null) cells (49). Moreover, treatment with RU486 blocked R5020-induced gene expression in T47D cells expressing either PR isoform (Fig. 3D). ChIP assays demonstrated recruitment of PR to progesterone response element (PRE)–containing regions in the WNT4, KLF4, and NOTCH2 promoters (6, 71, 72). After R5020 treatment, significantly greater PR-A was recruited to these promoters relative to PR-B (Fig. 3E). Taken together, these results have demonstrated that in 3D conditions favoring tumorsphere formation, PR-A promotes enhanced expression of CSC-associated target genes and drives ALDH+/CD44+/CD24− CSC biology relative to PR-B.
PR-A Ser294 is required for CSC expansion and CSC-associated gene expression
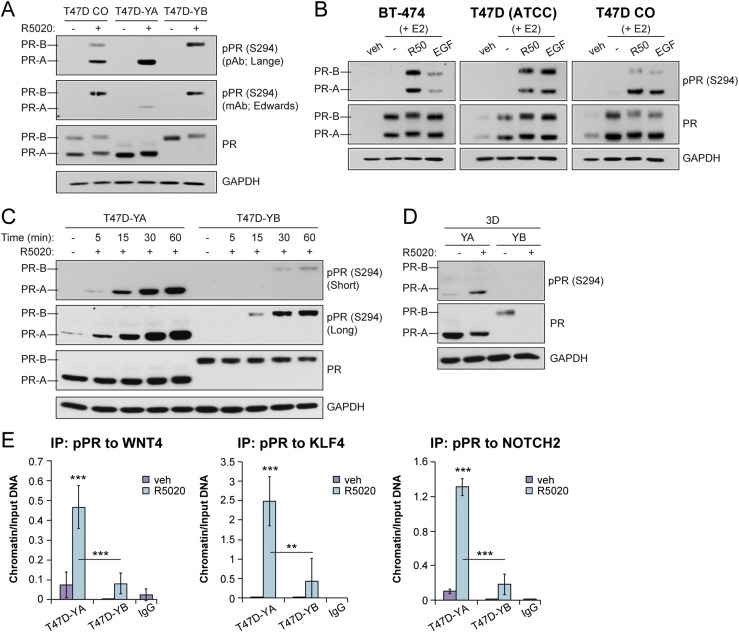
We previously implicated PR-B Ser294 phosphorylation in breast cancer CSC biology (27). We, and others, have reported that in breast cancer models, PR-B Ser294, but not the analogous residue on truncated PR-A, is uniquely phosphorylated by either MAPKs or cyclin-dependent kinase 2 (29, 73). We were surprised that PR-A is a dominant driver of breast CSC biology in isoform-specific T47D models. We, therefore, tested PR Ser294 phosphorylation in response to R5020 treatment in T47D (CO, YA, YB) cells using a previously characterized phosphorylated Ser294 monoclonal antibody (kind gift of Dean Edwards) (73) and our custom-made phosphorylated Ser294 polyclonal antibody (14). Only phosphorylated Ser294-specific polyclonal antibodies recognized both PR isoforms (Fig. 4A). PR-A and PR-B are both present in most ER+ breast cancer models; however, their expression has varied among the cell lines. Given that PR is a classic ER-target gene, we pretreated unmodified luminal breast cancer cell lines BT-474, T47D [ATCC], and T47Dco cells with E2 for 48 hours to induce robust PR expression (57). Ligand-activated PRs rapidly activate c-Src/Ras/Raf/MAPKs (74); thus, EGF was included as a positive control for MAPK-dependent PR Ser294 phosphorylation. With R5020 or EGF treatment, PR Ser294 phosphorylation was observed on both PR isoforms in all three unmodified breast cancer cell lines (Fig. 4B). This is consistent with our finding of PR Ser294 phosphorylation of both isoforms in ovarian cancer models (75). After R5020 treatment, PR-A Ser294 phosphorylation occurred within a more rapid (5- to 15-minute) time course relative to that of PR-B (15 to 60 minutes; Fig. 4C). Western blot analysis of PRs in T47D-YA and T47D-YB cells cultured in 3D tumorsphere conditions demonstrated that PR-A+ tumorspheres sustained higher levels of total and phosphorylated Ser294 PR in the absence and presence of progestin relative to PR-B+ spheres (Fig. 4D). We previously reported that PR-A is a more stable isoform relative to PR-B in 2D conditions (75). This difference in PR isoform stability/turnover might account for the dominant PR-A-driven tumorsphere formation relative to PR-B. To examine the levels of phosphorylated PR-A and phosphorylated PR-B recruitment to the promoter regions of CSC-associated genes, we performed ChIP-PCR using our custom polyclonal phosphorylated PR (S294) antibody (Fig. 4E). Consistent with our results showing PR-A dominance in regulating the expression of these genes, we observed significantly greater hormone-induced phosphorylated PR-A recruitment to PRE-containing regions of WNT4, KLF4, and NOTCH2 relative to phosphorylated PR-B recruitment (Fig. 4E). To the best of our knowledge, these results, together, provide the first evidence of regulated PR-A Ser294 phosphorylation in human breast cancer models.
Figure 4.
PR isoforms are phosphorylated at S294. (A) Western blot of phosphorylated and total PR in T47D cells (CO, YA, YB). Cells were treated with vehicle (veh; EtOH) or R5020 (10 nM) for 60 minutes. Phosphorylated PR (pPR; S294) antibodies were used from the Lange laboratory [polyclonal antibody (pAb)] and the Edwards laboratory [monoclonal antibody (mAB)]. (B) Western blot of phosphorylated and total PR in a panel of luminal breast cancer cell lines. Cells were pretreated with E2 for 48 hours, followed by R5020 (10 nM) for 1 hour or EGF (30 ng/mL) for 15 minutes. (C) Western blot of phosphorylated and total PR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; loading control) in T47D-YA and T47D-YB cells. The cells were treated with vehicle (veh; EtOH) or R5020 (10 nM) for the indicated times. (D) Western blot of phosphorylated and total PR and GAPDH (loading control) in T47D-YA and T47D-YB cells. The cells were cultured in tumorsphere conditions (i.e., suspension) and treated with vehicle (EtOH) or R5020 (10 nM). (E) ChIP assays showing phosphorylated PR (S294) recruitment to PRE-containing regions of the WNT4, KLF4, or NOTCH2 promoter. T47D-YA and T47D-YB cells were stimulated with vehicle (EtOH) or R5020 (10 nM) for 1 hour. Fixed lysates were subjected to ChIP assays using specific antibodies targeting phosphorylated PR (S294) or IgG control. Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
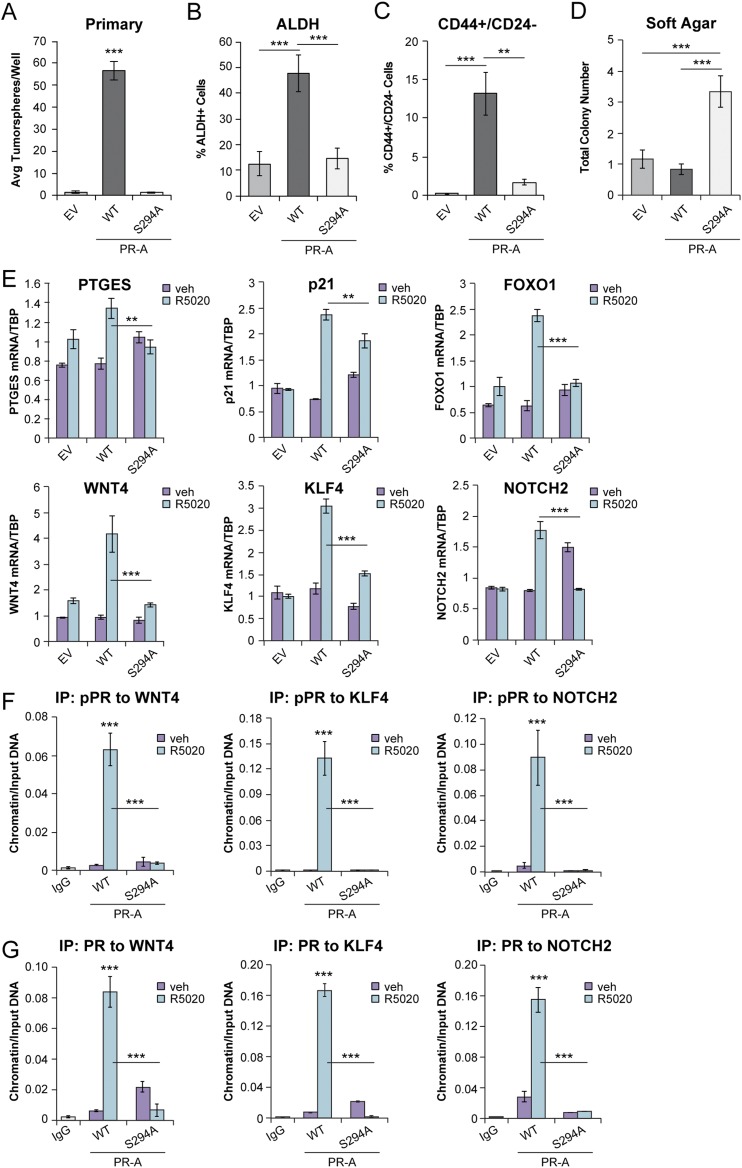
Given that growth factor signaling (i.e., EGF) promotes PR-driven tumorsphere formation (Fig. 2F and 2G) and the dominant role of PR-A in this biology (Fig. 3), we examined the requirement for Ser294 phosphorylation in PR-A–induced breast CSC phenotypes. Using PR-null parental T47D-Y cells, we generated EV controls and vector-matched models by re-expressing either WT PR-A or S294A PR-A. The PR-A+ cells formed abundant primary tumorspheres relative to PR-null controls. However, primary tumorsphere formation was greatly attenuated in cells expressing S294A PR-A, comparable to that of EV PR-null controls (Fig. 5A). Additionally, ALDH activity and CD44+/CD24− populations were markedly decreased in S294A PR-A+ tumorspheres relative to WT PR-A+ tumorspheres (Fig. 5B and 5C). We next performed soft agar assays to measure anchorage-independent proliferation. S294A PR-A+ cells exhibited increased basal soft agar colony formation compared with EV control cells and vector-matched cells expressing WT PR-A (Fig. 5D), indicating that PR-A+ cells do not require Ser294 for viability in low attachment; mutation of PR-A Ser294 to Ala increases proliferation. Consistent with the requirement for PR Ser294 in tumorsphere formation, T47D cells expressing S294A PR-A failed to induce numerous CSC-associated PR target genes relative to cells expressing WT PR-A (Fig. 5E). Analysis of TCGA public breast cancer database (49) showed that high expression of FOXO1 (P = 0.046) or NOTCH2 (P = 0.004) significantly decreased RFS. Furthermore, phosphorylated and total PR recruitment to CSC-associated genes was greatly abrogated in S294A PR-A+ cells relative to cells expressing WT PR-A (Fig. 5F and 5G). Collectively, these data indicate that PR-A Ser294 promotes expression of CSC-associated target genes and outgrowth of ALDH+/CD44+/CD24− breast CSCs in tumorsphere assays and revealed a role for PR Ser294 in the regulation of opposing proliferative vs CSC biological features.
Figure 5.
Ser294 contributes to PR-A–induced breast CSC phenotypes. (A) Primary tumorspheres from T47D (EV, WT PR-A, and S294A PR-A) cells. (B) ALDH activity in T47D (EV, WT PR-A, and S294A PR-A) primary tumorspheres. (C) Soft agar colony formation of T47D (EV, WT PR-A, and S294A PR-A) cells. (D) CD44+/CD24− populations in T47D (EV, WT PR-A, and S294A PR-A) primary tumorspheres. (E) mRNA levels of select genes (e.g.,PTGES, p21, FOXO1, WNT4, KLF4, NOTCH2) in T47D (EV, WT PR-A, and S294A PR-A) cells cultured in tumorsphere conditions. Cells were treated with vehicle (veh; EtOH) or R5020 (10 nM). (F) ChIP assays showing phosphorylated PR and (G) total PR recruitment to PRE-containing regions of the WNT4, KLF4, or NOTCH2 promoter. T47D (WT PR-A and S294 PR-A) cells were stimulated with vehicle (EtOH) or R5020 (10 nM) for 1 hour. Fixed lysates were subjected to ChIP assays using specific antibodies targeting phosphorylated PR (S294) or IgG control. Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. Avg, average.
PR-A target gene, FOXO1, augments PR-driven breast CSC phenotypes
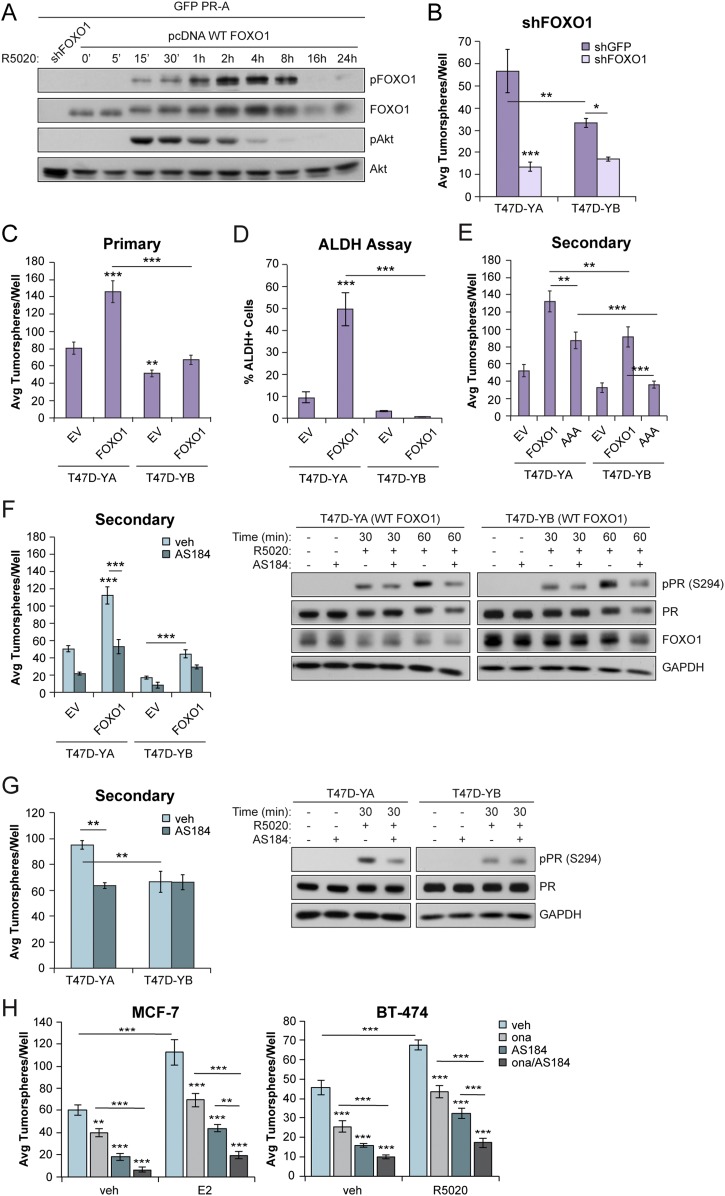
FOXO1 gene expression is repressed by PR-A, but activated by PR-B, in ovarian cancer models. Subsequent nuclear FOXO1/PR-B complexes promote increased p21 gene expression and drive cell cycle exit, accumulation of cells in G0, and cellular senescence (75). In contrast, PR-A+ breast cancer models carried as tumorspheres promote increased FOXO1 and p21 expression relative to PR-B+ tumorspheres (Fig. 3D). Forkhead box (FOX) transcription factors, including FOXO1, are regulated in part by AKT-dependent phosphorylation events that drive nuclear export and, consequently, inactivate nuclear FOX factor functions (76). Similar to ovarian cancer, we predict that FOXO1 is a key mediator of PR-regulated breast cancer cell fate transitions associated with poor outcomes. T47D cells exhibit constitutive AKT signaling, a hallmark of luminal breast cancer (77). To address direct regulation of FOXO1 by PR isoforms, we first transfected HeLa cells with green fluorescent protein-tagged PR-A or PR-B and pcDNA FOXO1 and performed a time course of R5020 treatment (5 minutes to 24 hours). Progestin treatment of PR-A+ cells rapidly activated AKT signaling (15 minutes) and increased the levels of phosphorylated FOXO1 over a similar period, with FOXO1 phosphorylation peaking at ∼8 hours (Fig. 6A). Similar results were observed in HeLa cells expressing PR-B (49). FOXO1 knockdown in T47D-YA and T47D-YB cells decreased primary tumorsphere formation (Fig. 6B). The yields from shFOXO1 cells were too low to progress to secondary tumorspheres. To address the role of FOXO1 in the modulation of PR isoform-specific actions in breast cancer models, we generated T47D-YA and T47D-YB cells overexpressing (control), WT FOXO1, or a phosphorylated mutant form of FOXO1 that cannot be phosphorylated by AKT (FOXO1-AAA). Again, PR-A+ cells exhibited increased tumorsphere formation relative to PR-B+ cells. Overexpression of WT FOXO1 enhanced primary and secondary tumorsphere formation in both PR-A+ and PR-B+ models relative to parental EV controls (Fig. 6C). Consistent with these results, ALDH activity was enriched on FOXO1 expression in PR-A+ cells (Fig. 6D). Overexpression of FOXO1-AAA weakly enhanced the formation of PR-A+, but not PR-B+, secondary tumorspheres relative to EV controls or WT FOXO1 (Fig. 6E). In support of these findings, AS1842856, a selective small-molecule FOXO1 inhibitor (78), blocked FOXO1-induced tumorsphere formation in T47D cells overexpressing WT FOXO1 (Fig. 6F) and in parental T47D-YA and T47D-YB cells (Fig. 6G). Treatment with AS1842856 (100 nM) had no appreciable effect on the total PR levels but attenuated R5020-induced PR Ser294 phosphorylation in these models (Fig. 6F and 6G; Right). Similarly, AS1842856 treatment of unmodified ER+/PR+ MCF-7 and BT-474 breast cancer cell lines attenuated tumorsphere formation, especially in combination with the anti-progestin onapristone (Fig. 6H). Collectively, these results have demonstrated that the phosphorylated PR-A target gene and cofactor, FOXO1, contributes to the maintenance of sustained phosphorylated PR Ser294 levels and drives PR-driven breast CSC biology as measured by tumorsphere formation. WT FOXO1 (i.e., the phosphorylated form) exhibited more potent cooperation with PRs relative to “inactive” or nuclear FOXO1-AAA.
Figure 6.
FOXO1 promoted breast CSC outgrowth in PR isoforms. (A) Western blot of FOXO1 (phosphorylated and total) and Akt (phosphorylated and total) in HeLa cells transfected with green fluorescent protein–tagged PR-A and with pcDNA FOXO1. Cells were treated with vehicle (veh; EtOH) or R5020 (10 nM) for the indicated times. shFOXO1 was included as a control. (B) Primary tumorsphere assays in T47D-YA and T47D-YB cells with FOXO1 knockdown. (C) Primary tumorsphere assays in T47D (YA and YB) cells stably expressing FOXO1. (D) ALDH activity in primary tumorspheres formed from T47D (YA and YB) FOXO1+ cells was assessed by flow cytometry. (E) Secondary tumorspheres in T47D (YA and YB) cells expressing WT FOXO1 or FOXO1-AAA. (F) Secondary tumorspheres in T47D (YA and YB) FOXO1+ cells treated with AS1842856 (100 nM). (Right) T47D (YA and YB) cells expressing FOXO1 were pretreated with AS1842856 (100 nM) for 60 minutes, followed by the indicated treatments with R5020 (10 nM). (G) Secondary tumorspheres in T47D (YA and YB) cells treated with AS1842856. (Right) T47D (YA and YB) cells were pretreated with AS1842856 (100 nM) for 60 minutes, followed by treatment with R5020 (10 nM). (H) MCF-7 and BT-474 primary tumorspheres treated with E2 (1 nM) or R5020 (10 nM), respectively, in the presence of onapristone (100 nM) or AS1842856 (100 nM), or both. Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. Avg, average.
Discussion
Relative to what is known about ER signaling, PR isoforms have been grossly understudied in luminal breast cancer biology. Our data have revealed opposing functions for the PR isoforms in proliferation vs breast CSC biology and identified phosphorylated Ser294 PR-A as a potent driver of breast CSC expansion. Our findings might, in part, explain why women with a diagnosis of ER+/PR+ tumors have an initially good response to endocrine therapies but remain at risk for late recurrence owing to long-lived CSCs capable of early dissemination and resistance to endocrine therapies (52, 79).
PR isoforms promote divergent breast CSC lineages
CSCs are nonproliferative, allowing them to readily evade standard therapies aimed at rapidly proliferating cancer cells and enabling tumor regrowth (80–82). PRs are emerging as major mediators of breast CSC biology (51, 83). Although only PR-B+ breast cancer cells will grow in soft agar, PR-A+ breast cancer cells will form increased numbers of tumorspheres enriched for ALDH+/CD44+/CD24− cell populations relative to PR-B+ breast cancer cells, suggesting a phenotypic “flip” from a proliferative to a CSC program (Figs. 2 and 3). PR-B+ cells form larger tumorspheres relative to PR-A+ cells, indicative of proliferative subpopulations primarily supported by PR-B. Consistent with our findings, studies of T47D xenografts have demonstrated that PR-A+ primary tumors will be smaller than their PR-B+ counterparts in vivo, albeit CSC biology was not considered (84). PR-A+ tumorspheres exhibited an enriched basal-like CSC phenotype (i.e., CD49f+/CD24−), indicative of increased clinical tumorigenicity, metastatic potential, and malignancy (60, 61). In contrast, PR-B tumorspheres were shifted toward a luminal phenotype (i.e., CD49f+/CD24+) relative to the PR-A tumorspheres. Our data suggest that although PR isoforms are both capable of modulating cancer stem-like cell behavior, they might do so by promoting divergent CSC lineages. Further studies using a larger array of known human breast CSC biomarkers are required to clearly define these lineages.
The unexpected finding that tumorsphere conditions favor PR-A–driven CSC behavior suggests that the 3D context somehow favors PR-A–driven expression of previously reported PR-B target genes, perhaps via altered signaling events. We noted persistent total and phosphorylated PR-A protein expression relative to total and phosphorylated PR-B, known to undergo rapid ligand-dependent downregulation (29). Consistent with this interpretation, ChIP assays demonstrated robust recruitment of phosphorylated and total PR-A but not PR-B to selected PR target genes with high CSC relevance (Figs. 3E and 4E). An increasing body of evidence has suggested that PR-induced expansion of the mammary stem cell compartment occurs via paracrine WNT4 signaling (31, 69). Similarly, progestin-mediated downregulation of miR-141 led to increased expression of KLF4 and reprogramming to a stem cell-like state (33). NOTCH2 has been identified through ChIP sequencing studies to contain PR-binding sites (71). PR-A predominance has been frequently reported in ER+ mammary carcinomas (85) and associated with higher tumor grade and poorer disease-free survival (25, 84). Phosphorylated Ser294 PR-A represents a unique biomarker with which to identify women whose tumors contain a high potential for CSC outgrowth and conversion to endocrine-resistant metastatic disease. Further studies in physiologically relevant conditions (i.e., suspension; 3D) are needed to elucidate the PR isoform-specific signaling events and unique gene programs driving expansion of breast CSC lineages.
PR isoforms mediate opposing biological functions via Ser294
Historically, PR-B has been characterized as a strong “positive regulator” of the effects of P4 and PR-A often as a ligand-independent mediator of gene repression. We found that soft agar growth is driven by PR-B and that PR-A+ cells failed to proliferate in soft agar (Fig. 2H). These data are supported by findings from previous studies demonstrating PR-B–driven proliferation in response to P4 or progestins in adherent (2D) culture systems (86, 87). When both isoforms are coexpressed (i.e., T47Dco) (49), soft agar colony formation is considerably dampened relative to that of cells exclusively expressing PR-B. PR-A trans-repression of PR-B might explain this observation, as has been reported in multiple models (88, 89). Hormone treatment with both E2 and R5020 ablates PR-B–induced soft agar growth (Fig. 2I), consistent with previous studies of breast cancer cell proliferative responses performed in adherent 2D conditions and in studies of tumor explants (6).
The mechanisms underlying imbalanced PR-A/PR-B ratios in human breast tumors relative to normal tissues remain unknown. Although most cells within a given breast tumor will express both PR isoforms, minority cell fractions have contained only activated PR-A (25%) or activated PR-B (23%) isoforms, suggesting the existence of potential lineage-specific roles in CSC biology (90). Although the expression of either PR isoform induced formation of primary tumorspheres that resulted in ALDH+/CD44+/CD24− tumorspheres, PR-A was the dominant isoform in tumorsphere conditions, perhaps owing to its increased stability relative to PR-B (75). An emerging concept in cancer data is that proliferative vs cancer stem-like cell behaviors are distinct biological processes governed by separate signaling pathways that often oppose each other (52, 91). In the present study, this opposition was apparent, with PR-B the dominant proliferative isoform in soft agar and PR-A a more potent driver of CSC biology. We observed that EGF withdrawal decreased PR-A tumorspheres (Fig. 2F and 2G), suggesting that growth factor-mediated signaling, rather than high hormone levels, provides strong input to the CSC outgrowth of PR+ breast cancer cells. Regulated phosphorylation of PR Ser294 links these opposing cancer cell biological decisions. We predict this occurs via profound changes in target gene selection. This observation requires further investigation such that PR isoform-specific cell fates can be selectively targeted to block breast CSC expansion.
Phosphorylated PR target gene FOXO1 is a mediator of cell fate transitions
FOXO1 is widely considered to be an important tumor suppressor gene but has also been implicated in hematopoietic and neural stem cell data (92–94). PR isoforms mediate cellular senescence and regulate mRNA expression of FOXO1 and the PR/FOXO1 target gene p21 in ES-2 ovarian cancer models (19). This occurs in an isoform-specific manner whereby FOXO1 and p21 are both repressed by PR-A but induced by ligand-bound PR-B. In sharp contrast, FOXO1 is induced by PR-A isoforms in a Ser294-dependent manner in breast cancer models (Figs. 3D and 5E). High FOXO1 expression in ER+ breast cancers predicts for shortened RFS (49). Using COS-1 cells, Rudd et al. (95) showed that constitutively active nuclear FOXO1 (FOXO1-AAA; phosphorylated mutant form that cannot undergo nuclear export) enhances ligand-dependent transcriptional activity of PR-A but not PR-B in PRE-luciferase assays. FOXO1 remains understudied in breast cancer models as a PR target gene and coregulator. To the best of our knowledge, our study is the first demonstration of FOXO1-dependent PR-A function in breast cancer models. Mechanistic linkage of FOXO1 (i.e., a phosphorylated PR-A target gene) to PR Ser294 phosphorylation and phosphorylated PR-driven CSC outgrowth represents a feed-forward signaling loop that ensures a sustained and decisive biological outcome. FOXO1 is emerging as a driver of advanced breast cancer phenotypes other than proliferation, including cancer cell migration, metastasis, and drug resistance (96, 97). Finak et al. (98) reported increased FOXO1 in invasive breast cancer relative to adjacent normal tissue in 59 paired microdissected specimens. Further studies are required to identify additional PR-binding partners and define their effect on PR-A and PR-B actions as they relate to ER+ breast cancer progression.
Conclusion
Elevation of therapy-resistant CSCs is a hallmark of aggressive breast cancer behavior and has been associated with metastasis and a poor prognosis. As we continue to delineate the factors underlying phosphorylated PR-mediated pathways in luminal breast cancer, it is clear that phosphorylated PRs could prove useful in the future as clinical biomarkers to identify patients with breast cancer in danger of widespread metastasis. Further investigation into the mechanism by which phosphorylated PR-A promotes CSC outgrowth and other related cancer phenotypes is warranted. A deeper understanding of PR isoform-specific actions, including PR-phosphorylated species and their target gene cofactors, might provide information on the mechanisms of late recurrence in luminal breast cancer and reveal new approaches to pharmacologically target phosphorylated PR isoforms as potent drivers of breast CSC biology.
Acknowledgments
We are grateful to Drs. Dean P. Edwards and Nancy L. Weigel (Baylor College of Medicine) for the kind gift of monoclonal phosphorylated Ser294 PR antibodies. We thank Drs. Julie H. Ostrander and Kathryn L. Schwertfeger for their critical reading of this manuscript.
Financial Support: The present study was supported by the National Institutes of Health (Grants R01 CA159712 to C.A.L., F32 CA210340 to T.H.T., and T32 HL007741 to T.H.T.), and the Tickle Family Land Grant Endowed Chair in Breast Cancer Research (to C.A.L.).
Author Contributions: T.H.T., A.R.D., C.H.D., K.M.H., and H.H. performed the experiments. T.H.T., A.R.D., and C.H.D. analyzed and interpreted the data. T.H.T., A.R.D., and C.A.L. conceived the study and wrote the report. C.A.L. supervised the study. All authors consented to publication of the report.
Disclosure Summary: C.A.L. serves on the board of scientific advisors for Context Therapeutics. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 2D
two-dimensional
- 3D
three-dimensional
- ALDH
aldehyde dehydrogenase
- ChIP
chromatin immunoprecipitation
- CSC
cancer stem cell
- E2
estradiol
- EGF
epidermal growth factor
- ER
estrogen receptor
- EtOH
ethanol
- EV
empty vector
- FOX
forkhead box
- P4
progesterone
- pcDNA
plasmid cytomegalovirus promoter DNA
- PR
progesterone receptor
- PRE
progesterone response element
- qPCR
quantitative PCR
- RFS
relapse-free survival
- TCGA
The Cancer Genome Atlas
- WT
wild-type
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Hilton HN, Santucci N, Silvestri A, Kantimm S, Huschtscha LI, Graham JD, Clarke CL. Progesterone stimulates progenitor cells in normal human breast and breast cancer cells. Breast Cancer Res Treat. 2014;143(3):423–433. [DOI] [PubMed] [Google Scholar]
- 3. Lippman ME, Allegra JC. Quantitative estrogen receptor analyses: the response to endocrine and cytotoxic chemotherapy in human breast cancer and the disease-free interval. Cancer. 1980;46(12, Suppl):2829–2834. [DOI] [PubMed] [Google Scholar]
- 4. Campbell FC, Blamey RW, Elston CW, Morris AH, Nicholson RI, Griffiths K, Haybittle JL. Quantitative oestradiol receptor values in primary breast cancer and response of metastases to endocrine therapy. Lancet. 1981;2(8259):1317–1319. [DOI] [PubMed] [Google Scholar]
- 5. Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995;13(2):513–529. [DOI] [PubMed] [Google Scholar]
- 6. Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, Menon S, Hadfield J, Pugh M, Raj GV, Brown GD, D’Santos C, Robinson JLL, Silva G, Launchbury R, Perou CM, Stingl J, Caldas C, Tilley WD, Carroll JS. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523(7560):313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Amicis F, Zupo S, Panno ML, Malivindi R, Giordano F, Barone I, Mauro L, Fuqua SAW, Andò S. Progesterone receptor B recruits a repressor complex to a half-PRE site of the estrogen receptor α gene promoter. Mol Endocrinol. 2009;23(4):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singhal H, Greene ME, Tarulli G, Zarnke AL, Bourgo RJ, Laine M, Chang Y-F, Ma S, Dembo AG, Raj GV, Hickey TE, Tilley WD, Greene GL. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;2(6):e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Burg B, Rutteman GR, Blankenstein MA, de Laat SW, van Zoelen EJJ. Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J Cell Physiol. 1988;134(1):101–108. [DOI] [PubMed] [Google Scholar]
- 10. Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer: potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273(47):31308–31316. [DOI] [PubMed] [Google Scholar]
- 11. Dressing GE, Lange CA. Integrated actions of PR and cell cycle machinery regulate breast cancer cell proliferation. Steroids. 2009;74:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol Cell Biol. 2011;31(12):2439–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D’Assoro AB, Ravindranathan P, Peng Y, Raj GV, Yee D, Lange CA. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2015;34(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14(3):R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kordon EC, Molinolo AA, Pasqualini CD, Charreau EH, Pazos P, Dran G, Lanari C. Progesterone induction of mammary carcinomas in BALB/c female mice: correlation between progestin dependence and morphology. Breast Cancer Res Treat. 1993;28(1):29–39. [DOI] [PubMed] [Google Scholar]
- 16. Lanari C, Lüthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, Sanjuan N, Merani S, Molinolo AA. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro; effects of estrogens and progestins. Cancer Res. 2001;61:293. [PubMed] [Google Scholar]
- 17. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. [DOI] [PubMed] [Google Scholar]
- 18. Singhal H, Greene ME, Zarnke AL, Laine M, Al Abosy R, Chang Y-F, Dembo AG, Schoenfelt K, Vadhi R, Qiu X, Rao P, Santhamma B, Nair HB, Nickisch KJ, Long HW, Becker L, Brown M, Greene GL. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget. 2017;9(4):4282–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diep CH, Knutson TP, Lange CA. Active FOXO1 is a key determinant of isoform-specific progesterone receptor transactivation and senescence programming. Mol Cancer Res. 2016;14(2):141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179(1-2):97–103. [DOI] [PubMed] [Google Scholar]
- 21. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGowan EM, Russell AJ, Boonyaratanakornkit V, Saunders DN, Lehrbach GM, Sergio CM, Musgrove EA, Edwards DP, Sutherland RL. Progestins reinitiate cell cycle progression in antiestrogen-arrested breast cancer cells through the B-isoform of progesterone receptor. Cancer Res. 2007;67(18):8942–8951. [DOI] [PubMed] [Google Scholar]
- 23. Aupperlee M, Kariagina A, Osuch J, Haslam SZ. Progestins and breast cancer. Breast Dis. 2006;24(1):37–57. [DOI] [PubMed] [Google Scholar]
- 24. Rojas PA, May M, Sequeira GR, Elia A, Alvarez M, Martínez P, Gonzalez P, Hewitt S, He X, Perou CM, Molinolo A, Gibbons L, Abba MC, Gass H, Lanari C. Progesterone receptor isoform ratio: a breast cancer prognostic and predictive factor for antiprogestin responsiveness. J Natl Cancer Inst. 2017;109(7):djw317–djw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–172. [DOI] [PubMed] [Google Scholar]
- 26. Mote PA, Gompel A, Howe C, Hilton HN, Sestak I, Cuzick J, Dowsett M, Hugol D, Forgez P, Byth K, Graham JD, Clarke CL. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res Treat. 2015;151(2):309–318. [DOI] [PubMed] [Google Scholar]
- 27. Knutson TP, Truong TH, Ma S, Brady NJ, Sullivan ME, Raj G, Schwertfeger KL, Lange CA. Posttranslationally modified progesterone receptors direct ligand-specific expression of breast cancer stem cell-associated gene programs. J Hematol Oncol. 2017;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21(12):2890–2906. [DOI] [PubMed] [Google Scholar]
- 29. Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97(3):1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward RD, Weigel NL. Steroid receptor phosphorylation: assigning function to site-specific phosphorylation. Biofactors. 2009;35(6):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 32. Arendt LM, Kuperwasser C. Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy. J Mammary Gland Biol Neoplasia. 2015;20(1-2):9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA, Richer JK. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2013;32(20):2555–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goodman CR, Sato T, Peck AR, Girondo MA, Yang N, Liu C, Yanac AF, Kovatich AJ, Hooke JA, Shriver CD, Mitchell EP, Hyslop T, Rui H. Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene. 2016;35(11):1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sartorius CA, Groshong SD, Miller LA, Powell RL, Tung L, Takimoto GS, Horwitz KB. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54(14):3868–3877. [PubMed] [Google Scholar]
- 36. Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20(23):8969–8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RRID:AB_2164331.
- 38.RRID:AB_2166687.
- 39.RRID:AB_329831.
- 40.RRID:AB_2106495.
- 41.RRID:AB_329825.
- 42.RRID:AB_329827.
- 43.RRID:AB_627678.
- 44.RRID:AB_11125142.
- 45.RRID:AB_11125547.
- 46. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. RStudio Team RStudio: Integrated Development for R. Boston, MA: RStudio, Inc.; 2015.
- 49.Truong TH, Dwyer AR, Diep CH, Hu H, Hagen KM, Lange CA. Data from: Phosphorylated progesterone receptor isoforms mediate opposing stem cell and proliferative breast cancer cell fates. figshare 2018. Deposited 14 December 2018. 10.6084/m9.figshare.7469948. [DOI] [PMC free article] [PubMed]
- 50. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. [DOI] [PubMed] [Google Scholar]
- 51. Finlay-Schultz J, Sartorius CA. Steroid hormones, steroid receptors, and breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2015;20(1-2):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hosseini H, Obradović MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Gužvić M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran F-A, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, Klein CA. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sartorius CA, Harvell DME, Shen T, Horwitz KB. Progestins initiate a luminal to myoepithelial switch in estrogen-dependent human breast tumors without altering growth. Cancer Res. 2005;65(21):9779–9788. [DOI] [PubMed] [Google Scholar]
- 54. Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10(3):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gass EK, Leonhardt SA, Nordeen SK, Edwards DP. The antagonists RU486 and ZK98299 stimulate progesterone receptor binding to deoxyribonucleic acid in vitro and in vivo, but have distinct effects on receptor conformation. Endocrinology. 1998;139(4):1905–1919. [DOI] [PubMed] [Google Scholar]
- 56. Horwitz KB, Mockus MB, Lessey BA. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell. 1982;28(3):633–642. [DOI] [PubMed] [Google Scholar]
- 57. Cho H, Aronica SM, Katzenellenbogen BS. Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells: a comparison of the effects of cyclic adenosine 3′,5′-monophosphate, estradiol, insulin-like growth factor-I, and serum factors. Endocrinology. 1994;134(2):658–664. [DOI] [PubMed] [Google Scholar]
- 58. Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28(11):1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ballaré C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23(6):1994–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stingl J, Eaves CJ, Watson CJ. Phenotypic characterization of mouse mammary epithelial stem and progenitor cells. Breast Cancer Research: BCR. 2006;8(Suppl 2):P2. [Google Scholar]
- 63. Lim E, Wu D, Pal B, Bouras T, Asselin-Labat M-L, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12(2):R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keller PJ, Lin AF, Arendt LM, Klebba I, Jones AD, Rudnick JA, DiMeo TA, Gilmore H, Jefferson DM, Graham RA, Naber SP, Schnitt S, Kuperwasser C. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 2010;12(5):R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sreekumar A, Toneff MJ, Toh E, Roarty K, Creighton CJ, Belka GK, Lee D-K, Xu J, Chodosh LA, Richards JS, Rosen JM. WNT-mediated regulation of FOXO1 constitutes a critical axis maintaining pubertal mammary stem cell homeostasis. Dev Cell. 2017;43(4):436–448.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jain MV, Jangamreddy JR, Grabarek J, Schweizer F, Klonisch T, Cieślar-Pobuda A, Łos MJ. Nuclear localized Akt enhances breast cancer stem-like cells through counter-regulation of p21(Waf1/Cip1) and p27(kip1). Cell Cycle. 2015;14(13):2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu F, Li J, Chen H, Fu J, Ray S, Huang S, Zheng H, Ai W. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30(18):2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Finetti F, Terzuoli E, Giachetti A, Santi R, Villari D, Hanaka H, Radmark O, Ziche M, Donnini S. mPGES-1 in prostate cancer controls stemness and amplifies epidermal growth factor receptor-driven oncogenicity. Endocr Relat Cancer. 2015;22(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rajaram RD, Buric D, Caikovski M, Ayyanan A, Rougemont J, Shan J, Vainio SJ, Yalcin-Ozuysal O, Brisken C. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34(5):641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat M-L, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3(4):429–441. [DOI] [PubMed] [Google Scholar]
- 71. Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS One. 2012;7(4):e35859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yin P, Roqueiro D, Huang L, Owen JK, Xie A, Navarro A, Monsivais D, Coon JS V, Kim JJ, Dai Y, Bulun SE. Genome-wide progesterone receptor binding: cell type-specific and shared mechanisms in T47D breast cancer cells and primary leiomyoma cells. PLoS One. 2012;7(1):e29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14(1):52–65. [DOI] [PubMed] [Google Scholar]
- 74. Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17(7):2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle. 2013;12(9):1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277(47):45276–45284. [DOI] [PubMed] [Google Scholar]
- 77. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. [DOI] [PubMed] [Google Scholar]
- 78. Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, Shibasaki M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol. 2010;78(5):961–970. [DOI] [PubMed] [Google Scholar]
- 79. Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SAW. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10(8):2751–2760. [DOI] [PubMed] [Google Scholar]
- 80. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. [DOI] [PubMed] [Google Scholar]
- 81. Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 83. Alferez DG, Simões BM, Howell SJ, Clarke RB. The role of steroid hormones in breast and effects on cancer stem cells. Curr Stem Cell Rep. 2018;4(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sartorius CA, Shen T, Horwitz KB. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat. 2003;79(3):287–299. [DOI] [PubMed] [Google Scholar]
- 85. Bamberger AM, Milde-Langosch K, Schulte HM, Löning T. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res. 2000;54(1):32–37. [DOI] [PubMed] [Google Scholar]
- 86. Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier Joffé E, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;21(6):1335–1358. [DOI] [PubMed] [Google Scholar]
- 88. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244–1255. [DOI] [PubMed] [Google Scholar]
- 89. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 90. Bonneterre J, Bosq J, Jamme P, Valent A, Gilles EM, Zukiwski AA, Fuqua SAW, Lange CA, O’Shaughnessy J. Tumour and cellular distribution of activated forms of PR in breast cancers: a novel immunohistochemical analysis of a large clinical cohort. ESMO Open. 2016;1(4):e000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, Yee D. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29(2):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Eijkelenboom A, Mokry M, de Wit E, Smits LM, Polderman PE, van Triest MH, van Boxtel R, Schulze A, de Laat W, Cuppen E, Burgering BMT. Genome-wide analysis of FOXO3 mediated transcription regulation through RNA polymerase II profiling. Mol Syst Biol. 2013;9(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae S-S, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Paik J-H, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rudd MD, Gonzalez-Robayna I, Hernandez-Gonzalez I, Weigel NL, Bingman WE III, Richards JS. Constitutively active FOXO1a and a DNA-binding domain mutant exhibit distinct co-regulatory functions to enhance progesterone receptor A activity. J Mol Endocrinol. 2007;38(6):673–690. [DOI] [PubMed] [Google Scholar]
- 96. Han C-Y, Cho K-B, Choi H-S, Han H-K, Kang K-W. Role of FoxO1 activation in MDR1 expression in Adriamycin-resistant breast cancer cells. Carcinogenesis. 2008;29(9):1837–1844. [DOI] [PubMed] [Google Scholar]
- 97. Procaccia S, Ordan M, Cohen I, Bendetz-Nezer S, Seger R. Direct binding of MEK1 and MEK2 to AKT induces Foxo1 phosphorylation, cellular migration and metastasis. Sci Rep. 2017;7(1):43078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. [DOI] [PubMed] [Google Scholar]