Abstract
Purpose
Acute exacerbations of COPD (AECOPD) are frequent and associated with a poor prognosis. A home discharge care bundle, the PRADO-BPCO program, has been set up by the French National Health System in order to reduce readmission rate after hospitalization for AECOPD. This program includes early consultations by the general practitioner, a nurse, and a physiotherapist after discharge. The aim of our study was to evaluate the effect of the PRADO-BPCO program on the 28-days readmission rate of COPD patients after hospitalization for AECOPD.
Patients and methods
This was a retrospective cohort study including all patients admitted for AECOPD in our center between November 2015 and January 2017. The readmission or death rate at 28 days after hospitalization for AECOPD was compared between patients included in the PRADO-BPCO program and patients with standard care after discharge. Inclusion in the program was decided by the physician in charge of the patient.
Results
A total of 62 patients were included in the PRADO-BPCO group and 202 in the control group. At baseline, patients in the PRADO group had a more severe COPD disease and more severe exacerbations than the control group and mean inpatient stay was shorter in the PRADO group: 8.6±4.3 vs 10.4±7.4 days (P=0.034). Readmission or death rate at 28 days was similar between groups: 10 (16.1%) in the PRADO group vs 30 (14.9%) in the control group (P=0.81). Ninety-days readmission or death rate and overall survival were similar in the two groups.
Conclusion
In our center, despite more severe COPD and a shorter hospitalization time, the PRADO-BPCO program failed to prove a benefit on the 28 days readmission or death rate when compared with standard care.
Keywords: COPD, exacerbation, patient readmission, patient care bundles, continuity of patient care
Introduction
Acute exacerbations (AE) are frequent in patients with COPD. They occur in 16.4% of patients with COPD and more frequently in patients with very severe disease.1 AECOPD alters quality of life2 and increases mortality.3–5 Hospitalization costs for AECOPD is 678 million euros in France.6 Previous AECOPD is a risk factor for further admissions for AECOPD.7–10 Therefore, preventing readmission after AECOPD hospitalization is a public health priority, and most hospitals are incited to reduce 28-days readmission rates.11,12 Strategies to identify patients at risk have been developed1,13–16 and discharge care bundles have sometimes been set at a national level. A meta-analysis showed that discharge care bundle may reduce hospital readmission with a risk ratio of 0.80.18 However, none of the analyzed studies was conducted in France and with the specific French Health Care Organization. Notably, 28-days readmission rate of exacerbated COPD patients is low in France (7.2%),18 when compared with that in UK (20%).19
Despite this seemingly positive result, the French social welfare (Sécurité Sociale) has developed a discharge care bundle named PRADO-BPCO (Programme d’accompagnement de retour à domicile) that begun in 2015. The discharge coordinator works for the social welfare and the patient is provided with a personalized treatment plan. At discharge, patients can be referred to the social welfare to benefit from this program. If so, a discharge coordinator performs a home visit after hospital discharge and organizes a follow-up that includes outpatient appointment with the general practitioner within 7 days and with a chest physician within 2 months. During these medical appointments, smoking cessation advice is given as well as a verification of their vaccinal status regarding influenzae and S. pneumoniae. It also includes home weekly therapeutic education sessions provided by a nurse. During these education sessions, the nurse provides a training on the disease and the warning signs, on inhalers technique, and provides smoking cessation advice. The program also includes 30-minute sessions of home-based rehabilitation by a physiotherapist for which the exercise program is defined by the physiotherapist. In case of recurrence of AECOPD symptoms, patients are instructed to call their nurse who will then liaise with the general practitioner. The discharge care bundle does not include self-managed antibiotics or steroids. The follow-up is for 2 months after discharge but can be extended by the patient’s general practitioner. Participation to PRADO is not mandatory.
The aim of this study was to assess the efficacy of PRADO discharge care bundle in patients admitted for AECOPD in our center. The primary outcome was 28-days readmission or death. The secondary outcomes were time to next severe AECOPD or death, 3-months’ readmission rates, number of severe AECOPDs following discharge, and adherence to PRADO discharge care bundle.
Patients and methods
This is a retrospective monocentric cohort study including all patients admitted for AECOPD to the Rouen University Hospital department of pulmonary and intensive respiratory care, between November 2015 and January 2017.
Patients were identified using hospital electronic database. They were included if they were admitted for an AECOPD and if they had a lung function test confirming the diagnosis of COPD according to GOLD guidelines. Patients admitted for another cause than AECOPD, patients who died during inpatient stay, or who were discharged to a rehabilitation and aftercare facility were not included. Medical history and management during inpatient stay were collected using the electronic medical record. Frequent exacerbators were defined as patients having two or more AECOPD within the last 12 months or having one severe AECOPD within the last 12 months. Severe AECOPD was defined as an AECOPD requiring hospitalization.
Data regarding follow-up and participation to the PRADO program were collected from the social welfare database. Referral to PRADO discharge bundle was decided by the senior clinician in charge of the patient. Hence, patients were allocated to the PRADO group, and were referred for the discharge care bundle, or were allocated to the control group in which they received only standard care. This study was approved by Rouen University Ethical Board (CERNI – approval E2018-65), and patients gave a written informed consent to participate. All patients’ data were anonymized.
Primary outcome was defined as readmission or death within 28 days following discharge for an AECOPD. Readmission for another cause than AECOPD was not considered.
Normal distribution was assessed using Shapiro–Wilk tests. Results were expressed as number and percentages, mean and SD when normally distributed, or medians and IQR when not normally distributed. Comparisons were performed using the student’s unpaired t-test for normally distributed continuous variable and a Mann–Whitney test for non-normally distributed continuous variable. Chi-squared test was used for categorical values. A log-binomial generalized linear model, adjusted on COPD severity (grade 3–4 vs grade 1–2) and exacerbator phenotype, was used for the multivariate analysis. The incidence rate of deaths/readmissions was estimated in a negative binomial regression. Profile-likelihood CI and likelihood ratio tests were used for statistical estimation. In order to take into account the very low number of events, a conservative exact conditional Poisson method was used to estimate the risk ratio of death at 28 days. Survival data were analyzed using the Kaplan–Meier method and Log-rank test. All tests were two-sided with type I error rate was set at 0.05. The analyses were performed using GraphPad Prism 6® for Mac OS X® (GraphPad Software, Inc., La Jolla, CA, USA), IBM SPSS® Statistics v20.0 (IBM Corporation, Armonk, NY, USA) and R (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria).
As this study was retrospective and monocentric, the sample size was not controlled. A post hoc power analysis was conducted. Assuming a 16% readmission rate in the control group, with 62 patients in the PRADO group and 202 patients in the control group, a 5% two-sided type I error rate, the statistical power to detect a 5% absolute readmission risk reduction (31% RR reduction) would have been 18% (normal approximation power analysis). As many readmissions are not preventable and that not all preventable readmissions would be prevented by PRADO, a larger risk reduction was not considered. For a smaller 3.2% absolute readmission risk reduction (20% RR reduction), the statistical power would have been 9.4%.
Results
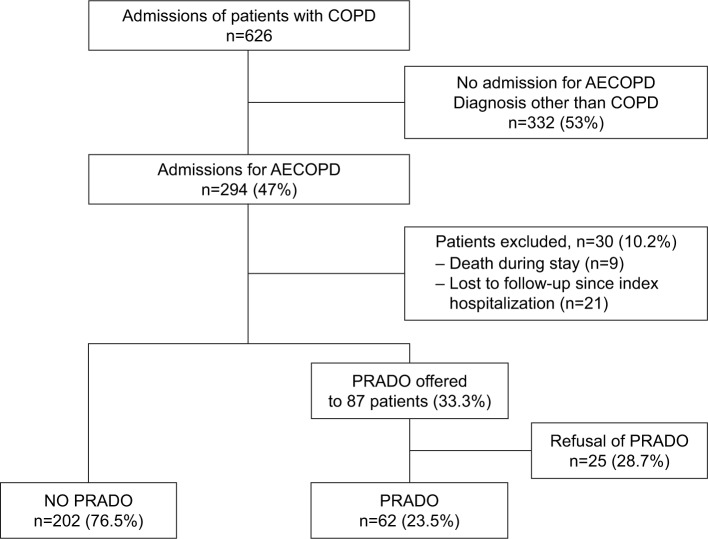
Six hundred twenty-six admissions of patients with COPD were identified during the inclusion period. Two-hundred ninety-four (47%) admissions were related to an AECOPD episode. Two-hundred sixty-four patients were included in the final analysis. PRADO discharge care bundle was offered to 87 (33%) patients. Among them, 25 patients declined, and 62 (23.5%) accepted to be enrolled in the PRADO discharge care bundle (Figure 1). Among the 25 patients who declined, 8 (32%) were readmitted within 28 days.
Figure 1.
Study flowchart.
Abbreviations: AECOPD, acute exacerbations of COPD; PRADO, Programme d’accompagnement de retour à domicile.
The characteristics of the study population are summarized in Table 1. Patients in the PRADO group had more severe COPD and more AECOPD prior to inclusion when compared with the control group (P=0.02, P=0.008, respectively). Inpatient management was similar between groups except for the use of diuretics, which was higher in the control group. Inpatient stay was shorter in the PRADO group compared with controls: 8.3±4.3 vs 10.4±7.4 days (P=0.034; Table 2). In the PRADO group, the median delay to the general practitioner appointment was 4.0 (3.0–6.0) days, 3.0 (1.5–5) days to the nurse appointment, and 3.0 (1–4) days to the physiotherapist, and the mean time to the chest physician appointment was 48.1±24.7 days. In the PRADO group, 53 (86%) had their general practitioner appointment within 7 days of discharge, 58 (94%) had appointment with a nurse within 7 days of discharge, 54 (87%) had a physiotherapist within 7 days of discharge, and 43 (70%) had a chest physician appointment within 2 months following discharge.
Table 1.
Characteristics of the patients with AECOPD
| Characteristics | PRADO (n=62) | Control (n=202) | P-value | ||
|---|---|---|---|---|---|
| Sex ratio (M/F) | 0.59 | (23/39) | 0.46 | (64/138) | 0.428 |
| Age, years, median (IQR) | 65.5 (61.4–75.3) | 70.1 (63.1–77.7) | 0.141 | ||
| Body mass index, kg/m2, mean ± SD | 25.2±7 | 26.4±7.3 | 0.269 | ||
| Diabetes, n, % | 12 | 19.3% | 40 | 19.8% | 0.938 |
| High blood pressure, n, % | 24 | 38.7% | 93 | 46.0% | 0.310 |
| Dyslipidemia, n, % | 17 | 27.4% | 61 | 30.2% | 0.095 |
| Chronic heart failure, n, % | 20 | 32.2% | 77 | 38.1% | 0.402 |
| LVEF, %, mean ± SD | 59±10.4 | 58.3±11.8 | 0.750 | ||
| Chronic renal failure, n, % | 6 | 9.5% | 20 | 9.9% | 0.668 |
| Cirrhosis, n, % | 1 | 1.6% | 4 | 2.0% | 0.853 |
| Depression, n, % | 8 | 12.9% | 37 | 18.3% | 0.321 |
| Cancer, n, % | 4 | 6.4% | 27 | 13.4% | 0.139 |
| Moderate-to-severe OSA, n, % | 15 | 24.2% | 37 | 18.3% | 0.309 |
| Excessive alcohol intake, n, % | 9 | 14.5% | 21 | 10.4% | 0.371 |
| Tobacco, n, % | 0.839 | ||||
| No tobacco history | 2 | 3.2% | 10 | 5.0% | |
| Active smoker | 24 | 38.7% | 79 | 39.1% | |
| Former smoker | 36 | 58.1% | 113 | 55.9% | |
| Pack-years of smoking, mean ± SD | 49.04±22.13 | 45.32±22.61 | 0.280 | ||
| Baseline mMRC dyspnea grade, mean ± SD | 2.36±1.25 | 2.50±1.64 | 0.668 | ||
| Functional respiratory exploration | |||||
| FEV1, L, mean ± SD | 0.99±0.48 | 1.10±0.47 | 0.126 | ||
| FEV1, % of predicted value, mean ± SD | 39.48±17.26 | 43.79±17.77 | 0.097 | ||
| FVC, L, mean ± SD | 2.20±0.84 | 2.19±0.81 | 0.946 | ||
| FVC, % of predicted value, mean ± SD | 67.23±20.07 | 67.83±21.91 | 0.854 | ||
| TLC, L, median (IQR) | 6.92 (5.62–8.42) | 6.81 (5.67–8.00) | 0.332 | ||
| TLC, % of predicted value, median (IQR) | 126 (105–148) | 116 (100–134) | 0.079 | ||
| RV, L, mean ± SD | 4.66±1.40 | 4.52±1.54 | 0.597 | ||
| RV, % of predicted value, median (IQR) | 225 (155–257) | 188 (146–240) | 0.074 | ||
| KCO, %, median (IQR) | 38.00 (28.00–58.50) | 41.50 (31.00–64.75) | 0.566 | ||
| Severity of COPD (GOLD 2011), n, % | 0.021 | ||||
| Mild | 1 | 1.6% | 7 | 3.5% | |
| Moderate | 14 | 22.6% | 55 | 27.2% | |
| Severe | 22 | 35.5% | 91 | 45.0% | |
| Very severe | 24 | 38.7% | 45 | 22.3% | |
| COPD diagnosis >5 years, n, % | 29 | 46.8% | 99 | 49.0% | 0.758 |
| Prior Pseudomonas aeruginosa isolation, n, % | 9 | 14.5% | 36 | 17.8% | 0.545 |
| Prior Mycobacteria isolation, n, % | 5 | 8.1% | 20 | 9.9% | 0.666 |
| Prior Aspergillus fumigatus isolation, n, % | 11 | 17.7% | 20 | 9.9% | 0.093 |
| Pulmonary rehabilitation program, n, % | 8 | 12.9% | 20 | 9.9% | 0.502 |
| Frequent-exacerbation phenotype | 37 | 59.7% | 86 | 42.6% | 0.018 |
| Number of AECOPDs within previous 12 months, n, % | 1.83±1.63 | 1.17±1.58 | 0.008 | ||
| Hospitalized AECOPDs within previous 12 months, n, % | 1.26±1.49 | 0.64±1.15 | 0.001 | ||
Abbreviations: AECOPD, acute exacerbation of COPD; F, female; KCO, carbon monoxide transfer coefficient; LVEF, left ventricular ejection fraction; M, male; mMRC, modified British Medical Research Council dyspnea scale; OSA, obstructive sleep apnea syndrome; PRADO, Programme d’accompagnement de retour à domicile; RV, residual volume; TLC, total lung capacity.
Table 2.
Characteristics of index hospitalization
| Characteristics | PRADO | Non PRADO | P-value | ||
|---|---|---|---|---|---|
| n=62 | n=202 | ||||
| Length of stay in hospital, days, mean ± SD | 8.3±4.3 | 10.4±7.4 | 0.034 | ||
| ICU during stay, n, % | 22 | 35.5% | 94 | 46.5% | 0.125 |
| Arterial blood gas | |||||
| pH, mean ± SD | 7.38±0.08 | 7.37±0.09 | 0.554 | ||
| PaO2, kPa, mean ± SD | 10.4±2.6 | 11.2±4.7 | 0.208 | ||
| PaCO2, kPa, mean ± SD | 7.3±2.1 | 7.4±2.8 | 0.741 | ||
| HCO3-, mmol/L, mean ± SD | 31.3±7.3 | 30.3±6.9 | 0.313 | ||
| Oxygen flow rate, L/min, median (IQR) | 2 (1–3) | 2.5 (0.75–4.25) | 0.391 | ||
| Antibiotics, n, % | 47 | 75.8% | 160 | 79.2% | 0.569 |
| Oral corticosteroids, n, % | 25 | 40.3% | 59 | 29.2% | 0.100 |
| Diuretics, n, % | 11 | 17.7% | 64 | 31.7% | 0.033 |
Abbreviations: ICU, intensive care unit; PRADO, Programme d’accompagnement de retour à domicile.
Twenty-eight days’ readmission and death rate were similar between groups with 10 (16.1%) patients reaching primary end point in the PRADO group and 30 (14.9%) in the control group. Patients in the PRADO group did not have a significant lower risk for readmission (RR =1.09 [95% CI: 0.53–2.01], P=0.81). During the 28 days following hospitalization discharge, two (3.2%) patients died in the PRADO group and seven (3.5%) patients died in the control group (RR =0.93 [95% CI: 0.09–4.89], P=1.00). Excluding patients who died within 28 days, with or without readmission, 8 (13.3%) patients in the PRADO group and 23 (11.8%) patients in the control group had a readmission within 28 days after hospital discharge (RR =1.13 [95% CI: 0.50–2.29], P=0.75). In the multivariate analysis, inclusion in the PRADO group was neither significantly associated with 28-days readmission or death (RR adjusted [RRa] =0.99 [95% CI: 0.48–1.85], P=0.97) nor with the frequent exacerbator phenotype (RRa =1.09 [95% CI: 0.61–1.96], P=0.78), but was significantly associated with stage 3 and 4 COPD (RRa =2.89 [95% CI: 1.28–8.24], P=0.008).
Similar results were found at 3 months: 18 (29.0%) read-missions or deaths in the PRADO group and 55 (27.2%) in the control group (RR =1.07 [95% CI: 0.66–1.63], P=0.78). In the multivariate analysis, inclusion in the PRADO group (RRa =0.91 [95% CI: 0.56–1.38], P=0.66) was not significantly associated with 90-days, readmission or death while having a stage 3 or 4 COPD (RRa =1.63 [95% CI: 1.01–2.88], P=0.0475) and having a frequent exacerbator phenotype (RRa =1.66 [95% CI: 1.11–2.53], P=0.014) were significantly associated. At 3 months, 5 (8.1%) patients died in the PRADO group and 18 (8.9%) died in the control group (RR =0.91 [95% CI: 0.31–2.16], P=0.83).
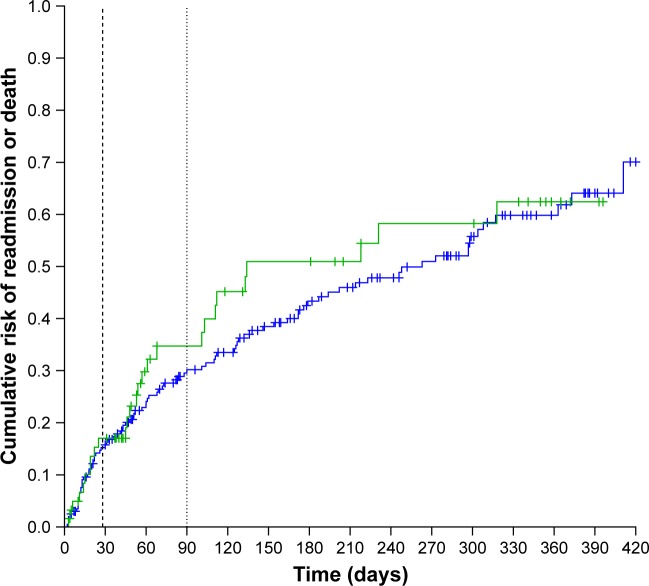
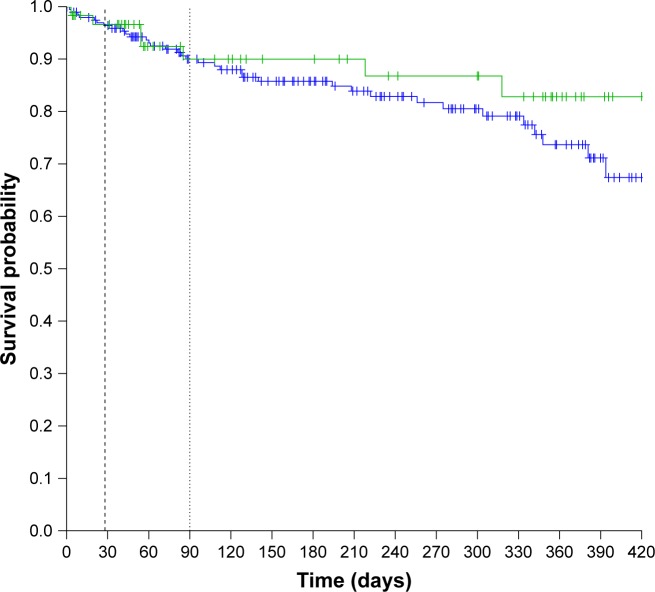
The median time to readmission for AECOPD or death was not significantly different between the PRADO and the control groups: 134 (95% CI: 103–Infinity) days in the PRADO group vs 263 (95% CI: 179–363) days in the control group (HR =1.14 [95% CI: 0.74–1.76], P=0.55; Figure 2). Median overall survival time was not met. Overall survival was not significantly different between the PRADO and the control groups (HR =0.66 [95% CI: 0.29–1.48], P=0.31, Figure 3). Adjustment on stage 3/4 and on exacerbator phenotype had little effect (Hazard Ratio adjusted (HRa) =0.61 [95% CI: 0.27–1.37], P=0.23). The annualized number of deaths/ exacerbations was not significantly different in the two groups: 1.52 (95% CI: 1.12–2.05) readmission or death/person/year in the PRADO group vs 1.18 (95% CI: 0.99–1.41) in the control group (P=0.16). The median follow-up times for the overall survival in the PRADO and control groups were 228 (Q1=101, Q3=362) and 226 (Q1=58, Q3=359), respectively.
Figure 2.
Cumulative risk of readmission or death in the PRADO group (green line) and in the control group (blue line) (black dashed line: 28 days – black dot line: 90 days; Log-rank: 0.37, P: 0.55).
Figure 3.
Probability of survival in the PRADO group (green line) and in the control group (blue line) (black dashed line: 28 days – black dot line: 90 days; Log-rank: 1.04, P: 0.31).
Discussion
In this retrospective cohort study, the PRADO discharge care bundle did not significantly improve 28-days readmission rate and mortality. However, patients in the PRADO group had a more severe COPD with more frequent exacerbations than those in the control group. Adjustment on these confounding had a minor effect on results.
This is the first study to assess the feasibility of a home discharge care bundle in France for the follow-up of patients hospitalized for AECOPD. Lack of access to community support following an AECOPD is a risk factor for new AECOPD.20,21 Our results show that the French health care organization can provide rapid access to community support. Indeed, on average, all patients in the PRADO group were referred to the general practitioner, the nurse, and the physiotherapist within 7 days after discharge.
Our study did not show a significant improvement in early (28-days) and late (90-days) readmission or mortality rate in the PRADO group when compared with controls. This could be explained by a lack of power as the post hoc power analysis shows. Furthermore, patients in the PRADO group also had a significantly higher number of severe AECOPDs prior to inclusion. Considering the retrospective design of this study, and as inclusion to the PRADO discharge care bundle was left to physician’s judgment, we hypothesize that physicians were more likely to offer such care bundle to the most severe patients.
As this was a retrospective study, we were not able to perform a detailed phenotyping of patients during AECOPD. In contrast with stable COPD,23 AECOPD phenotypes are not well characterized.24 As patients with COPD often have comorbidities;25–27 such phenotyping could be useful to identify patients more likely to benefit from a home discharge care bundle. Indeed, in our population, patients in the control group were more likely to receive diuretics when compared with the PRADO group, emphasizing a different clinical presentation. Several prognostic factors for readmission have been developed,13,15,18 as well as noninvasive physiological assessment.16,28 These tools need to be evaluated to identify patients at high risk for readmission, who are more likely to benefit from a home discharge care bundle.
This study shows that patients in the PRADO group had a shorter length of stay than the control group despite being more severe. This shorter stay may have been achieved because hospital physicians were confident with the organized outpatient’s follow-up. This result is important because it could contribute to the cost-effectiveness of discharge care bundle programs and such evaluation should be integrated in the future. Indeed, the trials included in the meta-analysis17 had variable interventions with variable costs. Length of stay was shorter in the PRADO group but remains higher than the average one in UK22 and Canada.29 It is similar to the usual French inpatient stay duration for AECOPD, 9.9 days,6 illustrating another French paradox, as this longer inpatient stay could explain a lower readmission rate.18
Given health care organization in France, all patients included in the program had full access to the care. The PRADO program shows that the health care system provides rapid access to general practitioners as well as health care provided in the week following discharge. This access to care can explain why our discharge care bundle does not include self-managed antibiotic or steroids.
This study has several limitations given its retrospective design. The PRADO group is not comparable to the control group with respect to the phenotype and COPD severity of patients. This is due to a selection bias as physicians were more likely to refer patients with severe COPD to the PRADO program. However, even after adjustments for the cofounding factor, we did not find any significant difference between groups. Another limitation is that patients were recruited from the initiation of the PRADO program. In 2015, this program was new and relied on community physicians, nurses, and physiotherapists who may not have been fully aware of the program. Hence, community services may not have taken full advantage from the help of the social welfare discharge coordinator. This may have led to under-emphasize the efficacy of the program.
Conclusion
In our center, a home discharge care bundle failed to prove its efficacy on 28-days readmission and mortality after hospitalization for AECOPD. Considering longer inpatient stay for AECOPD in France compared with other countries, future randomized controlled trials evaluating the care bundle program should focus on early discharge and cost-effectiveness.
Acknowledgments
The authors thank Dr Valérie Buvat (Service médical de l’Assurance Maladie Normandie) and Virginie Illien (Caisse primaire d’Assurance Maladie de Rouen-Elbeuf-Dieppe) for their help in the management of PRADO procedure and patients.
Footnotes
Author contributions
SC: conception, acquisition, analysis, interpretation, drafting the work; MS, LT: interpretation and revising critically; AC: conception, interpretation, revising critically; MP: conception, acquisition, analysis, drafting the work, revising critically. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Santibáñez M, Garrastazu R, Ruiz-Nuñez M, et al. Predictors of hospitalized exacerbations and mortality in chronic obstructive pulmonary disease. PLoS One. 2016;11(6):e0158727. doi: 10.1371/journal.pone.0158727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solem CT, Sun SX, Sudharshan L, Macahilig C, Katyal M, Gao X. Exacerbation-related impairment of quality of life and work productivity in severe and very severe chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:641–652. doi: 10.2147/COPD.S51245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinari N, Chanez P, Roche N, Ahmed E, Vachier I, Bourdin A. Rising total costs and mortality rates associated with admissions due to COPD exacerbations. Respir Res. 2016;17(1):149. doi: 10.1186/s12931-016-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Aymerich J, Farrero E, Félez MA, et al. Estudi del Factors de Risc d’Agudització de la MPOC investigators Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi: 10.2147/COPD.S34186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20–28. doi: 10.1378/chest.06-1316. [DOI] [PubMed] [Google Scholar]
- 10.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 11.Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173(8):695–698. doi: 10.1001/jamainternmed.2013.171. [DOI] [PubMed] [Google Scholar]
- 12.Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634–639. doi: 10.1164/rccm.201308-1541PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echevarria C, Steer J, Heslop-Marshall K, et al. The PEARL score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPD. Thorax. 2017;72(8):686–693. doi: 10.1136/thoraxjnl-2016-209298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahadori K, Fitzgerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation – systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241–251. [PMC free article] [PubMed] [Google Scholar]
- 15.Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi: 10.2147/COPD.S122382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suh ES, Mandal S, Harding R, et al. Neural respiratory drive predicts clinical deterioration and safe discharge in exacerbations of COPD. Thorax. 2015;70(12):1123–1130. doi: 10.1136/thoraxjnl-2015-207188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ospina MB, Mrklas K, Deuchar L, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax. 2017;72(1):31–39. doi: 10.1136/thoraxjnl-2016-208820. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman C, Moutengou E, Roche N, Delmas MC. Prognostic factors after hospitalization for COPD exacerbation. Rev Mal Respir. 2017;34(1):1–18. doi: 10.1016/j.rmr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Steer J, Norman EM, Afolabi OA, Gibson GJ, Bourke SC. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117–121. doi: 10.1136/thoraxjnl-2011-200332. [DOI] [PubMed] [Google Scholar]
- 20.Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(18):1664–1670. doi: 10.1001/archinternmed.2010.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif R, Parekh TM, Pierson KS, Kuo YF, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospital-ized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):685–694. doi: 10.1513/AnnalsATS.201310-358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National COPD Audit 2008 . RCP; London: [Accessed April 17, 2017]. Published August 20, 2015. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-copd-audit-2008. [Google Scholar]
- 23.Burgel PR, Roche N, Paillasseur JL, et al. Clinical COPD phenotypes identified by cluster analysis: validation with mortality. Eur Respir J. 2012;40(2):495–496. doi: 10.1183/09031936.00228511. [DOI] [PubMed] [Google Scholar]
- 24.Zhou A, Zhou Z, Zhao Y, Chen P. The recent advances of phenotypes in acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1009–1018. doi: 10.2147/COPD.S128604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888. doi: 10.2147/COPD.S49621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi: 10.1183/09059180.00008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divo MJ, Casanova C, Marin JM, et al. COPD comorbidities network. Eur Respir J. 2015;46(3):640–650. doi: 10.1183/09031936.00171614. [DOI] [PubMed] [Google Scholar]
- 28.Murphy PB, Kumar A, Reilly C, et al. Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax. 2011;66(7):602–608. doi: 10.1136/thx.2010.151332. [DOI] [PubMed] [Google Scholar]
- 29.Mulpuru S, McKay J, Ronksley PE, Thavorn K, Kobewka DM, Forster AJ. Factors contributing to high-cost hospital care for patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:989–995. doi: 10.2147/COPD.S126607. [DOI] [PMC free article] [PubMed] [Google Scholar]