Abstract
Ubiquitin (Ub)-conjugating enzymes and Ub ligases control protein degradation and regulate many cellular processes in eukaryotes. Cellular inhibitor of apoptosis protein-1 (cIAP1) plays a central role in apoptosis and tumor necrosis factor signaling. It harbors a C-terminal RING domain that homodimerizes to recruit E2∼Ub (where ∼ denotes a thioester bond) complex to catalyze Ub transfer. Noncovalent Ub binding to the backside of the E2 Ub-conjugating enzyme UbcH5 has previously been shown to enhance RING domain activity, but the molecular basis for this enhancement is unclear. To investigate how dimeric cIAP1 RING activates E2∼Ub for Ub transfer and what role noncovalently bound Ub has in Ub transfer, here we determined the crystal structure of the cIAP1 RING dimer bound to both UbcH5B covalently linked to Ub (UbcH5B–Ub) and a noncovalent Ub to 1.7 Å resolution. The structure along with biochemical analyses revealed that the cIAP1 RING domain interacts with UbcH5B–Ub and thereby promotes the formation of a closed UbcH5B–Ub conformation that primes the thioester bond for Ub transfer. We observed that the noncovalent Ub binds to the backside of UbcH5B and abuts UbcH5B's α1β1-loop, which, in turn, stabilizes the closed UbcH5B–Ub conformation. Our results disclose the mechanism by which cIAP1 RING dimer activates UbcH5B∼Ub and indicate that noncovalent Ub binding further stabilizes the cIAP1-UbcH5B∼Ub complex in the active conformation to stimulate Ub transfer.
Keywords: ubiquitin, ubiquitin ligase, allosteric regulation, structural biology, ubiquitin-conjugating enzyme (E2 enzyme), ubiquitylation (ubiquitination), E3 ubiquitin ligase, activation, cIAP1, non-covalent, RING E3, UbcH5B, activation, protein degradation, apoptosis
Introduction
Post-translational modification of proteins by ubiquitin (Ub),2 achieved via the sequential actions of Ub-activating enzyme (E1), Ub-conjugating enzyme (E2), and Ub-ligase (E3), governs vast arrays of eukaryotic cellular processes (1, 2). E1 activates and transfers the C terminus of Ub to the E2's catalytic cysteine to produce an E2∼Ub thioester intermediate (where ∼ denotes a thioester bond). E3 binds E2∼Ub and substrate to promote Ub transfer from E2 to a nucleophile, which is usually a lysine side chain. There are three major types of E3s: RING, HECT, and RING-in-between-RING (RBR) (3, 4). RING E3s harbor a RING domain that binds and activates E2∼Ub to promote the direct transfer of Ub from E2 to the substrate. In contrast, HECT E3s contain a catalytic cysteine and catalyze a two-step Ub transfer reaction in which Ub is initially transferred from E2 to HECT E3's catalytic cysteine and then to the substrate. RBR E3s share common features from both RING and HECT E3s, where a RING-like domain (RING1) recruits E2∼Ub and transfers Ub to the catalytic cysteine on RING2 prior to transfer to substrate.
Cellular inhibitor of apoptosis protein-1 (cIAP1) is a RING-type E3 and belongs to the inhibitor of apoptosis (IAP) family of proteins. The RING-mediated ubiquitin ligase activity of cIAP1 is essential for its function in both cell death and survival pathways. In cell death pathways, cIAP1 inhibits apoptosis by sequestering and ubiquitinating second mitochondria-derived activator of caspase (SMAC) for degradation by the proteasome, thereby freeing XIAP to bind and inhibit caspases (5–7). Moreover, cIAP1 has been shown to target caspases for ubiquitination and degradation by the proteasome (8). In the cell survival pathway, tumor necrosis factor receptor 1 signaling complex recruits RIP kinase 1 (RIPK1) and various adaptor proteins, including TRADD, TRAF2, and TRAF5, that lead to the recruitment of cIAP1 and cIAP2 (9). cIAP1 and cIAP2 ubiquitinate RIPK1 and components within this complex to enable the recruitment of a linear Ub chain assembly complex that ultimately activates NF-κB signaling (10–16).
cIAP1 contains three N-terminal baculoviral IAP repeat domains (BIR1–3), followed by a Ub-associated domain (UBA), a caspase-recruiting domain (CARD), and a C-terminal RING domain. Dimerization of its C-terminal RING domain is important for E2∼Ub recruitment and ligase activity (17, 18). Studies showed that the N-terminal BIR3-UBA-CARD domain sequesters the RING domain in an inactive conformation to prevent RING dimerization (19, 20). The addition of SMAC or SMAC mimetic induces conformational changes that restore activity by allowing RING dimerization (19, 21). Currently, how RING dimerization activates cIAP1's ligase activity, and the structure of cIAP1 RING domain bound to E2∼Ub, are not known. However, there are several structures of RING E3s bound to E2 covalently linked to Ub (E2–Ub; en dash denotes covalent linkage) (22–32). Collectively, these structures show that the RING domain binds and stabilizes E2–Ub in a closed conformation such that the thioester bond is optimized for Ub transfer (33). For dimeric RING E3s, such as BIRC7, an IAP family protein, the C-terminal tails of each subunit of the RING dimer function to stabilize the closed E2–Ub conformation to enhance ligase activity (23). It seems likely that cIAP1 RING dimer utilizes a similar mechanism for activating E2–Ub.
cIAP1 has been shown to function with the UbcH5 family of E2s to catalyze substrate ubiquitination (34, 35). This family of E2s has a noncovalent Ub binding site on its backside. This backside Ub-UbcH5 interaction is important for processivity of poly-Ub chain formation (25, 36–39). Our recent structural study on the monomeric RING E3 RNF38 showed that backside-bound Ub (UbB) stimulates RNF38-catalyzed Ub transfer by restricting the flexibility of UbcH5B's α1 and α1β1-loop to stabilize the closed active RNF38 RING-UbcH5B–Ub complex, thereby enhancing the rate of catalysis (25). It remains unclear whether this mechanism is conserved for dimeric RING E3s.
To better understand how dimeric cIAP1 RING domain (cIAP1R) activates E2∼Ub for Ub transfer and how UbB could influence this process, we present a crystal structure of cIAP1R bound to UbcH5B–Ub and UbB. Structural and biochemical analyses showed that cIAP1R forms multiple contacts with UbcH5B–Ub to stabilize it in a closed conformation. Notably, the C-terminal tail of cIAP1R functions in trans to stabilize the closed UbcH5B–Ub conformation, thereby explaining the importance of RING domain dimerization, and consistent with prior examples of dimeric RING E3s. Last, UbB restrains UbcH5B's α1β1-loop conformation to stabilize contacts with donor Ub (i.e. Ub conjugated to UbcH5B; hereafter UbD). This interaction augments stabilization of the closed UbcH5B–Ub conformation, thereby enhancing Ub transfer. Our results reveal a conserved UbB-stimulatory mechanism for both monomeric and dimeric RING E3s in mediating UbcH5B∼Ub transfer.
Results
UbB stimulates cIAP1R-mediated Ub transfer
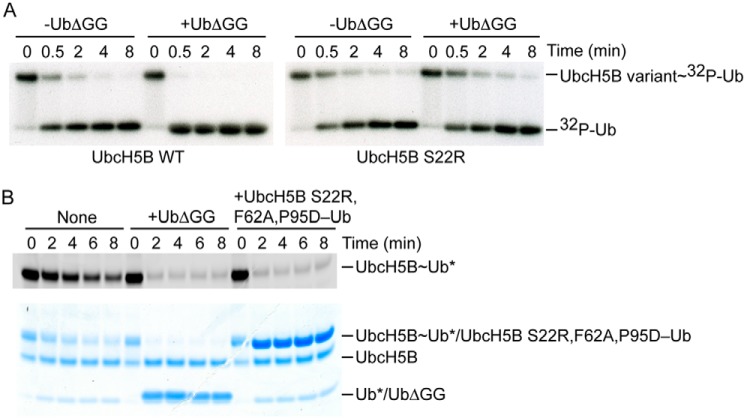
Previously, we showed that the addition of UbΔGG (lacking the C-terminal diglycine motif) can serve as UbB and bind to UbcH5B's backside to stimulate UbcH5B∼Ub discharge catalyzed by the monomeric RING E3 RNF38 and dimeric RING E3 XIAP. To assess whether UbB can exert similar effects on cIAP1R-catalyzed Ub transfer, we performed single-turnover lysine discharge assays using WT and S22R UbcH5B. S22R substitution abrogates the UbB-UbcH5B interaction and was therefore used as a control (25, 36). UbcH5B variants were precharged with equimolar concentrations of 32P-Ub and then chased by the addition of cIAP1R alone and in the presence of UbΔGG, which cannot be charged by E1 but can still bind to the backside of UbcH5B WT. The addition of 300 μm UbΔGG stimulated the discharge of UbcH5B∼Ub but had no effect on UbcH5B S22R∼Ub (Fig. 1A), indicating that UbB stimulates cIAP1R-catalyzed Ub transfer.
Figure 1.
UbB stimulates cIAP1R-catalyzed Ub transfer. A, nonreduced autoradiograms of lysine discharge reactions showing the disappearance of UbcH5B variant∼32P-Ub over time in the presence and absence of UbΔGG (300 μm) catalyzed by cIAP1R. B, nonreduced SDS-PAGE showing the cIAP1R-mediated discharge of fluorescently labeled UbcH5B∼Ub to l-lysine over time in the presence of UbΔGG (20 μm) or UbcH5B S22R,F62A,P95D–Ub (20 μm) visualized with a LI-COR Odyssey scanner (top) followed by staining with InstantBlue (bottom). *, fluorescently labeled Ub.
Synergistic binding enhancement between UbB, cIAP1R, and UbcH5B–Ub
Our prior study showed that UbB stimulates RNF38 and XIAP-catalyzed Ub transfer by enhancing RING E3 affinity for UbcH5B–Ub by ∼5–10-fold (25). To determine whether UbB functions in a similar manner to stimulate cIAP1R-catalyzed Ub transfer, we performed surface plasmon resonance (SPR) experiments to investigate the effects of UbB on cIAP1R's affinity for UbcH5B–Ub. We generated stable UbcH5B–Ub complex by mutating UbcH5B's catalytic cysteine (Cys85) to lysine, thereby forming a stable amide linkage that mimics the thioester linkage (22). UbcH5B C85K and UbcH5B S22R C85K covalently linked to Ub (hereafter referred to as UbcH5B–Ub and UbcH5BS22R–Ub, respectively) were generated to assess the effect of backside binding. cIAP1R exhibited weak binding affinity for UbcH5B alone, but displayed ∼270-fold higher binding affinity for UbcH5B–Ub (Table 1 and Fig. S1), suggesting that UbD contributes to RING domain binding, consistent with previous observations with other RING E3s (23, 25). The addition of excess UbΔGG (0.6 mm; Kd for UbB-UbcH5B is ∼280 μm (25)) further enhanced cIAP1R's affinity for UbcH5B–Ub by ∼4-fold (Table 1 and Fig. S1). In contrast, the addition of excess UbΔGG had no effect on cIAP1R's affinity for UbcH5BS22R–Ub, suggesting that UbB-UbcH5B interaction enhances cIAP1R's affinity for UbcH5B–Ub.
Table 1.
Kd values for interactions between cIAP1R, UbcH5B, UbcH5B–Ub variants, and Ub
| Immobilized protein | Analyte | Kd |
|---|---|---|
| μm | ||
| GST-cIAP1R | UbcH5B | 223 ± 4 |
| GST-cIAP1R | UbcH5B–Ub | 0.83 ± 0.05 |
| GST-cIAP1R | UbcH5B–Ub + 0.6 mm UbΔGG | 0.22 ± 0.01 |
| GST-cIAP1R | UbcH5BS22R–Ub | 0.90 ± 0.01 |
| GST-cIAP1R | UbcH5BS22R–Ub + 0.6 mm UbΔGG | 0.99 ± 0.05 |
| GST-Ub | UbcH5B–Ub + excess cIAP1R | 13 ± 2 |
We showed previously that UbB binds UbcH5B–Ub with a Kd of ∼280 μm, but, in the presence of the monomeric RING E3 RNF38, the Kd improved by 20-fold (Kd of 14 μm), revealing a synergistic effect in RNF38-UbcH5B–Ub and UbB-UbcH5B binding (25). Similar to our prior observation, we found that Ub displayed a Kd of 13 μm for UbcH5B–Ub in the presence of cIAP1R (Table 1 and Fig. S1), suggesting that this binding synergy is conserved.
To verify the improved UbB-UbcH5B interaction in the presence of cIAP1R, we performed single-turnover lysine discharge assays using 20 μm UbΔGG, which is just above the Kd of 13 μm, and showed that it was sufficient to stimulate cIAP1R-catalyzed Ub transfer (Fig. 1B). Furthermore, we showed that UbcH5B S22R,F62A,P95D–Ub, a stable isopeptide conjugate that cannot bind RING E3 or UbB but can serve as the UbB source (25), also stimulated cIAP1R-catalyzed Ub transfer at 20 μm (Fig. 1B).
Overall structure of cIAP1R-UbcH5B–Ub-UbB complex
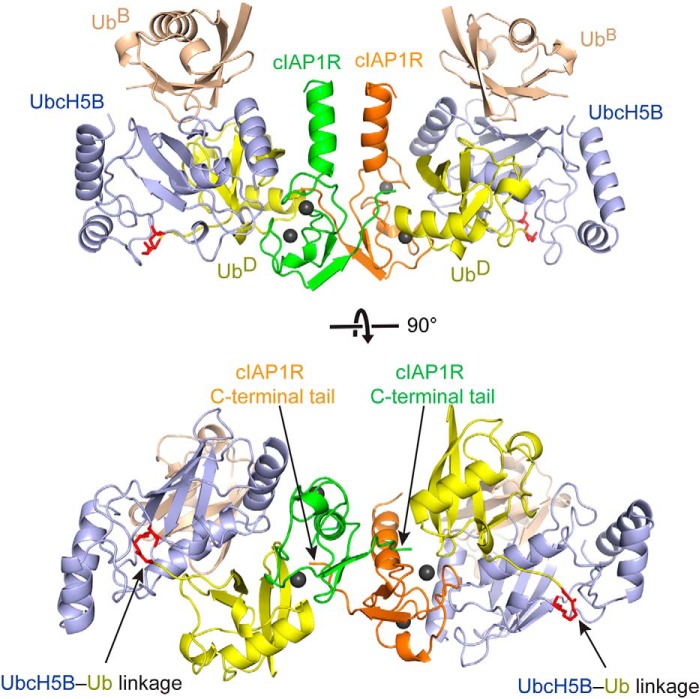
To gain insight into how UbB enhances cIAP1R-mediated UbcH5B∼Ub transfer, we crystallized and determined the structure of cIAP1R bound to UbcH5B–Ub and UbB. The cIAP1R-UbcH5B–Ub-UbB complex crystals belong to space group C21 with one copy of cIAP1R-UbcH5B–Ub-UbB complex in the asymmetric unit. The structure was refined to a resolution of 1.7 Å (Table 2). Because cIAP1 exists as a biological homodimer via the RING domain (6, 18, 40), we used crystallographic symmetry to generate the structure of dimeric cIAP1R-UbcH5B–Ub-UbB complex (Fig. 2). The structure shows that cIAP1R dimerizes via the RING domain, the C-terminal tail, and a helix that precedes the RING domain similar to other IAP family RING E3s, such as cIAP2, XIAP, and BIRC7 (17, 23, 41). cIAP1R's RING domain binds both UbcH5B and UbD and stabilizes the UbcH5B–Ub complex in a closed conformation. Additionally, the C-terminal tail of the second subunit in the cIAP1R dimer packs against UbD in trans to stabilize the closed UbcH5B–Ub conformation. These features are similar to those observed in other structures of dimeric RING E3-E2–Ub complexes, such as BIRC7, RNF4, and MDM2-MDMX (22, 23, 30). In our structure, UbB binds to the backside of UbcH5B centering on the Ser22 surface, as reported previously (25, 36).
Table 2.
Data collection and refinement statistics
| Data collection | cIAP1R-UbcH5B–Ub-UbB complex |
| Space group | C 1 2 1 |
| Cell dimensions | |
| a, b, c (Å) | 79.19, 53.60, 78.54 |
| α, β, γ (degrees) | 90, 107.57, 90 |
| Resolution (Å) | 23.52–1.70 (1.74–1.70) |
| Rmerge | 0.063 (0.539)a |
| I/σ | 13.8 (2.0) |
| Completeness (%) | 98.8 (94.7) |
| Redundancy | 3.3 (2.7) |
| Refinement | |
| Resolution (Å) | 23.52–1.70 |
| No. of reflections | 34,206 |
| Rwork/Rfree | 0.170/0.197 |
| No. of atoms | |
| Protein | 2794 |
| Ions | 2 |
| Water | 222 |
| B factor | |
| Protein | 26.2 |
| Ion | 18.2 |
| Water | 33.0 |
| RMSDs | |
| Bond length (Å) | 0.007 |
| Bond angles (degrees) | 0.922 |
| Ramachandran | |
| Mostly favored (%) | 97.8 |
| Outliers (%) | 0 |
a Values in parenthesis are for the highest-resolution shell.
Figure 2.
Crystal structure of cIAP1R-UbcH5B–Ub-UbB complex. Shown is a cartoon representation of homodimeric cIAP1R-UbcH5B–Ub-UbB complex generated from crystallographic symmetry. The top and bottom panels are related by 90° rotation about the x axis. The two protomers of cIAP1R are colored green and orange. UbcH5B is shown in light blue, UbD in yellow, and UbB in wheat. Zn2+ ions are shown as gray spheres. UbcH5B–Ub linkage is shown in red and is indicated by arrows. cIAP1R's C-terminal tails are indicated by arrows.
Interactions important for the closed UbcH5B–Ub conformation
Because this is the first structure of cIAP1R bound to E2–Ub, we investigated how cIAP1R stabilizes the closed UbcH5B–Ub conformation to promote Ub transfer. The closed UbcH5B–Ub conformation is stabilized by multiple contacts involving 1) cIAP1R-UbcH5B, 2) cIAP1R-UbD, 3) cIAP1R tail-UbD, and 4) UbD-UbcH5B interactions.
The cIAP1R-UbcH5B interaction closely resembled that observed in the structure of cIAP2R-UbcH5B complex (17), which was expected because cIAP1R and cIAP2R share ∼90% sequence identity. The interaction primarily involves cIAP1R's Met575 and the hydrophobic core surrounding Val573 contacting UbcH5B's α1-helix and L1 and L2 loops (Fig. 3A). Despite having nearly identical RING domain sequences, the cIAP1R-UbcH5B portion of the structure and the cIAP2R-UbcH5B structure only superpose with a root mean square deviation (RMSD) of ∼1.0 Å for all Cα atoms. When superimposition was performed using only the RING domain (RMSD of 0.62 Å for Cα atoms), the oblong shape of UbcH5B tilts ∼8°, suggesting subtle differences in UbcH5B-RING domain contacts (Fig. 3B). Similar E2 shifts were also observed in the structures of TRAF6 (from human)-Ubc13 and TRAF6 (from Danio rerio)-Ubc13–Ub complexes (31, 42). It is unclear whether this E2 movement results from formation of the closed E2–Ub conformation or is due to crystal packing. Nonetheless, the primary RING-E2 interaction is maintained.
Figure 3.
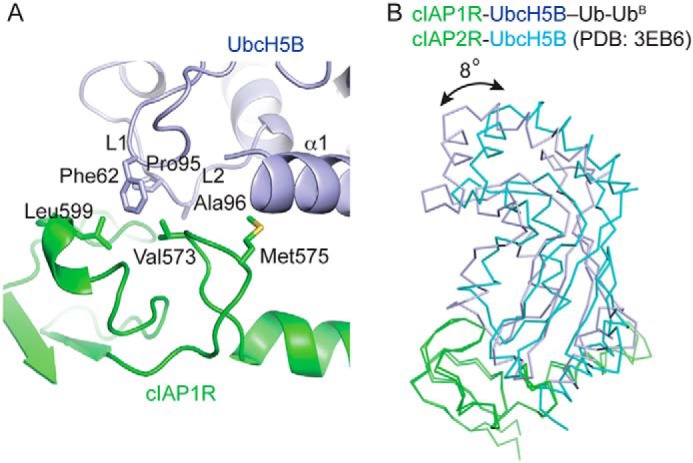
cIAP1R-UbcH5B interactions. A, close-up view of cIAP1R-UbcH5B interactions. UbcH5B's α1, L1, and L2 loops are indicated. All coloring is the same as in Fig. 2. B, superimposition of cIAP1R portion of structure in cIAP1R-UbcH5B–Ub-UbB complex with cIAP2R portion of structure in cIAP2R-UbcH5B complex (PDB entry 3EB6). cIAP1R and cIAP2R are colored in green. UbcH5B from cIAP1R-UbcH5B–Ub-UbB and cIAP2R-UbcH5B complexes are colored in light blue and cyan, respectively.
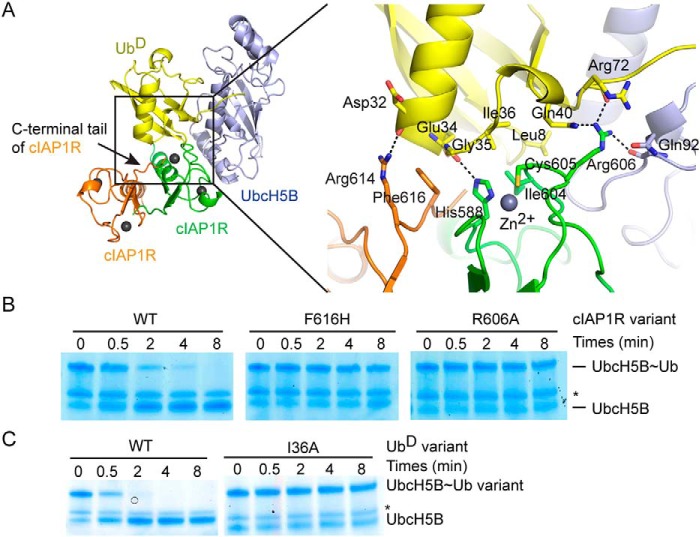
Our structure shows that cIAP1R's C-terminal tail, RING domain, and UbcH5B stabilize the closed UbD conformation. cIAP1R's C-terminal tail interactions involve Arg614 and Phe616 from the other cIAP1R protomer in the dimer. Arg614 forms a hydrogen bond with the carbonyl oxygen of UbD's Asp32, and Phe616 packs against UbD's Gly35 surface (Fig. 4A). This trans tail packing arrangement is similar to those observed in the structures of BIRC7, RNF4, and MDM2-MDMX bound to UbcH5–Ub (22, 23, 30). These RING E3s all contain a Phe or Tyr corresponding to Phe616 on cIAP1R that disrupted ligase activity when substituted with histidine or alanine. Likewise, substitution on the corresponding Phe in cIAP2 also disrupted activity (17, 23). To determine the importance of this residue, we mutated cIAP1R's Phe616 to His and performed lysine discharge assays to assess the effect on Ub transfer. cIAP1R F616H was defective in discharging UbcH5B∼Ub (Fig. 4B), consistent with an earlier study showing that deletion of cIAP1's C-terminal residues abrogates activity (20). Thus, the trans tail-UbD interaction explains the importance of RING domain dimerization.
Figure 4.
cIAP1R-UbD interactions. A, cartoon representation of the catalytic competent cIAP1R dimer bound to UbcH5B–Ub (left) and close-up view of cIAP1R-UbD interactions (right). All coloring is the same as in Fig. 2. Hydrogen bonds are shown as dotted lines. B, nonreduced SDS-PAGE of lysine discharge reactions showing the disappearance of UbcH5B∼Ub band over time catalyzed by cIAP1R variants. C, nonreduced SDS-PAGE of lysine discharge reactions showing the disappearance of UbcH5B∼Ub variant bands over time catalyzed by cIAP1R. *, contaminating band from other reaction components.
The cIAP1R-UbD interactions primarily involve His588, Ile604, and Cys605 from cIAP1R's RING domain contacting Leu8 and Ile36 patches of UbD. Crucially, cIAP1R's Arg606 forms hydrogen bonds with the carbonyl oxygen of Arg72 and the side chain of Gln40 from UbD and the carbonyl oxygen of Gln92 from UbcH5B (Fig. 4A). This Arg606 is commonly known as the “linchpin Arg” (33), and its interaction network is conserved in several structures of RING E3-E2–Ub complexes (22–30). To assess the importance of this interaction in cIAP1R, we generated Ub I36A and cIAP1R R606A and tested their effects in UbcH5B∼Ub discharge assays. Although charging of UbcH5B∼Ub I36A was incomplete, as observed previously (23, 25), in the presence of cIAP1R, UbcH5B∼Ub I36A discharged slower than the WT UbcH5B∼Ub (Fig. 4C). Similarly, cIAP1R R606A was defective in discharging UbcH5B∼Ub (Fig. 4B).
The UbD-UbcH5B interaction involves UbD's Ile44 patch contacting the Ser108 region in UbcH5B's α2-helix (Fig. 5A). Additional interactions are also observed between Lys48 and Arg42 of UbD and UbcH5B's Asp42, Lys101, Leu104, and Asp112 (Fig. 5A). To investigate the importance of these interactions, we performed UbcH5B∼Ub discharge assays using Ub I44A and UbcH5B S108R. In both cases, cIAP1R-mediated Ub transfer was impaired (Fig. 5B).
Figure 5.
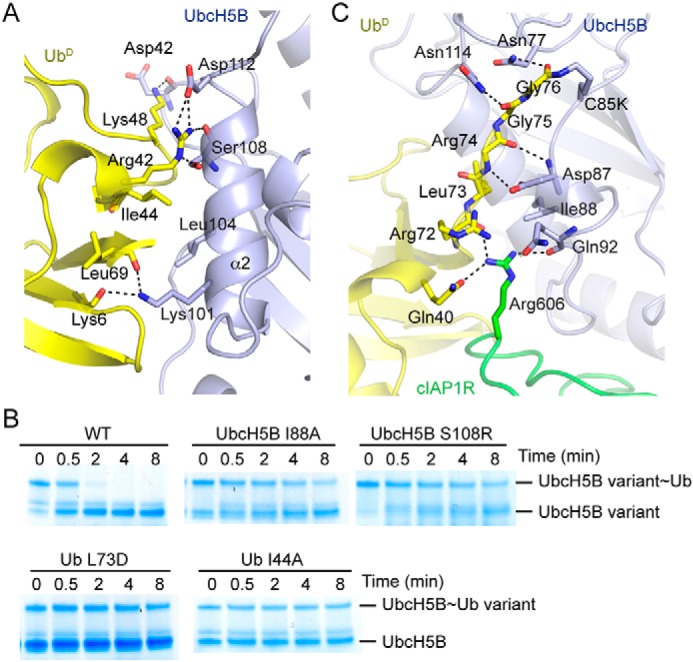
UbD-UbcH5B interactions. A, close-up view of UbD-UbcH5B interactions. B, nonreduced SDS-PAGE of lysine discharge reactions showing the disappearance of UbcH5B variant∼Ub or UbcH5B∼Ub variant band over time catalyzed by cIAP1R. C, close-up view of UbD's C-terminal tail interactions. All coloring in A and B is the same as in Fig. 2. Hydrogen bonds are shown as dotted lines in A and C.
The C-terminal tail of UbD is extended and lies along UbcH5B's active site cleft (Fig. 5C). The C-terminal tail of UbD is stabilized by hydrophobic interactions between UbcH5B's Ile88 and UbD's Leu73 and numerous hydrogen bonds involving UbcH5B's Asn77, Asp87, and Asn114 and UbD's C-terminal tail. To validate the importance of these interactions, we generated Ub L73D and UbcH5B I88A and assessed their effects in UbcH5B∼Ub discharge assays. UbcH5B loaded with Ub L73D and UbcH5B I88A charged with WT Ub were defective in discharge catalyzed by cIAP1R (Fig. 5B). Collectively, our data showed that cIAP1R initiates multiple contacts to stabilize UbcH5B–Ub in the closed conformation to promote Ub transfer similar to other RING E3s (22–32).
UbB-stimulatory mechanism in dimeric cIAP1R-mediated Ub transfer
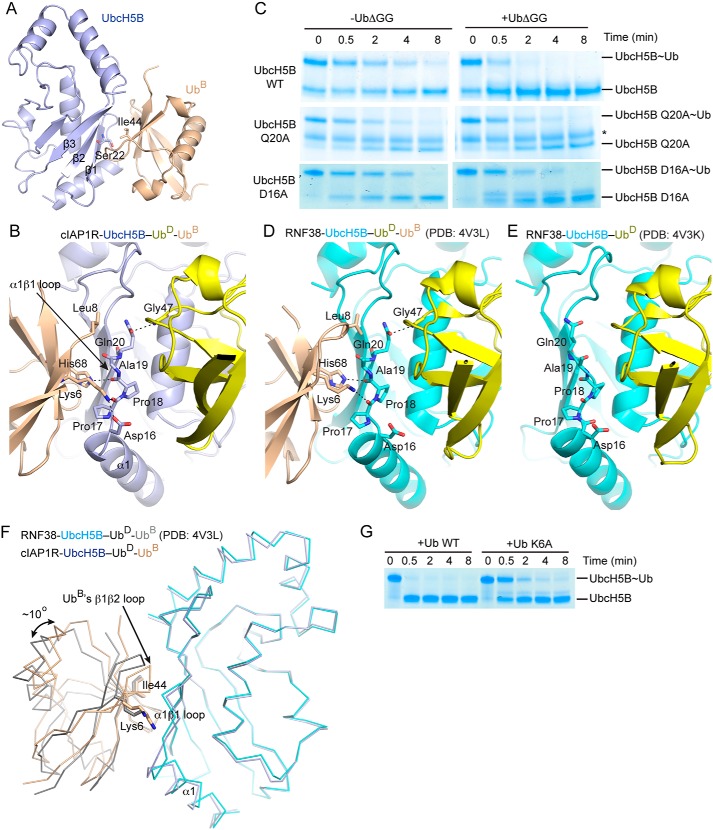
UbB binds UbcH5B via the Ile44 hydrophobic patch of UbB and UbcH5B's β1–3 surface surrounding Ser22 (Fig. 6A). This binding mode resembles other available structures of UbcH5 family E2s bound to UbB (25, 36, 39, 43). In our structure, UbB does not contact cIAP1R or UbD (Fig. 2). In addition to UbcH5B's Ser22 surface, UbB also contacts UbcH5B's α1β1-loop, which in turn packs against UbD (Fig. 6, A and B). Here, UbB's Lys6 and His68 form hydrogen bonds with carbonyl oxygens of UbcH5B's Pro17 and Pro18, respectively, and Leu8 packs against UbcH5B's Gln20, thereby placing Gln20 within hydrogen-bonding distance of the backbone amide of UbD's Gly47 (Fig. 6B). To test the importance of Gln20, we used UbcH5B Q20A to perform cIAP1R-mediated UbcH5B∼Ub discharge assays. The discharge of UbcH5B Q20A∼Ub in the presence and absence of excess of UbΔGG remained similar, suggesting that Gln20 plays an important role in UbB-mediated stimulation of Ub transfer (Fig. 6C).
Figure 6.
UbB interactions. A, cartoon representation showing the UbcH5B–UbB portion of the structure from the cIAP1R-UbcH5B–Ub-UbB complex. Ile44 of UbB and Ser22 of UbcH5B are indicated. B, close-up view of UbB-UbcH5B–UbD binding interface. UbcH5B's α1β1-loop is indicated by an arrow. Hydrogen bonds are shown as dotted lines. All coloring in A and B is the same as in Fig. 2. C, nonreduced SDS-PAGE of lysine discharge reactions showing the disappearance of UbcH5B variant∼Ub bands over time in the presence and absence of UbΔGG catalyzed by cIAP1R. *, contaminating band from other reaction components. D, close-up view of UbB-UbcH5B–UbD binding interface in the structure of RNF38-UbcH5B–Ub-UbB complex (PDB entry 4V3L). UbcH5B is shown in cyan, UbD in yellow, and UbB in wheat. E, close-up view of UbcH5B's α1β1-loop in the structure of RNF38-UbcH5B–Ub complex (PDB entry 4V3K). UbcH5B is shown in cyan and UbD in yellow. D and E are shown in the same orientation as in B. F, comparison of UbB conformations in the structures of cIAP1R-UbcH5B–Ub-UbB and RNF38-UbcH5B–Ub-UbB complexes (PDB entry 4V3L). Superimposition was performed on all Cα atoms of the UbcH5B portion of the structure. Ribbon representations of the UbcH5B-UbB portion from both structures are shown. UbB's β1β2 loop is indicated by an arrow. UbcH5B and UbB from cIAP1R-UbcH5B–Ub-UbB structure are colored as in Fig. 2. UbcH5B and UbB from RNF38-UbcH5B–Ub-UbB structure are colored in cyan and gray, respectively. G, nonreduced SDS-PAGE of lysine discharge reactions showing the disappearance of the UbcH5B∼Ub band over time in the presence of excess WT Ub or Ub K6A catalyzed by cIAP1R.
Previously, we have determined the structures of a monomeric RING E3, RNF38, bound to UbcH5B–Ub alone and in complex with UbB (25). These structures showed that in the absence of UbB, UbcH5B's α1β1-loop adopts various conformations that are not optimal for interaction with UbD. The presence of UbB locks UbcH5B's α1β1-loop into a conformation that helps optimize UbD for transfer (Fig. 6, D and E) (25). Superimposition of the structures of cIAP1R-UbcH5B–Ub-UbB and RNF38-UbcH5B–Ub-UbB complexes by overlaying the UbcH5B structure reveals that UbB in cIAP1R-UbcH5B–Ub-UbB rotates by ∼10° and shifts by ∼1.5–4 Å in different regions across UbB (Fig. 6F). Whereas the UbB Ile44 and UbcH5B Ser22 interacting interface is largely maintained, UbB's β1β2-loop packs more closely to UbcH5B's α1β1-loop in cIAP1R-UbcH5B–Ub-UbB (Fig. 6F). In this manner, UbB's Lys6 moves closer to UbcH5B's α1β1-loop and forms an additional hydrogen bond with UbcH5B's Asp16 located at the C terminus of α1; this interaction was not observed in RNF38-UbcH5B–Ub-UbB (Fig. 6, B and D). To test the importance of the UbB Lys6-UbcH5B Asp16 interaction in UbB-mediated stimulation of Ub transfer, we generated UbcH5B D16A and Ub K6A and performed cIAP1R-mediated UbcH5B∼Ub discharge assays. The discharge of UbcH5B D16A∼Ub remained similar in the presence or absence of excess of UbΔGG (Fig. 6C), suggesting that UbcH5B's Asp16 plays a role in UbB-mediated stimulation of Ub transfer. Correspondingly, the addition of excess Ub K6A to precharged UbcH5B∼Ub was slower than WT Ub in stimulating cIAP1R-mediated UbcH5B∼Ub discharge (Fig. 6G). Thus, the additional contact between UbB Lys6 and UbcH5B Asp16 contributes to UbB-mediated stimulation of Ub transfer. Despite this slight difference, the conformation of UbcH5B's α1β1-loop is nearly identical in both structures, which further supports our proposed UbB-stimulatory mechanism, whereby UbB binding reorganizes UbcH5B's α1β1-loop to help stabilize UbD in a conformation primed for transfer.
Discussion
The structure of cIAP1R-UbcH5B–Ub-UbB reported here provides insight into the UbB-stimulatory mechanism of dimeric RING E3-catalyzed Ub transfer. The cIAP1 RING domain forms a homodimer and utilizes a general mechanism that is shared by other RING E3s to stabilize UbcH5B–Ub in a closed conformation to activate the thioester bond for catalysis (3). UbB functions by reorganizing UbcH5B's α1β1-loop conformation to reinforce UbD in the closed conformation, thereby enhancing Ub transfer in a manner consistent with our prior study with the monomeric RING E3 RNF38 (25). Our current work demonstrates that the UbB-stimulatory mechanism is conserved in both monomeric and dimeric RING E3-catalyzed reactions with the UbcH5 family of E2s.
The closed E2∼Ub conformation has been shown to be important for Ub transfer, and the role of the RING domain is to promote the transition to this conformation to enhance the rate of Ub transfer (22, 23, 33, 44, 45). In addition to the established contacts between RING-E2, RING-UbD, and UbD-E2, several RING E3s have evolved different mechanisms to facilitate this process (3). For cIAP1, the RING dimer arrangement enables cIAP1R to utilize the C-terminal tail of the other dimeric cIAP1R protomer to stabilize UbD. This mechanism is observed in several dimeric RING E3s containing a Phe or Tyr residue in their C-terminal tail, such as BIRC7, RNF4, and MDM2-MDMX (22, 23, 30).
Noncovalent Ub binding to the backside of UbcH5 family E2 has been shown to increase the processivity of Ub transfer (25, 36–39). Mechanistically, we have recently shown that UbB binding improved RING E3's affinity for the E2∼Ub complex and that the RING E3-E2∼Ub complex displayed higher affinity for UbB using the monomeric RING E3 RNF38 (25). Here we observed a similar synergistic effect with the dimeric RING E3, cIAP1. We have shown previously that the Kd for the UbB-UbcH5B interaction was ∼280 μm (25). In the presence of the cIAP1R, UbcH5B–Ub complex is primed into the closed conformation, and the Kd for UbB-UbcH5B binding improved to ∼13 μm (Table 1). Our structure showed that the closed UbcH5B–Ub conformation stabilizes UbcH5B's α1β1-loop, which in turn forms optimal interaction with UbB and could explain the drop in Kd. The total cellular Ub concentration is ∼20–85 μm, depending on cell type. Within this total concentration, Ub presents as a mixture of monoubiquitinated substrates, free Ub, thioester intermediates of ligation machinery, and poly-Ub chains (46, 47). A previous study (25) and our current study showed that these forms of Ub can serve as sources of UbB, and hence the total cellular Ub concentration could serve as the guide for the availability of UbB. The formation of cIAP1-UbcH5B∼Ub complex lowers the Kd for the UbB-UbcH5B interaction to a value in which the UbB interaction would be favorable in cells. We anticipate that noncovalent Ub binding would have an impact on cIAP1-UbcH5–catalyzed ubiquitination in cells. In both crystal structures of cIAP1R-UbcH5B–Ub-UbB and RNF38-UbcH5B–Ub-UbB (25) complexes, UbB alters UbcH5B's α1β1-loop into a nearly identical configuration to buttress UbD in the closed conformation. The subtle differences in UbB conformations seen in the two structures could potentially arise from crystal packing. Nonetheless, the cIAP1R-UbcH5B–Ub-UbB structure presented here provides a more detailed view of how UbB could make an additional contact with UbcH5B's α1 C terminus and α1β1-loop to optimize these elements in stabilizing the closed UbD conformation. In conclusion, our work shows that UbB serves as an allosteric activator of RING E3-E2∼Ub complexes and that the UbB-stimulatory mechanism is conserved for both monomeric and dimeric RING E3s.
Experimental procedures
Protein expression and purification
All constructs were expressed in Escherichia coli BL21 (DE3) Gold (Stratagene). All proteins used are from humans unless otherwise specified. cIAP1 RING domain (residues 556-C; cIAP1R) was cloned into pGEX4T1 (GE Healthcare), which contains an N-terminal GST tag followed by a tobacco etch virus protease cleavage site. cIAP1R was purified by GSH affinity chromatography, followed by tobacco etch virus cleavage to release the GST tag. The released GST tag was removed by GSH affinity chromatography, and the cleaved cIAP1R was purified by size-exclusion chromatography. Arabidopsis thaliana Uba1, untagged UbcH5B variants, 32P-Ub, Ub, and Ub lacking the C-terminal diglycine motif (UbΔGG) were prepared as described previously (25). Fluorescently labeled Ub was prepared as described previously (30). UbcH5B–Ub, UbcH5BS22R–Ub, and UbcH5B S22R,F62A,P95D–Ub were generated and purified as described previously (25). Protein concentrations were determined by Bradford assay using BSA as a standard. Ub concentration was determined by measuring the absorbance at 280 nm and the molar extinction coefficient calculated from the protein sequence. Proteins were stored in 25 mm Tris-HCl (pH 7.6), 0.15 m NaCl, and 1 mm DTT at −80 °C.
Crystallization
cIAP1R-UbcH5B–Ub-UbB complex was assembled by mixing cIAP1R (8.5 mg/ml), UbcH5B–Ub (20 mg/ml), and Ub (100 mg/ml) at 1:1:1.2 molar ratio. Crystals were obtained by mixing protein complex with an equal volume of reservoir solution containing 0.2 m ammonium fluoride and 15% (w/v) PEG 3350 using sitting drop vapor diffusion at 19 °C. The crystals were harvested and flash-frozen in 0.2 m ammonium fluoride, 18% (w/v) PEG 3350, and 20% (v/v) ethylene glycol.
Data collection and processing
Data were collected at beamline I03 at Diamond Light Source, processed using xia2 pipeline (48), and integrated with automated XDS (49). Initial phases of cIAP1R-UbcH5B–Ub-UbB complex were obtained by molecular replacement with PHASER (50) using UbcH5B and Ub from PDB entry 3ZNI and cIAP2 RING from PDB entry 3EB6. All models were built in COOT (51) and refined using PHENIX (52). cIAP1R-UbcH5B–Ub-UbB complex was refined to a resolution of 1.7 Å. The final model contains one copy of cIAP1R (chain A, residues 556-C), one copy of UbB (chain B residues 1–74), one copy of UbcH5B (chain C residues 2–147), and one copy of UbD (chain D, residues 1–76). All figure models were generated using PyMOL.
Lysine discharge assays
UbcH5B variant (15 μm) was charged with equimolar Ub variant (15 μm), 32P-Ub (15 μm), or fluorescently labeled Ub (15 μm) in a reaction containing 50 mm Tris-HCl, pH 7.6, 50 mm NaCl, Arabidopsis Uba1 (1 μm), BSA (1 mg/ml), 5 mm MgCl2, and 5 mm ATP for 15 min at 23 °C as described previously (25). The charged reaction was stopped by adding 0.01 units/ml apyrase and 30 mm EDTA for 2 min at 23 °C. The lysine discharge reaction was initiated by adding a mixture containing 50 mm Tris-HCl, pH 7.6, 50 mm NaCl, BSA (1 mg/ml), l-lysine (20 mm), and cIAP1R variant (0.5 μm) in the presence or absence of UbΔGG (300 μm for Figs. 1A, 4 (B and C), 5B, and 6C; 20 μm for Fig. 1B) or UbcH5B S22R,F62A,P95D–Ub (20 μm; Fig. 1B). WT Ub (300 μm) and K6A Ub (300 μm) were used to perform lysine discharge assays in Fig. 6G. Final concentrations are shown in parenthesis except for UbcH5B and Ub variants, which were ∼5 μm. Reactions were quenched with 2× SDS-loading buffer at the indicated time points and resolved by SDS-PAGE and visualized by staining with InstantBlue. Reactions performed using 32P-Ub were dried and visualized using autoradiography. Fluorescently labeled UbcH5B∼Ub was visualized by a LI-COR Odyssey scanner, followed by staining with InstantBlue.
SPR
All SPR experiments were performed at 25 °C on a Biacore T200 system with a CM-5 chip. For cIAP1R-UbcH5B and cIAP1R-UbcH5B–Ub variant binding experiments, GST-cIAP1R was coupled to CM-5 chips as described previously (25). UbcH5B and UbcH5B–Ub variants were serially diluted in running buffer containing 25 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.1 mg/ml BSA, 1 mm DTT, and 0.005% (v/v) Tween 20. For experiments with UbΔGG, UbcH5B–Ub variants were serially diluted in running buffer containing 0.6 mm UbΔGG. For the UbB-UbcH5B backside binding experiment, GST-Ub was captured on a CM-5 chip, and UbcH5B–Ub was mixed with a 2.4-fold molar excess of cIAP1R (100 μm UbcH5B–Ub and 240 μm cIAP1R) and then serially diluted in running buffer containing 10 μm cIAP1R to ensure that all UbcH5B–Ub concentration ranges were saturated with cIAP1R (cIAP1R binds UbcH5B–Ub with a Kd of ∼0.83 μm; Table 1). Binding was measured at the indicated concentration ranges as in Fig. S1. Data reported are the differences in SPR signal between GST-cIAP1R and GST alone or GST-Ub and GST alone. The data were analyzed by steady-state affinity analysis using Biacore T200 BIAevaluation software (GE Healthcare) and Scrubber2 (BioLogic Software).
Author contributions
A. P. purified proteins and performed ubiquitination assays and crystallization. G. S. performed and analyzed SPR experiments. A. P. and D. T. H. determined the structure and wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Lori Buetow for discussion and Diamond Light Source for access to stations I03 (BAG allocation mx8659).
This work was supported by Cancer Research UK Grant A23278 and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant Agreement 647849). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1.
The atomic coordinates and structure factors (code 6HPR) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- Ub
- ubiquitin
- PDB
- Protein Data Bank
- cIAP1 and cIAP2
- cellular inhibitor of apoptosis protein-1 and -2, respectively
- IAP
- inhibitor of apoptosis
- E1
- Ub-activating enzyme
- E2
- Ub-conjugating enzyme
- E3
- Ub-ligase
- E2∼Ub
- E2∼ubiquitin conjugate
- UbcH5B–Ub
- UbcH5B covalently linked to Ub
- RING
- really interesting new gene
- HECT
- homologous to E6-AP C terminus
- SMAC
- second mitochondria-derived activator of caspase
- XIAP
- X-linked inhibitor of apoptosis protein
- TRAF
- TNF receptor–associated factor protein
- RIPK1
- receptor-interacting serine/threonine-protein kinase 1
- UBA
- Ub-associated domain
- BIR
- baculoviral IAP repeat domain
- CARD
- caspase-recruiting domain
- UbB
- backside-bound Ub
- UbD
- donor Ub
- cIAP1R
- cIAP1 RING domain
- SPR
- surface plasmon resonance
- RSMD
- root mean square deviation
- RBR
- RING-in-between-RING
- GST
- glutathione S-transferase.
References
- 1. Hershko A., and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- 2. Pickart C. M., and Eddins M. J. (2004) Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 10.1016/j.bbamcr.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 3. Buetow L., and Huang D. T. (2016) Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 17, 626–642 10.1038/nrm.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dye B. T., and Schulman B. A. (2007) Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu. Rev. Biophys. Biomol. Struct. 36, 131–150 10.1146/annurev.biophys.36.040306.132820 [DOI] [PubMed] [Google Scholar]
- 5. Dynek J. N., and Vucic D. (2013) Antagonists of IAP proteins as cancer therapeutics. Cancer Lett 332, 206–214 10.1016/j.canlet.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 6. Hu S., and Yang X. (2003) Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J. Biol. Chem. 278, 10055–10060 10.1074/jbc.M207197200 [DOI] [PubMed] [Google Scholar]
- 7. Eckelman B. P., and Salvesen G. S. (2006) The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J. Biol. Chem. 281, 3254–3260 10.1074/jbc.M510863200 [DOI] [PubMed] [Google Scholar]
- 8. Choi Y. E., Butterworth M., Malladi S., Duckett C. S., Cohen G. M., and Bratton S. B. (2009) The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J. Biol. Chem. 284, 12772–12782 10.1074/jbc.M807550200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peltzer N., Darding M., and Walczak H. (2016) Holding RIPK1 on the Ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 26, 445–461 10.1016/j.tcb.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 10. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., and Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 10.1016/j.molcel.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 11. Varfolomeev E., Goncharov T., Fedorova A. V., Dynek J. N., Zobel K., Deshayes K., Fairbrother W. J., and Vucic D. (2008) c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 10.1074/jbc.C800128200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haas T. L., Emmerich C. H., Gerlach B., Schmukle A. C., Cordier S. M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T., Koschny R., Komander D., Silke J., and Walczak H. (2009) Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 10.1016/j.molcel.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 13. Tokunaga F., Sakata S., Saeki Y., Satomi Y., Kirisako T., Kamei K., Nakagawa T., Kato M., Murata S., Yamaoka S., Yamamoto M., Akira S., Takao T., Tanaka K., and Iwai K. (2009) Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat. Cell Biol. 11, 123–132 10.1038/ncb1821 [DOI] [PubMed] [Google Scholar]
- 14. Gerlach B., Cordier S. M., Schmukle A. C., Emmerich C. H., Rieser E., Haas T. L., Webb A. I., Rickard J. A., Anderton H., Wong W. W., Nachbur U., Gangoda L., Warnken U., Purcell A. W., Silke J., and Walczak H. (2011) Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 10.1038/nature09816 [DOI] [PubMed] [Google Scholar]
- 15. Tokunaga F., Nakagawa T., Nakahara M., Saeki Y., Taniguchi M., Sakata S., Tanaka K., Nakano H., and Iwai K. (2011) SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature 471, 633–636 10.1038/nature09815 [DOI] [PubMed] [Google Scholar]
- 16. Ikeda F., Deribe Y. L., Skånland S. S., Stieglitz B., Grabbe C., Franz-Wachtel M., van Wijk S. J., Goswami P., Nagy V., Terzic J., Tokunaga F., Androulidaki A., Nakagawa T., Pasparakis M., Iwai K., et al. (2011) SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471, 637–641 10.1038/nature09814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mace P. D., Linke K., Feltham R., Schumacher F. R., Smith C. A., Vaux D. L., Silke J., and Day C. L. (2008) Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 283, 31633–31640 10.1074/jbc.M804753200 [DOI] [PubMed] [Google Scholar]
- 18. Silke J., Kratina T., Chu D., Ekert P. G., Day C. L., Pakusch M., Huang D. C., and Vaux D. L. (2005) Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc. Natl. Acad. Sci. U.S.A. 102, 16182–16187 10.1073/pnas.0502828102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dueber E. C., Schoeffler A. J., Lingel A., Elliott J. M., Fedorova A. V., Giannetti A. M., Zobel K., Maurer B., Varfolomeev E., Wu P., Wallweber H. J., Hymowitz S. G., Deshayes K., Vucic D., and Fairbrother W. J. (2011) Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science 334, 376–380 10.1126/science.1207862 [DOI] [PubMed] [Google Scholar]
- 20. Lopez J., John S. W., Tenev T., Rautureau G. J., Hinds M. G., Francalanci F., Wilson R., Broemer M., Santoro M. M., Day C. L., and Meier P. (2011) CARD-mediated autoinhibition of cIAP1's E3 ligase activity suppresses cell proliferation and migration. Mol. Cell 42, 569–583 10.1016/j.molcel.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 21. Feltham R., Bettjeman B., Budhidarmo R., Mace P. D., Shirley S., Condon S. M., Chunduru S. K., McKinlay M. A., Vaux D. L., Silke J., and Day C. L. (2011) Smac mimetics activate the E3 ligase activity of cIAP1 protein by promoting RING domain dimerization. J. Biol. Chem. 286, 17015–17028 10.1074/jbc.M111.222919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Plechanovová A., Jaffray E. G., Tatham M. H., Naismith J. H., and Hay R. T. (2012) Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 10.1038/nature11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dou H., Buetow L., Sibbet G. J., Cameron K., and Huang D. T. (2012) BIRC7-E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 19, 876–883 10.1038/nsmb.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dou H., Buetow L., Sibbet G. J., Cameron K., and Huang D. T. (2013) Essentiality of a non-RING element in priming donor ubiquitin for catalysis by a monomeric E3. Nat. Struct. Mol. Biol. 20, 982–986 10.1038/nsmb.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buetow L., Gabrielsen M., Anthony N. G., Dou H., Patel A., Aitkenhead H., Sibbet G. J., Smith B. O., and Huang D. T. (2015) Activation of a primed RING E3-E2-ubiquitin complex by non-covalent ubiquitin. Mol. Cell 58, 297–310 10.1016/j.molcel.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 26. Branigan E., Plechanovová A., Jaffray E. G., Naismith J. H., and Hay R. T. (2015) Structural basis for the RING-catalyzed synthesis of K63-linked ubiquitin chains. Nat. Struct. Mol. Biol. 22, 597–602 10.1038/nsmb.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koliopoulos M. G., Esposito D., Christodoulou E., Taylor I. A., and Rittinger K. (2016) Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity. EMBO J. 35, 1204–1218 10.15252/embj.201593741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanchez J. G., Chiang J. J., Sparrer K. M. J., Alam S. L., Chi M., Roganowicz M. D., Sankaran B., Gack M. U., and Pornillos O. (2016) Mechanism of TRIM25 catalytic activation in the antiviral RIG-I pathway. Cell Rep. 16, 1315–1325 10.1016/j.celrep.2016.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dawidziak D. M., Sanchez J. G., Wagner J. M., Ganser-Pornillos B. K., and Pornillos O. (2017) Structure and catalytic activation of the TRIM23 RING E3 ubiquitin ligase. Proteins 85, 1957–1961 10.1002/prot.25348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nomura K., Klejnot M., Kowalczyk D., Hock A. K., Sibbet G. J., Vousden K. H., and Huang D. T. (2017) Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity. Nat. Struct. Mol. Biol. 24, 578–587 10.1038/nsmb.3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Middleton A. J., Budhidarmo R., Das A., Zhu J., Foglizzo M., Mace P. D., and Day C. L. (2017) The activity of TRAF RING homo- and heterodimers is regulated by zinc finger 1. Nat. Commun. 8, 1788 10.1038/s41467-017-01665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright J. D., Mace P. D., and Day C. L. (2016) Secondary ubiquitin-RING docking enhances Arkadia and Ark2C E3 ligase activity. Nat. Struct. Mol. Biol. 23, 45–52 10.1038/nsmb.3142 [DOI] [PubMed] [Google Scholar]
- 33. Pruneda J. N., Littlefield P. J., Soss S. E., Nordquist K. A., Chazin W. J., Brzovic P. S., and Klevit R. E. (2012) Structure of an E3:E2∼Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell 47, 933–942 10.1016/j.molcel.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bertrand M. J., Lippens S., Staes A., Gilbert B., Roelandt R., De Medts J., Gevaert K., Declercq W., and Vandenabeele P. (2011) cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor interacting proteins kinases 1 to 4 (RIP1–4). PLoS One 6, e22356 10.1371/journal.pone.0022356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dynek J. N., Goncharov T., Dueber E. C., Fedorova A. V., Izrael-Tomasevic A., Phu L., Helgason E., Fairbrother W. J., Deshayes K., Kirkpatrick D. S., and Vucic D. (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 29, 4198–4209 10.1038/emboj.2010.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brzovic P. S., Lissounov A., Christensen D. E., Hoyt D. W., and Klevit R. E. (2006) A UbcH5/ubiquitin noncovalent complex is required for processive BRCA1-directed ubiquitination. Mol Cell 21, 873–880 10.1016/j.molcel.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 37. Ranaweera R. S., and Yang X. (2013) Auto-ubiquitination of Mdm2 enhances its substrate ubiquitin ligase activity. J. Biol. Chem. 288, 18939–18946 10.1074/jbc.M113.454470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li S., Liang Y. H., Mariano J., Metzger M. B., Stringer D. K., Hristova V. A., Li J., Randazzo P. A., Tsai Y. C., Ji X., and Weissman A. M. (2015) Insights into ubiquitination from the unique clamp-like binding of the RING E3 AO7 to the E2 UbcH5B. J. Biol. Chem. 290, 30225–30239 10.1074/jbc.M115.685867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakata E., Satoh T., Yamamoto S., Yamaguchi Y., Yagi-Utsumi M., Kurimoto E., Tanaka K., Wakatsuki S., and Kato K. (2010) Crystal structure of UbcH5b∼ubiquitin intermediate: insight into the formation of the self-assembled E2∼Ub conjugates. Structure 18, 138–147 10.1016/j.str.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 40. Cheung H. H., Plenchette S., Kern C. J., Mahoney D. J., and Korneluk R. G. (2008) The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways. Mol. Biol. Cell 19, 2729–2740 10.1091/mbc.e08-01-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakatani Y., Kleffmann T., Linke K., Condon S. M., Hinds M. G., and Day C. L. (2013) Regulation of ubiquitin transfer by XIAP, a dimeric RING E3 ligase. Biochem. J. 450, 629–638 10.1042/BJ20121702 [DOI] [PubMed] [Google Scholar]
- 42. Yin Q., Lin S. C., Lamothe B., Lu M., Lo Y. C., Hura G., Zheng L., Rich R. L., Campos A. D., Myszka D. G., Lenardo M. J., Darnay B. G., and Wu H. (2009) E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 16, 658–666 10.1038/nsmb.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bosanac I., Phu L., Pan B., Zilberleyb I., Maurer B., Dixit V. M., Hymowitz S. G., and Kirkpatrick D. S. (2011) Modulation of K11-linkage formation by variable loop residues within UbcH5A. J. Mol. Biol. 408, 420–431 10.1016/j.jmb.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 44. Saha A., Lewis S., Kleiger G., Kuhlman B., and Deshaies R. J. (2011) Essential role for ubiquitin-ubiquitin-conjugating enzyme interaction in ubiquitin discharge from Cdc34 to substrate. Mol. Cell 42, 75–83 10.1016/j.molcel.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wickliffe K. E., Lorenz S., Wemmer D. E., Kuriyan J., and Rape M. (2011) The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell 144, 769–781 10.1016/j.cell.2011.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaiser S. E., Riley B. E., Shaler T. A., Trevino R. S., Becker C. H., Schulman H., and Kopito R. R. (2011) Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat. Methods 8, 691–696 10.1038/nmeth.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siepmann T. J., Bohnsack R. N., Tokgöz Z., Baboshina O. V., and Haas A. L. (2003) Protein interactions within the N-end rule ubiquitin ligation pathway. J. Biol. Chem. 278, 9448–9457 10.1074/jbc.M211240200 [DOI] [PubMed] [Google Scholar]
- 48. Winter G. (2010) xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Cryst. 43, 186–190 10.1107/S0021889809045701 [DOI] [Google Scholar]
- 49. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 52. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., and Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 10.1107/S0907444902016657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.