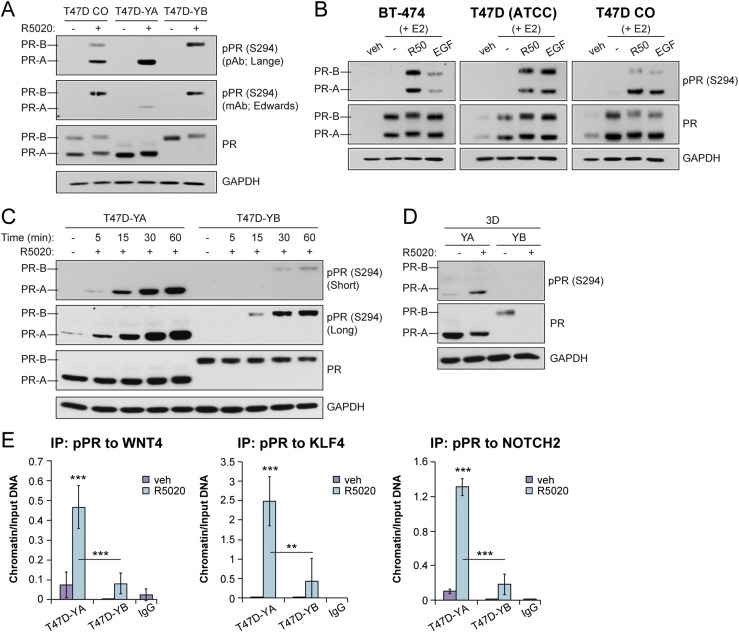
Figure 4.
PR isoforms are phosphorylated at S294. (A) Western blot of phosphorylated and total PR in T47D cells (CO, YA, YB). Cells were treated with vehicle (veh; EtOH) or R5020 (10 nM) for 60 minutes. Phosphorylated PR (pPR; S294) antibodies were used from the Lange laboratory [polyclonal antibody (pAb)] and the Edwards laboratory [monoclonal antibody (mAB)]. (B) Western blot of phosphorylated and total PR in a panel of luminal breast cancer cell lines. Cells were pretreated with E2 for 48 hours, followed by R5020 (10 nM) for 1 hour or EGF (30 ng/mL) for 15 minutes. (C) Western blot of phosphorylated and total PR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; loading control) in T47D-YA and T47D-YB cells. The cells were treated with vehicle (veh; EtOH) or R5020 (10 nM) for the indicated times. (D) Western blot of phosphorylated and total PR and GAPDH (loading control) in T47D-YA and T47D-YB cells. The cells were cultured in tumorsphere conditions (i.e., suspension) and treated with vehicle (EtOH) or R5020 (10 nM). (E) ChIP assays showing phosphorylated PR (S294) recruitment to PRE-containing regions of the WNT4, KLF4, or NOTCH2 promoter. T47D-YA and T47D-YB cells were stimulated with vehicle (EtOH) or R5020 (10 nM) for 1 hour. Fixed lysates were subjected to ChIP assays using specific antibodies targeting phosphorylated PR (S294) or IgG control. Graphed data represent the mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.