Abstract
Propionic acidemia (PA) is an autosomal recessive metabolic disorder. PA is characterized by deficiency of the mitochondrial enzyme propionyl CoA carboxylase (PCC) that results in the accumulation of propionic acid. Alpha and beta subunits of the PCC enzyme are encoded by the PCCA and PCCB genes, respectively. Pathogenic variants in PCCA or PCCB disrupt the function of the PCC enzyme preventing the proper breakdown of certain amino acids and metabolites. To determine the frequency of pathogenic variants in PA in our population, 84 Saudi Arabian patients affected with PA were sequenced for both the PCCA and PCCB genes. We found that variants in PCCA accounted for 81% of our cohort (68 patients), while variants in PCCB only accounted for 19% (16 patients). In total, sixteen different sequence variants were detected in the study, where 7 were found in PCCA and 9 in PCCB. The pathogenic variant (c.425G > A; p.Gly142Asp) in PCCA is the most common cause of PA in our cohort and was found in 59 families (70.2%), followed by the frameshift variant (c.990dupT; p.E331Xfs*1) in PCCB that was found in 7 families (8.3%). The p.Gly142Asp missense variant is likely to be a founder pathogenic variant in patients of Saudi Arabian tribal origin and is associated with a severe phenotype. All variants were inherited in a homozygous state except for one family who was compound heterozygous. A total of 11 novel pathogenic variants were detected in this study thereby increasing the known spectrum of pathogenic variants in the PCCA and PCCB genes.
Keywords: Propionic acidemia, PCCA, PCCB, Founder mutation, Genotype-phenotype correlation
1. Introduction
Propionic acidemia (PA; OMIM 606054) is an autosomal recessive metabolic disorder caused by the deficient activity of the propionyl coenzyme A carboxylase (PCC) [1]. Most PA patients have an early onset (diagnosed <3 months of age). Classically, this group presents in the neonatal period with lethargy, vomiting, refusal to feed, hypotonia and less frequently, seizures. Biochemical features include metabolic acidosis, hyperammonemia, hypoglycaemia, bone marrow suppression and ketonuria. The later course is dominated by recurrent metabolic decompensation characterized by the same above symptoms and biochemical abnormalities. Recurrent pancreatitis, cardiomyopathy and abnormal movements are also seen. Almost all PA patients surviving into childhood and adolescence have some degree of intellectual disability [1]. PA is a life threatening disease and has been reported in Saudi Arabia and other Arab countries [[2], [3], [4]] with most patients showing an early age of onset [5,6]. In previously reported Saudi PA patients, poor feeding, vomiting and lethargy were the most common clinical features sometimes in parallel with ketoacidosis and hyperammonemia [5,7].
PA is a reported to be a rare condition with incidence ranges from 1:50,000 to 1:100,000 worldwide (http://www.ncbi.nlm.nih.gov/books/NBK92946). PA is however the most frequently encountered organic aciduria in newborn screening in Saudi Arabia with an estimated incidence of 1 per 12,500 live births [2,8], which is much higher than the previously reported prevalence estimated to be 1 in 27,264 live births [9]. The incidence of PA is even higher in certain tribes of Saudi Arabia in the range of 1:2000 to 1:5000 [10].
PCC is a mitochondrial biotin-dependent enzyme that catalyses the conversion of propionyl-CoA to D-methylmalonyl-CoA. Deficiency of PCC leads to accumulation of propionic acid leading to PA. PCC comprises of six α-subunits that form binding sites and six β-subunits that form a central core of the enzyme, in addition to a biotin domain that located on α-subunits [11]. Alpha and beta subunits of the PCC enzyme are encoded by the PCCA and PCCB genes, respectively.
The PCCA gene is located on chromosome 13q32 [12], spans >360 kb and consists of 24 exons encoding 728 amino acids. The alpha subunit encoded by PCCA is responsible for the formation of carboxybiotin upon hydrolysis of ATP and it contains a C-terminus biotin-binding domain and a biotin carboxylase domain [13]. In fibroblasts, the level of PCCA expression does not normally limit the activity of PCC enzyme or propionate flux [14]. Pathogenic variants in PCCA account approximately for 50% of molecularly diagnosed individuals with PA [15]. According to the Human Gene Mutation Database; HGMD (http://www.hgmd.org); currently there are 126 pathogenic variants were reported in PCCA gene implicated in causing PA including gross deletions. Most variants in PCCA gene cause protein instability [16]. PCCB gene was mapped to chromosome 3q22.3 and contains 15 exons [17] coding for 539 amino acids. Functional analysis of PCCB sequence variants revealed 3 effects: retention of PCC activity, null or very low activity [18]. To date, there are 112 pathogenic variants in PCCB gene reported to cause PA; HGMD (http://www.hgmd.org). Large deletions in PCCB gene are far less frequent than in the PCCA gene. The beta subunit of PCC enzyme contains a propionyl-CoA binding site that is responsible for transferring the carboxyl group to propionyl-CoA and most pathogenic variants in PCCB are predicted to alter the active site and reduce PCC activity [16].
In newborn screening programs, plasma acylcarnitine analysis using tandem mass spectrometry is the initial step in the diagnosis of PA [19] and is followed by urine organic acid analysis using gas chromatography/mass spectrometry [20]. Further confirmation can be pursued by measuring the PCC enzyme activity in leucocytes or fibroblasts and sequencing the PCCA and PCCB genes for pathogenic variants [13,21,22]. The Clavero et al. study also allows for genotype- phenotype correlation and prevention through reproductive genetic counselling. Apart from newborn screening, the diagnosis is usually established through clinical assessment and suspicion during a metabolic crisis and confirmed using the same biochemical and molecular tests described above [23].
Despite its high prevalence, many reports from Saudi Arabia describing PA and the corresponding molecular causes were in singleton cases [[24], [25], [26]]. In this study, we screened for sequence variants in both the PCCA and PCCB genes implicated in PA in 84 Saudi Arabian patients. To date, this is the largest cohort to be studied from this part of the world. The Saudi Arabian population has a tribal structure and the overall rate of consanguineous marriage is reported to be over 55%, with regional variations [27]. In such a population, the identification of a molecular genetic cause of PA allows both a confirmatory diagnosis, cascade carrier screening and prenatal testing for at-risk relatives.
Our study is aimed to describe the molecular basis of PA in Saudi Arabian patients and to determine possible founder variants in this population. Although there has been no significant genotype-phenotype correlation described in our population to date, this study attempts to draw a relevant relationship between the genotype and clinical phenotype in our patient cohort.
2. Materials and methods
2.1. Study cohort
The cohort consists of 84 Saudi Arabian patients from individual 84 families. PA diagnosis was based on newborn screening programme where elevation of C3-carnitine and C3-/C2-carnitine ratio was recorded in addition to clinical phenotype that include poor feeding, vomiting, lethargy, metabolic acidosis, and hyperammonemia. The study has been approved by the IRB of King Faisal Specialist Hospital, Riyadh, Saudi Arabia (RAC# 2020 011). DNA was extracted from peripheral blood cells using the Gentra Systems PUREGENE DNA Isolation kit (Qiagen, Valencia, California, USA).
2.2. Sequence variants analysis
Screening for sequence variants was undertaken of known genes implicated in PA; PCCA gene (RefSeq: NM_000282.3) and PCCB gene (RefSeq: NM_000532.4). Direct sequencing of all coding exons and exon–intron boundaries was performed for both genes. Oligonucleotide primers for PCR amplification of genomic DNA were designed using Primer3 software (http://frodo.wi.mit.edu/) and synthesized by Metabion International AG (Munich, Germany). PCR was performed in a final volume of 25 μl containing approximately 20 ng of genomic DNA and Qiagen (Manchester, UK) master mix kit (including 1× PCR buffer, 100 mmol l−1 dNTP, and 1 U per reaction HotStar Taq polymerase) and 0.5 mmol l−1 primer. PCR products were treated with the Agencourt AMPure PCR purification system (Agencourt Bioscience Corporation, Beverly, MA, USA). PCR products were sequenced using BigDye Terminator Cycle Sequencing kit (PE Applied Biosystems, Beverly, MA, USA) as described by the manufacturer. Sequences were analysed using Mutation Surveyor software Version 3.24 (SoftGenetics LLC, State College, PA, USA) and SeqMan II software 6.1 (DNAStar, Madison, WI, USA). For predicting the damaging effect of novel missense variants, three in silico prediction tools were used: PolyPhen-2 (http://genetics.bwh.harvard.edu/pph/), Provean (http://provean.jcvi.org/index.php), and Mutation Taster (http://www.mutationtaster.org/). PolyPhen-2 scores range from 0 to 1, the higher the score the more damaging the amino-acid substitution. Provean scores range from 0 to 1. The amino-acid substitution is predicted damaging if the score is ≤0.05, and tolerated if the score is >0.05. Allele frequency of all novel sequence variants was determined using a local database (SGP737) of 550 patients containing Arab-specific variants and the ExAC database (http://exac.broadinstitute.org/). Evolutionary conservation was determined from sequence alignments using Mutation Taster and UCSC (https://genome.ucsc.edu).
Protein folding predictions were generated with the Phyre2 application [28] (http://www.sbg.bio.ic.ac.uk/phyre2) using the ‘intensive’ modelling mode.
3. Results and discusion
Eighty four individual cases affected with PA representing 84 families from Saudi Arabia were screened for sequence variants in the PCCA and PCCB genes. A molecular genetic diagnosis was established in all families studied. A total of sixteen different sequence variants were detected in the study, where 7 pathogenic variants were detected in PCCA and 9 in PCCB (Table 1). Pathogenic variants in PCCA represent over 80% of genetically proven PA in this population. Previously published data from a world-wide cohort suggested that ~50% of PA is caused by pathogenic variants in PCCA gene and other 50% by variants in PCCB gene [15]. Although it has been reported that most PCCA pathogenic variants are private [15]; the missense variant (c.425G > A; p.Gly142Asp) in PCCA is the most common cause of PA in our cohort and was found in 59 patients (70.2%). All sequence variants detected in our cohort were in homozygous state except one family who was compound heterozygous.
Table 1.
Mutations in PCCA and PCCB genes identified in Saudi Arabian cases with Propionic Acidemia (PA).
| Gene | Nucleotide change | Amino acid change | Number of families | Reference |
|---|---|---|---|---|
| PCCA | c.111_130del20 | Cys44Leufs*6 | 3 | This study |
| PCCA | c.266C > G | Thr89Arg | 1 | This study |
| PCCA | c.425G > A | Gly142Asp | 59 | [26] |
| PCCA | c.955C > T | Gln319* | 1 | This study |
| PCCA | c.1288C > T | Arg430* | 2 | [29] |
| PCCA | c.1375_1391dup + c.1391ins | 1 | This study | |
| PCCA | c.2120 T > G | Val707Gly | 1 | This study |
| PCCB | c.90_108del(19N)ins(14N) | Ser30Argfs*25 | 1 | This study |
| PCCB | c.337C > T | Arg113* | 1 | [41] |
| PCCB | c.518_543del26 | Leu173* | 1 | This study |
| PCCB | c.629 T > C | Leu210Pro | 1 | This study |
| PCCB | c.866G > C | Arg289Pro | 1 | This study |
| PCCB | c.990dupT | Glu331* | 7 | [16] |
| PCCB | c.1088C > T | Ser363Leu | 1 | This study |
| PCCB | c.1163 T > A | Leu388His | 2 | This study |
| PCCB | c.1210G > A | Glu404Lys | 1 | [26] |
Novel mutations are in bold
Of the novel homozygous or compound heterozygous sequence variants in coding regions (Table 2 and Fig. 1), we identified 1 novel nonsense variant, 4 frameshift variants and 6 missense variants (11 novel disease causing variants). In silico predictions suggested that the missense changes are indeed pathogenic affecting the function of PCC (Table 2). A detailed analysis of the results for PCCA and PCCB genes is given below:
Table 2.
Novel genetic variants detected in PCCA and PCCB genes and in silico analysis of pathogenicity.
| Gender | Date of birth | Gene | Nucleotide change | Amino-acid change | Zygosity | Mutation category | PolyPhen-2 | SIFT | Provean | MutationTaster | Age of diagnosis | Clinical Features | Laboratory findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 06 Jul 2014 | PCCA | c.111_130del20 | Tyr39Asnfs*5 | Homozygous | frame-shift | N/A | N/A | N/A | N/A | Neonatal period | ID Hypotonia, Recurrent MC, ECEPH and pancreatitis | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia and increased serum lipase |
| Male | 12 Aug 2007 | PCCA | c.266C > G | Thr89Arg | Homozygous | Missense | Probably damaging | Damaging | Deleterious | Disease causing | ID Hypotonia, Recurrent MC, ECEPH. infrequent pancreatitis, SZ disorder | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia and increased serum lipase | |
| Male | 01 Oct 2013 | PCCA | c.955C > T | Gln319* | Homozygous | Nonsense | N/A | N/A | N/A | N/A | Mild ID, rare MC | metabolic acidosis | |
| Male | 05 May 2009 | PCCA | c.1375_1391dup + c.1391ins | Homozygous | frame-shift | N/A | N/A | N/A | N/A | Neonatal period | ID Hypotonia, Recurrent MC, ECEPH pancreatitis | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia and increased serum lipase | |
| Female | 01 Aug 1991 | PCCA | c.2120 T > G | Val707Gly | Homozygous | Missense | Probably damaging | Damaging | Deleterious | Disease causing | ID, Hypotonia, Recurrent MC ECEPH | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia | |
| Male | 1993 | PCCB | c.90_108delins14 | Ser30Argfs*25 | Homozygous | frame-shift | N/A | N/A | N/A | N/A | ID, Hypotonia, Recurrent MC, ECEPH, Pancreatitis | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia | |
| Male | 04 Oct 2010 | PCCB | c.518_543del26 | Leu173* | Homozygous | frame-shift | N/A | N/A | N/A | N/A | Neonatal period | ID, Hypotonia, Recurrent MC, ECEPH pancreatitis choreoathetoid movements | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia |
| Female | 19 Oct 2010 | PCCB | c.629 T > C | Leu210Pro | homozygous | Missense | Probably damaging | Damaging | Deleterious | Disease causing | Neonatal period | ID, Hypotonia, Recurrent MC, ECEPH pancreatitis choreoathetoid movements | Recurrent; metabolic acidosis, hyperammonemia, hypoglycemia ketonuria. Pancytopenia increased serum lipase |
| Male | 22 Oct 2000 | PCCB | c.866G > C/c.990dupT | Arg289Pro Glu331*/ | Compound heterozygous | Missense | Probably damaging | Tolerated | Deleterious | Disease causing | ID, Hypotonia, Recurrent MC less with age, ECEPH infrequent pancreatitis, SZ disorder, | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia increased serum lipase | |
| Male | 12 Sep 2011 | PCCB | c.1088C > T | Ser363Leu | Homozygous | Missense | Possibly damaging | Damaging | Deleterious | Disease causing | Neonatal period | ID, Hypotonia, Recurrent MC, ECEPH pancreatitis preterm. | Recurrent; metabolic acidosis, hyperammonemia, hypoglycemia ketonuria. Pancytopenia increased serum lipase |
| Female | 15 Apr 2004 | PCCB | c.1163 T > A | Leu388His | Homozygous | Missense | Probably damaging | Damaging | Deleterious | Disease causing | ID, Hypotonia, Recurrent MC ECEPH | Recurrent; metabolic acidosis, hyperammonemia, ketonuria. Pancytopenia |
ID: Intellectual Disability, SZ: Seizure, MC: MC, ECEPH: encephalopathy
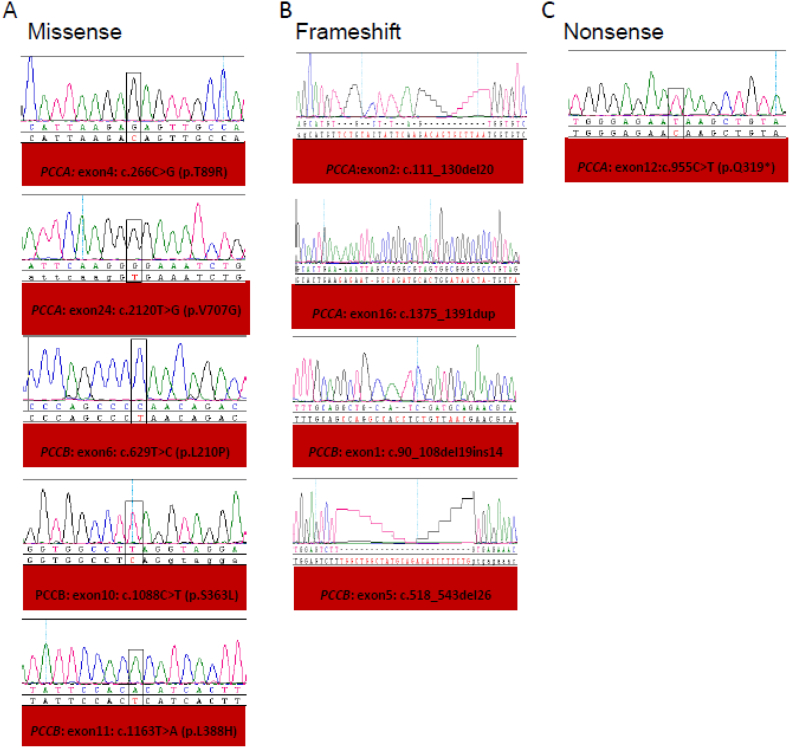
Fig. 1.
Novel mutations identified in cohort with PA phenotypes in Saudi population (A) Five missense (B) Four frame shift and (C) One nonnense novel mutations (boxed) were detected homozygously in patients. Normal sequence is show alongside.
3.1. PCCA gene mutational analysis
All variants in affected individuals were found in a homozygous state consistent with known parental consanguinity. Variants detected in PCCA accounted for around 81% of all the variants detected in our cohort. In this gene we found 2 known pathogenic variants [26,29] and 5 novel homozygous sequence variants predicted to affect PCC function (Table 1, Table 2). The novel pathogenic variants included two frameshift variants, two missense variants and one nonsense variant (each variant was found privately in one family only). The novel c.955C > T nonsense variant (p.Gln319*) was located near a previously characterized nonsense variant p.Arg313* [30] that was reported to show a significance response to different readthrough drugs [31]. Interestingly, the patient homozygous for the novel nonsense variant (p.Gln319*) showed a milder phenotype in comparison to other similar variants detected. However, the patient passed away at age of 4 years with cardiopulmonary arrest. The c.111_130del20 frameshift variant (p.Cys44Leufs*6) is the most premature truncating variant reported so far in PCCA. The other c.1375_1391dup17 frameshift variant is a complex insertion/duplication variant. The c.1375_1391dup17 frameshift variant is a complex insertion/duplication variant. This complex variant involves a duplication of 17 nucleotides in exon 16 of the PCCA gene starting at nucleotide 1375 and between the duplicated regions a large fragment with poly (A) rich sequence (~200 bp) is inserted. This variant is predicted to be disease-causing. Finally, the c.1288C > T nonsense variant (p.Arg430*) that has been reported previously [29] was found in two families of our cohort (Table 1).
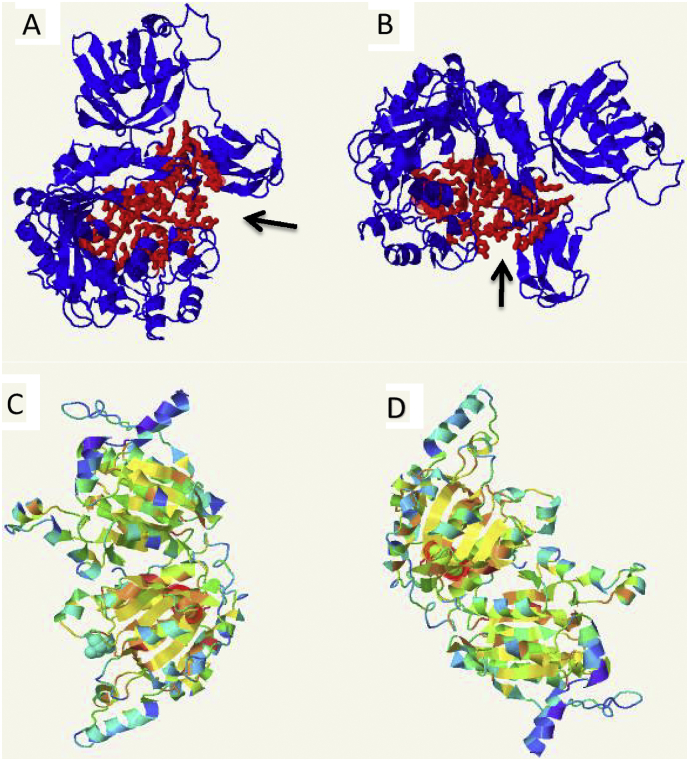
In silico analysis suggested the pathogenicity of two of the novel (p.Thr89Arg and p.Val707Gly) missense variants detected in the study (Table 2). Both sequence variants were not found in our local database; SGP737 (http://shgp.kfshrc.edu.sa/bioinf/db/variants/mendliome/) nor found in the ExAC database or 1000 Genome. Among our PA cohort there were 59 patients (Table 1) with the pathogenic missense variant (c.425G > A; p.Gly142Asp). This pathogenic variant has been reported previously by colleagues in two PA patients in association with visual hallucinations [26] and by another group [8]. The variant p.Gly142Asp is located in the “A” subdomain of biotin carboxylase domain of the PCC enzyme. The A and C subdomains form the active site of PCCA subunit where p.Gly142 is part of active site and thus predicted to affect the catalysis or substrate binding [11]. Using fpocket2 program [32] where large pockets are frequently found to be the location of active site, the protein folding predicted for the p.Gly142Asp variant is showing a conformational change in the location of the active site of the protein when compared to normal folding (Fig. 2A & B). The p.Gly142Asp variant is the most common pathogenic variant in our cohort and would be considered as a founder pathogenic variant in Arabs.
Fig. 2.
Peptide modelling of 2 missense mutations in PCCA and PCCB genes; Arrows in A & B are showing predected active site (in red) in PCCA where A represent wild type PCCA and B represent PCCA with the mutation Gly142Asp. C is showing normal configration of PCCB while D is showing the configration with changing the amino acid Leu to His at position 388. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
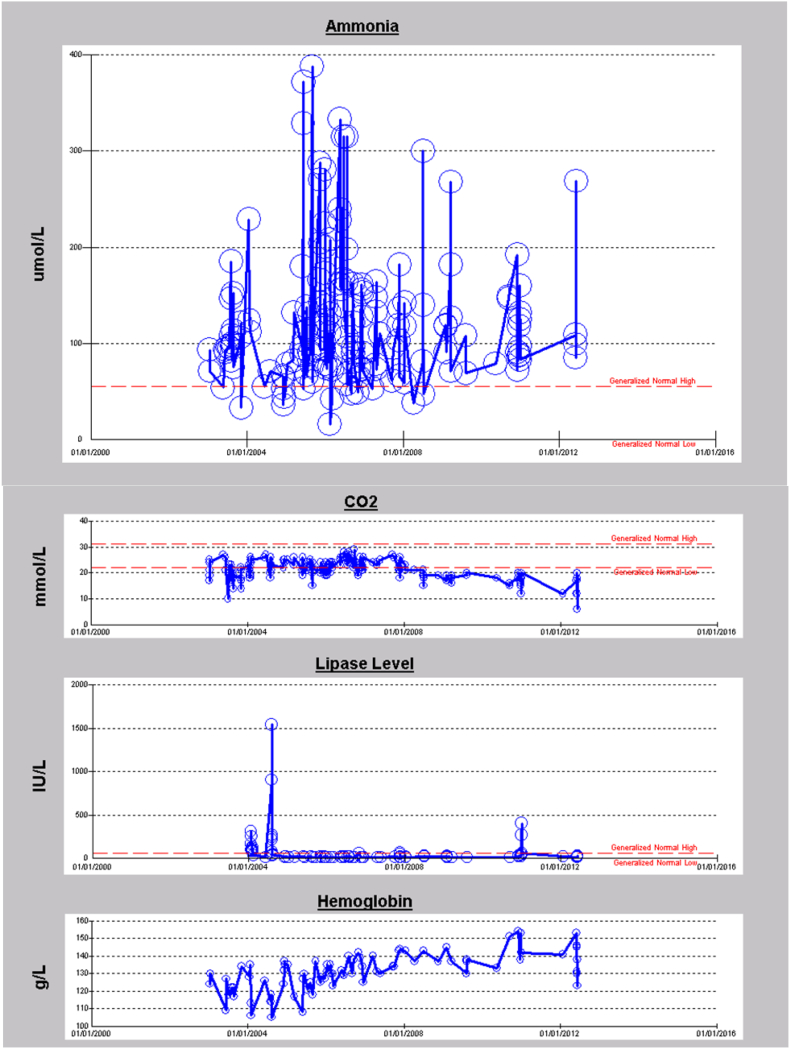
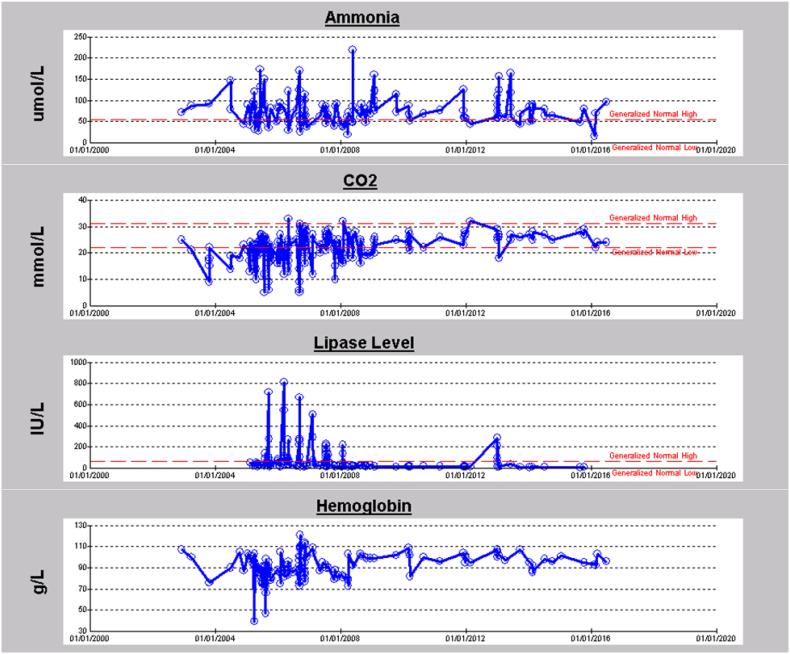
Patients with the p.Gly142Asp variant presented with a moderate-severe phenotype including developmental delay, metabolic crisis, hypotonia and pancreatitis. Fig. 3A and B represent longitudinal data of two PA patients with a homozygous p.Gly142Asp variant. Biochemical measurements of ammonia (NH3), carbon dioxide (CO2), lipase enzyme level, and haemoglobin for eight years period for the first patient (Fig. 3A) are shown. The patient had frequent episodes of hyperammonemia, recurrent metabolic acidosis, mild anemia and infrequently elevated lipase levels as indicative of pancreatitis. The patient had a sudden death at 17 years of age while admitted with a mild crisis. The second patient (Fig. 3B) presented with recurrent hyperammonemia, metabolic acidosis, pancreatitis, mild anemia, intellectual disability, and attention deficit hyperactivity disorder (ADHD). The patient is currently 15 years old and still alive, and the corresponding data shown is for almost a 14 year period. Although most variants reported in PCCA and PCCB genes are private, we report here the first founder pathogenic variant in PCCA gene in our population.
Fig. 3.
A. 8 years longitudinal data for first patient with a homozygous p.Gly142Asp mutation in PCCA gene. B. 14 years longitudinal data for second patient with a homozygous p.Gly142Asp mutation in PCCA gene.
3.2. Genotype-phenotype correlation for the missense variant (c.425G > A; p.Gly142Asp)
Although the overall clinical presentation of patients with this genotype is not too distinct from the reported classical clinical course of early onset PA [[33], [34], [35]], several observations are summarized below based on the longitudinal follow up of patients up to almost 20 years: a) all patients present in the neonatal period with symptoms of poor feeding, vomiting, lethargy, hypotonia, tachypnoea, variable metabolic acidosis compensated by respiratory alkalosis. The condition proceeds rapidly to acute encephalopathy characterized by variably depressed level of consciousness eventually leading to coma. Hyperammonemia is a constant feature in the neonatal period and can be severe enough to require hemofiltration. If treated early and aggressively the majority of the patients recover; b) and then present with an unpredictable recurrent metabolic crisis characterized with variable degree of hyperammonemia, metabolic acidosis, and pancreatitis with elevated lipase and amylase. Metabolic acidosis is often more severe when pancreatitis is present and requires longer period of hydration and hospitalization for recovery. Anemia, which often becomes chronic with acute exacerbations during crisis secondary to bone marrow suppression and frequent blood extraction, is commonly observed (Fig. 3A and B). Leukopenia and thrombocytopenia is often but not always observed, it usually appears a few days into crisis; c) presentation with only metabolic acidosis or pancreatitis is also observed. Presentation with only hyperammonemia is also observed, however much more commonly in older patients; d) another common observation is the dissociation of hyperammonemia and clinical symptoms as patients grow older. It seems that the tolerance of neurons to hyperammonemia increases with age. It is common to see patients with serum ammonia of over 200 μmol/l who are completely active with no symptoms or are oligosymptomatic with only nausea and a few episodes of vomiting; e) visual hallucinations and autism has also been reported in patients with this genotype [24,26] f) survival is usually limited to 20 years of age. Triphasic mortality is observed. Some patients die in the neonatal period due to severe crisis, although this is becoming less common with newborn screening and precautionary measures for at risk neonates, death around the first decade and then 2nd decade of life. The cause of death in the later years is usually a combination of poor overall health due to chronic disease, intercurrent infection often related to an indwelling central line, aspiration pneumonia, metabolic acidosis, pancreatitis, metabolic encephalopathy with hyperammonemia, stroke involving the basal ganglia and brain oedema. This relatively homogeneous PA patient population may provide an excellent opportunity for clinical trials for emerging therapies for this disease.
3.3. PCCB gene mutational analysis
Among our PA cohort there were 16 families with PCCB sequence variants (Table 1). All variants in affected individuals were in a homozygous state except one family where a compound heterozygous variant was found. Pathogenic variants detected in PCCB accounted for around 19% of all the variants detected in our cohort. A total of 9 variants were detected in PCCB in this cohort. Six of these variants are novel and not reported previously; four of which missense and 2 frameshift variants (Table 2). The c.90_108delins14 frameshift variant was found in one family and it involves the deletion of 19 nucleotides and insertion of 14 nucleotides (gctgcatcgatgca), resulting in premature protein truncation (p. S30Rfs*25). The second novel c.518_543del26 frameshift variant (p.Leu173*) was also found in one family leading to protein truncation. Interestingly, Lévesque et al. [36] described a smaller deletion at same position c.517_518delTT (p.L173Gfs*56) causing truncation.
All missense variants detected were located at the C-terminus of propionyl-CoA carboxylase beta chain apart from the c.629 T > C variant (p.Leu210Pro), located at the N-terminus of the beta chain of PCC. The c.1163 T > A variant (p.Leu388His) was detected in two different families while 6 other novel PCCB variants were found in one family each. Modelling of p.Leu388His using Phyer2 showed configuration change of the protein in comparison to the normal protein (Fig. 2C & D).The novel c.866G > C missense variant (p.Arg289Pro) was detected in a compound heterozygous state with another reported c.990dupT frameshift variant (p.Glu331X) [37]. Regarding the novel c.1088C > T missense variant (p.Ser363Leu), the serine residue at position 363 (p.S363) is mutated to leucine in our cohort but was shown to be mutated to proline in another study [38]. The reported variant c.990dupT (p.Glu331*) was found in 7 families and accounted for 44% of variants in PCCB. In our cohort, there are 4 PCCB variants detected leading to premature protein truncation; Ser30Argfs*25, Arg113*, Leu173*, and Glu331*.
4. Conclusions
To date, this study is the largest to describe molecular basis of PA in the Saudi Arabian population. To summarize, all patients with identified variants in PA associated genes were from consanguineous marriages and most of the pathogenic variants identified were in the homozygous state. In our study, which included 84 families, pathogenic variants were detected in all cases. Such a high mutation detection rate would not be achieved without a comprehensive and confirmatory clinical assessment and biochemical analysis. All sequence variants detected were considered to be pathogenic; we carefully checked for the presence of additional variants in both genes (PCCA and PCCB) and confirmed the variant co-segregated, where parental samples were available. The allele frequency of all novel sequence variants was zero in all databases checked. Most missense variants detected in PCCA and PCCB in the altered amino acids in conserved regions of the protein. The p.Gly142Asp variant in PCCA is the most common cause of PA in our population followed by the Glu331* variant in PCCB, while all other variants detected were private familial variants. The p.Gly142Asp affects almost 70% of our PA patients and no such variant frequency has been reported in other populations studied so far [15,[38], [39], [40]]. The presence of a common pathogenic variant in PCCA signifies the homogeneity of Saudi Arabian population. The molecular genetic diagnosis of PA is a vital aid to the clinical management of families with PA, cascade carrier screening and prenatal testing for at-risk relatives.
In conclusion, in a Saudi Arabian cohort of families with PA we were able to detect disease causing variants in all families in the known propionic acidemia genes; PCCA and PCCB. Pathogenic variants in PCCA were the most common molecular genetic cause of PA in our cohort. Our study confirms the c.425G > A (p.Gly142Asp) variant to be the most common and a founder variant causing PA in the Saudi Arabian population. A clear insight into a genotype-phenotype correlation for this pathogenic variant is documented here.
Disclosure statement
The authors declare no conflicts of interest.
Acknowledgments
We thank the families for participating in the study. Also we thank sequencing core facility at Genetics Department at the Research Centre, King Faisal Specialist Hospital and Research Centre for doing sequencing.
References
- 1.Hsia Y.E., Scully K.J., Rosenberg L.E. Inherited propionyl-Coa carboxylase deficiency in “ketotic hyperglycinemia”. J. Clin. Invest. 1971;50:127–130. doi: 10.1172/JCI106466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rashed M., Ozand P.T., al Aqeel A., Gascon G.G. Experience of king faisal specialist hospital and research center with Saudi organic acid disorders. Brain Dev. 1994;16(Suppl):1–6. doi: 10.1016/0387-7604(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 3.Al Essa M., Rahbeeni Z., Jumaah S., Joshi S., Al Jishi E., Rashed M.S., Al Amoudi M., Ozand P.T. Infectious complications of propionic acidemia in Saudia Arabia. Clin. Genet. 1998;54:90–94. doi: 10.1111/j.1399-0004.1998.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Odaib A.N., Abu-Amero K.K., Ozand P.T., Al-Hellani A.M. A new era for preventive genetic programs in the Arabian Peninsula. Saudi Med. J. 2003;24:1168–1175. [PubMed] [Google Scholar]
- 5.Rafique M. Propionic acidaemia: demographic characteristics and complications. J. Pediatr. Endocrinol. Metab. 2013;26:497–501. doi: 10.1515/jpem-2013-0031. [DOI] [PubMed] [Google Scholar]
- 6.Al-Rikabi A.C., Al-Homsi H.I. Propionic acidemia and zinc deficiency presenting as necrolytic migratory erythema. Saudi Med. J. 2004;25:660–662. [PubMed] [Google Scholar]
- 7.Moammar H., Cheriyan G., Mathew R., Al-Sannaa N. Incidence and patterns of inborn errors of metabolism in the Eastern Province of Saudi Arabia, 1983–2008. Ann. Saudi Med. 2010;30:271–277. doi: 10.4103/0256-4947.65254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfadhel M., Benmeakel M., Hossain M.A., Al Mutairi F., Al Othaim A., Alfares A.A., Al Balwi M., Alzaben A., Eyaid W. Thirteen year retrospective review of the spectrum of inborn errors of metabolism presenting in a tertiary center in Saudi Arabia. Orphanet J. Rare Dis. 2016;11:126. doi: 10.1186/s13023-016-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zayed H. Propionic acidemia in the Arab World. Gene. 2015;564:119–124. doi: 10.1016/j.gene.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Ozand P.T., Rashed M., Gascon G.G., Youssef N.G., Harfi H., Rahbeeni Z., al Garawi S., al Aqeel A. vol. 16 Suppl. 1994. Unusual Presentations of Propionic Acidemia Brain & Development; pp. 46–57. [DOI] [PubMed] [Google Scholar]
- 11.Huang C.S., Sadre-Bazzaz K., Shen Y., Deng B., Zhou Z.H., Tong L. Crystal structure of the alpha(6)beta(6) holoenzyme of propionyl-coenzyme A carboxylase. Nature. 2010;466:1001–1005. doi: 10.1038/nature09302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennerknecht I., Suormala T., Barbi G., Baumgartner E.R. The gene coding for the alpha-chain of human propionyl-CoA carboxylase maps to chromosome band 13q32. Hum. Genet. 1990;86:238–240. doi: 10.1007/BF00197713. [DOI] [PubMed] [Google Scholar]
- 13.Clavero S., Martinez M.A., Perez B., Perez-Cerda C., Ugarte M., Desviat L.R. Functional characterization of PCCA mutations causing propionic acidemia. Biochim. Biophys. Acta. 2002;1588:119–125. doi: 10.1016/s0925-4439(02)00155-2. [DOI] [PubMed] [Google Scholar]
- 14.Stankovics J., Ledley F.D. Cloning of functional alpha propionyl CoA carboxylase and correction of enzyme deficiency in pccA fibroblasts. Am. J. Hum. Genet. 1993;52:144–151. [PMC free article] [PubMed] [Google Scholar]
- 15.Kraus J.P., Spector E., Venezia S., Estes P., Chiang P.W., Creadon-Swindell G., Mullerleile S., de Silva L., Barth M., Walter M., Walter K., Meissner T., Lindner M., Ensenauer R., Santer R., Bodamer O.A., Baumgartner M.R., Brunner-Krainz M., Karall D., Haase C., Knerr I., Marquardt T., Hennermann J.B., Steinfeld R., Beblo S., Koch H.G., Konstantopoulou V., Scholl-Burgi S., van Teeffelen-Heithoff A., Suormala T., Ugarte M., Sperl W., Superti-Furga A., Schwab K.O., Grunert S.C., Sass J.O. Mutation analysis in 54 propionic acidemia patients. J. Inherit. Metab. Dis. 2012;35:51–63. doi: 10.1007/s10545-011-9399-0. [DOI] [PubMed] [Google Scholar]
- 16.Desviat L.R., Perez B., Perez-Cerda C., Rodriguez-Pombo P., Clavero S., Ugarte M. Propionic acidemia: mutation update and functional and structural effects of the variant alleles. Mol. Genet. Metab. 2004;83:28–37. doi: 10.1016/j.ymgme.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Pombo P., Hoenicka J., Muro S., Perez B., Perez-Cerda C., Richard E., Desviat L.R., Ugarte M. Human propionyl-CoA carboxylase beta subunit gene: exon-intron definition and mutation spectrum in Spanish and Latin American propionic acidemia patients. Am. J. Hum. Genet. 1998;63:360–369. doi: 10.1086/301970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Cerda C., Clavero S., Perez B., Rodriguez-Pombo P., Desviat L.R., Ugarte M. Functional analysis of PCCB mutations causing propionic acidemia based on expression studies in deficient human skin fibroblasts. Biochim. Biophys. Acta. 2003;1638:43–49. doi: 10.1016/s0925-4439(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 19.Rashed M.S., Ozand P.T., Bucknall M.P., Little D. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr. Res. 1995;38:324–331. doi: 10.1203/00006450-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Wojtowicz P., Zrostlikova J., Kovalczuk T., Schurek J., Adam T. Evaluation of comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the diagnosis of inherited metabolic disorders using an automated data processing strategy. J. Chromatogr. A. 2010;1217:8054–8061. doi: 10.1016/j.chroma.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 21.Gravel R.A., Lam K.F., Scully K.J., Hsia Y. Genetic complementation of propionyl-CoA carboxylase deficiency in cultured human fibroblasts. Am. J. Hum. Genet. 1977;29:378–388. [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders M., Sweetman L., Robinson B., Roth K., Cohn R., Gravel R.A. Biotin-response organicaciduria. Multiple carboxylase defects and complementation studies with propionicacidemia in cultured fibroblasts. J. Clin. Invest. 1979;64:1695–1702. doi: 10.1172/JCI109632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner M.R., Horster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A., Huemer M., Hochuli M., Assoun M., Ballhausen D., Burlina A., Fowler B., Grunert S.C., Grunewald S., Honzik T., Merinero B., Perez-Cerda C., Scholl-Burgi S., Skovby F., Wijburg F., MacDonald A., Martinelli D., Sass J.O., Valayannopoulos V., Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Owain M., Kaya N., Al-Shamrani H., Al-Bakheet A., Qari A., Al-Muaigl S., Ghaziuddin M. Autism spectrum disorder in a child with propionic acidemia. JIMD Rep. 2013;7:63–66. doi: 10.1007/8904_2012_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaya N., Al-Owain M., Albakheet A., Colak D., Al-Odaib A., Imtiaz F., Coskun S., Al-Sayed M., Al-Hassnan Z., Al-Zaidan H., Meyer B., Ozand P. Array comparative genomic hybridization (aCGH) reveals the largest novel deletion in PCCA found in a Saudi family with propionic acidemia. Eur. J. Med. Genet. 2008;51:558–565. doi: 10.1016/j.ejmg.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Shuaib T., Al-Hashmi N., Ghaziuddin M., Megdad E., Abebe D., Al-Saif A., Doubi A., Aldhalaan H., Abouzied M.E., Al-Owain M. Propionic acidemia associated with visual hallucinations. J. Child Neurol. 2012;27:799–803. doi: 10.1177/0883073811426929. [DOI] [PubMed] [Google Scholar]
- 27.Al-Abdulkareem A.A., Ballal S.G. Consanguineous marriage in an urban area of Saudi Arabia: rates and adverse health effects on the offspring. J. Community Health. 1998;23:75–83. doi: 10.1023/a:1018727005707. [DOI] [PubMed] [Google Scholar]
- 28.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nizon M., Ottolenghi C., Valayannopoulos V., Arnoux J.B., Barbier V., Habarou F., Desguerre I., Boddaert N., Bonnefont J.P., Acquaviva C., Benoist J.F., Rabier D., Touati G., de Lonlay P. Long-term neurological outcome of a cohort of 80 patients with classical organic acidurias. Orphanet J. Rare Dis. 2013;8:148. doi: 10.1186/1750-1172-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Alcudia R., Perez B., Ugarte M., Desviat L.R. Feasibility of nonsense mutation readthrough as a novel therapeutical approach in propionic acidemia. Hum. Mutat. 2012;33:973–980. doi: 10.1002/humu.22047. [DOI] [PubMed] [Google Scholar]
- 31.Perez B., Rodriguez-Pombo P., Ugarte M., Desviat L.R. Readthrough strategies for therapeutic suppression of nonsense mutations in inherited metabolic disease. Mol. Syndromology. 2012;3:230–236. doi: 10.1159/000343086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Guilloux V., Schmidtke P., Tuffery P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinforma. 2009;10:168. doi: 10.1186/1471-2105-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafique M. Clinical spectrum of propionic acidaemia. J. Nutr. Metab. 2013;2013 doi: 10.1155/2013/975964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafique M. Emerging trends in management of propionic acidemia. Arquivos brasileiros de endocrinologia e metabologia. 2014;58:237–242. doi: 10.1590/0004-2730000002821. [DOI] [PubMed] [Google Scholar]
- 35.Selim L.A., Hassan S.A., Salem F., Orabi A., Hassan F.A., El-Mougy F., Mahmoud I.G., El-Badawy A., Girgis M.Y., Elmonem M.A., Mehaney D. Selective screening for inborn errors of metabolism by tandem mass spectrometry in Egyptian children: a 5 year report. Clin. Biochem. 2014;47:823–828. doi: 10.1016/j.clinbiochem.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Levesque S., Lambert M., Karalis A., Melancon S., Russell L., Braverman N. Short-term outcome of propionic aciduria treated at presentation with N-carbamylglutamate: a retrospective review of four patients. JIMD Rep. 2012;2:97–102. doi: 10.1007/8904_2011_54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez B., Desviat L.R., Rodriguez-Pombo P., Clavero S., Navarrete R., Perez-Cerda C., Ugarte M. Propionic acidemia: identification of twenty-four novel mutations in Europe and North America. Mol. Genet. Metab. 2003;78:59–67. doi: 10.1016/s1096-7192(02)00197-x. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z., Wen P., Wang G., Hu Y., Liu X., Chen L., Chen S., Wan L., Cui D., Shang Y., Li C. Analysis of PCCA and PCCB gene mutations in patients with propionic acidemia. Zhonghua yi xue yi chuan xue za zhi. 2015;32:26–30. doi: 10.3760/cma.j.issn.1003-9406.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Rivera-Barahona A., Navarrete R., Garcia-Rodriguez R., Richard E., Ugarte M., Perez-Cerda C., Perez B., Gamez A., Desviat L.R. Identification of 34 novel mutations in propionic acidemia: functional characterization of missense variants and phenotype associations. Mol. Genet. Metab. 2018;125(3):266–275. doi: 10.1016/j.ymgme.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Gupta D., Bijarnia-Mahay S., Kohli S., Saxena R., Puri R.D., Shigematsu Y., Yamaguchi S., Sakamoto O., Gupta N., Kabra M., Thakur S., Deb R., Verma I.C. Seventeen novel mutations in PCCA and PCCB genes in Indian propionic acidemia patients, and their outcomes. Genet. Test. Mol. Biomark. 2016;20:373–382. doi: 10.1089/gtmb.2016.0017. [DOI] [PubMed] [Google Scholar]
- 41.Brosch S. [Propionic acidemia and sensorineural hearing loss: is there a connection at the molecular genetics level?] HNO. 2008;56(1):37–42. doi: 10.1007/s00106-007-1560-6. [DOI] [PubMed] [Google Scholar]