Abstract
Objectives:
Cannabis smokers often report that they use the drug to relax or to relieve emotional stress. However, few clinical studies have shown evidence of the stress-relieving effects of cannabis or cannabinoid agonists. In this study, we sought to assess the influence of delta-9-tetrahydrocannabinol (THC), a main active ingredient of cannabis, upon emotional responses to an acute psychosocial stressor among healthy young adults.
Methods:
Healthy volunteers (N=42) participated in two experimental sessions, one with psychosocial stress (Trier Social Stress Test, TSST) and another with a non-stressful task, after receiving 0 (N=13), 7.5 mg (N=14) or 12.5mg (N=15) oral THC. Capsules were administered under randomized, double blind conditions, 2.5-h before the tasks began. We measured subjective mood and drug effects, vital signs and salivary cortisol before and at repeated times after the capsule and tasks. Subjects also appraised the tasks, before and after completion.
Results:
In comparison to placebo, 7.5mg THC significantly reduced self-reported subjective distress after the TSST and attenuated post-task appraisals of the TSST as threatening and challenging. By contrast, 12.5mg THC increased negative mood overall i.e., both before and throughout the tasks, and pre-task ratings of the TSST as threatening and challenging. It also impaired TSST performance and attenuated blood pressure reactivity to the stressor.
Conclusions:
Our findings suggest that a low dose of THC produces subjective stress-relieving effects in line with those commonly reported among cannabis users, but that higher doses may non-specifically increase negative mood.
Keywords: THC, TSST, acute stress, subjective effects, anxiety, stress-coping
1. Introduction
It is well established that people take drugs for the subjective experiences that they produce. One of the most commonly cited motives for cannabis use is for stress-relief (tension-reduction) or relaxation (Hyman and Sinha, 2009, Lee et al., 2007), yet there are few empirical studies that demonstrate calming subjective effects of cannabis. In fact, there is clinical and preclinical evidence that delta-9-tetrahydrocannabinol (THC), the main active ingredient of cannabis, can produce anxiety-like behaviors and anxiogenic effects (D’Souza et al., 2004; Onaivi et al., 1990). In this study, we sought to evaluate the purported stress-relieving effects of THC by assessing the influence of the drug upon emotional responses to acute psychosocial stress among non-daily cannabis users.
Cannabis users express a range of motives for use of the drug (Hyman and Sinha, 2008; Simons et al., 2000) including for enhancement (e.g. of positive mood, to get high), coping (e.g. to reduce negative mood, cope with worries, relax), recreational and social (e.g. to be sociable, ‘partying’), conformity (e.g. so others won’t kid me), and expansion (e.g. to expand awareness, be more creative). One of the most commonly expressed reasons for use is for stress-coping or relaxation, and this is remarkably higher than that reported for other drugs (Segal et al., 1982). Coping motives are associated with use and a greater percentage of daily users than non-daily users (72% vs. 53% respectively) report using the drug to relax or relieve tension (Johnston and O’Malley, 1986). Considering these widespread reports of calming effects of cannabis, it is surprising that few studies have specifically sought to empirically measure these subjective effects in controlled studies.
Clinical studies with cannabis or THC most often report that the drugs increase feelings of anxiety and tension (Crippa et al., 2009). In a study using intravenous THC (2.5, 5mg), D’Souza et al., 2004 reported increases in anxiety among individuals with a wide range of cannabis use history (i.e., from <5 to >100 times lifetime use). More recently, Hunault et al., (2014) reported dose-dependent effects of cannabis cigarettes (29–69mg THC) upon anxiety (increased) and calmness (decreased) among occasional users (2–9 cannabis cigarettes/month). Yet, others have reported an increase in feelings of relaxation after smoked cannabis (3–4% THC) and oral THC (20mg) among daily users (Hart et al., 2001, 2002). One review of the evidence concluded that anxiogenic effects of cannabis and THC are greater at high doses and among naïve users (Crippa et al., 2009). Nevertheless, the relaxing effects of cannabis and THC per se have been relatively understudied in empirical experiments perhaps because few studies include measures of calmness or feeling “mellow”. Moreover, the effects of THC upon situational distress i.e., emotional responses to an acute stressor, are also unclear.
Some studies have assessed the effects of THC on behavioral responses to an aversive stimulus. In laboratory animals, THC (0.075–1mg/kg) reduces anxiety-like behaviors in the elevated plus maze and light-dark box, and increases active coping responses in the forced swim stress test (Bambico et al., 2012; Berrendero and Maldonado, 2002; Braida et al., 2007). In one clinical study with non-daily cannabis users, 15mg THC reduced recognition of threatening faces (Ballard et al., 2012). In an imaging study, 7.5mg THC reduced amygdala responses to threatening faces and anterior cingulate responses to negative emotional images (Phan et al., 2008; Rabinak et al., 2012). THC (9mg) also reduced regional brain activity, including amygdala activity, when participants (non-daily users) viewed threatening faces and increased activity when they viewed happy faces (Bossong et al., 2013). The amygdala and cingulate are regions associated with fear and anxiety in both animal and human studies (Davis and Whalen, 2001; LeDoux, 2000), and also contain high concentrations of CB1 receptors (Katona, 2009). These brain areas are also involved in the regulation of hypothalamic pituitary adrenal axis (HPAA) responses to acute stressors (Herman et al., 2016). Together the evidence suggests that THC may dampen behavioral and neural responses to aversive stimuli, however, to date no studies have examined the effects of THC upon a tangible stress-inducing stimulus i.e., one that produces obvious emotional distress. It is important to evaluate the effects of THC upon an emotionally distressing experience to further investigate the widely reported stress-relieving effects of cannabis among smokers.
In the present study we examined the effect of THC upon emotional responses to a standardized laboratory test of acute psychosocial stressor, the Trier Social Stress Test (TSST; Kirschbaum et al., 1993). The TSST is a well-validated and widely used laboratory procedure that reliably increases subjective distress, cortisol, heart rate and blood pressure (Dickerson and Kemeny, 2004), and is sensitive to anxiolytic drugs (McNair et al., 1982). Healthy male and female participants received oral doses of THC (7.5 or 12.5 mg) or placebo before participating in the TSST or a non-stressful control task. We obtained measures of subjective distress, anticipatory and retrospective task appraisal ratings, heart rate, blood pressure and salivary cortisol before and at repeated times after participating in the tasks. Our primary hypothesis was that THC would attenuate negative emotional responses (i.e., subjective distress, retrospective threat appraisals) to the TSST. Secondary hypotheses concerned the effects of THC upon cardiovascular and cortisol responses to the TSST. First, in view of the established stimulatory effects of THC upon heart rate and blood pressure (Jones, 2002), we hypothesized that THC would potentiate cardiovascular responses to the TSST. Second, in line with reports suggesting that endocannabinoids inhibit HPAA activity (Hill and McEwen, 2010), we hypothesized that THC would also attenuate cortisol responses to the TSST.
2. Materials and Methods
2.1. Subjects
Healthy men and women (N=42) with some history of cannabis use were recruited from the community, and attended an in-person interview for psychological and medical screening. Eligibility requirements included age 18–40, good health, body mass index of 19–29 kg/m2 and ≥3 lifetime uses of cannabis, use in the past year, but current use not exceeding once a week. These criteria are similar to those used in previous clinical studies and limit the possibility of adverse reactions or tolerance to THC. Exclusion criteria were >20 tobacco cigarettes a week (Ginty et al., 2014), serious medical conditions, current or past year Axis I disorder (APA, 2013), substance dependence history (except nicotine), history of cannabis abuse (APA, 2013), abnormal electrocardiogram, prescription medications including hormonal contraceptives (Roche et al., 2013), or night shift work. Individuals who reported adverse effects of cannabis use (e.g., anxiety, racing heart) were excluded for ethical reasons and also to negate overly aversive reactions to the TSST. Participants had to abstain from cannabis use for 1-week before sessions and test negative for recent use at each session.
2.2. Procedure
The experiment was conducted according to the World Medical Association Declaration of Helsinki and the protocol was approved by the University of Chicago Hospital Institutional Review Committee (#11–0377). Written consent was obtained from all participants at an initial orientation (enrollment) session; the consent form stated that the study aimed to examine drug effects upon responses to verbal tasks. For blinding purposes it stated that participants might receive one of the following drug classes; 1) stimulant, 2) sedative, 3) cannabinoid, 4) opiate, or 5) placebo.
Participants attended two 4-h sessions, one with a psychosocial stress task (the TSST) and one with a non-stressful task (Control), in counterbalanced order. The sessions were conducted from 1pm to 5pm at the Human Behavioral Pharmacology Laboratory and separated by five days. Participants spent most of the sessions (when not performing the tasks) in comfortable testing rooms with a sofa, easy chair, television, desk, and a computer for administration of questionnaires. Figure 1 shows the timeline of procedures during each experimental session. Upon arrival, participants provided breath and urine samples to detect recent drug use. No one tested positive. Participants then relaxed for 20-min before providing baseline measures of subjective mood, vital signs and salivary cortisol. Five minutes later, they consumed a capsule containing 0, 7.5, or 12.5mg THC administered under double-blind conditions. Subjects were randomly assigned to dose conditions and subjects received the same dose at both sessions (TSST, Control). The doses were selected because they are behaviorally active without producing adverse effects or pronounced subjective or cardiovascular effects (Ballard et al., 2011; Curran et al., 2002) which could confound the interpretation of their effects on the task. Participants then relaxed for 2-h to allow drug absorption, providing further measures of subjective effects and vital signs at 60- and 120-min post-capsule. At 3pm (90-min post-capsule), they were given a heart rate monitor (watch and chest band) to wear that measured cardiovascular activity continuously throughout the rest of the session. At 120-min subjects provided salivary cortisol samples and pre-task assessments of subjective distress. The tasks were scheduled to begin 2.5-h post-capsule, at the time when plasma levels were expected to peak (Curran et al., 2002; Karschner et al., 2011). The doses were expected to produce plasma levels comparable to those attained 30-min after smoking cannabis (5–25ng/ml, Cone and Huestis, 1993; Karschner et al., 2011). At 135-min (2.25h) after capsule administration, task procedures began. First, the research assistant read the task instructions (TSST or Control) to the participant. Participants were given 10-min to prepare for the task. For the TSST condition only, subjects heard an audible “ticking” throughout, and alarm at the end of, the 10-min. At the end of the preparatory period for both TSST and Control conditions, participants rated their perceptions of the upcoming task on a pre-task appraisal questionnaire. They were then escorted to a separate room to perform the tasks. The TSST consisted of a 5-min speech followed by 5-min arithmetic (serial subtraction) performed in front of two interviewers who were unknown to the participant. Subjects also viewed a video display of their interview. The 10-min Control task consisted of a 5-min conversation with the research assistant about a favorite book, movie or television program followed by a 5-min computer game (Solitaire), without a video camera. During both tasks, subjects completed the subjective distress questionnaire between the speaking and arithmetic portions. After the tasks, participants were escorted back to the original testing room where they completed further measures after the task. Blood pressure and subjective distress were assessed 0, 30 and 60-min after the task, saliva samples were collected at 10, 20, and 60-min after the task, and post-task appraisals were obtained, immediately after the task. At a separate visit after completing both sessions, participants were debriefed about the study drugs and received payment.
Figure 1:
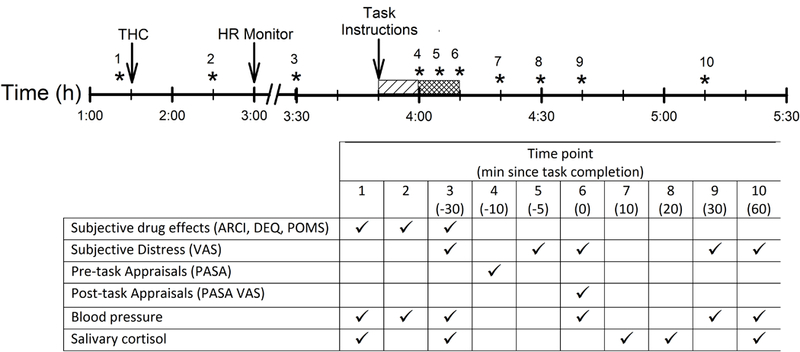
Time line of procedures during each experimental session. Asterisks indicate when dependent measures were collected, and the table indicates which measures were collected at each time point. Lined and hatched bars indicate the preparatory (3:50–4:00 pm) and task performance (4:00–4:10 pm) periods respectively.
The primary outcome measures were subjective distress and pre- and post-task appraisals. Secondary measures included heart rate, blood pressure, salivary cortisol, subjective drug responses, and task performance scores.
2.3. Dependent measures
2.3.1. Subjective distress.
Emotional responses to the tasks were assessed using a questionnaire that consisted of 3 items, each associated with a 100mm line (“I feel stressed”, “I feel tense”, “I feel insecure”) anchored at one end with “not at all” and at the other with “extremely”. Individuals were instructed to place a line bisecting the scale that represented how they felt at that time. Item scores were summed to yield an overall measure of subjective distress. Scale reliability was assessed using Cronbach’s Alpha; coefficients at each time point were >0.8 indicating high internal consistency.
2.3.2. Pre-task appraisals.
Subjects completed the Primary Appraisal Secondary Appraisal rating scale (PASA; Gaab et al., 2005) after the 10-min preparatory period and before task performance to assess THC effects upon perception of the tasks. This 16-item questionnaire assesses anticipatory cognitive appraisals of tasks. Subjects rate the extent to which they agree or disagree with statements regarding how threatening and challenging they perceive the task (Primary Appraisal) and their ability to perform the task and control the task outcome (Secondary Appraisal). Three items (#4, 8, 16) were excluded from the questionnaire administered before the Control task as they were irrelevant to the task, thus only scores upon the “Threat”, “Challenge” and “Self-Efficacy” scales could be compared between the tasks.
2.3.3. Post-task appraisals.
Subjects completed a post-task appraisal questionnaire (PASA VAS; Gaab et al., 2005) immediately after completion of the tasks that consisted of 4 items associated with VAS scales; “I found the task stressful”, “I found the task challenging”, “I knew how to influence the task”, and “I was able to influence the task”. We also added a fifth item, “I was satisfied with my performance”, to assess participants’ satisfaction with their performance in the task.
2.3.4. Physiological.
Blood pressure and heart rate were assessed using a monitor (Critikon Dinamap Plus Vital Signs Monitor, GE Healthcare Technologies, Waukesha, WI). Mean arterial pressure (MAP) was calculated using the formula MAP=2/3*Diastolic + 1/3*Systolic. Minute-to-minute changes in heart rate during the tasks were assessed using a Polar chest band and monitor (Mini-Logger, Mini Mitter/ Respironics, Bend, OR). Heart rate data was averaged over consecutive 10-min periods (baseline, preparation, task, recovery 1, recovery 2, recovery 3). Salivary cortisol samples were collected using Salivette® cotton wads (Sarstedt Inc., Newton, NC) and analyzed by the Core Laboratory at the University of Chicago Hospitals General Clinical Research Center (Salimetrics LLC, State College, PA, sensitivity=0.003 ug/dL). Outliers who exhibited changes ±3SD from the mean were excluded.
2.3.5. Subjective drug effects.
Drug effects before the tasks were assessed using the Addiction Research Center Inventory (ARCI; Martin et al., 1971), the Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth, 1980), and the Profile of Mood States (POMS; McNair et al., 1971).
2.3.6. TSST performance:
While subjects performed the TSST, one of the interviewers documented measures of performance including the number of pauses >5s and the total length of time for which subjects paused. During the arithmetic portion, the interviewer recorded the number of correct and incorrect answers.
2.3.7. Other.
At the initial orientation (enrollment) session before the study began, participants completed standardized questionnaires to provide measures of trait anxiety (State Trait Anxiety Inventory, STAI, Spielberger et al., 1970), current stress levels (Perceived Stress Scale, PSS, Cohen et al., 1983) and perceived stress reactivity (Perceived Stress Reactivity Scale, PSRS, Schlotz et al., 2011).
2.4. Drugs
THC capsules (2.5, 5 or 10 mg dronabinol, Marinol®; Solvay Pharmaceuticals, Marietta, Georgia) were placed inside opaque size 00 capsules with dextrose filler. Placebo capsules contained only dextrose filler.
2.5. Statistical analysis
2.5.1. Demographic Characteristics.
To ensure that the groups were comparable, we examined demographic characteristics, trait anxiety and stress reactivity, past month levels of perceived stress and all dependent measures obtained at baseline (pre-drug) between the groups using one-factor (Dose Group) analyses of variance (ANOVA, for continuous variables) and chi-squared analysis (for categorical variables). The groups did not differ on any of these measures. Trait measures of anxiety (STAI), stress reactivity (PSRS) and past month perceived stress (PSS) were included in later analyses as covariates but were removed if there were no significant effects.
2.5.2. Efficacy of the TSST.
First, we confirmed the efficacy of the TSST among the placebo-treated group using two-factor (Task*Time) repeated measures (rm) ANOVA (time points 3–10, Figure 1) upon heart rate, blood pressure, cortisol and subjective distress. Summary measures of task-induced changes in the outcomes (peak change - greatest change from time point 3) are displayed in Table 2 and were analyzed using paired t-tests. The analyses of summary measures are not discussed unless the findings differed to those of the rmANOVA. Differences in pre- and post-task appraisals between the TSST and Control sessions were analyzed using paired t-tests.
Table 2:
Change in outcome measures after the TSST and Control tasks among placebo-treated participants (data represent pre- to post-task changes compared between tasks using paired t-test).Asterisks indicate significant outcomes (*p<0.05, **p<0.01, ***p<0.001).
| Control | TSST | t(df) | |
|---|---|---|---|
| Physiological | |||
| Heart rate (bpm) | 4.3±3.0 | 17.4±3.2 | 2.5(6)* |
| Mean arterial pressure (mmHg) | 1.6±2.3 | 12.0±3.3 | −2.3(13)* |
| Cortisol (ug/dL) | 0.02±0.02 | 0.24±0.09 | −2.4(8)* |
| Subjective distress (VAS) | 6.2±10.8 | 141±21.2 | −6.2(13)*** |
| Pre-task Appraisals (PASA) | |||
| Self-efficacy | 4.8±0.2 | 3.9±0.2 | 3.7(12)** |
| Threat | 3.2±0.2 | 3.3±0.2 | −0.3(12) |
| Challenge | 3.6±0.1 | 3.5±0.1 | 0.3(12) |
| Post-task Appraisals (PASA VAS) | |||
| Stressful | 7.3±2.4 | 62.9±7.5 | −7.4(13)*** |
| Challenging | 12.3±4.2 | 71.4±5.3 | −9.4(13)*** |
| Know how to influence | 85.4±6.7 | 66.9±8.2 | 1.7(13) |
| Ability to influence | 84.4±6.8 | 56.4±7.8 | 2.4(13)* |
| Satisfaction | 51.6±3.8 | 36.1±6.8 | 8.3(13)*** |
2.5.3. Effects of THC before the tasks.
Next, we evaluated the effects of THC before the tasks using two-factor (Time*Group) rmANOVA upon subjective drug effects (ARCI, DEQ, POMS) and physiological (heart rate, blood pressure, cortisol) measures (time points 1–3, Figure 1) averaged across the two testing sessions (TSST and Control). Summary measures of drug-induced changes in the outcomes (time point 3 minus time point 1) are displayed in Table 3 and were analyzed between the groups using one-factor (Group) ANOVA. The analyses of these summary measures are not discussed unless the findings differed to those of the rmANOVA. Post-drug (pre-task) ratings of subjective distress (at time point 3, Figure 1) were compared between groups using one-factor (Group) ANOVA. The low and high doses of THC produced different effects upon subjective distress before task performance (high>low, placebo), thus we also compared changes in subjective distress separately for the low THC dose group compared to the placebo group as they did not differ significantly on the measure before tasks were performed.
Table 3:
Change in outcome measures for effects of THC before the tasks (data represent pre- to post-capsule changes compared between groups using ANOVA). Asterisks indicate significant outcomes (*p<0.05, **p<0.01, ***p<0.001). Symbols indicate difference from #0mg, §7.5mg (post-hoc Bonferroni test).
| 0mg | 7.5mg | 12.5mg | Group F(df) | |
|---|---|---|---|---|
| ARCI | ||||
| Amphetamine | 0.2±0.5 | 0.4±0.2 | 0.0±0.5 | 0.2(2,41) |
| Benzedrine | −0.5±0.7 | −2.3±0.5 | −2.2±0.5 | 2.9(2,41) |
| Morphine-Benzedrine | −0.2±0.5 | −0.3±0.6 | −0.3±0.8 | 0.01(2,41) |
| LSD | 0.4±0.5 | 2.2±0.7 | 2.2±0.9 | 2.4(2,41) |
| Pentobarbital-Chlorpromazine-Alcohol | 1.7±0.8 | 4.5±0.8# | 4.7±0.7# | 4.5(2,41)* |
| Marijuana | 0.5±0.4 | 3.4±0.6# | 3.3±0.8# | 7.3(2,41)** |
| DEQ | ||||
| I feel some drug effects | 10.5±2.8 | 37.2±6.6# | 39.6±7.3# | 7.5(2,41)** |
| I like the effects I am feeling | 14.1±4.3 | 27.1±4.2 | 25.5±6.2 | 2.1(2,41) |
| I dislike the effects I am feeling | 21.9±6.2 | 26.3±5.8 | 36.5±7.0 | 1.3(2,41) |
| I feel high | 5.5±2.0 | 33.1±6.5# | 35.4±8.1# | 7.5(2,41)** |
| I would like more of what I consumed | 5.0±11.9 | 8.8±6.4 | 15.7±6.0 | 0.3(2,41) |
| POMS | ||||
| Anxiety | −0.06±0.04 | 0.11±0.10 | 0.29±0.09# | 4.4(2,41)* |
| Depression | −0.08±0.05 | 0.01±0.03 | 0.16±0.08# | 4.6(2,41)* |
| Elation | −0.18±0.11 | −0.46±0.11 | −0.31±0.13 | 1.5(2,41) |
| Confusion | −0.13±0.09 | 0.44±0.09# | 0.63±0.14# | 13.8(2,41)*** |
| Vigor | −0.29±0.10 | −0.69±0.17 | −0.56±0.10 | 2.5(2,41) |
| Fatigue | 0.10±0.11 | 0.32±0.15 | 0.51±0.15 | 2.1(2,41) |
| Anger | −0.05±0.04 | 0.00±0.01 | 0.10±0.07 | 2.5(2,41) |
| Friendliness | −0.20±0.08 | −0.54±0.11 | −0.35±0.14 | 2.5(2,41) |
| Physiological | ||||
| Heart rate (bpm) | −4.9±1.5 | −0.8±1.3 | 1.9±2.2# | 4.2(2,41)* |
| Mean arterial pressure (mmHg) | 0.4±1.7 | −3.5±1.4 | 0.1±1.4 | 2.2(2,41) |
| Cortisol (ug/dL) | −0.10±0.02 | −0.09±0.03 | −0.07±0.01 | 0.3(2,38) |
| VAS | ||||
| Subjective distress | 11.6±2.7 | 27.6±6.1 | 58.2±13.9#§ | 7.4(2,41)** |
2.5.4. Effects of THC upon task reactivity.
Finally, differences in subjective distress, heart rate, blood pressure and salivary cortisol during TSST and control sessions were compared across the groups using three-factor (Task*Time*Group) rmANOVA (time points 3–10, Figure 1). Pre- and post-task appraisals were analyzed using two-factor (Task*Group) rmANOVA. We also analyzed summary measures of responses, peak change (greatest change from baseline at any time point) and area under the curve (AUC, relative to baseline using the trapezoid method, Altman, 1991) to interpret responses for the time course analyses described above. For the most part, analyses of summary measures (Group*Task rmANOVAs) mirrored the analyses across time, thus summary measure values are presented for information (Table 4) and to interpret significant effects in the analyses across time.
Table 4:
Change in outcome measures for effects of THC upon acute stress responses (data represent AUC relative to the pre-task post-drug baseline, except for task appraisals and performance measures which are raw scores, compared using Task*Group rmANOVAs). 1Analysis performed for 0 vs. 7.5mg THC (due to true baseline group difference in subjective distress for 12.5mg THC before task performance). Asterisks indicate significant outcomes (*p<0.05, **p<0.01, ***p<0.001). Symbols indicate difference from #0mg, §7.5mg (post-hoc Bonferroni test).
| Control | TSST | ||||||
|---|---|---|---|---|---|---|---|
| 0mg | 7.5mg | 12.5mg | 0mg | 7.5mg | 12.5mg | F(df) | |
| VAS | |||||||
| Subjective distress1 | −11.4±13.2 | 2.6±8.6 | (−56.0±39.7) | 183.1±29.0 | 102.1±26.9# | (65.3±43.4) | Task*Group=5.3(1,27)* |
| Physiological | |||||||
| Heart rate | 1.8±2.0 | 0.7±1.9 | 2.6±2.3 | 4.6±1.3 | 12.2±2.6 | 5.8±2.7 | All n.s. |
| Mean arterial pressure | 2.5±1.8 | 2.9±1.9 | −0.5±1.8 | 3.9±2.9 | 8.7±1.3 | −0.4±1.5 | Group=5.5(2,30)** (0, 7.5>12.5) |
| Cortisol | 1.4±1.0 | −1.2±1.4 | 2.5±1.2 | 9.5±3.5 | 5.0±2.4 | 4.8±1.0 | All n.s. |
| Pre-task Appraisals (PASA) | |||||||
| Self-efficacy | 4.8±0.2 | 3.9±0.2# | 4.5±0.2 | 3.9±0.2 | 3.4±0.2 | 3.8±0.3 | Group=5.1(2,38)* (0, 12.5>7.5) |
| Threat | 3.2±0.2 | 3.5±0.1 | 2.3±0.3#§ | 3.3±0.2 | 3.3±0.1 | 4.0±0.3 | Task*Group=12.1(2,38)*** |
| Challenge | 3.6±0.1 | 3.4±0.1 | 3.2±0.3 | 3.5±0.1 | 3.6±0.1 | 4.0±0.3 | Task*Group=3.2(2,38)* |
| Post-task Appraisals (PASA VAS) | |||||||
| Stressful | 7.3±2.4 | 18.9±6.0 | 16.5±4.1 | 62.9±7.5 | 44.9±7.2 | 58.9±9.1 | Task*Group=3.9(2,39)* |
| Challenging | 12.3±4.2 | 27.0±7.5 | 34.8±7.5 | 71.4±5.3 | 54.1±7.4 | 70.0±5.4 | Task*Group=4.3(2,39)* |
| Know how to influence | 85.4±6.7 | 67.8±6.9 | 67.9±7.6 | 66.9±8.2 | 43.5±7.5 | 34.6±6.7 | Group=5.9(2,39)** (0>7.5, 12.5) |
| Ability to influence | 84.4±6.8 | 67.3±6.0 | 61.9±7.2 | 56.4±7.8 | 50.0±8.8 | 43.6±9.2 | All n.s. |
| Satisfaction | 81.6±3.8 | 62.5±7.7 | 64.0±6.2 | 36.1±6.8 | 45.7±7.4 | 43.0±10.2 | Task*Group=3.7(2,39)* |
| TSST Performance | |||||||
| # Pauses (speech phase) | 0.7±0.2 | 1.7±0.4 | 2.2±0.6 | Group=3.2(2,41)* (12.5>0) | |||
| % Correct (arithmetic phase) | 88.7±2.7 | 86.3±2.2 | 87.3±3.3 | All n.s. | |||
Analyses were performed using SPSS 24 for Windows. Differences were considered significant at p<0.05. Significant main effects and interactions were followed by post-hoc tests with Bonferroni correction. Effect sizes are reported using partial eta-squared (ηρ2), a standardized measure of effect size, where 0.01, 0.06 and 0.16 represent small, medium and large effect sizes respectively. Partial eta-squared represents the ratio of variance accounted for by an effect and that effect plus its associated error variance. It is a more appropriate measure of effect size for studies with repeated measures designs and can also be used to compare effects across studies.
3. Results
3.1. Demographic characteristics
Most participants were White (62%) in their early twenties (23.6 ± 0.7 years) who reported using cannabis on average once per month (1.0 ± 0.2 times per month). The dose groups did not differ on demographic, trait or drug use characteristics (Table 1).
Table 1:
Demographic and drug use characteristics of participants in each group. 1Perceived Stress Scale, 2State Trait anxiety Inventory, 3Perceived Stress Reactivity Scale.
| 0 mg | 7.5 mg | 12.5 mg | |
|---|---|---|---|
| N (Male/Female) | 14 (9/5) | 15 (9/6) | 13 (11/2) |
| Race* (N/%) | |||
| Asian | 1/7 | 1/7 | 0/0 |
| Black or African American | 1/7 | 2/13 | 2/15 |
| White | 8/57 | 9/60 | 9/70 |
| Other | 4/29 | 2/20 | 2/15 |
| Age (yrs) | 23.8 ± 1.4 | 23.2 ± 0.9 | 23.9 ± 1.3 |
| Body mass index (kg/m2) | 21.7 ± 0.5 | 22.8 ± 0.5 | 23.3 ± 0.4 |
| Current Drug Use | |||
| Caffeine (drinks/wk) | 7.8 ± 1.4 | 6.1 ± 1.4 | 9.4 ± 1.6 |
| Alcohol (drinks/wk) | 4.3 ± 1.0 | 6.7 ± 1.5 | 8.0 ± 1.5 |
| Cigarettes (per wk) | 4.8 ± 2.9 | 0.9 ± 0.9 | 4.1 ± 2.6 |
| Cannabis (times/mo) | 1.5 ± 0.5 | 0.8 ± 0.3 | 0.7 ± 0.2 |
| Recreational Drug Use History (% ever used) | |||
| Stimulants | 36 | 40 | 39 |
| Opiates | 7 | 7 | 8 |
| Club Drugs | 29 | 27 | 23 |
| Hallucinogens | 29 | 40 | 31 |
| Tranquilizers | 0 | 0 | 8 |
| Inhalants | 7 | 13 | 8 |
| Current Stress (PSS1) | 10.7 ± 1.3 | 8.1 ± 1.0 | 8.2 ± 1.4 |
| Trait Anxiety (STAI2) | 30.6 ± 2.2 | 29.7 ± 1.8 | 35.8 ± 2.6 |
| Stress Reactivity (PSRS3) | 12.0 ± 1.7 | 11.4 ± 1.2 | 13.8 ± 1.5 |
Participants self-identified their Race by selecting one or more of the following categories; “American Indian or Alaska Native”, “Asian”, “Black or African American”, “Native Hawaiian or Other Pacific Islander”, “White”, “More than one Race”. There were small numbers of participants in categories other than “Black or African American”, “White” or “Asian”, thus these individuals were grouped as “Other”.
3.2. Efficacy of the TSST among placebo-treated participants
The TSST produced characteristic effects upon mood, cardiovascular measures and salivary cortisol in line with previous reports (see Childs et al., 2010 and Figure 2). Pre- to post-task changes in the subjective and physiological measures are shown in Table 2. Self-report measures of trait anxiety (STAI), current stress (PSS), and perceived stress reactivity (PSRS) did not influence responses to stress and were removed from the models. In comparison to the control task, the TSST significantly increased subjective distress [Task*Time F(3,11)=7.6 p<0.01, ηρ2=0.67], blood pressure [MAP Task*Time F(2,12)=16.2 p<0.001, ηρ2=0.73], heart rate [Task F(1,9)=9.6 p<0.05, ηρ2=0.52], and salivary cortisol [Task*Time F(3,9)=5.8 p<0.05 ηρ2=0.66] among participants in the placebo group. Peak emotional and heart rate effects were observed during the 10-min task and returned to baseline within 30-min. Blood pressure peaked immediately after the task and returned to baseline within 30-min. Peak cortisol increases were observed 10-min after the task and returned to baseline within 60-min.
Figure 2:
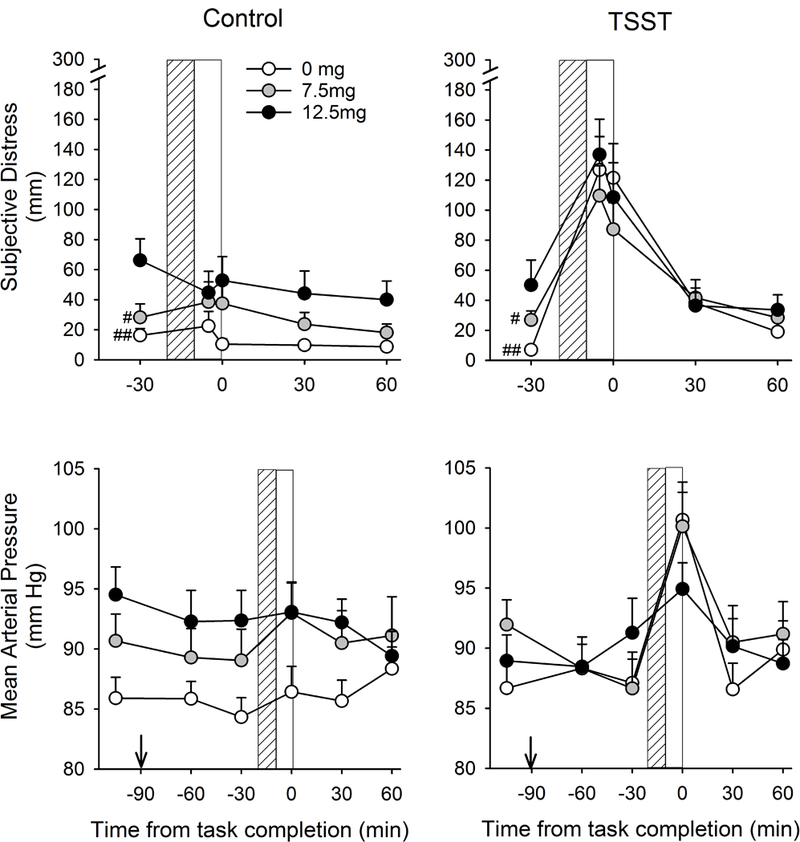
Changes in subjective distress (VAS) and mean arterial pressure across time after Control and TSST tasks among subjects treated with 0, 7.5 or 12.5 mg THC. Lined and open bars indicate task preparation and performance periods respectively. #Indicates difference from 12.5mg THC #p<0.05 ##p<0.01 (Bonferroni post hoc test). Arrow indicates drug administration.
Task appraisals obtained immediately before the tasks showed that individuals’ Self-Efficacy ratings were significantly lower before the TSST in comparison to the Control task [t(12)=3.72 p<0.01]. However, their appraisals of Threat [t(12)=−0.28 n.s.] and Challenge [t(12)=0.26 n.s.] before the tasks did not differ between the TSST and Control tasks (Table 2).
Task appraisals obtained immediately after task performance showed that the TSST significantly increased ratings of Stressful [t(13)=−7.4 p<0.001], and Challenging [t(13)=−9.4 p<0.001], and decreased ratings of Ability to Influence (the task) [t(13)=−2.4 p<0.05] and Satisfaction (with performance) [t(13)=8.3 p<0.001] in comparison to the Control task (Table 2).
3.3. Effects of THC before the tasks
Summary scores of THC-induced changes in the measures for each group during the 2-h pre-treatment period (averaged across sessions) are shown in Table 3. In comparison to placebo, THC significantly increased DEQ “I feel some drug effects” [Time*Group F(4,78)=7.5 p<0.001 ηρ2=0.28; 0 vs. 7.5 p<0.05, 0 vs. 12.5 p=0.001], “I like the effects I am feeling” [Time*Group F(4,78)=2.9 p<0.05 ηρ2=0.18; post hoc n.s.], and “I feel high” [Time*Group F(4,78)=7.5 p<0.001 ηρ2=0.28; 0 vs. 7.5 p<0.05, 0 vs. 12.5 p=0.001]. Treatments did not alter DEQ “I dislike the effects I am feeling” or “I want more of what I just consumed”.
On the ARCI, THC significantly increased Pentobarbital-Chlorpromazine-Alcohol [Time*Group F(4,78)=3.0 p<0.05 ηρ2=0.13; 0 vs. 12.5 p<0.05] and Marijuana [Time*Group F(4,78)=7.7 p<0.001 ηρ2=0.28; 0 vs. 12.5 p<0.05] scale scores in comparison to placebo. Treatments did not change ARCI Amphetamine, Benzedrine, Morphine-Benzedrine and LSD scale scores.
For the POMS, 12.5mg THC significantly increased Depression [Time*Group F(4,78)=4.5 p<0.01 ηρ2=0.19; post hoc n.s.], Anxiety [Time*Group F(4,78)=3.3 p<0.05 ηρ2=0.14; 0 vs. 12.5 p<0.05], and Confusion [Time*Group F(4,78)=10.9 p<0.001 ηρ2=0.36; 0 vs. 12.5 p=0.01] scale scores in comparison to placebo. Elation, Vigor, Fatigue, Anger and Friendliness scale scores were unaffected.
THC (12.5 mg) increased VAS ratings of subjective distress before the task compared to both 0 and 7.5mg THC [Group F(2,39)=7.4 p<0.01; 12.5 vs. 0 p<0.001, 12.5 vs. 7.5 p<0.05].
In comparison to placebo, THC did not dose-dependently alter MAP or salivary cortisol during the 2-h pre-treatment period. The analysis of heart rate across the pre-treatment period showed a trend toward THC-induced elevation [Group*Time F(4,78)=2.3 p<0.07 ηρ2=0.11] and analysis of change scores at time point 3 showed a significant effect of 7.5mg THC upon heart rate [Group F(2,41)=4.2 p<0.05].
3.4. Effects of THC upon task responses
Table 4 shows summary measures (AUC) for subjective and physiological measures and raw scores for pre- and post-task appraisals for each group during the Control and TSST sessions. Self-report measures of trait anxiety (STAI), current stress (PSS), and perceived stress reactivity (PSRS) did not influence THC effects on responses to the tasks and were removed from the models.
In comparison to placebo and 7.5mg THC, 12.5 mg THC increased ratings of subjective distress during both Control and TSST sessions [Group F(2,39)=4.7 p<0.05 ηρ2=0.19, post hoc Bonferroni correction p<0.05, Figure 2]. Because there were differences in subjective distress before the tasks began between the 12.5mg group and the other groups (12.5mg > 0, 7.5mg, Table 3), we performed a separate analysis to compare responses between the 0 vs. 7.5mg groups (in whom pre-task subjective distress did not differ). This revealed that 7.5mg THC significantly attenuated TSST-induced increases in subjective distress [Task*Group F(1,27)=5.3 p<0.05 ηρ2=0.16, Figure 2] and this appeared to occur during the recovery period after the task; Analysis of peak change scores showed that the TSST significantly increased subjective distress similarly in both 0- and 7.5mg-treated participants [Control mean±SEM: 0mg=6.2±10.8, 7.5mg=5.2±6.7; TSST 0mg=141.1±21.2, 7.5mg=98.8±19.0; Task*Group F(1,27)=1.9 p=0.18 ηρ2=0.07], however 7.5mg THC decreased AUC measures of subjective distress compared to placebo [Table 4, mean AUC difference between TSST and Control sessions for 0mg and 7.5mg was respectively 194.4±28.4 and 99.5±39.7, t(27)=2.3 p<0.05].
For pre-task appraisals, 12.5mg THC differentially altered ratings of Threat [Task*Group F(2,38)=12.1 p<0.001 ηρ2=0.39] and Challenge [Task*Group F(2,38)=3.2 p=0.05 ηρ2=0.15], for the TSST and Control sessions. Post-hoc tests [one-factor (Group) ANOVAs conducted separately for Control and TSST] showed that 12.5mg significantly decreased ratings of Threat before the Control task, yet increased ratings before the TSST [Control, Group F(2,41)=9.5 p<0.001, 12.5 vs. 0, 7.5 ps<0.05; TSST, Group F(2,41)=5.5 p<0.01; 12.5 vs. 0 p<0.05]. Although the pattern of effects for Challenge was similar to threat, these effects did not reach statistical significance. THC (7.5mg) decreased ratings of Self-Efficacy before both tasks.
For post-task appraisals, THC significantly influenced ratings of the tasks as Stressful [Task*Group F(2,39)=3.9 p<0.05 ηρ2=0.17;], and Challenging [Task*Group F(2,39)=4.3 p<0.05 ηρ2=0.18], and also altered ratings of Satisfaction (with performance) [Task*Group F(2,39)=3.7 p<0.05 ηρ2=0.16]. Follow-up analyses revealed that, in comparison to placebo, 7.5mg THC dampened TSST-induced increases in task ratings of Stressful [Task*Group F(1,27)=10.6 p<0.01 ηρ2=0.28] and Challenging [Task*Group F(1,27)=8.9 p<0.01 ηρ2=0.25], while 12.5mg THC did not (ps>0.3). For ratings of Satisfaction (with performance), both 7.5 and 12.5mg THC decreased ratings after the Control task but not the TSST (Table 4). Finally, in comparison to placebo, THC (7.5, 12.5mg) significantly decreased ratings of “I knew what to do to influence the task” after both the Control task and TSST [Group F(2,39)=5.9 p<0.01; 0 vs. 7.5, 12.5 ps<0.05].
THC altered MAP in a task and dose-dependent manner (Task*Group interaction [F(2.39)=3.2 p=0.05 ηρ2=0.14, Figure 2]. The higher dose of THC (12.5 mg) dampened the TSST-induced increase in MAP so that the TSST no longer increased MAP as it did in both the placebo and the 7.5 mg conditions (i.e., Task*Time for 12.5 mg condition F(3,36)=0.7 p=0.6; Task*Time for both 0mg and 7.5mg THC: ps<0.01]. When summary measures of task responses were compared, 12.5mg THC significantly dampened peak increases in MAP after the TSST only [Task*Group F(2,39)=3.7 p<0.05 ηρ2=0.16] and attenuated AUCs after both tasks (Table 4). Neither dose of THC significantly altered task-induced changes in heart rate or salivary cortisol.
THC (12.5mg) marginally increased the number of pauses during the speaking portion of the TSST [Group F(2,41)=3.2 p=0.05, post hoc Bonferroni correction 0 vs. 12.5mg p=0.052]. The mean (±SEM) number of pauses during the TSST was 0.7±0.2 in the placebo condition and 2.2±0.6 in the 12.5mg THC condition. The lower dose did not affect pauses, and neither dose of THC significantly influenced performance during the mental arithmetic portion of the task.
4. Discussion
The main conclusion of this study is that a low dose of THC (7.5mg) mitigated the negative emotional effects of a psychosocial stressor among healthy young non-daily cannabis users. Using a well-validated psychosocial stress task (TSST) we found that 7.5mg THC reduced the duration of negative emotional responses to acute psychosocial stress and participants’ post-task appraisals of how threatening and challenging they found the stressor. These results are consistent with the common claim that cannabis is used to reduce subjective stress and relieve tension and anxiety. In contrast, the higher dose of THC (12.5mg) produced small but significant increases in anxiety, negative mood and subjective distress at baseline before the tasks began, and throughout both the TSST and Control tasks, which is consistent with previously reported anxiogenic effects of THC in controlled human laboratory studies. Together, these findings are in line with clinical and experimental reports that THC can both decrease and increase negative mood states.
The finding that 7.5mg THC attenuated post-task emotional responses is consistent with previous clinical studies showing that this dose reduces brain reactivity to negative stimuli. Phan and colleagues previously showed that 7.5mg THC reduced amygdala reactivity to social threat and anterior cingulate reactivity to negative images among non-daily users (Phan et al., 2008; Rabinak et al., 2012). These brain regions are strongly associated with fear and anxiety in both animal and human studies (Davis and Whalen, 2001; LeDoux, 2000), and also contain high concentrations of CB1 receptors (Katona, 2009). In those previous studies, it was reported that THC did not alter self-reported emotional responses to the relatively mild visual images. Yet, here we show that in the presence of a potent stimulus (public speaking) that produces marked subjective distress, the same dose of THC significantly attenuates self-reported emotional responses. Our findings are also in line with a recent study which showed that rimonabant, a CB1 antagonist, elevated anxiety induced by a public speaking task (Bergamaschi et al., 2014). Thus, the subjective stress-relieving properties of 7.5mg THC demonstrated in the present study may be mediated via CB1 receptors in the amygdala and associated limbic frontal cortex.
In contrast, 12.5mg THC produced mild but significant negative affect before the tasks began, and overall it increased ratings of subjective distress throughout both the TSST and Control conditions. Unfavorable effects of THC were also evident upon pre-task (Control, TSST) appraisals of Self-Efficacy (7.5mg), post-task (Control, TSST) appraisals of ability to influence the tasks (7.5, 12.5mg), post-Control task ratings of satisfaction (7.5, 12.5mg), and task performance; 12.5mg THC marginally increased the number of pauses during the public speaking portion of the TSST in comparison to placebo. Overall, our findings support previous evidence of bi-directional effects of different THC doses upon anxiety-like behavior in animals (Rey et al., 2012; Viveros et al., 2005) and non-linear effects of the drug on subjective mood (Ballard et al., 2012; Bedi et al., 2013). Our findings add to this literature of bi-directional THC effects and highlight the need to carefully select an appropriate dose range in research studies with the drug.
The subjective effects of THC have been shown to be comparable to those of cannabis (Wachtel et al., 2002), however it is important to note that there are many other active constituents of the cannabis plant which impact its overall profile of effects. In particular, cannabidiol is another major constituent that has psychoactive properties. Cannabidiol produces anxiolytic effects in both preclinical and clinical studies (Crippa et al., 2004; Guimarães et al., 1990) and blunts the anxiogenic effects of THC (Zuardi et al., 1982). Importantly, cannabidiol has been shown to reduce anxiety in a public speaking task similar to that used in the current study (Zuardi et al., 1993). Thus, the bi-directional effects of the low and high THC doses demonstrated in the present study may differ to effects of the same doses in combination with cannabidiol, and this should be investigated in future studies.
With regard to our secondary hypotheses, the highest dose of THC significantly attenuated blood pressure responsivity to the TSST. It is well known that THC and cannabinoids tend to stimulate cardiovascular activity (Montecucco and Di Marzo, 2012) but their effects upon task reactivity are less clear. One study previously reported that smoked THC increased blood pressure responsivity to a mentally challenging computer task (Capriotti et al., 1988). However, THC is also known to decrease vascular resistance and impair sympathetic vascular reflexes (Benowitz et al., 1979; Sidney, 2002) which may explain the effect upon blood pressure reactivity observed in this study. In contrast to our hypothesis regarding HPAA responses, neither dose of THC influenced TSST-induced cortisol responses. Nevertheless, considering the large inherent variability in cortisol responses to acute stress, the effects of THC upon cortisol responses should be investigated in a larger sample. Alternatively, previous studies have demonstrated effects of THC on total cortisol levels measured in serum (Goodwin et al., 2012; Ranganathan et al., 2009) and this may also be a more sensitive measure of THC effects on acute stress-induced increases in cortisol.
There were several limitations to this study. First, the group size was relatively small to detect between-subjects differences in cortisol responses to acute stress that are inherently variable. Thus, THC effects on cortisol responses to acute stress might be detected with larger samples in each treatment condition. A second limitation is the dose of THC selected. We selected relatively low doses because the effects of THC in emotionally distressing situations have not been routinely investigated, and it is likely that higher doses may produce greater negative mood effects in this population of non-daily users (Ballard et al., 2012). Finally, the sample was relatively homogeneous with regard to age, education, body weight and prior drug use, and they reported few psychiatric symptoms. Whether THC would have different effects in a more heterogeneous population is not known. It is possible that participants with more varied psychiatric or drug use history, a wider age range, some prior history of adverse responses to cannabis, more frequent use (i.e. daily), or those with cannabis use disorder may reveal a different profile of effects. Cannabis use for coping motives is higher among daily users, yet these self-reported reasons are still considerably high (19%) even among individuals reporting very infrequent use (<10 lifetime uses, Johnston and O’Malley, 1986). There is also evidence of tolerance to certain subjective effects with increasing frequency of use (e.g. sedation, Kirk and de Wit, 1999) and daily users could potentially exhibit altered responses to acute stress per se. Thus, the acute stress-relieving effects of THC demonstrated here, should be investigated in studies with individuals who report more frequent use of cannabis.
In summary, we found evidence that a low dose of THC, which produced negligible subjective and physiological effects by itself, counteracted the negative emotional effects of a standardized psychosocial stress task without influencing performance. In contrast a higher THC dose increased negative mood and subjective distress, impaired task performance, and attenuated blood pressure responsivity to acute stress. Overall, the observed non-linear effects of THC on emotional responses to psychosocial stress highlight the complexity of the effects of this drug even within a relatively homogeneous population of healthy non-daily cannabis smokers.
Acknowledgements
We thank Summer Thompson, Celina Joos, Justin Birnholz, Les Sidney, Jon Solamillo and Michael Helzer for technical assistance, and Dr Royce Lee for medical support. We offer many thanks to the participants.
Role of Funding Source
This research was supported by a grant from the National Institute on Drug Abuse (DA02812, PI: deWit). This article is the sole responsibility of the authors and does not reflect the views of the National Institute on Drug Abuse.
Footnotes
Human participant protection statement
These experiments complied with US laws.
Financial Disclosure
No financial disclosure to report.
Conflict of Interest
No conflicts of interest to report.
References:
- Altman DG, 1991. Practical Statistics for Medical Research, 1st ed New York.: Chapman and Hall. [Google Scholar]
- APA, American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (5th ed.). APA: Washington DC. [Google Scholar]
- Ballard ME, Bedi G, de Wit H, 2012. Effects of delta-9-tetrahydrocannabinol on evaluation of emotional images. J Psychopharmacol. 26, 1289–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, Gallo DA, de Wit H, 2011. Psychoactive drugs and false memory: comparison of dextroamphetamine and delta-9-tetrahydrocannabinol on false recognition. Psychopharmacology (Berl) 219, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Hattan PR, Garant JP, Gobbi G, 2012. Effect of delta-9-tetrahydrocannabinol on behavioral despair and on pre- and postsynaptic serotonergic transmission. Prog. Neuropsychopharmacol. Biol. Psychiatry.38, 88–96. [DOI] [PubMed] [Google Scholar]
- Bedi G, Cooper ZD, Haney M 2013. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. 18:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT, 1979. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin. Pharmacol. Ther.25, 440–6. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, Linares IM, Arrais KC, de Oliveira DC, Queiroz ME, Nardi AE, Huestis MA, Hallak JE, Zuardi AW, Moreira FA, Crippa JA 2014. Rimonabant effects on anxiety induced by simulated public speaking in healthy humans: a preliminary report. Hum. Psychopharmacol. 29, 94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R, 2002. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 163, 111–117. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM, 2013. The endocannabinoid system and emotional processing: a pharmacological fMRI study with Δ9-tetrahydrocannabinol. Eur. Neuropsychopharmacol. 23, 1687–97. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, Sala M, 2007. 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur. J. Pharmacol. 555, 156–63. [DOI] [PubMed] [Google Scholar]
- Capriotti RM, Foltin RW, Brady JV, Fischman MW, 1988. Effects of marijuana on the task-elicited physiological response. Drug Alcohol Depend. 21, 183–7. [DOI] [PubMed] [Google Scholar]
- Childs E, Dlugos A, de Wit H, 2010. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 47, 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. [PubMed] [Google Scholar]
- Cone EJ, Huestis MA,1993. Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Ther. Drug Monit. 15, 527–32. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho Busatto G 2004. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 29, 417–26. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P, 2009. Cannabis and anxiety: a critical review of the evidence. Hum. Psychopharmacol. 24, 515–523. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J, 2002. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 164, 61–70. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH, 2004. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 29, 1558–72.. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ, 2001. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–91. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U, 2005. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology 30, 599–610. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, de Rooij SR, 2014. Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology 48, 87–97. [DOI] [PubMed] [Google Scholar]
- Goodwin RS, Baumann MH, Gorelick DA, Schwilke E, Schwope DM, Darwin WD, Kelly DL, Schroeder JR, Ortemann-Renon C, Bonnet D, Huestis MA 2012. CB1 - cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. Am. J. Drug Alcohol Abuse.38,114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães FS, Chiaretti TM, Graeff F, Zuardi AW 1990. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl). 100, 558–9. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW 2002. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl). 164:407–15. [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW 2001. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 25:757–65. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS, 2010. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Böcker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J 2014. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology (Berl). 231, 4723–33. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Sinha R, 2009. Stress-related factors in cannabis use and misuse: implications for prevention and treatment. J. Subst. Abuse Treat. 36, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH, 1980. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 71, 269–273. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM 1986. Why do the Nation’s Students Use Drugs and Alcohol? Self-Reported Reasons From Nine National Surveys. J. Drug Issues. 16, 29–66. [Google Scholar]
- Jones RT, 2002. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 42, 58S–63S. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA, 2011. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 57, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, 2009. Endocannabinoid receptors: CNS localization of the CB(1) cannabinoid receptor. Curr. Top. Behav. Neurosci. 1, 65–86. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H 1999. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol. Biochem. Behav. 63, 137–42. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 2000. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lee CM, Neighbors C, Woods BA, 2007. Marijuana motives: young adults’ reasons for using marijuana. Addict. Behav. 32, 1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR, 1971. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical Pharmacology & Therapeutics 12, 245–258. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L, 1971. Profile of Mood States. San Diego: Educational and Industrial Testing Service. [Google Scholar]
- McNair DM, Frankenthaler LM, Czerlinsky T, White TW, Sasson S, Fisher S 1982. Simulated public speaking as a model of clinical anxiety. Psychopharmacology (Berl). 77, 7–10. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Di Marzo V, 2012. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol. Sci. 33, 331–340. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR 1990. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 253:1002–9. [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H, 2008. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J. Neurosci. 28, 2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Sripada CS, Angstadt M, de Wit H, Phan KL, 2012. Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J. Neural Transm. 119, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, D’Souza DC, 2008. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl). 203, 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B, 2012. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37, 2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, King AC, Cohoon AJ, Lovallo WR, 2013. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol. Biochem. Behav. 109, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Yim IS, Zoccola PM, Jansen L, Schulz P, 2011. The Perceived Stress Reactivity Scale: measurement invariance, stability, and validity in three countries. Psychol. Assess. 23, 80–94. [DOI] [PubMed] [Google Scholar]
- Segal B, Cromer F, Hobfoll SS, Wasserman PZ 1982. Patterns of reasons for drug use among detained and adjudicated juveniles. Int. J. Addict. 17, 1117–30. [DOI] [PubMed] [Google Scholar]
- Sidney S, 2002. Cardiovascular consequences of marijuana use. J. Clin. Pharmacol. 42, 64S–70S. [DOI] [PubMed] [Google Scholar]
- Simons J, Correia CJ, Carey KB 2000. A comparison of motives for marijuana and alcohol use among experienced users. Addict. Behav. 25, 153–60. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, 1970. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press: Palo Alto, CA. [Google Scholar]
- Viveros MP, Marco EM, File SE 2005. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 81:331–342 [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H 2002. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl). 161, 331–9. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Cosme RA, Graeff FG, Guimarães FS 1993. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 7, S82–8. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Cosme RA, Graeff FG, Guimarães FS 1993. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 7:82–8. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG 1982. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl). 76, 245–50. [DOI] [PubMed] [Google Scholar]


