Abstract
Voltage-gated Kv7.2 potassium channels regulate neuronal excitability. The gating of these channels is tightly controlled by various mediators and neurotransmitters acting via G protein-coupled receptors; the underlying signaling cascades involve phosphatidylinositol-4,5-bisphosphate (PIP2), Ca2+/calmodulin, and phosphorylation. Recent studies found that the PIP2 sensitivity of Kv7.2 channels is affected by two posttranslational modifications, phosphorylation and methylation, harboured within putative PIP2-binding domains. In this study, we updated phosphorylation and methylation sites in Kv7.2 either heterologously expressed in mammalian cells or as GST-fusion proteins exposed to recombinant protein kinases by using LC–MS/MS. In vitro kinase assays revealed that CDK5, protein kinase C (PKC) alpha, PKA, p38 MAPK, CamKIIα, and GSK3β could mediate phosphorylation. Taken together, we provided a comprehensive map of phosphorylation and methylation in Kv7.2 within protein–protein and protein–lipid interaction domains. This may help to interpret the functional roles of individual PTM sites in Kv7.2 channels. All MS data are available via ProteomeXchange with the identifier PXD005567.
Keywords: Kv7.2, methylation, phosphorylation, posttranslational modification
Kv7.2 channels slowly activate at subthreshold voltages and play a fundamental role in the control of neuronal excitability.[1] Several mutations of this channel gene (KCNQ2) were reported to cause early onset epilepsy.[2] The gating of Kv7.2 channels depends on the availability of phosphatidylinositol- 4,5-bisphosphate (PIP2), the membrane contents of which are controlled by G protein-coupled receptors (GPCRs).[3] In addition, phosphorylation of Kv7.2 can contribute to its functional regulation: cyclic AMP augments Kv7 currents through phosphorylation at S52 in Kv7.2,[4] and protein kinase C (PKC) anchored to Kv7.2 via the scaffold protein AKAP150 is involved in the suppression of Kv7 currents via M1 muscarinic acetylcholine receptors.[5] Moreover, in Xenopus oocytes the conductance–voltage relation of recombinant Kv7.2 was shifted in a depolarizing direction by muscarinic acetylcholine receptor agonists as well as direct PKC activation.[6] Interestingly, an analogous effect was not observed in HEK293 cells, but the application of a phosphatase caused an opposite effect. This indicates that Kv7.2 channels are phosphorylated in mammalian cells under basal conditions.[6]
MS-based proteomics is a powerful tool to study PTM-mediated modulation of proteins including ion channels.[7,8] A potential phosphorylation site was proposed at T217 in Kv7.2 by MS.[9] Later on, global phosphoproteome studies presented a number of phosphorylation sites in Kv7.2 channels.[10–15] Recently, we have reported five phosphorylation sites in one of the PIP2-binding domains of Kv7.2 channels and revealed their implication in the PIP2 sensitivity of Kv7.2.[16] In contrast to the identification of phosphorylation sites, only few MS studies have reported methylation sites in ion channels.[17–19] Interestingly, two arginine methylation sites were also identified in the putative PIP2-binding domains of Kv7.2, and PTMs therein determine apparent PIP2 affinities.[19] Here, we updated in vivo and in vitro phosphorylation and methylation sites in Kv7.2 proteins purified from transfected cells and in GST-fusion proteins containing the N- and C-terminal ends of Kv7.2 by using LC–MS/MS. In addition, we revised the list of protein kinases responsible for the phosphorylation of Kv7.2 GST-fusion proteins when incubated with recombinant enzymes.
To obtain a high yield of Kv7.2 protein, we first generated HEK293 cells stably expressing CFP-tagged human Kv7.2 channel (isoform 4; KCNQ2/pECFP-C1) proteins by selecting for G418 resistance (250 μg/mL; Merck) and then immunopurified Kv7.2 proteins via an anti-GFP antibody (Invitrogen), which was more efficient in immunoprecipitation than anti-Kv7.2 antibodies (data not shown). After size fractionation by SDS-PAGE, the colloidal blue-stained bands were subjected to in-gel digestion with trypsin at 37°C for 2 to 18 h. The digested samples were separated on an Ultimate 3000 nano-HPLC system (Dionex) with a PepMap100 C-18 analytic column (75 μm ×150 mm).[16] Eluted peptides were then directly sprayed into an ESI-Q-TOF (Compact, Bruker) or ESI-ITMS (HCT and amaZon, Bruker). MS spectra were recorded and interpreted with the Mascot search engine (version 2.4.1, Matrix Science, London, UK) against a customized database with 551 766 sequences including Swiss-Prot (551 705 sequences, released in August 2016) and all isoforms of Kv7.2 proteins. In addition, we added a modified protein sequence for our transfected Kv7.2 based on the translated protein sequence deduced from the DNA sequencing result of KCNQ2/pECFP-C1 (containing K668E and R823C, Supporting Information Figure 1). The taxonomy was restricted to Homo sapiens (human; 20 235 sequences) for Kv7.2 from cell lines and Kv7.2 GST fusion proteins and to Rodentia (rodents; 26 483 sequences) for Kv7.2 proteins purified from the rat brain. The search parameters were used with a mass tolerance of 20 ppm (Q-TOF) or 0.5 Da (ITMS), an MS/MS tolerance of 0.1 Da (Q-TOF) or 0.5 Da (ITMS) and carbamidomethylation on Cys, oxidation on Met, phosphorylation on Ser (pS)/Thr (pT), and mono- and dimethylation on Arg were initially allowed. As a competition for the identification of methylation sites, monomethylation on Arg (R), Lys (K), and Asp (D)/Glu (E) and di-methylation on Arg and Lys were also allowed. Each filtered MS/MS spectrum exhibiting possible phosphorylation and methylation was carefully examined based on the existence of a neutral loss of H3PO4 (97.977 Da) and the mass shift of MS peaks by 14.015 Da (monomethylation) or 28.031 Da (dimethylation), respectively.[20,21]
LC–MS/MS unambiguously identified Kv7.2 (Swiss-Prot: O43526-4) with 64.33% sequence coverage. When applying the modified protein sequence from KCNQ2/pECFP-C1, a total coverage of 69.32% was obtained with 79.12 and 87.90% coverage of cytosolic N- and C-termini, respectively (Supporting Information Figure 2). Mascot database searches and subsequent manual validation determined 17 phosphorylation sites to Kv7.2 purified from stably transfected HEK293 cells (Table 1, Supporting Information Figure 3), including 12 sites previously shown in our study.[16] Additionally identified phosphorylation sites in Kv7.2 were pS416, pS442, pS448, pT699, and pS707 as well as double phosphorylation pS438/pS446. At the same time, arginine methylation sites were determined by Mascot database searches. When MS/MS spectra were examined manually to detect mass shifts by methylated arginine, some of MS/MS peaks were not correctly shifted in the presence of Lys, Glu, or Asp that can be methylated as well. For example, when searches were done only for arginine methylation, the Mascot database suggested R418 as a monomethylation site based on increased masses of a parent ion and y ions (y7-y11) by 14.01 Da (407KDPPPEPSPSPR418; "R418" column in Supporting Information Figure 4C). However, by comparison with the MS/MS spectrum of the nonmethylated peptide (Supporting Information Figure 4A vs. 4B), the y6 ion at m/z 640.3 was not shifted in the methylated peptide (Supporting Information Figure 4B). Then, methylated K and D/E were allowed together with R for the competition of database searches, and the Mascot database assigned E412 (407KDPPPEPSPSPR418) as methylated residue instead of R418. Further manual inspection confirmed that mass shifts by methylation were only observed downstream of E412 (Supporting Information Figure 4B). By competition searches, we identified mono- and dimethylation on ten arginines, three lysines, 16 glutamic acids, and six aspartic acids in Kv7.2 purified from stably transfected cells (Table 1, Supporting Information Table 1 and Supporting Information Figure 5). However, here we only considered arginine and lysine methylation because we used 20% methanol in the colloidal staining of SDS-PAGE gels and this can cause artefactual methylation on D/E.[21,22] It has been reported before that methylation at Arg/Lys hinders trypsin cleavage.[22] Therefore, we carefully monitored tryptic peptides for methylation-mediated mass shifts of b and y ions (Supporting Information Figure 5). Thereby, we identified dimethylation at K449 that apparently prevented cleavage by trypsin (RSPSADQSLEDSPSKdi-metVPK; Supporting Information Figure 5I). In addition, some related tryptic peptides displayed C-terminal dimethylated K449 residues (RSPSADQSLEDSPSKdi-met and SPSADQSLEDSPSKdi-met; Supporting Information Figure 6). Based on these findings and previous studies,[23–25] we conclude that methylation does not completely prevent tryptic cleavage at Arg/Lys, but instead reduces its efficiency.
Table 1. Phosphorylation and methylation sites identified in human Kv7.2 by MS.
| Phosphorylation | In vivoa | In vitroa | Methylation | In vivoa | In vitroa |
|---|---|---|---|---|---|
| pS52 | + | PKCα, PKA | R36 (di-) | + | − |
| pS414 | + | CDK5, p38 MAPK | R345 (mono-) | − | + |
| pS416 | + | GSK3β | R345 (di-) | + | + |
| pS427 | + | CDK5b, p38 MAPKb | R353 (di-) | − | + |
| pS436 | + | p38 MAPKb | R383 (di-) | + | + |
| pS438 | + | PKAb,CaMKIIαb | R418 (di-) | + | + |
| pS442 | + | b | R434 (di-) | + | + |
| pS446 | + | CDK5b, p38 MAPKb | R435 (di-) | + | + |
| pS448 | + | CDK5 | K449 (di-) | + | + |
| pS438/pS446 | + | − | R550 (di-) | − | + |
| pS455 | + | PKAb | K575 (mono-) | + | + |
| pS477 | + | PKCα, PKA, CaMKIIα | R591 (di-) | − | + |
| pS641 | + | N.D. | R646 (di-) | + | + |
| pT699 | + | − | R694 (mono-) | − | + |
| pS700 | + | − | R694 (di-) | + | − |
| pS707 | + | PKCα | R710 (di-) | + | − |
| pS722 | − | PKCα, PKA | R744 (mono-) | + | + |
| pS722/pS731 | − | PKA | K780 (di-) | − | + |
| pS731 | + | PKA | K798 (mono-) | + | + |
| pS772 | − | PKA | |||
| pS779 | + | − |
“+”=Identified by MS/MS, “−”=No identification
As shown in ref.16.
N.D., not determined due to no sequence coverage.
To check the identified methylation sites in native Kv7.2 channels, we researched the Mascot database with LC–MS/MS data of purified rat Kv7.2 proteins obtained in our previous study.[16] Among the peptides that have been found, the following two homologous sequences verified the presence of several of the nine known isoforms of Kv7.2 in the rat brain: sequence 1: KEPQPEPSPSQK for isoforms 1, 3, 7, 9 vs. KEPQPEPSPSPR for isoform 2 vs. KEPQPEPSPSK for isoforms 4, 5, 6, 8; sequence 2: QNSEEASLPGEDIVEDNK for isoforms 1, 2, 3, 5, 7, 8 vs. QNSEASLPGEDIVEDNK for isoforms 4, 6, 9 (Supporting Information Figure 7). Interestingly, our MS analysis identified the following two amino acid conflicts: all nine isoforms of rat Kv7.2 show conserved isoleucine (I) at position 372 and methionine (M) at position 393 (residue number in the rat isoform 1; Swiss-Prot O88944), but our MS analysis identified tyrosine (Y) and leucine (L) instead (Supporting Information Figure 8A and B). With these newly identified amino acids, all Kv7.2 proteins in three given species (human, rat, mouse) have conserved Y and L residues in these positions (Supporting Information Figure 8C). Finally, we observed two methylation sites at R406 (or K407; residue numbers in the rat isoform 1; 401SGLTFRKEPQPEPSPSQK418) and R430 (425VFSSPRGVAAK435). However, these were only shown with low confidence. Restricted numbers of methylation sites with low confidence and reproducibility may be related to differences between single experiments employing Q-TOF or ITMS, differences between anti-Kv7.2 antibodies used for IP, potentially low amounts of Kv7.2 protein, and/or variable trypsin digestion periods.
To elucidate the protein kinases that might be responsible for the phosphorylation sites identified, we purified GST-fusion proteins of Kv7.2 N- and C-termini (GST-Kv7.2N and GST-Kv7C) for in vitro phosphorylation analyses using recombinant protein kinases (Supporting Information Figure 9).[16] GST-Kv7.2C (residues 317–841) was phosphorylated by CDK5, p38 MAPK, CamKIIα, PKA, GSK3β, and PKCα; GST-Kv7.2N (residues 1–91) was phosphorylated by PKA and PKCα. Table 1 shows in vitro phosphorylation sites identified subsequent to incubation with individual protein kinases. Residue S52, which had been indicated to get phosphorylated by PKA through a functional mutation analysis,[4] was confirmed here by MS. Additionally, PKCα phosphorylated the same residue. S414, S427, S438, S446, S477, and S722 were also phosphorylated by two or three protein kinases (Table 1 and Supporting Information Table 2), showing possible orchestration of multiple kinases at specific residues. At the same time, 15 arginine and lysine methylation sites were assigned by the Mascot database (Table 1, Supporting Information Table 1 and Supporting Information Figure 5).
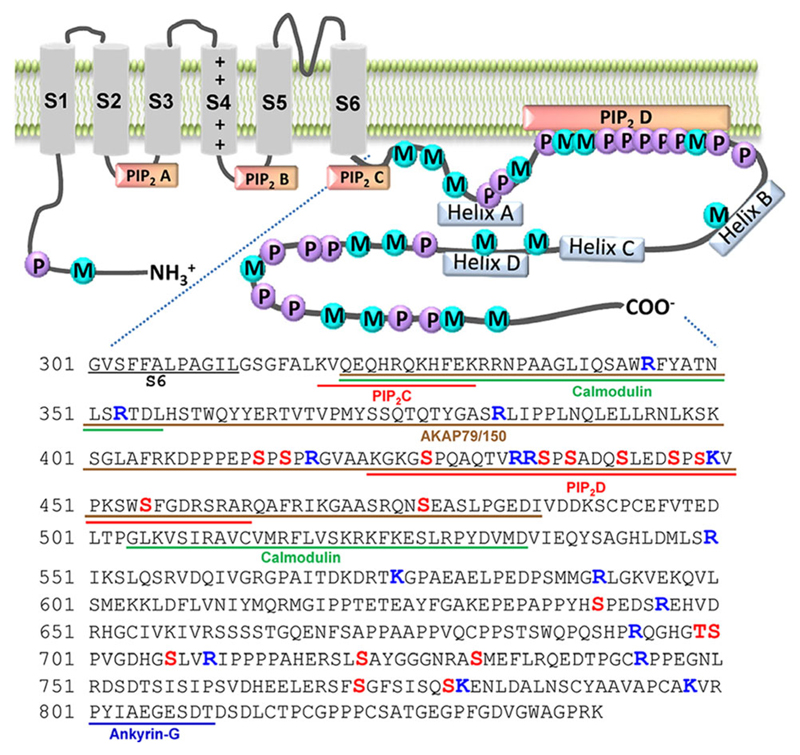
In this study, we updated phosphorylation and methylation sites in Kv7.2 channel proteins by using LC–MS/MS and presented the protein kinases responsible for the phosphorylated residues. Using all the phosphorylation and Arg/Lys methylation sites detected, we generated a phosphorylation and methylation map of Kv7.2 protein (Figure 1); this map depicts four newly identified phosphorylation and methylation sites (R434, S442, S448, and K449) within the PIP2-binding domain located in the Helix A-B linker, where six modification sites (S427, R435, S436, S438, S446, and S455) have been reported previously to regulate PIP2 sensitivity.[16,19] This cluster of two PTMs may prove essential for the dynamic regulation of Kv7.2 through changes in PIP2 sensitivity.
Figure 1.
Membrane topology of Kv7.2 with identified phosphorylation (P) and methylation (M) sites. Putative protein- and PIP2-binding domains are underlined and phosphorylation (S/T) and methylation (K/R) sites are indicated in bold.
Supplementary Material
Supporting Information is available from the Wiley Online Library or from the author.
Acknowledgements
The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE[26] partner repository with the dataset identifier PXD005567. We are grateful to Dr. Mark Shapiro (San Antonio, TX, USA) for providing KCNQ2/pECFP-C1 cDNA and Dr. Aner Gurvitz for the critical reading of the manuscript. This work was supported by the Austrian Science Fund (FWF): W1205 (to S.B.) and P23670 (to J.-W.Y.). I.S. is a member of the doctoral program “Cell Communication in Health and Disease” (CCHD; co-financed by FWF and the Medical University of Vienna).
Abbreviations
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PKC
protein kinase C
Footnotes
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/pmic.201700015
Conflict of Interest
The authors declare no competing financial interests.
References
- [1].Brown DA, Passmore GM. Br J Pharmacol. 2009;156:1185. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, et al. Nat Genet. 1998;18:25. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- [3].Suh BC, Inoue T, Meyer T, Hille B. Science. 2006;314:1454. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Nature. 1998;396:687. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- [5].Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. Nat Neurosci. 2003;6:564. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakajo K, Kubo Y. J Physiol. 2005;569:59. doi: 10.1113/jphysiol.2005.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Olsen JV, Mann M. Mol Cell Proteomics. 2013;12:3444. doi: 10.1074/mcp.O113.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park KS, Yang JW, Seikel E, Trimmer JS. Physiology. 2008;23:49. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- [9].Surti TS, Huang L, Jan YN, Jan LY, Cooper EC. Proc Natl Acad Sci U S A. 2005;102:17828. doi: 10.1073/pnas.0509122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. Cell. 2010;143:1174. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, Olsen JV. Nat Commun. 2012;3:876. doi: 10.1038/ncomms1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, Burlingame AL. Mol Cell Proteomics. 2012;11:215. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Trinidad JC, Thalhammer A, Specht CG, Lynn AJ, Baker PR, Schoepfer R, Burlingame AL. Mol Cell Proteomics. 2008;7:684. doi: 10.1074/mcp.M700170-MCP200. [DOI] [PubMed] [Google Scholar]
- [14].Tweedie-Cullen RY, Reck JM, Mansuy IM. J Proteome Res. 2009;8:4966. doi: 10.1021/pr9003739. [DOI] [PubMed] [Google Scholar]
- [15].Wisniewski JR, Nagaraj N, Zougman A, Gnad F, Mann M. J Proteome Res. 2010;9:3280. doi: 10.1021/pr1002214. [DOI] [PubMed] [Google Scholar]
- [16].Salzer I, Erdem FA, Chen WQ, Heo S, Koenig X, Schicker KW, Kubista H, Lubec G, Boehm S, Yang JW. J Physiol. 2017;595:759. doi: 10.1113/JP273274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baek JH, Rubinstein M, Scheuer T, Trimmer JS. J Biol Chem. 2014;289:15363. doi: 10.1074/jbc.M114.562785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beltran-Alvarez P, Pagans S, Brugada R. J Proteome Res. 2011;10:3712. doi: 10.1021/pr200339n. [DOI] [PubMed] [Google Scholar]
- [19].Kim HJ, Jeong MH, Kim KR, Jung CY, Lee SY, Kim H, Koh J, Vuong TA, Jung S, Yang H, Park SK, et al. Elife. 2016;5:e17159. doi: 10.7554/eLife.17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DeGnore JP, Qin J. J Am Soc Mass Spectrom. 1998;9:1175. doi: 10.1016/S1044-0305(98)00088-9. [DOI] [PubMed] [Google Scholar]
- [21].Jung SY, Li Y, Wang Y, Chen Y, Zhao Y, Qin J. Anal Chem. 2008;80:1721. doi: 10.1021/ac7021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hart-Smith G, Yagoub D, Tay AP, Pickford R, Wilkins MR. Mol Cell Proteomics. 2016;15:989. doi: 10.1074/mcp.M115.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Azkargorta M, Wojtas MN, Abrescia NG, Elortza F. J Proteome Res. 2014;13:2637. doi: 10.1021/pr500084p. [DOI] [PubMed] [Google Scholar]
- [24].Couttas TA, Raftery MJ, Padula MP, Herbert BR, Wilkins MR. Proteomics. 2012;12:960. doi: 10.1002/pmic.201100570. [DOI] [PubMed] [Google Scholar]
- [25].Ong SE, Mittler G, Mann M. Nat Methods. 2004;1:119. doi: 10.1038/nmeth715. [DOI] [PubMed] [Google Scholar]
- [26].Vizcaino JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, et al. Nat Biotechnol. 2014;32:223. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.