Abstract
Cellular membrane trafficking mediated by the clathrin adaptor protein complex-1 (AP-1) is important for the proper composition and function of organelles of the endolysosomal system. Normal AP-1 function requires proteins of the HEAT repeat–containing 5 (HEATR5) family. Although HEATR5 proteins were first identified based on their ability to interact with AP-1, the functional significance of this interaction was unknown. We used bioinformatics-based phenotypic profiling and information from genome-wide fluorescence microscopy studies in the budding yeast Saccharomyces cerevisiae to identify a protein, Laa2, that mediates the interaction between AP-1 and the yeast HEATR5 protein Laa1. Further characterization of Laa2 revealed that it binds to both Laa1 and AP-1. Laa2 contains a motif similar to the characterized γ-ear–binding sites found in other AP-1–binding proteins. This motif in Laa2 is essential for the Laa1–AP-1 interaction. Moreover, mutation of this motif disrupted AP-1 localization and function and caused effects similar to mutations that remove the γ-ear of AP-1. These results indicate that Laa2 mediates the interaction between Laa1 and AP-1 and reveal that this interaction promotes the stable association of AP-1 with membranes in yeast.
Keywords: clathrin, protein trafficking (Golgi), endosome, membrane trafficking, adaptor protein, γ-ear, CLBA, HEAT repeat–containing 5, HEATR5, lysosome
Introduction
Clathrin-dependent traffic is a central feature of all eukaryotic cells. Clathrin is important for endocytosis and traffic at internal compartments. At these locations, it is the major structural component of a protein coat that assembles on membranes that is thought to generate vesicles, larger membrane compartments, and/or tubules that mediate traffic (1). Clathrin function requires adaptors, which recruit clathrin to the membrane and link it to the membrane protein cargo that will be trafficked (2). Adaptors also recruit accessory proteins important for traffic and may contribute mechanistically to traffic through membrane deformation and other activities (3, 4).
AP-1 is a highly conserved clathrin adaptor that functions at internal organelles including the trans-Golgi network (TGN),2 endosomes, and lysosome-related organelles (5). AP-1 plays pivotal roles in lysosomal protein sorting and recycling from the endosomes and nascent lysosome-related organelles. It is made of four subunits: β, γ, μ, and σ. The N termini of the β- and γ-subunits together with the μ- and σ-subunits form the cargo and membrane-binding “core” (6), whereas appendages on the β- and γ-subunits bind clathrin and several accessory factors (7). Therefore, the interaction of AP-1 with cargo sets in motion a process that assembles a coat and ultimately dictates where cargo will be directed. For this reason, AP-1 association with cargo is highly regulated.
In budding yeast, AP-1 localizes to the TGN (8, 9). In yeast, the TGN is a dynamic compartment that forms from the maturation of the Golgi and dissipates through the action of recycling, secretion, and vacuolar protein sorting (10, 11). In this continuum, AP-1 participates in one of the last acting events at the TGN as monitored by time-lapse microscopy (12, 13). It is still unclear how the cell orchestrates the precise temporal specificity of AP-1 recruitment to this late stage.
AP-1 recruitment depends, in part, on physical interaction with cargo, the small GTPase Arf1, and phosphatidyl-inositol-4-phosphate (PI4P). Structural studies suggest that AP-1 can bind all three simultaneously, and in vitro, purified AP-1 binds synthetic liposomes containing all three factors (14, 15). This cooperative binding does not completely explain the temporal specificity of AP-1 recruitment, because AP-1 is recruited after the peak of Arf1 and PI4P, suggesting additional factors may play a role in AP-1 recruitment in vivo (13). One candidate for such a factor is Laa1, a member of the conserved HEATR5 family, which was previously reported to contribute to AP-1 localization (16).
HEATR5 proteins are large proteins made of many tandem HEAT repeats. The HEAT repeat is a simple fold often found in scaffolding proteins (17). Based on this property, HEATR5 proteins may have many binding partners. The founding member of this family, HEATR5b (also known as p200a), was identified in a proteomics study of proteins that interact with the AP-1 γ subunit appendage domain known as the γ-ear (18). HEATR5b and homologs in Drosophila melanogaster (CG2747), Caenorhabditis elegans (SOAP-1), Schizosaccharomyces pombe (Sip1), and Saccharomyces cerevisiae (Laa1) are required for many AP-1 functions (16, 19–22). Although disruption of HEATR5 genes causes strong defects in AP-1 recruitment in flies, nematodes, fission, and budding yeast, it was unclear whether HEATR5 played a direct role in recruiting AP-1 and whether this required the interaction between HEATR5 and AP-1 (16, 21, 22).
One challenge was that there was no way to selectively disrupt HEATR5 binding with AP-1 to test the functional significance of the interaction in AP-1 localization. This is because the interaction between AP-1 and HEATR5 proteins is thought to be indirect. HEATR5 proteins lack canonical AP-1 γ-ear–binding sites. In mammalian cells, HEATR5b binds two proteins, aftiphilin and γ-synergin, that themselves interact directly with the γ-ear (19, 23, 24). These proteins are thought to mediate the interaction between HEATR5b and AP-1. However, this has yet to be tested because HEATR5, aftiphilin, and γ-synergin stabilize one another; thus knocking down any one destabilizes the other two (19, 25). Furthermore, the functional orthologs of aftiphilin and γ-synergin were unknown in other, more genetically and biochemically tractable organisms. Here we present the identification of the protein Ybl010c in budding yeast as the bridge between the HEATR5 protein Laa1 and AP-1. We identify a single canonical γ-ear–binding site in Ybl010c that is required for the interaction between Laa1 and AP-1. Moreover, disruption of this motif disrupts AP-1 localization and function. Together, these results argue that in budding yeast, HEATR5 cooperates with cargo, Arf1, and PI4P to recruit AP-1.
Results
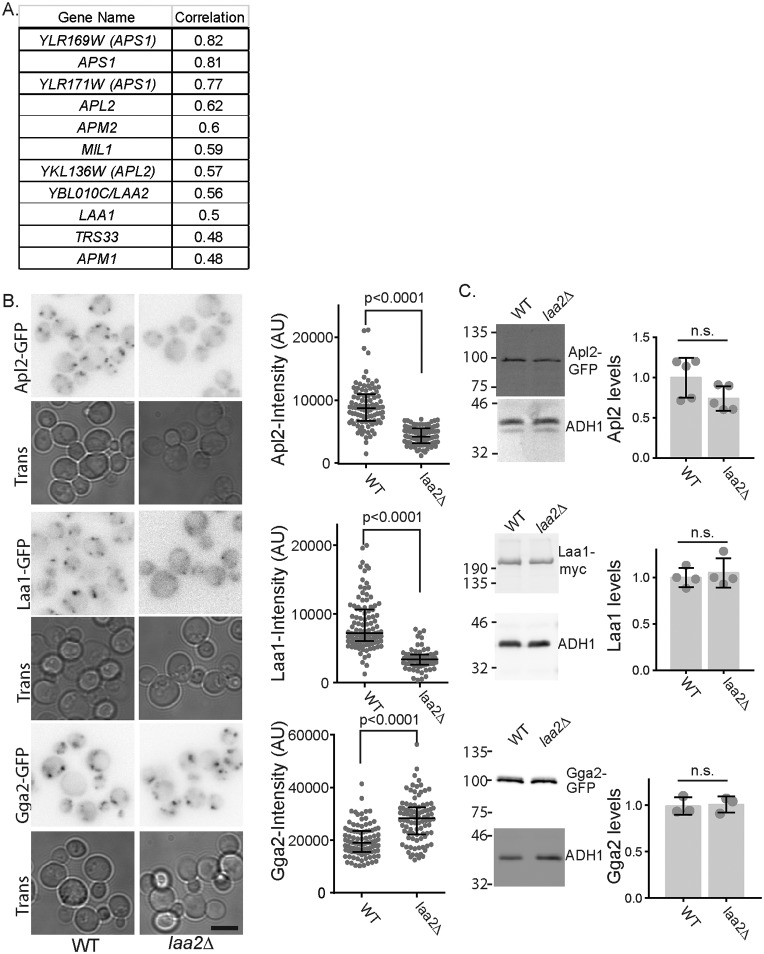
Budding yeast is an excellent system to understand gene networks important for membrane traffic. It is amenable to both genetic and biochemical approaches. The genome and membrane trafficking pathways are simpler, yet many of the gene functions are conserved (26). Moreover, as the first eukaryote for which a systematic deletion/depletion system was developed, there is a wealth of genome-wide phenotypic data to inform on gene function (27). We took advantage of these data sets to search for new genes important for AP-1 function. We mined an existing database that contains information about the effect of gene deletions on growth under ∼1800 different chemical treatments (28). These data were subjected to global two-way hierarchical clustering to identify gene deletions whose sensitivity and resistance profiles were highly correlated (28). We analyzed the gene deletions whose profiles correlated with the AP-1 γ-subunit gene APL4. As expected, deletions that disrupted other subunits of the AP-1 complex were correlated with APL4. The top correlated genes also included deletion of MIL1, a recently identified co-factor for AP-1, LAA1, and TRS33, a core component of the TRAPP tethering complexes that function at the Golgi and endosomes (Fig. 1A) (29, 30). The top hits also included one uncharacterized gene YBL010c; on the basis of the results described below, we refer to YBL010c as LAA2 (Large Adaptin Accessory 2).
Figure 1.
Identification of Laa2 as a potential AP-1 accessory factor. A, LAA2 (YBL010C) was identified by homozygous deletion library profile correlation analysis as similar to genes important for AP-1 dependent traffic. The table shows genes correlated with APL2 and correlation values from Hoepfner et al. (28). Genes indicated in parentheses are AP-1 subunit genes that overlap with the indicated dubious open reading frames. B, effect of laa2Δ on localization of indicated adaptors. WT or laa2Δ cells expressing the indicated GFP-tagged genes from their endogenous loci were imaged by fluorescence microscopy. Bar is 5 μm. Charts show the intensity of individual puncta, representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis. C, expression levels of Apl2, Laa1, and Gga2 are unaffected in laa2Δ. Locations of molecular weight standards are indicated. Charts show the expression levels normalized to WT. Individual replicates are plotted individually. Bars show mean and standard deviation. Significance was determined with a two-tailed Student's t test. n.s., not significant.
In addition to unbiased phenotypic correlation with AP-1 subunit genes, Laa2–GFP was reported to co-localize with clathrin at internal structures in a high-throughput data set (31). Based on these data, we first asked whether LAA2 was important for AP-1 localization. To do this, we visualized GFP-tagged β-subunit (Apl2) expressed from endogenous locus in WT and laa2Δ (Fig. 1B). In WT cells, Apl2 localizes to several bright puncta per cell. In the laa2Δ cells, Apl2 localized to fewer puncta, and these puncta were substantially dimmer. When intensity values of individual puncta were determined, those in laa2Δ were on average half as intense as those in WT cells, and the maximum intensity of puncta in laa2Δ cells was lower than the median intensity in WT cells. This defect was not due to a significant loss of Apl2 expression, as determined by Western blotting analysis, indicating a severe defect in AP-1 localization (Fig. 1C).
To determine whether this effect was specific to AP-1, we examined the effect of laa2Δ on the localization of Laa1 and on Gga2, a clathrin adaptor that localizes to the TGN. Similar to AP-1, we found that Laa1 localization was disrupted in laa2Δ cells. Like AP-1, it localized to fewer puncta, and the puncta were dimmer than in WT (Fig. 1B). In contrast, Gga2 localized to many puncta, and the puncta intensities were slightly higher in the laa2Δ cells than in WT cells (Fig. 1B). As with Apl2, loss of Laa2 did not significantly affect the expression of either Laa1 or Gga2 (Fig. 1C). The fluorescence of Gga2 argues that the decrease in GFP fluorescence seen with AP-1 and Laa1 was not due to changes in cytosolic chemistry that reduces GFP fluorescence. Together, these results argue Laa2 is important for the localization of both AP-1 and Laa1, but based on the localization of Gga2, laa2Δ does not disrupt the TGN, indicating a specific role in AP-1 and Laa1 localization.
The effect of Laa2 on Laa1 localization led us to investigate whether Laa2 interacts with Laa1. We found that Laa2–Myc expressed from its endogenous locus co-immunoprecipitated Laa1–Flag expressed from its endogenous locus, indicating that the two interact (Fig. 2A). We next asked whether Laa2 localization depends on Laa1. We first established that Laa2–GFP expressed from its endogenous locus co-localized with AP-1 (Fig. 2B). We then examined Laa2 localization in cells lacking Laa1. We found that in cells lacking Laa1, Laa2 localization was disrupted. Laa2–GFP was localized to fewer, dimmer puncta in laa1Δ compared with WT cells (Fig. 2C). Notably, this was associated with a dramatic decrease in Laa2 levels, suggesting that Laa2 requires Laa1 for stability (Fig. 2D). This indicates Laa2 is an obligate binding partner for Laa1. However, Laa1 levels were unaffected in cells lacking Laa2, suggesting that Laa1 does not require Laa2 for stability in vivo (Fig. 1C).
Figure 2.
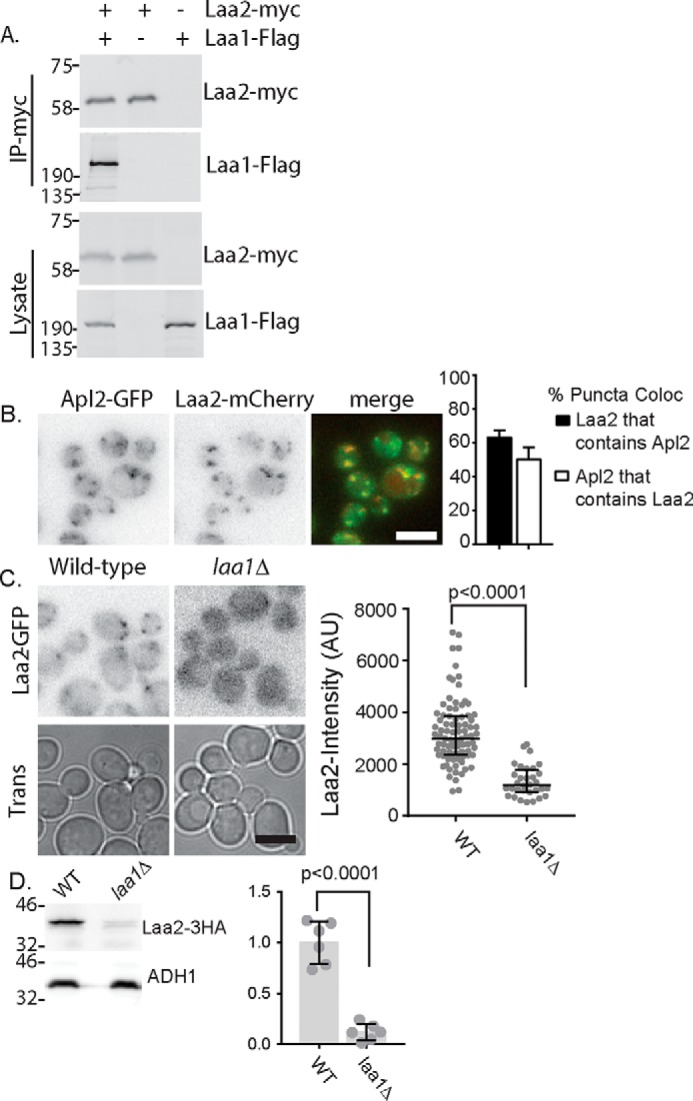
Laa2 binds Laa1. A, indicated fusion proteins were expressed at their endogenous loci and subjected to co-immunoprecipitation. Lysates are 2% of immunoprecipitation reactions. The data are representative of three repeats. B, Laa2 co-localizes with AP-1. Indicated fusion proteins were expressed at their endogenous loci and imaged by fluorescence microscopy. Right, quantification of the percentage of Laa2-mCherry regions that contain Apl4-GFP and vice versa. Error bars indicate standard deviation, n = 5. C, Laa2 is delocalized in cells lacking Laa1. The indicated strains were imaged by fluorescence microscopy. Charts show the intensity of individual puncta, representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis. D, Laa2 is destabilized in cells lacking Laa1. Western blotting of lysates from cells expressing Laa2-HA from its endogenous locus in WT and laa1Δ cells. The data are representative of three repeats. Approximate molecular weights are indicated. Bars in micrographs are 5 μm. Chart shows expression levels normalized to WT. Individual replicates are plotted individually. Bars show mean and standard deviation. Significance was determined with a two-tailed Student's t test.
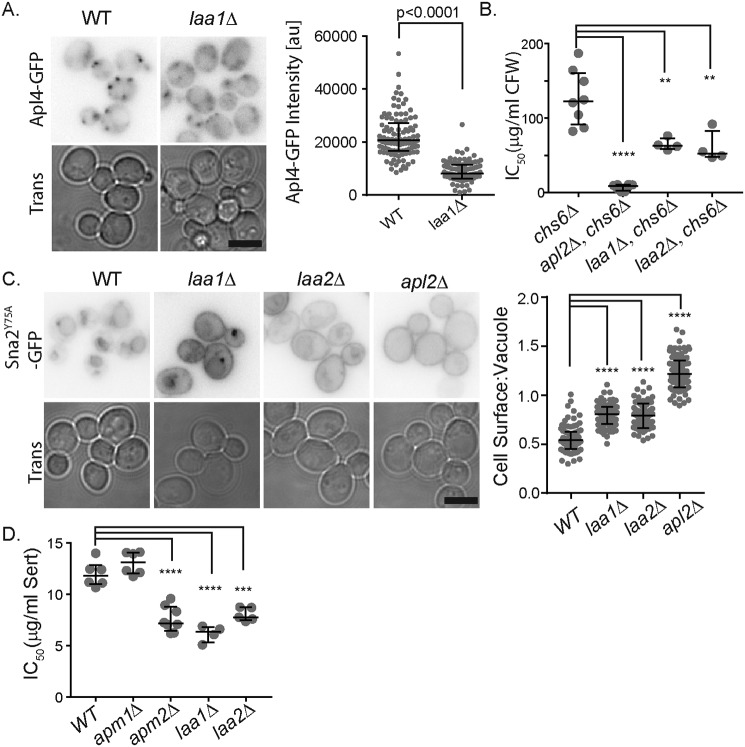
Based on the obligate role of Laa1 for Laa2 stability, we asked whether loss of the two genes caused similar effects on AP-1 localization and function. Using quantitative fluorescence microscopy, we found that like laa2Δ, laa1Δ reduced the number of AP-1 puncta and reduced the intensity by ∼50% (Fig. 3A). Next, we investigated effects of laa2Δ and laa1Δ on AP-1 functions. In yeast, AP-1 forms two functionally distinct complexes defined by their incorporation of one of two alternate μ-subunits (29). The more well-studied AP-1 complex in yeast (referred to here as AP-1C for conventional) contains the μ-subunit encoded by Apm1, whereas the newly characterized AP-1 complex (AP-1R) contains the μ-subunit encoded by Apm2 (29). These complexes recognize distinct cargo and can be assayed independently. To monitor AP-1C, we used calcofluor white (CFW) sensitivity in cells lacking the exomer subunit Chs6. This assay is thought to monitor the AP-1c–dependent retention of the chitin synthase Chs3 in the TGN when its normal traffic out of the TGN is disrupted (29, 32). We found that laa2Δ and laa1Δ caused similar increases in the CFW sensitivity in cells lacking Chs6, suggesting a similar defect in AP-1C–mediated traffic (Fig. 3B). To monitor AP-1R, we used localization of Sna2Y75A and sertraline sensitivity. Sna2Y75A is a vacuolar membrane protein whose delivery to the vacuole depends on AP-1R (29). Sertraline is a cationic amphiphilic drug that is thought to interfere with membrane trafficking pathways by intercalating into phospholipid bilayers (33). It selectively inhibits the growth of cells lacking Apm2 (29). We found that laa2Δ and laa1Δ mis-sorted Sna2Y75A to the plasma membrane to a similar extent and caused similar levels of sensitivity to sertraline. These results indicate that laa2Δ and laa1Δ cause similar defects in AP-1R–mediated traffic. Consistent with the partial localization of AP-1 in the laa2Δ and laa1Δ deletions strains, complete loss of AP-1 caused stronger effects on CFW sensitivity and Sna2Y75A mis-sorting than laa2Δ and laa1Δ. In contrast, all three strains were similarly sensitive to sertraline. However, cationic amphipathic drugs such as sertraline are thought to concentrate in cellular membranes, which may lead to nonlinear effects explaining why the three mutants appear similarly sensitive (33). Together, these results indicate that Laa2 and Laa1 are important for the function of both AP-1C and AP-1R consistent with their strong effect on the localization of Apl2 and Apl4, which are components of both complexes.
Figure 3.
laa1Δ and laa2Δ cause similar phenotypes. A, WT or laa1Δ cell expressing Apl4-GFP from its endogenous locus were imaged by fluorescence microscopy. Charts show the intensity of individual puncta, representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis. B, IC50 values for CFW in indicated strains. Individual repeats are plotted. Bars indicate median and interquartile range. p values were determined by two-tailed Student's t test. ****, p < 0.0001; **, p < 0.01 C, indicated cells expressing Sna2Y75A-GFP from a plasmid were imaged by fluorescence microscopy. Charts show ratio of intensity of vacuolar membrane to plasma membrane for individual cells. The data are representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis ****, p < 0.0001. D, IC50 values for Sertraline in indicated strains. Individual repeats are plotted. Bars indicate median and interquartile range. p values were determined by two-tailed Student's t test. ****, p < 0.0001; ***, p = 0.0001. For micrographs, a bar indicates 5 μm.
Laa2 is a small protein with no identifiable homology to known folded protein domains. However, it contains two regions of similarity to aftiphilin, a HEATR5b-binding protein that interacts with both AP-1 and clathrin. Notably, one region of similarity is in the canonical AP-1 γ-ear–binding motif found in many AP-1–binding proteins (Fig. 4A) (18, 24, 34, 35). Based on the presence of this motif, we tested whether Laa2 is an AP-1 γ-ear–binding protein. We found that Laa2 bound the γ-ear encoded in the C terminus of Apl4 using yeast two-hybrid and recombinant proteins (Fig. 4, B and C). In yeast lysates, recombinant AP-1 γ-ear interacted with Laa2–3HA (Fig. 4D). Moreover, mutation of two amino acids of the γ-ear–binding motif in Laa2 abolished this interaction, suggesting that this motif is essential for the interaction. We next asked whether the interaction of Laa2 with AP-1 was important for the interaction of Laa1 with the γ-ear. We found in cells lacking Laa2, Laa1 did not interact with AP-1 by co-immunoprecipitation (Fig. 4E). Furthermore, in lysates from cells expressing the ear-binding mutant form of Laa2 (laa2–ΔDF24AA) as the sole copy of Laa2, Laa1–Myc was unable to bind the AP-1 γ-ear (Fig. 4F). Notably, this mutation in LAA2 did not alter the interaction of the γ-ear with Ent3 or Ent5, clathrin adaptors that contain their own γ-ear–binding motifs (Fig. 4, D and F) (34). This indicates that Laa2 γ-ear binding is specifically required for Laa1 γ-ear binding.
Figure 4.
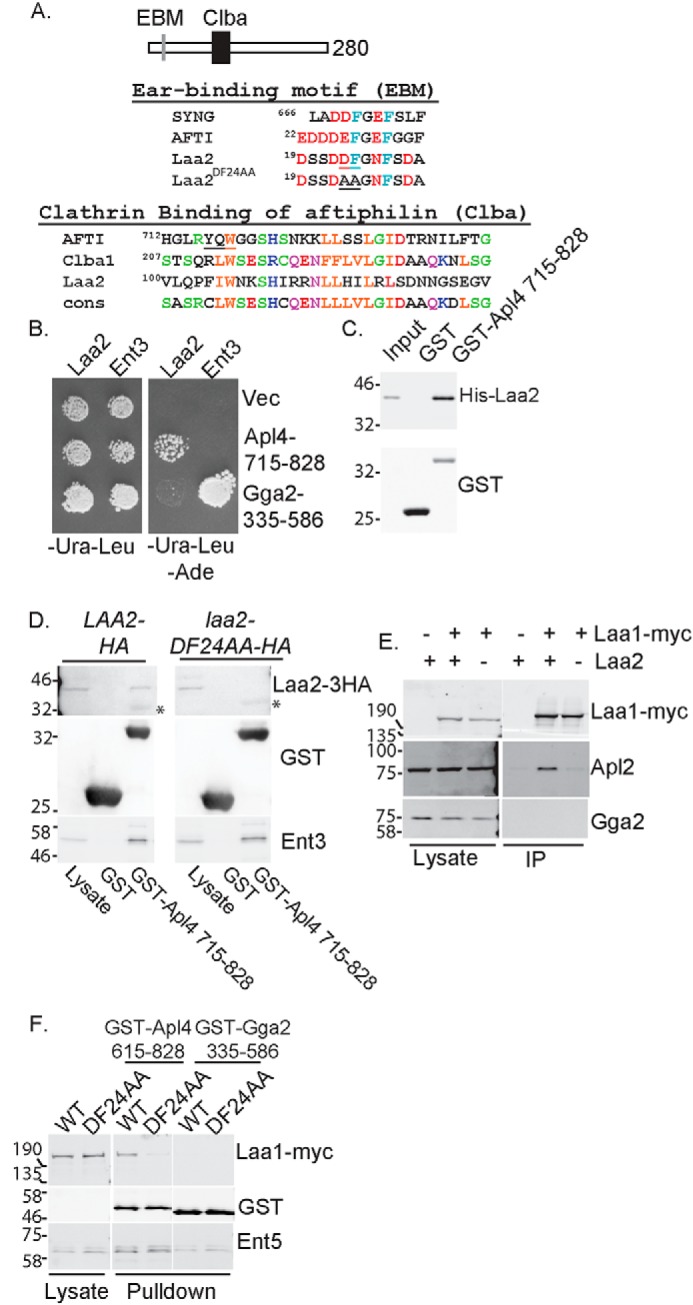
Laa2 ear-binding motif mediates the interaction with AP-1 γ-ear. A, schematic of Laa2 (top). Alignment of ear-binding motif (EBM) with those of γ-synergin and aftiphilin is shown. Underlined residues were mutated to alanines to generate laa2–DF24AA (middle). Alignment of Laa2 with Clba domains of aftiphilin, uncharacterized human protein Clba1, and the consensus motif for the domain defined in Pfam is shown. Underlined residues in aftiphilin are required for clathrin binding (bottom). B, Laa2 interacts with the γ-ear of AP-1 but not that of Gga2 in two-hybrid analysis. Cell expressing activation domain fusions to full-length Laa2 or Ent3, as a positive control, and DNA-binding domain fusions to indicated regions of Apl4 or Gga2 or empty vector were spotted on medium that selected for the plasmids (left) or medium that selected for interaction (right). C, Laa2 interacts directly with the γ-ear of AP-1. Recombinant Laa2 was subjected to GST pulldown using recombinant γ-ear of AP-1 or GST. Input is 10%. The data are representative of three repeats. D, Laa2 ear-binding motif is required for interaction with the γ-ear of AP-1 in GST pulldown experiments. Asterisks denote nonspecific binding of antibodies to GST. E, Laa2 is required for Laa1 interaction with AP-1. Laa1–Myc was immunoprecipitated from WT or laa2Δ cells. Endogenous AP-1 or Gga2 were detected with polyclonal antibodies. Lysates are 4% of immunoprecipitations. Data shown are representative of at least three repeats. F, Laa2 binding to AP-1 is required for Laa1 interaction with the γ-ear of AP-1. Cells expressing indicated allele of Laa2 and Laa1 from their endogenous loci were subjected to GST pulldown using the γ-ear of AP-1, or the γ-ear of Gga2, or GST as a negative control. Samples were probed for GST, HA, Myc, and the adaptor-interacting proteins Ent5 or Ent3, as a positive control. Lysates are 5 and 6% of pulldown without any GST protein for D and E, respectively. Locations of molecular weight standards are indicated. The data shown are representative of at least three repeats.
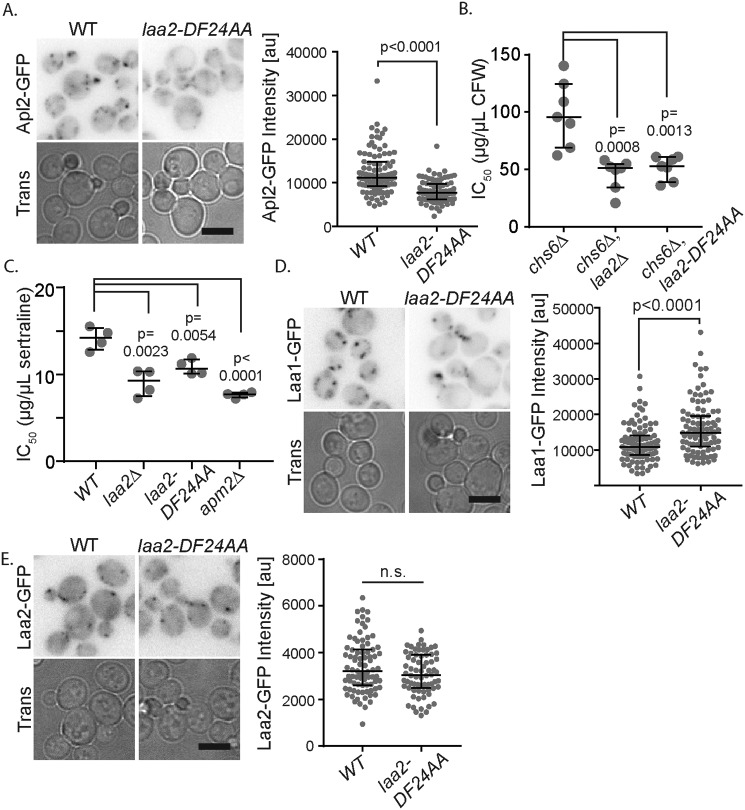
We next tested the functional significance of the interaction between Laa2 and the γ-ear. We found the ear-binding mutant form of Laa2 (laa2–ΔDF24AA) was unable to maintain normal levels of AP-1 localization (Fig. 5A) and function, as assessed with CFW and sertraline sensitivity (Fig. 5, A–C). The defects caused by laa2–DF24AA were nearly as strong as those caused by the complete deletion of LAA2 (compare Figs. 5, A–C, and 3, A, B, and D). This indicates that Laa2 γ-ear binding is required for AP-1 localization and function. Importantly, the localization of Laa1 and Laa2 were unaffected in these cells (Fig. 5, D and E). Because their localization is unaffected, these data strongly suggest that Laa2 directly mediates the interaction between Laa1 and AP-1 and that this interaction is required for AP-1 localization.
Figure 5.
Laa2 ear-binding motif is important for AP-1 localization and function but not Laa1 localization. A, WT or laa2–DF24AA cells expressing Apl2-GFP from its endogenous locus were imaged by fluorescence microscopy. B, IC50 values for CFW in indicated strains. C, IC50 values for sertraline in indicated strains. D, WT or laa2-DF24 expressing Laa1-GFP from its endogenous locus were imaged by fluorescence microscopy. E, GFP-tagged WT or laa2–DF24AA was expressed from its endogenous locus and imaged by fluorescence microscopy. For microscopy analysis, charts show the intensity of individual puncta, representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis. Scale bars are 5 μm. For IC50 values, charts show individual repeats, bars indicate median and interquartile range. p values were determined by two-tailed Student's t test. n.s., not significant.
Finally, we determined whether additional γ-ear–binding proteins contribute to AP-1 localization and function. We reasoned that if additional γ-ear–binding proteins contribute to AP-1 localization, deletion of the γ-ear would cause stronger defects than loss of laa1Δ or laa2Δ. We first examined the localization and function of AP-1 lacking the γ-ear. Deletion of the γ-ear reduced AP-1 puncta number and intensity to a level similar to that of laa2–ΔDF24AA, laa2Δ, and laa1Δ (Fig. 6A). In CFW assays, deletion of the γ-ear reduced CFW resistance to a level similar to that of laa2–ΔDF24AA, laa2Δ, and laa1Δ (Fig. 6B). In sertraline sensitivity assays, deletion of the γ-ear had a weaker effect than laa2–ΔDF24AA, laa2Δ, and laa1Δ (Fig. 6C). These data indicate that other γ-ear–binding proteins cannot compensate for the loss Laa complex in terms of AP-1 localization and function and suggest that the Laa complex is the primary γ-ear–binding factor required for AP-1 localization.
Figure 6.
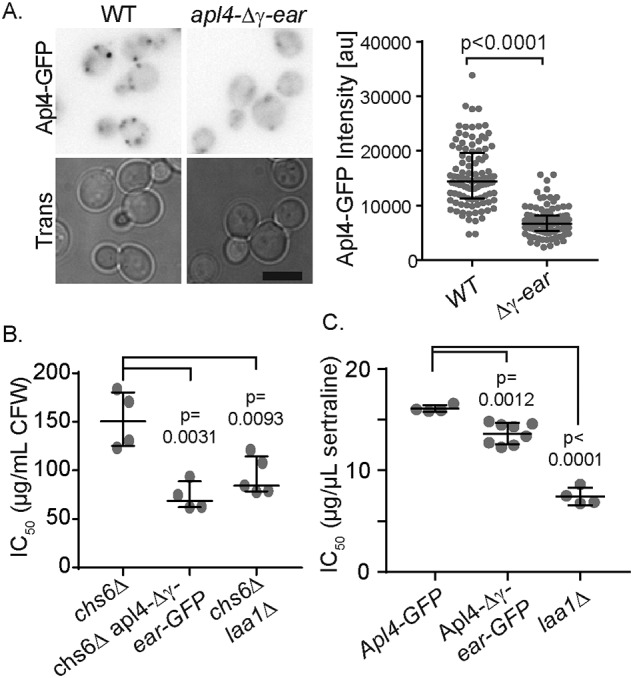
Apl4 γ-ear causes effects similar to the loss of the Laa1–Laa2 complex. A, GFP-tagged WT or apl4-Δγear was imaged by fluorescence microscopy. B, IC50 values for sertraline in indicated strains. C, IC50 values for sertraline in indicated strains. For microscopy analysis, charts show the intensity of individual puncta, representative of three repeats. Bars indicate median and interquartile ranges. p values were determined by Mann–Whitney rank-sum analysis. Scale bars are 5 μm. For IC50 values, charts show individual repeats. Bars indicate median and standard deviation. p values were determined by two-tailed Student's t test.
Discussion
Proteins of the HEATR5 family are important for AP-1 function in diverse organisms (16, 19–22, 36). Despite their original identification as AP-1–associated proteins, the functional significance of their interaction with AP-1 was unclear in any system until now (19). Our identification of Laa2 as the critical factor that bridges the interaction between the HEATR5 protein Laa1 and AP-1 in yeast has allowed us to test the functional significance of the interaction for the first time in any system. We found that a point mutation that disrupts the single γ-ear–binding site in Laa2 disrupts the interaction between Laa1 and AP-1 and also disrupts AP-1 localization without altering Laa1 localization. These results indicate that binding of the Laa1–Laa2 complex to AP-1 plays a direct role in recruiting and/or stabilizing AP-1 on membranes.
Our results further indicate that like mammalian HEATR5b, Laa1 functions in the context of a protein complex. We propose that the Laa1–Laa2 protein complex cooperates with Arf1 and PI4P synthesis to confer temporal specificity to AP-1 recruitment. In this light, it is noteworthy that Laa1 depends on Arf1 for localization (16). This could explain why AP-1 recruitment peaks after the peak recruitment of the Arf-GEF Sec7 (12, 13). In this case, the temporal lag of AP-1 is explained by the time required to recruit Laa1 in response to active Arf1 and then the time required for Laa1 to recruit AP-1. The concept that an additional γ-ear–binding factor would cooperate with cargo, Arf1, and PI4P is supported by early studies with overexpressed AP-1 γ-subunits in mammalian cells, which showed a dramatic loss of membrane association in the absence of the γ-ear (37). It is appealing to speculate that HEATR5 complexes may be the key γ-ear–binding factors important for AP-1 localization in mammalian cells.
Although it seems that the requirement for a co-factor to mediate the interaction between AP-1 and HEATR5 proteins is conserved, it is not yet clear what features of Laa2 are conserved. Like the mammalian HEATR5-binding proteins aftiphilin and γ-synergin, Laa2 binds to AP-1 using a canonical γ-ear–binding motif (18, 23, 24, 38). Moreover, Laa2 is similar to a section of aftiphilin known as pfam15045 or the Clba (clathrin box of aftiphilin) domain. The Clba domain is an ∼80-amino acid region that spans a highly conserved tryptophan residue that is required for clathrin binding in aftiphilin (19). This tryptophan residue and flanking residues are similar in Laa2, raising the possibility Laa2 shares activities with aftiphilin in addition to γ-ear binding. However, aftiphilin is three times larger than Laa2. This suggests that if Laa2 is the functional ortholog of aftiphilin, then aftiphilin may have additional functions that are lacking in Laa2. Moreover, aftiphilin is required for HEATR5b stability, whereas Laa2 does not appear to stabilize Laa1 (19, 25). This could indicate that Laa2 and aftiphilin bind to different regions of HEATR5 and that they are not homologs. In support of the possibility that Laa2 represents a novel type of HEATR5-binding protein, we identified several additional proteins annotated as containing the Clba domain in diverse species, which may represent Laa2 homologs (Fig. 4A). Like Laa2, these additional Clba domain–containing proteins are small proteins, with sequences that match the γ-ear–binding motif. However, it remains to be determined whether any of these proteins bind clathrin, AP-1, or HEATR5 or perform any functions similar to Laa2.
In summary, these findings reveal Laa2 as a critical factor important for AP-1 function. Laa2 provides the major γ-ear–binding activity for the HEATR5 protein Laa1 in yeast. The disruption of γ-ear binding reveals that one major function of the Laa1 complex in yeast is to recruit or stabilize AP-1 on membranes. Further work will be needed to determine whether this complex performs additional activities that contribute to normal membrane traffic before or after AP-1 is recruited.
Materials and methods
The strains, plasmids, and oligonucleotides used for this study are listed in Tables S1–S3 (39) (40). Tags and deletions were generated using a one-step PCR-based method (41, 42). LAA2::URA3 and laa2–DF24AA::URA3 were made by gene replacement of laa2Δ with pMD368 and pMD369. Yeast cells were grown in YPD yeast/peptone medium supplemented with 2% glucose and a mixture of adenine, uracil, and tryptophan or synthetic medium SD supplemented with 2% glucose and an amino acid mix as previously described (43). CFW was from Sigma. Sertraline was from Fisher Scientific. CFW (10 mg/ml) was prepared in water. Sertraline (10 mm) was prepared in DMSO. Antibodies against Myc (9E10, RRID:CVCL_L708) were from Biolegend, ADH1 (ab34680, RRID:AB_722702) was from Abcam, and HA (12CA5, RRID:AB_514505) and Flag (M2, RRID:AB_439685) were from Sigma. Alexa Fluor 647 (RRID:AB_141698) and horseradish peroxidase-conjugated goat anti-mouse (RRID:AB_258167) antibodies were from Life Technologies and Sigma, respectively. Alexa Fluor 647 goat anti-rabbit (RRID:AB_141663) antibody was from Life Technologies. Antibodies against Apl2, Ent5, and GST were described previously (44, 45).
Imaging and analysis was performed as described previously (46). Briefly, cells were cultured to logarithmic phase in SD medium, centrifuged briefly, and mounted on an uncoated coverslip in growth medium. Images were collected with a Nikon Ti-E inverted microscope with a 1.4 numerical aperture/100× oil immersion objective. The Lumencor LED light engine (472/20 nm for GFP and 575/20 nm for mCherry) was used for fluorophore excitation. Filters were ET/GFP-mCherry (59,002×), excitation dichroic (89019bs), and emission-side dichroic (T560lpxr), and for emission filters, ET525/50 m and ET595/50 m (Chroma).
Measurement of fluorescence intensities of puncta was conducted using a previously described method using a custom graphical user interface written in MATLAB (47). Briefly, the signal region was a 6 × 6-pixel box centered on the brightest four pixels of a manually selected spot in the micrograph. The background fluorescence was defined by a concentric 8 × 8-pixel box surrounding signal region. The background intensity was the median value of pixel intensities in the outermost ring of this box. The fluorescence signal was the sum of all 36 pixels in the 6 × 6 box. Intensity analysis was performed on structures from a minimum of 50 cells. The data shown come from analysis performed on a single day; intensity variance between days was minimal. To determine co-localization of Laa2 and AP-1, images were denoised using SpatTrackV2 (48). From the denoised images, ImageJ.v5.1K (National Institutes of Health) was used to threshold the images using Otsu's method and identify particles (49). Co-localization was defined as a particle where at least 20% of the area of a particle defined in one channel was also positive for the second channel.
Whole-cell yeast extracts were generated using glass bead lysis as previously described (50), with the exception of Fig. 2D. For Fig. 2D, cell pellets were resuspended in 0.2 m NaOH and incubated on ice for 10 min. TCA was added to 10%, and cells were disrupted by glass bead lysis. Precipitated proteins were pelleted, rinsed in ice cold acetone, and then resuspended in Laemmli sample buffer with 0.5 m Tris, pH 7, and boiled before loading. For immunoprecipitation, spheroplasts were first generated by resuspending cells in 100 mm Tris-SO4, pH 9.5, 2% glucose, and 5 mm DTT for 10 min. The cells were then resuspended in YP medium supplemented with 0.5% glucose, 10 mm Tris-HCl, 1.2 m sorbitol, and 120 units of lyticase (Sigma). The cells were gently agitated for 15 min at 30 °C and resuspended in HEKG5 (20 mm Hepes, pH 7.5, 1 mm EDTA, 150 mm KCl, 5% glycerol) with protease inhibitor mixture without EDTA (Promega). The cells were disrupted by glass-bead lysis, followed by the addition of 0.3% CHAPS. The lysates were clarified by centrifugation at 13,000 rpm for 10 min at 4 °C. The lysates were incubated overnight at 4 °C with 50 μl of 20% protein A–Sepharose slurry (GE LifeSciences) and 0.5 μl of antibody. The beads were washed three times with ice-cold HEKG5 with CHAPS, and the bound proteins were eluted with SDS sample buffer.
Cell lysates for GST-tagged protein interaction studies were generated by liquid nitrogen blending as previously described (44). Powder was thawed at a ratio of 0.15 g of lysate to 0.85 ml of HEKG5 with protease inhibitor mixture without EDTA (Promega). The lysate was clarified by centrifugation at 13,000 rpm for 10 min, and the resulting supernatants were used for binding studies. Purification of GST and γ-ear constructs were described previously (44). His-tagged Laa2 was purified using Talon resin according to the manufacturer's instructions (Clontech). For binding studies, GST-tagged proteins were prebound to GSH–Sepharose (GE LifeSciences) in 1 ml of PBS with 0.1 mg/ml BSA for 1 h at room temperature, washed twice with PBS, and resuspended in 1 ml lysate or in 1 ml of PBS containing purified His-tagged Laa2. Protein binding proceeded for 2 h at 4 °C, and beads were washed. For Fig. 4 (C and D), beads were washed three times in PBS. For Fig. 4E, beads were wash twice with HEKG5 with 0.3% CHAPS and once with HEKG5 without CHAPS. The beads were resuspended in 100 mm Tris, pH 9, 200 mm NaCl, 5 mm DTT, 20 mm reduced GSH and incubated for 20 min at room temperature. The supernatants were taken as the bound fraction. Two-hybrid interactions were monitored as previously described (34).
For immunoblotting, after SDS-PAGE, samples were transferred to nitrocellulose, blocked with 5% milk in TBS-T (137 mm NaCl, 15.2 mm Tris-HCl, 4.54 mm Tris, 0.896 mm Tween 20), and then probed with primary and fluorescent secondary antibodies. Fluorescence and chemiluminescent signals were detected on an Azure c600 imaging system (Azure Biosciences). For chemiluminescence, Immobilon Western reagents were used (Millipore).
CFW and sertraline sensitivity assays were performed as previously described (51). Briefly, a log phase culture medium was diluted to 0.02 (A600) in yeast/peptone and transferred to a sterile reagent reservoir. The diluted culture was distributed into the wells of a sterile 96-well assay plate. An equal volume of chemical in yeast/peptone media was added to the prepared 96-well plate. An assay plate with lid was placed in a Spectra Max 340PC plate reader (Molecular Devices). The cells were incubated at 30 °C without shaking. Absorbance at 600 nm was collected every 30 min for 18 h. IC50 values, halfway between the maximal and the minimal inhibition, were calculated from a sigmoidal dose-response curve (variable slope, four parameters) using Prism 7 (GraphPad). All other statistical analyses were performed using Prism 7 (GraphPad).
Author contributions
C. J. Z., S. L., F. T. J., J. Y. M.-M., and M. C. D. conceptualization; C. J. Z., S. L., F. T. J., J. Y. M.-M., and M. C. D. investigation; C. J. Z., S. L., F. T. J., J. Y. M.-M., and J. W. T. writing-review and editing; J. Y. M.-M. and M. C. D. methodology; J. W. T. and M. C. D. formal analysis; M. C. D. supervision; M. C. D. funding acquisition; M. C. D. writing-original draft; M. C. D. project administration.
Supplementary Material
Acknowledgments
We thank Ajit P. Joglekar for use of microscopes used in this study. We thank Elizabeth Conibear for reagents and useful discussions.
This work was supported by National Institutes of Health Grants R01 GM092741 (to M. C. D.) and F31-GM112470 (J. Y. M.-M) and by funds from the Michigan Protein Folding Disease Initiative (to M. C. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Tables S1–S3.
- TGN
- trans-Golgi network
- CFW
- calcofluor white
- EBM
- ear-binding motif
- PI4P
- phosphatidyl-inositol-4-phosphate
- AP-1
- adaptor protein complex-1
- HEATR
- HEAT repeat.
References
- 1. Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., and Wakeham D. E. (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568 10.1146/annurev.cellbio.17.1.517 [DOI] [PubMed] [Google Scholar]
- 2. Kirchhausen T., Owen D., and Harrison S. C. (2014) Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725 10.1101/cshperspect.a016725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owen D. J., Collins B. M., and Evans P. R. (2004) Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153–191 10.1146/annurev.cellbio.20.010403.104543 [DOI] [PubMed] [Google Scholar]
- 4. Paczkowski J. E., Richardson B. C., and Fromme J. C. (2015) Cargo adaptors: structures illuminate mechanisms regulating vesicle biogenesis. Trends Cell Biol. 25, 408–416 10.1016/j.tcb.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson M. S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 10.1016/j.tcb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 6. Canagarajah B. J., Ren X., Bonifacino J. S., and Hurley J. H. (2013) The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci. 22, 517–529 10.1002/pro.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Traub L. M. (2005) Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta 1744, 415–437 10.1016/j.bbamcr.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 8. Stepp J. D., Pellicena-Palle A., Hamilton S., Kirchhausen T., and Lemmon S. K. (1995) A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol. Biol. Cell 6, 41–58 10.1091/mbc.6.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rad M. R., Phan H. L., Kirchrath L., Tan P. K., Kirchhausen T., Hollenberg C. P., and Payne G. S. (1995) Saccharomyces cerevisiae Apl2p, a homologue of the mammalian clathrin AP β subunit, plays a role in clathrin-dependent Golgi functions. J. Cell Sci. 108, 1605–1615 [DOI] [PubMed] [Google Scholar]
- 10. Matsuura-Tokita K., Takeuchi M., Ichihara A., Mikuriya K., and Nakano A. (2006) Live imaging of yeast Golgi cisternal maturation. Nature 441, 1007–1010 10.1038/nature04737 [DOI] [PubMed] [Google Scholar]
- 11. Losev E., Reinke C. A., Jellen J., Strongin D. E., Bevis B. J., and Glick B. S. (2006) Golgi maturation visualized in living yeast. Nature 441, 1002–1006 10.1038/nature04717 [DOI] [PubMed] [Google Scholar]
- 12. Day K. J., Casler J. C., and Glick B. S. (2018) Budding yeast has a minimal endomembrane system. Dev. Cell 44, 56–72.e4 10.1016/j.devcel.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daboussi L., Costaguta G., and Payne G. S. (2012) Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat. Cell Biol. 14, 239–248 10.1038/ncb2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crottet P., Meyer D. M., Rohrer J., and Spiess M. (2002) ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell 13, 3672–3682 10.1091/mbc.e02-05-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren X., Farías G. G., Canagarajah B. J., Bonifacino J. S., and Hurley J. H. (2013) Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 152, 755–767 10.1016/j.cell.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernández G. E., and Payne G. S. (2006) Laa1p, a conserved AP-1 accessory protein important for AP-1 localization in yeast. Mol. Biol. Cell 17, 3304–3317 10.1091/mbc.e06-02-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimura S. H., and Hirano T. (2016) HEAT repeats: versatile arrays of amphiphilic helices working in crowded environments? J. Cell Sci. 129, 3963–3970 [DOI] [PubMed] [Google Scholar]
- 18. Lui W. W., Collins B. M., Hirst J., Motley A., Millar C., Schu P., Owen D. J., and Robinson M. S. (2003) Binding partners for the COOH-terminal appendage domains of the GGAs and γ-adaptin. Mol. Biol. Cell 14, 2385–2398 10.1091/mbc.e02-11-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirst J., Borner G. H., Harbour M., and Robinson M. S. (2005) The aftiphilin/p200/γ-synergin complex. Mol. Biol. Cell 16, 2554–2565 10.1091/mbc.e04-12-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Bras S., Rondanino C., Kriegel-Taki G., Dussert A., and Le Borgne R. (2012) Genetic identification of intracellular trafficking regulators involved in Notch-dependent binary cell fate acquisition following asymmetric cell division. J. Cell Sci. 125, 4886–4901 10.1242/jcs.110171 [DOI] [PubMed] [Google Scholar]
- 21. Gillard G., Shafaq-Zadah M., Nicolle O., Damaj R., Pécréaux J., and Michaux G. (2015) Control of E-cadherin apical localisation and morphogenesis by a SOAP-1/AP-1/clathrin pathway in C. elegans epidermal cells. Development 142, 1684–1694 10.1242/dev.118216 [DOI] [PubMed] [Google Scholar]
- 22. Yu Y., Kita A., Udo M., Katayama Y., Shintani M., Park K., Hagihara K., Umeda N., and Sugiura R. (2012) Sip1, a conserved AP-1 accessory protein, is important for Golgi/endosome trafficking in fission yeast. PLoS One 7, e45324 10.1371/journal.pone.0045324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takatsu H., Yoshino K., and Nakayama K. (2000) Adaptor γ ear homology domain conserved in γ-adaptin and GGA proteins that interact with γ-synergin. Biochem. Biophys. Res. Commun. 271, 719–725 10.1006/bbrc.2000.2700 [DOI] [PubMed] [Google Scholar]
- 24. Mattera R., Ritter B., Sidhu S. S., McPherson P. S., and Bonifacino J. S. (2004) Definition of the consensus motif recognized by γ-adaptin ear domains. J. Biol. Chem. 279, 8018–8028 10.1074/jbc.M311873200 [DOI] [PubMed] [Google Scholar]
- 25. Lui-Roberts W. W., Ferraro F., Nightingale T. D., and Cutler D. F. (2008) Aftiphilin and γ-synergin are required for secretagogue sensitivity of Weibel–Palade bodies in endothelial cells. Mol. Biol. Cell 19, 5072–5081 10.1091/mbc.e08-03-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karathia H., Vilaprinyo E., Sorribas A., and Alves R. (2011) Saccharomyces cerevisiae as a model organism: a comparative study. PLoS One 6, e16015 10.1371/journal.pone.0016015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giaever G., and Nislow C. (2014) The yeast deletion collection: a decade of functional genomics. Genetics 197, 451–465 10.1534/genetics.114.161620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoepfner D., Helliwell S. B., Sadlish H., Schuierer S., Filipuzzi I., Brachat S., Bhullar B., Plikat U., Abraham Y., Altorfer M., Aust T., Baeriswyl L., Cerino R., Chang L., Estoppey D., et al. (2014) High-resolution chemical dissection of a model eukaryote reveals targets, pathways and gene functions. Microbiol. Res. 169, 107–120 10.1016/j.micres.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 29. Whitfield S. T., Burston H. E., Bean B. D., Raghuram N., Maldonado-Báez L., Davey M., Wendland B., and Conibear E. (2016) The alternate AP-1 adaptor subunit Apm2 interacts with the Mil1 regulatory protein and confers differential cargo sorting. Mol. Biol. Cell 27, 588–598 10.1091/mbc.e15-09-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yip C. K., Berscheminski J., and Walz T. (2010) Molecular architecture of the TRAPPII complex and implications for vesicle tethering. Nat. Struct. Mol. Biol. 17, 1298–1304 10.1038/nsmb.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., and O'Shea E. K. (2003) Global analysis of protein localization in budding yeast. Nature 425, 686–691 10.1038/nature02026 [DOI] [PubMed] [Google Scholar]
- 32. Valdivia R. H., Baggott D., Chuang J. S., and Schekman R. W. (2002) The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2, 283–294 10.1016/S1534-5807(02)00127-2 [DOI] [PubMed] [Google Scholar]
- 33. Rainey M. M., Korostyshevsky D., Lee S., and Perlstein E. O. (2010) The antidepressant sertraline targets intracellular vesiculogenic membranes in yeast. Genetics 185, 1221–1233 10.1534/genetics.110.117846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duncan M. C., Costaguta G., and Payne G. S. (2003) Yeast epsin-related proteins required for Golgi-endosome traffic define a γ-adaptin ear-binding motif. Nat. Cell Biol. 5, 77–81 10.1038/ncb901 [DOI] [PubMed] [Google Scholar]
- 35. Duncan M. C., and Payne G. S. (2003) ENTH/ANTH domains expand to the Golgi. Trends Cell Biol. 13, 211–215 10.1016/S0962-8924(03)00076-X [DOI] [PubMed] [Google Scholar]
- 36. Yu Y., Li C., Kita A., Katayama Y., Kubouchi K., Udo M., Imanaka Y., Ueda S., Masuko T., and Sugiura R. (2013) Sip1, an AP-1 accessory protein in fission yeast, is required for localization of Rho3 GTPase. PLoS One 8, e68488 10.1371/journal.pone.0068488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson M. S. (1993) Assembly and targeting of adaptin chimeras in transfected cells. J. Cell Biol. 123, 67–77 10.1083/jcb.123.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nogi T., Shiba Y., Kawasaki M., Shiba T., Matsugaki N., Igarashi N., Suzuki M., Kato R., Takatsu H., Nakayama K., and Wakatsuki S. (2002) Structural basis for the accessory protein recruitment by the γ-adaptin ear domain. Nat. Struct. Biol. 9, 527–531 [DOI] [PubMed] [Google Scholar]
- 39. Renard H. F., Demaegd D., Guerriat B., and Morsomme P. (2010) Efficient ER exit and vacuole targeting of yeast Sna2p require two tyrosine-based sorting motifs. Traffic 11, 931–946 10.1111/j.1600-0854.2010.01070.x [DOI] [PubMed] [Google Scholar]
- 40. James P., Halladay J., and Craig E. A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Longtine M. S., McKenzie A. 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., and Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 10.1002/(SICI)1097-0061(199807)14:10%3C953::AID-YEA293%3E3.0.CO%3B2-U [DOI] [PubMed] [Google Scholar]
- 42. Noguchi C., Garabedian M. V., Malik M., and Noguchi E. (2008) A vector system for genomic FLAG epitope-tagging in Schizosaccharomyces pombe. Biotechnol. J. 3, 1280–1285 10.1002/biot.200800140 [DOI] [PubMed] [Google Scholar]
- 43. Lang M. J., Martinez-Marquez J. Y., Prosser D. C., Ganser L. R., Buelto D., Wendland B., and Duncan M. C. (2014) Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling. J. Biol. Chem. 289, 16736–16747 10.1074/jbc.M113.525782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hung C. W., Aoh Q. L., Joglekar A. P., Payne G. S., and Duncan M. C. (2012) Adaptor autoregulation promotes coordinated binding within clathrin coats. J. Biol. Chem. 287, 17398–17407 10.1074/jbc.M112.349035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeung B. G., Phan H. L., and Payne G. S. (1999) Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 10, 3643–3659 10.1091/mbc.10.11.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hung C. W., and Duncan M. C. (2016) Clathrin binding by the adaptor Ent5 promotes late stages of clathrin coat maturation. Mol. Biol. Cell 27, 1143–1153 10.1091/mbc.E15-08-0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aravamudhan P., Felzer-Kim I., Gurunathan K., and Joglekar A. P. (2014) Assembling the protein architecture of the budding yeast kinetochore-microtubule attachment using FRET. Curr. Biol. 24, 1437–1446 10.1016/j.cub.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lund F. W., Jensen M. L., Christensen T., Nielsen G. K., Heegaard C. W., and Wüstner D. (2014) SpatTrack: an imaging toolbox for analysis of vesicle motility and distribution in living cells. Traffic 15, 1406–1429 10.1111/tra.12228 [DOI] [PubMed] [Google Scholar]
- 49. Otsu N. (1979) Threshold selection method from gray-level histograms. IEEE T. Syst. Man. Cyb. 9, 62–66 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- 50. Aoh Q. L., Graves L. M., and Duncan M. C. (2011) Glucose regulates clathrin adaptors at the trans-Golgi network and endosomes. Mol. Biol. Cell 22, 3671–3683 10.1091/mbc.e11-04-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hung C. W., Martínez-Márquez J. Y., Javed F. T., and Duncan M. C. (2018) A simple and inexpensive quantitative technique for determining chemical sensitivity in Saccharomyces cerevisiae. Sci. Rep. 8, 11919 10.1038/s41598-018-30305-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.