Abstract
CXXC5 is a member of the CXXC‐type zinc‐finger protein family. Proteins in this family play a pivotal role in epigenetic regulation by binding to unmethylated CpG islands in gene promoters through their characteristic CXXC domain. CXXC5 is a short protein (322 amino acids in length) that does not have any catalytic domain, but is able to bind to DNA and act as a transcription factor and epigenetic factor through protein‐protein interactions. Intriguingly, increasing evidence indicates that expression of the CXXC5 gene is controlled by multiple signaling pathways and a variety of transcription factors, positioning CXXC5 as an important signal integrator. In addition, CXXC5 is capable of regulating various signal transduction processes, including the TGF‐β, Wnt and ATM‐p53 pathways, thereby acting as a novel and crucial signaling coordinator. CXXC5 plays an important role in embryonic development and adult tissue homeostasis by regulating cell proliferation, differentiation and apoptosis. In keeping with these functions, aberrant expression or altered activity of CXXC5 has been shown to be involved in several human diseases including tumourigenesis. This review summarizes the current understanding of CXXC5 as a transcription factor and signaling regulator and coordinator.
Keywords: CXXC domain, CXXC5, signaling coordinator, transcription factor, tumour suppressor
1. INTRODUCTION
CXXC5, also known as retinoid‐inducible nuclear factor, is a member of the CXXC‐type zinc‐finger protein family. Members of this family are characterized by a specialized CXXC domain and include CXXC1/CFP1, CXXC2/KDM2B, TET1/3 and DNMT1, among others.1, 2, 3 The CXXC domain binds to unmethylated cytosine‐guanine (CpG) dinucleotides, especially those within short (500‐2000 bp) CpG islands and contiguous CpG‐rich DNA sequences in promoter regions. Intriguingly, these CpG islands are generally refractory to DNA methylation. Accordingly, many CXXC family members are involved in epigenetic regulation and therefore play pivotal roles in embryonic development, tissue homeostasis and pathological alterations.1, 2, 4
The CXXC5 protein, though much less understood, has been shown to act as a transcriptional regulator by binding directly to DNA.5, 6 Intriguingly, accumulating evidence indicates that CXXC5 gene expression is regulated by various secreted cytokines and intracellular transcription factors.7, 8, 9, 10 In addition, the CXXC5 protein is implicated in regulating and coordinating multiple signaling pathways including those initiated by transforming growth factor beta (TGF‐β), bone and morphogenetic proteins (BMPs), Wnt, ATM/p53 and others.8, 10, 11, 12 This review will first summarize the characteristics of the CXXC‐type zinc‐finger protein family and the role of CXXC5 as a transcriptional regulator. Next, we will give special attention to the mechanisms by which the CXXC5 protein regulates and coordinates different signaling pathways, thereby exerting multi‐faceted pathophysiological functions.
2. CXXC‐TYPE ZINC‐FINGER PROTEIN FAMILY
The combined pattern of DNA and histone modifications confers an epigenetic code that can profoundly affect both chromatin structure and gene expression.13 In vertebrates, DNA methylation mainly occurs on cytosine within the CpG dinucleotides, which are frequently found in mammalian gene promoters and are associated with a compacted chromatin structure and transcriptional repression.14 Previous studies have demonstrated that proteins harbouring methyl‐CpG‐binding domains (MBDs) play a significant role in this process.14, 15 However, as mentioned above, methylation‐resistant CpG islands are prevalent in many promoters, up to 60% of human gene promoters.1, 2, 13 It is reasonable to assume that these unmethylated CpG islands are also targeted by epigenetic factors and are associated with an open chromatin state and transcriptional activation. Indeed, the specialized CXXC‐type zinc‐finger domain has been shown to bind unmethylated CpG in vitro.16 Further, ChIP‐sequencing (ChIP‐Seq) analyses have demonstrated that some of the CXXC domain‐containing proteins are capable of binding to CpG at the genomic scale in various in vivo contexts.1, 4, 17
Twelve CXXC zinc‐finger protein family members have been identified in mammalians (Table 1).1, 2, 3, 18 The CXXC‐type zinc‐finger domain is characterized by two conserved cysteine‐rich motifs (ie, CxxCxxCx4‐5CxxCxxC and CxxRxC, wherein x indicates residues other than cysteine), with three cysteines from the first motif and one from the latter together coordinating one Zn2+ ion, therefore forming two zinc‐finger structures.2, 14 However, the linker region between the two motifs is less conserved and classifies the family members into different subgroups. CFP1/CXXC1, KDM2A/CXXC8, KDM2B/CXXC2, FBXL19/CXXC11, DNMT1/CXXC9, MLL1/CXXC7, MLL2/CXXC10 and MBD1/CXXC3 share a conserved KFGG motif and fall into the same subgroup. Meanwhile, TET1/CXXC6, TET3, IDAX/CXXC4 and CXXC5 are shorter, lack the KFGG motif, and constitute a second subgroup. The majority of CXXC family members exhibit chromatin‐modifying activity due to the existence of functional domains. CXXC4 and CXXC5, though structurally similar, lack these types of domains.11, 19 The CXXC5 protein is 322 amino acids in length, has a molecular weight of 32.98 kDa, and contains a typical CXXC zinc‐finger domain (amino acids 257‐302) near the C‐terminus (Figure 1). A nuclear localization sequence (NLS) exists at the N‐terminus of the CXXC domain, between amino acids 257 and 262 (KKKRKR).3, 11, 12
Table 1.
CXXC‐type zinc‐finger protein family in human
| Member | Alias | Length (aa) | Catalytic activity |
|---|---|---|---|
| CXXC1 | CGBP, CFP1 | 660 | H3K4 methyltransferase |
| CXXC2 | KDM2B, FBXL10 | 1336 | H3K36 demethylase |
| CXXC3 | MBD1 | 605 | Interacts with and recruits the H3K9 methyltransferase SETDB1 |
| CXXC4 | IDAX | 367 | Interacts with and recruits TET2 |
| CXXC5 | RINF, WID | 322 | Interacts with and recruits TET2 |
| CXXC6 | TET1, LCX | 2136 | 5‐methylcytosine dioxygenase |
| CXXC7 | MLL1, KMT2A | 3969 | H3K4 methyltransferase |
| CXXC8 | KDM2A, JHDM1a, FBXL11 | 1162 | H3K36 demethylase |
| CXXC9 | DNMT1 | 1616 | DNA methyltransferase |
| CXXC10 | MLL2, KMT2B | 2715 | H3K4 methyltransferase |
| CXXC11 | FBXL19 | 694 | A component of the Skp1‐Cullin‐F‐box family of E3 ubiquitin ligases |
| TET3 | 1660 | 5‐methylcytosine dioxygenase |
Figure 1.
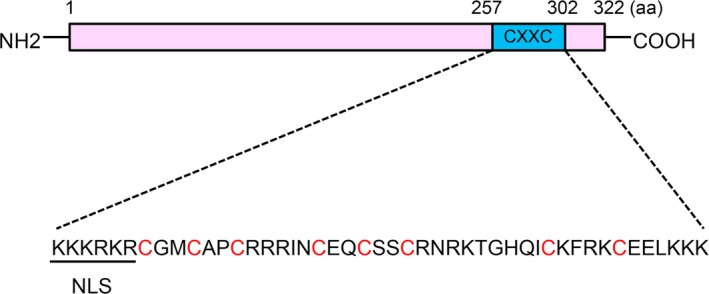
Diagrammatic presentation of the human CXXC5 protein. The sequence of the CXXC domain (amino acids 257‐302) is shown, and the nuclear localization sequence (NLS) (amino acids 257‐262) and the cysteine residues that are involved in the formation of zinc‐finger structures are underscored
3. CXXC5 ACTS AS A TRANSCRIPTION FACTOR AND/OR EPIGENETIC FACTOR
Although CXXC4 and CXXC5 do not have any catalytic domains, it is interesting that both have been found to interact with and recruit TET2, which functions in a similar way as TET1/3 to promote DNA demethylation but is unable to bind DNA directly due to the absence of the CXXC domain.4, 5 ChIP‐Seq analysis indicated that the CXXC domain of CXXC4 mainly interacts with unmethylated and CpG‐rich promoters throughout the genome in HEK293T cells.4 Intriguingly, CXXC4 interacts directly with the TET2 catalytic domain, leading to caspase‐mediated downregulation of TET2 protein levels in mouse embryonic stem cells (mESCs) and monocytic cells. Similarly, CXXC5 has recently been shown to recruit TET2. Genetic ablation of the CXXC5 gene resulted in a series of epigenetic alterations in the IRF7 gene region, including decreased levels of promoter CpG methylation as well as H3K4m3, H3K36m3, H3K27ac and H4ac histone modifications in plasmacytoid dendritic cells, thus contributing to TLR7/9‐ and virus‐induced IFN responses.5
Several other reports have provided additional evidence that CXXC5 could act as a transcription factor and/or epigenetic factor by binding to DNA via its CXXC domain (Table 2). In neural stem cells, Wnt3a‐stimulated CXXC5 directly interacts with the promoter region (−720 to −511 bp) and promotes the expression of the myelin basic protein gene, which is a molecular marker for oligodendrocyte differentiation.20 In endothelial cells, the CXXC5 protein interacts with the Flk‐1 promoter through its CXXC domain, activating Flk‐1 expression and promoting endothelial cell differentiation, migration and vessel formation.7 In C2C12 myoblasts, CXXC5 is able to increase the activities of gene promoters that drive the expression of muscle creatine kinase, minimal thymidine kinase and myosin heavy chain, thus promoting skeletal muscle differentiation.21
Table 2.
Genes directly regulated by CXXC5
| Gene | Regulation by CXXC5 | Function | References |
|---|---|---|---|
| IRF7 | Up | Contributes to TLR7/9‐ and virus‐induced IFN responses | 5 |
| MBP | Up | Promotes differentiation of neural stem cells into oligodendrocytes | 20 |
| Flk‐1 | Up | Promotes endothelial cell differentiation, migration and vessel formation | 7 |
| MCK, MHC | Up | Promotes skeletal muscle differentiation | 21 |
| Cd40L | Down | Inhibits differentiation into helper‐lineage T cells | 22 |
| COX4I2 | Down | Inhibits energy production in hypoxic conditions | 6 |
MBP, myelin basic protein; MCK, muscle creatine kinase; MHC, myosin heavy chain.
In other contexts, CXXC5 may repress gene expression. For instance, in CD8+ Th cells, CXXC5 could associate with the histone‐lysine N‐methyltransferase SUV39H1, inducing histone H3K9 methylation in the Cd40L gene promoter region and inhibiting CD40 ligand expression.22 COX4 is one subunit of the Cytochrome c oxidase (COX) complex. An oxygen responsive element (ORE) has been identified in the promoter region of the COX4I2 gene isoform. In mammalian cell lines, CXXC5 interacts with the ORE to inhibit COX4I2 expression under hypoxic conditions.6 Together, these observations indicate that CXXC5 acts as a transcription factor and/or epigenetic factor to promote or repress gene expression, depending on cell types and contexts (Table 2).
4. CXXC5 FUNCTIONS AS A SIGNALING REGULATOR AND MEDIATOR
4.1. CXXC5 in TGF‐β/BMP signaling
Transforming growth factor‐β is a multi‐functional peptide that plays a critical role in regulating various cellular functions and exerts a diverse array of physiological functions. Deregulation of TGF‐β signaling has been connected with several major human diseases, including developmental defects, tumourigenesis, cardiovascular disorders and metabolic syndromes.23, 24, 25, 26, 27, 28, 29 TGF‐β is the founding member of the TGF‐β cytokine family, which includes TGF‐β itself, Activin, Nodal, Lefty, BMPs, GDFs (growth and differentiation factors), AMH (anti‐Müllerian hormone) and others. These peptides are roughly grouped into two subclasses based on structural similarities and signaling properties. TGF‐β, Activin, Nodal and Lefty fall into the TGF‐β subclass, while the majority of BMPs, GDFs and AMH constitute the BMP subclass. All of these cytokines transduce signals by binding to serine/threonine kinase receptors on the cell membrane, leading to the phosphorylation and activation of Smad proteins.30, 31, 32, 33, 34, 35 In total, there are eight Smad proteins in mammalians, which are functionally classified into three subgroups: R‐Smads (receptor‐regulated Smads), Co‐Smad (common Smad, Smad4) and inhibitory Smads (I‐Smads, Smad6 and Smad7).33, 36 TGF‐β subfamily cytokines utilize Smad2 and Smad3 as R‐Smads, while BMPs activate Smad1/5/8. Activated R‐Smads form a complex with Smad4, and together they translocate into the nucleus to regulate target gene transcription in collaboration with other transcription factors or co‐factors.37, 38 In addition, TGF‐β is capable of activating other signaling pathways mediated by PI3K/Akt, MAPKs (EKR1/2, JNKs and p38), PAK2 and Cdc42/RhoA GTPases, among others.39, 40, 41
One major cellular response to cytokine stimulation is transcriptional alteration. Target gene identification and their functional analyses are crucial for understanding the context‐dependent biological roles of specific cytokines, including TGF‐β.38, 42 With this regard, transcriptional expression of the CXXC5 gene is induced by BMP4 in embryonic telencephalic neural stem cells, mESC‐derived endothelial cells and human umbilical vein endothelial cells.7, 11 Functionally, CXXC5 mediates BMP4‐induced repression of Wnt signaling in neural stem cells, as well as endothelial cell migration and vessel formation. In addition, we have previously reported that CXXC5 is a target gene and functional mediator of TGF‐β in hepatocellular carcinoma (HCC) cells, contributing to the tumour‐suppressive functions of TGF‐β by promoting cell cycle arrest and apoptosis.10
Another important point to understand the pleiotropic nature of TGF‐β is the spatiotemporal regulatory mechanisms of the signal transduction process.31, 34, 43 Feedback regulation plays a pivotal role, fine‐tuning signaling robustness and duration.32, 44 With respect to it, the two inhibitory Smads have been shown critical in controlling TGF‐β/BMP signaling through negative feedback mechanisms.36, 45, 46, 47, 48 We found that overexpression of the CXXC5 protein in both HCC cells and normal hepatocyte cell lines promotes TGF‐β‐induced luciferase reporter expression, while knockdown of CXXC5 gene ameliorates it. Moreover, transcriptional profiling indicates that depletion of CXXC5 in Hep3B HCC cells is able to attenuate the expression of a significant portion of TGF‐β target genes, suggesting that CXXC5 is a novel positive feedback regulator of TGF‐β signaling.10 Moreover, the CXXC5 protein associates with the histone deacetylase HDAC1 in HCC cells and competes for Smad2/3 binding, therefore alleviating HDAC1‐mediated signaling inhibition. Intriguingly, CXXC5 was also suggested to interact with Smad2/3 and Smad4 to promote TNF‐α‐induced apoptosis of primary cortical neurons and normal heart development and function in zebrafish.49, 50
4.2. CXXC5 in Wnt/β‐catenin signaling
The Wnt family of cytokines plays a critical role in embryonic development and adult tissue homeostasis in multicellular animals.51, 52, 53 In the Wnt/β‐catenin pathway, the β‐catenin protein is sequestered in a multi‐protein complex in the absence of ligand stimulation. This complex is comprised of the scaffold proteins Axin and APC as well as two constitutively active serine‐threonine protein kinases, GSK3β and CK1. Sequestration of β‐catenin leads to its phosphorylation and subsequent poly‐ubiquitination and degradation by the F‐box/WD repeat protein β‐TrCP, part of an E3 ubiquitin ligase complex.51, 52, 54, 55 Upon ligand binding, the seven‐pass transmembrane receptor Frizzled (Fzd) and the single‐pass receptor LRP5/6 undergo conformational changes and phosphorylation. This is followed by recruitment of the Axin and Dvl proteins and breakdown of the destruction complex, leading to release and stabilization of β‐catenin, which then enters the nucleus and acts as a transcriptional activator for TCF/LEF transcription factors, thus controlling the expression of a diverse assay of target genes. In addition to this canonical signaling, some Wnt ligands are capable of initiating signaling pathways independent of β‐catenin, including the PCP pathway and those involving release of calcium ions or activation of JNK, Src or other molecules.56, 57
Not surprisingly, the Wnt/β‐catenin pathway is also subject to intense regulation.54, 55, 58 Within the CXXC‐type zinc‐finger protein family, CXXC4 has been shown to interact with Dvl, resulting in inhibition of β‐catenin signaling.19, 59, 60 Similarly, CXXC5 is also able to inhibit β‐catenin signaling by interacting with the PDZ domain of the Dvl protein via its C‐terminal CXXC domain, thereby inhibiting Wnt3a‐induced osteoblast differentiation, bone formation, cutaneous wound healing, in addition to disturbing embryonic kidney development.8, 9, 61 These results suggest that CXXC4 and CXXC5 not only share structural similarities but are also functionally conserved in regulating Wnt signaling. Intriguingly, Wnt3a is able to induce CXXC5 expression in pre‐osteoblast MC3T3E1 cells, human dermal fibroblasts and neural stem cells, making CXXC5 a negative feedback regulator.8, 9, 20
4.3. CXXC5 in other signaling pathways
The p53 tumour suppressor acts as a major defence against cancer by inducing DNA repair, cell cycle arrest, senescence or apoptosis in response to diverse cellular stresses.62 The ataxia telangiectasia mutated (ATM) protein kinase, a member of the PI3/PI4‐kinase family, phosphorylates p53 upon DNA damage, leading to stabilization and activation of the p53 protein, predisposing it for DNA damage repair.63, 64 Interestingly, the CXXC5 protein has been found to interact with ATM and is required for DNA damage‐induced ATM phosphorylation, p53 stabilization and activation, and subsequent DNA damage response.12
1α,25‐dihydroxyvitamin D3 (1,25D) is the active hormonal metabolite of vitamin D. It incites a variety of biological functions by binding the vitamin D receptor (VDR), thus stimulating its transcriptional activity.65 In a yeast two‐hybrid screening, CXXC5 was identified as a novel VDR‐interacting protein, promoting VDR‐mediated transcription from selected Vitamin D‐responsive DNA elements (VDREs).66 Given that VDR has been shown to inhibit Wnt signaling by associating with β‐catenin and restricting its nuclear localization,67, 68 an interesting question to consider is whether VDR and CXXC5 might cooperate with one another to restrain Wnt signaling. FoxL2 is a transcription factor belonging to the large family of winged‐helix forkhead transcription factors. This protein has been implicated in numerous cellular processes including apoptosis, cell cycle control, steroidogenesis and reactive oxygen species (ROS) detoxification.69 Both CXXC4 and CXXC5 can interact with FoxL2, attenuating its transcriptional activity in luciferase reporter assays while facilitating its pro‐apoptosis activity via unknown mechanisms.70 The known interacting partners of CXXC5 are summarized in Table 3.
Table 3.
Interacting partners of CXXC5 protein
| Interacting partner | Interaction site | Functional consequence of the interaction | References |
|---|---|---|---|
| Dishevelled | Cytoplasm | Inhibits Wnt/β‐catenin signaling | 8, 9, 11, 61 |
| HDAC1 | Nd. | Removes HDAC1 from Smad2/3 and promotes TGF‐β signaling | 10 |
| Smad2/3/4 | Predominantly nucleus | Mediates TNF‐α‐induced apoptosis and regulates zebrafish heart development | 49, 50 |
| ATM | Mainly nucleus | Contributes to p53 activation and DNA damage response | 12 |
| VDR | Nd. | Stimulates VDR transcriptional activity | 66 |
| SUV39H1 | Nd. | Represses CD40 ligand expression in CD8+ cytotoxic T cells | 22 |
| RBPJ | Nd. | Inhibits COX4I2 gene transcription | 6 |
| Tet2 | Nucleus | Contributes to IFN response by activating IRF7 expression | 5 |
Nd., not determined; ATM, ataxia telangiectasia mutated; VDR, vitamin D receptor; TGF‐β, transforming growth factor beta.
5. CXXC5 FUNCTIONS AT THE CROSSROADS OF MULTIPLE SIGNALING PATHWAYS
The CXXC5 gene is evolutionarily conserved from Xenopus and zebrafish to mammals. It is located on chromosome 5q31.3 in human, spans 35.5 kb and contains 4 exons, with an mRNA product of approximately 1.4 kb.71, 72 CXXC5 is ubiquitously expressed across human development and adult tissues, though its expression level varies.49 Another study examined CXXC5 expression in human tissues and found that it is highly expressed in placenta, heart and kidney, and moderately expressed in liver, lung and testis.3
As mentioned above, the expression of the CXXC5 gene is transcriptionally induced by TGF‐β, BMP4 and Wnt3a, which rely on Smad proteins and β‐catenin respectively.7, 9, 10 In turn, the CXXC5 protein is able to inhibit Wnt signaling or facilitate TGF‐β signaling, forming distinct feedback regulatory loops. Considering that the three pathways often crosstalk with one another in different contexts including embryonic development, homeostatic adult tissues and pathological conditions,73, 74 it is conceivable that CXXC5 could be a novel crosstalk mediator of these pathways. Indeed, BMP4 induces CXXC5 gene expression in neural stem cells, to antagonize Wnt/β‐catenin signaling and its target gene expression.11 In addition, CXXC5 and BMP4 expression are closely correlated in the dorsal telencephalon of mouse forebrain, just adjacent to Wnt3a expression, strongly suggesting an in vivo functional link.11
Several transcription factors have been shown to bind to the CXXC5 promoter or enhancer, thereby regulating its transcription (Table 4). The zinc‐finger transcription factor Wilms tumour 1 has been shown to activate CXXC5 gene transcription in mammalian cell lines by directly interacting with DNA sequences in the upstream enhancer region.61 GATA2 is a DNA‐binding transcription factor that is important for hematopoietic stem cell proliferation. Mutations in the GATA2 gene have been associated with familial myelodysplastic syndrome and acute myeloid leukaemia (AML).75, 76 Interestingly, GATA2 can bind to the promoter of the CXXC5 gene, increasing its expression. Accordingly, CXXC5 mRNA levels have been found significantly downregulated in cells from AML patients with GATA2 mutations.71 In addition, CXXC5 can be silenced by promoter hypermethylation in prostate cancer cells.77 Therefore, epigenetic alterations or transcription factor mutations could contribute to aberrant CXXC5 expression in cancer. KANK1 is a potential tumour suppressor gene and is downregulated in more than half of human malignant peripheral nerve sheath tumours (MPNSTs).78 Overexpression of KANK1 could induce CXXC5 expression to promote cell apoptosis in MPNSTs.79 The retinoic acid receptor (RARα) acts as a transcription factor when bound to all‐trans retinoic acid, facilitating terminal maturation of leukaemic blasts.80 The CXXC5 promoter contains a retinoid‐responsive element at −3116 bp. Ligand‐bound RARα directly binds to this regulatory element in NB4 acute promyelocytic leukaemia cells, promoting CXXC5 gene expression and leukaemia cell differentiation.3 17β‐estradiol (E2)‐ERα signaling plays a critical role in both physiological functions and malignant transformation of breast tissue.81 E2‐ERα is able to interact with an oestrogen‐responsive element (ERE) (GGTCAnnnTGACC) in the CXXC5 gene promoter, inducing its gene expression in breast cancer cells.82 Conversely, ThPOK, a zinc‐finger family transcription factor encoded by the Zbtb7b gene, represses CXXC5 gene expression in CD8+ cytotoxic T cells.22 Interestingly, NUDT21 (Nudix Hydrolase 21), one subunit of a cleavage factor required for 3’ RNA cleavage and polyadenylation, was found to repress CXXC5 expression by inducing alternative polyadenylation in liver cancer cells.83
Table 4.
Transcriptional regulation of the CXXC5 gene
| Cytokine or transcription factor | CXXC5 expression | Cell type and context | References |
|---|---|---|---|
| TGF‐β | Up | Hepatocellular carcinoma cells and normal liver cells | 10 |
| BMP4 | Up | Embryonic neural stem cells, endothelial cells and HUVECs | 7, 11 |
| Wnt3a/β‐catenin | Up | Pre‐osteoblast MC3T3E1 cells, human dermal fibroblasts and neural stem cells | 8, 9, 20 |
| E2/ERα | Up | Breast cancer cells | 82 |
| Retinoid/RARα | Up | NB4 myeloid cells | 3 |
| WT1 | Up | Zebrafish embryonic kidney development | 61 |
| KANK1 | Up | Embryonic kidney | 79 |
| GATA2 | Up | Acute myeloid leukemia | 71 |
| ThPOK | Down | CD8+ cytotoxic T cells | 22 |
| NUDT21 | Down | Liver cancer cells | 83 |
TGF‐β, transforming growth factor beta; BMPs, bone and morphogenetic proteins.
Together, different cytokines and transcription factors converge on the CXXC5 gene to regulate its expression, suggesting that CXXC5 may act as an important downstream integrator and mediator of various signal inputs. Taking into account its ability to fine‐tune various signal transduction processes, CXXC5 is likely to function as a crucial coordinator of multiple cellular signaling pathways (Figure 2).
Figure 2.
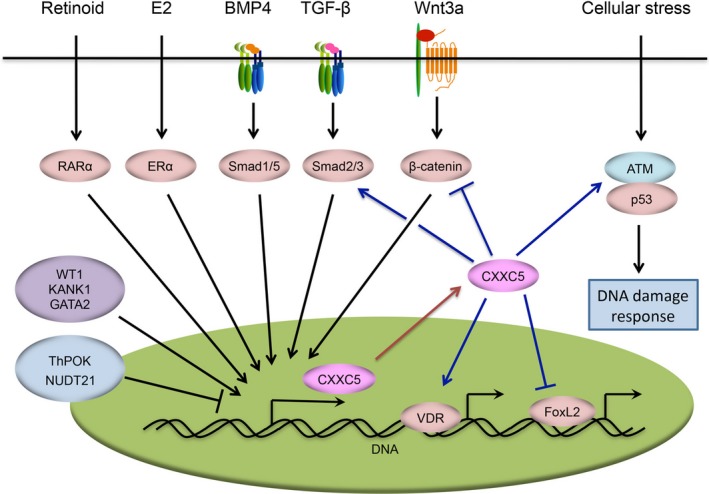
CXXC5 is an integrator and coordinator of cellular signaling networks. TGF‐β, BMP4 and Wnt3a induce CXXC5 gene expression in different contexts. The protein product of this gene can in turn inhibit Wnt signaling or facilitate TGF‐β signaling, forming distinct regulatory feedback loops. The expression of CXXC5 is also transcriptionally induced by Retinoid/RARα and E2/ERα signaling as well as by some transcription factors, including WT1, KANK1 and GATA2. Conversely, the transcription factor ThPOK and the RNA cleavage factor NUDT21 are capable of repressing CXXC5 gene expression. In addition to TGF‐β and Wnt signaling, CXXC5 promotes ATM‐mediated activation of the p53 protein and DNA damage response. CXXC5 also enhances the transcriptional activity of VDR and ameliorates that of FoxL2. TGF‐β, transforming growth factor beta; BMPs, bone and morphogenetic proteins; ATM, ataxia telangiectasia mutated; VDR, vitamin D receptor
6. PATHOPHYSIOLOGICAL FUNCTIONS OF CXXC5
Considering its roles in regulating gene expression and coordinating cellular signaling, it is not surprising that CXXC5 regulates a diverse array of cellular events including cell proliferation, differentiation and apoptosis in developmental processes, adult tissue maintenance and pathological circumstances.
With respect to cell differentiation, retinoid‐induced CXXC5 has been shown to play a role in normal primary leukaemia cell differentiation and tumoral myelopoiesis.3 BMP4‐activated CXXC5 promotes the differentiation of mESCs into endothelial cells.7 In mice, both BMP4‐ and Wnt‐induced CXXC5 can promote the differentiation of neural stem cells into oligodendrocytes, contributing to brain development and maturation.11, 20 In addition, CXXC5 facilitates skeletal myogenic differentiation in vitro by promoting the expression of related genes.21 Conversely, CXXC5 inhibits Wnt‐induced myofibroblast differentiation of dermal fibroblasts in cutaneous wound healing, and osteoblast differentiation and bone formation in mice.8, 9 Interestingly, a peptide that competitively inhibits the CXXC5‐Dvl interaction is capable of alleviating CXXC5‐mediated inhibition of Wnt signaling and accelerating both osteoblast differentiation and cutaneous wound healing.8, 9 In CD8+ cytotoxic T cells, CXXC5 represses CD40L expression, thus preventing the differentiation of helper‐lineage T cells.22 Importantly, gene‐targeting studies in mice have verified the in vivo functions of CXXC5 in regulating cell differentiation.7, 8, 9, 20
CXXC5 is involved in regulating cell proliferation and death in various cell types. In primary leukaemia cells, CXXC5 not only promotes differentiation, as mentioned above, but it also contributes to retinoid‐induced cell cycle arrest and cell death.3 CXXC5 is capable of promoting cell cycle arrest, DNA repair or apoptosis by activating the ATM‐p53 signaling axis.12 In addition, CXXC5 associates with FoxL2 to enhance its pro‐apoptotic activity in mammalian cells,70 and may mediate TNF‐α‐induced apoptosis of primary cortical neurons by associating with Smad proteins.49
Aberrant expression or altered activity of CXXC5 is closely linked to multiple human diseases, especially tumourigenesis (Figure 3). The CXXC5 gene locates in a chromosomal DNA region that is frequently deleted in myeloid leukaemia (AML and myelodysplasia).3 Indeed, CXXC5 is often downregulated in clinical AML samples and may serve as an independent prognostic factor in patients.71, 72 CXXC5 downregulation leads to augmentation of Wnt signaling and impairment of DNA damage‐induced and p53‐dependent cell cycle arrest.71 We found that CXXC5 is also downregulated in the majority of HCC tissue samples, leading to breakdown of CXXC5‐mediated positive feedback regulation of TGF‐β signaling and attenuation of its anti‐proliferative effects.10 In addition, CXXC5 is involved in KANK1‐induced cell growth inhibition and apoptosis in MPNSTs.79 However, it has also been shown that CXXC5 is overexpressed in ER+ breast cancer and associated with poor prognosis in patients.84 This is in agreement with the observation that CXXC5 is upregulated by E2‐ERα.82 Together, these findings suggest that the role of CXXC5 in cancer initiation and progression could rely on cancer types.
Figure 3.
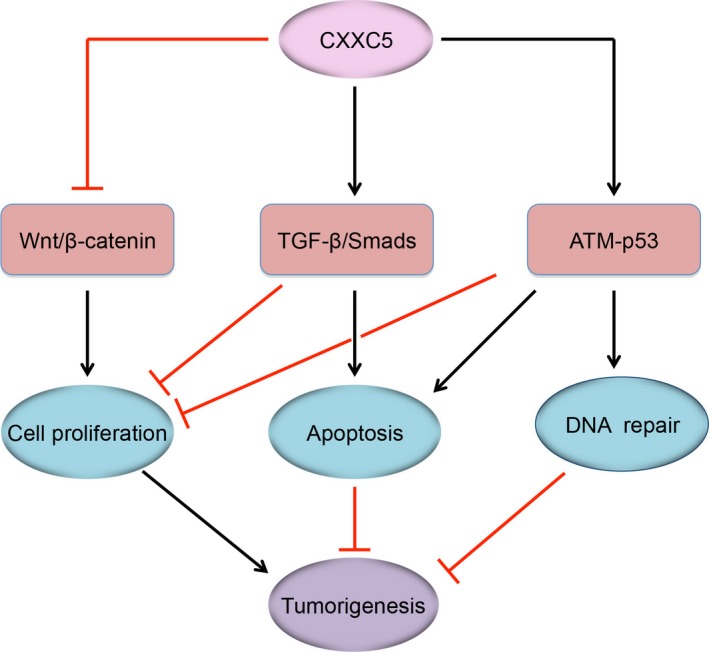
The tumour‐suppressive roles of CXXC5. CXXC5 is able to inhibit leukemia cell proliferation and tumourigenesis by inhibiting Wnt/β‐catenin signaling or facilitating ATM‐p53‐induced DNA damage response. In hepatocellular carcinoma cells, CXXC5 may act as a tumor suppressor by enhancing TGF‐β‐induced cell cycle arrest and apoptosis. TGF‐β, transforming growth factor beta; ATM, ataxia telangiectasia mutated
CXXC5 has also been observed to play a role in some other pathological processes. Aberrant Wnt signaling has been found to be associated with hair loss (alopecia) and anabolic osteoporosis. A previous study demonstrated that expression of CXXC5 is upregulated and is inversely correlated with that of β‐catenin in both haired and bald individuals.85 In mice, CXXC5 knockout or competitive peptide‐mediated disruption of the CXXC5‐Dvl interaction restores Wnt signaling and accelerates hair regrowth and wound‐induced hair follicle neogenesis.85 Similarly, targeting the CXXC5‐Dvl interaction with small molecules has been suggested as a new therapeutic approach for the treatment of anabolic osteoporosis.86, 87
7. PERSPECTIVES
CXXC5 shares a conserved CXXC domain with other CXXC‐type zinc‐finger protein family members, which utilize this domain to recognize unmethylated CpG dinucleotides, especially those within CpG islands in gene promoters. Therefore, the majority of the proteins in this family, including CXXC5, act as transcription factors and epigenetic regulators.1, 2 Intriguingly, CXXC5 is emerging as a pivotal regulator of cellular signaling networks, as it not only receives various signal inputs and changes its expression accordingly, but also regulates and coordinates different signaling pathways. Although much progress has been made in understanding the molecular biology and pathophysiology of CXXC5, several interesting and important questions remain unanswered.
First, although CXXC5 is capable of associating with unmethylated CpG islands via its CXXC domain and regulating the expression of certain genes, the specific genomic sites at which CXXC5 binds to in a given cell type or context remain to be determined.
Second, CXXC5 is regulated by various cytokines and transcription factors and in turn regulates the intensity and duration of several cellular pathways. However, it is unknown whether and how the different signals converge on CXXC5 expression and how CXXC5 coordinates various signaling pathways in a certain circumstance.
Third, the CXXC domain plays a crucial role in CXXC5‐mediated regulation of gene expression or signal transduction. However, as this domain is conserved in other CXXC‐type zinc‐finger proteins, it remains unclear how the functional specificity of CXXC5 is achieved and what functions the amino acids out of the CXXC domain may exert.
Fourth, targeting of CXXC5, for example through inhibition of its interaction with the Dvl protein using competitive peptides or small molecules, is emerging as a promising means of therapeutic intervention in animal models. Given that aberrant CXXC5 expression is involved in various human diseases, especially cancer, it is reasonable to believe that targeting CXXC5 could be an effective clinical approach to treat these diseases.
8. COMPLIANCE WITH ETHICS GUIDELINES
The authors declare that they have no conflicts of interest. This is a review of previous studies and it does not contain any original human or animal studies performed by any of the authors.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (nos. 31671460 and 31871378 to X.H.Y., and no. 81760509 to X.Y.X.), and the Natural Science Foundation of Jiangxi Province of China (no. 20171ACB21004 to X.H.Y. and no. 20181BAB205043 to X.Y.X.).
Xiong X, Tu S, Wang J, Luo S, Yan X. CXXC5: A novel regulator and coordinator of TGF‐β, BMP and Wnt signaling. J. Cell. Mol. Med. 2019;23:740–749. 10.1111/jcmm.14046
REFERENCES
- 1. Blackledge NP, Thomson JP, Skene PJ. CpG island chromatin is shaped by recruitment of ZF‐CxxC proteins. Cold Spring Harb Perspect Biol. 2013;5(11):a018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long HK, Blackledge NP, Klose RJ. ZF‐CxxC domain‐containing proteins, CpG islands and the chromatin connection. Biochem Soc Trans. 2013;41(3):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pendino F, Nguyen E, Jonassen I, et al. Functional involvement of RINF, retinoid‐inducible nuclear factor (CXXC5), in normal and tumoral human myelopoiesis. Blood. 2009;113(14):3172–3181. [DOI] [PubMed] [Google Scholar]
- 4. Ko M, An J, Bandukwala HS, et al. Modulation of TET2 expression and 5‐methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497(7447):122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma S, Wan X, Deng Z, et al. Epigenetic regulator CXXC5 recruits DNA demethylase Tet2 to regulate TLR7/9‐elicited IFN response in pDCs. J Exp Med. 2017;214(5):1471–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aras S, Pak O, Sommer N, et al. Oxygen‐dependent expression of cytochrome c oxidase subunit 4–2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res. 2013;41(4):2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HY, Yang DH, Shin SW, et al. CXXC5 is a transcriptional activator of Flk‐1 and mediates bone morphogenic protein‐induced endothelial cell differentiation and vessel formation. FASEB J. 2014;28(2):615–626. [DOI] [PubMed] [Google Scholar]
- 8. Kim HY, Yoon JY, Yun JH, et al. CXXC5 is a negative‐feedback regulator of the Wnt/beta‐catenin pathway involved in osteoblast differentiation. Cell Death Differ. 2015;22(6):912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee SH, Kim MY, Kim HY, et al. The dishevelled‐binding protein CXXC5 negatively regulates cutaneous wound healing. J Exp Med. 2015;212(7):1061–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan X, Wu J, Jiang Q, Cheng H, Han JJ, Chen YG. CXXC5 suppresses hepatocellular carcinoma by promoting TGF‐beta‐induced cell cycle arrest and apoptosis. J Mol Cell Biol. 2018;10(1):48–59. [DOI] [PubMed] [Google Scholar]
- 11. Andersson T, Sodersten E, Duckworth JK, et al. CXXC5 is a novel BMP4‐regulated modulator of Wnt signaling in neural stem cells. J Biol Chem. 2009;284(6):3672–3681. [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, Wang R, Wang Y, et al. The CXXC finger 5 protein is required for DNA damage‐induced p53 activation. Sci China C Life Sci. 2009;52(6):528–538. [DOI] [PubMed] [Google Scholar]
- 13. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21. [DOI] [PubMed] [Google Scholar]
- 14. Hashimoto H, Vertino PM, Cheng X. Molecular coupling of DNA methylation and histone methylation. Epigenomics. 2010;2(5):657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Zhang X, Blumenthal RM, Cheng X. A common mode of recognition for methylated CpG. Trends Biochem Sci. 2013;38(4):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voo KS, Carlone DL, Jacobsen BM, Flodin A, Skalnik DG. Cloning of a mammalian transcriptional activator that binds unmethylated CpG motifs and shares a CXXC domain with DNA methyltransferase, human trithorax, and methyl‐CpG binding domain protein 1. Mol Cell Biol. 2000;20(6):2108–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ. CpG islands recruit a histone H3 lysine 36 demethylase. Mol Cell. 2010;38(2):179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeltsch A, Jurkowska RZ. Allosteric control of mammalian DNA methyltransferases – a new regulatory paradigm. Nucleic Acids Res. 2016;44(18):8556–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hino S, Kishida S, Michiue T, et al. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl‐binding protein. Mol Cell Biol. 2001;21(1):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim MY, Kim HY, Hong J, et al. CXXC5 plays a role as a transcription activator for myelin genes on oligodendrocyte differentiation. Glia. 2016;64(3):350–362. [DOI] [PubMed] [Google Scholar]
- 21. Li G, Ye X, Peng X, et al. CXXC5 regulates differentiation of C2C12 myoblasts into myocytes. J Muscle Res Cell Motil. 2014;35(5–6):259–265. [DOI] [PubMed] [Google Scholar]
- 22. Tsuchiya Y, Naito T, Tenno M, et al. ThPOK represses CXXC5, which induces methylation of histone H3 lysine 9 in Cd40lg promoter by association with SUV39H1: implications in repression of CD40L expression in CD8+ cytotoxic T cells. J Leukoc Biol. 2016;100(2):327–338. [DOI] [PubMed] [Google Scholar]
- 23. Yan X, Zhang L. A special issue on TGF‐beta signaling: regulation, crosstalk, and biology. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):1–2. [DOI] [PubMed] [Google Scholar]
- 24. Kashima R, Hata A. The role of TGF‐beta superfamily signaling in neurological disorders. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Massague J. TGFbeta in cancer. Cell. 2008;134(2):215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morikawa M, Derynck R, Miyazono K. TGF‐beta and the TGF‐beta family: context‐dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8(5):a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Alexander PB, Wang XF. TGF‐beta family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9(4):a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim KK, Sheppard D, Chapman HA. TGF‐beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2017;10(4):a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song J, Shi W. The concomitant apoptosis and EMT underlie the fundamental functions of TGF‐beta. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):91–97. [DOI] [PubMed] [Google Scholar]
- 30. Nickel J, Ten Dijke P, Mueller TD. TGF‐beta family co‐receptor function and signaling. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):12–36. [DOI] [PubMed] [Google Scholar]
- 31. Yakymovych I, Yakymovych M, Heldin CH. Intracellular trafficking of transforming growth factor beta receptors. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):3–11. [DOI] [PubMed] [Google Scholar]
- 32. Yan X, Xiong X, Chen YG. Feedback regulation of TGF‐beta signaling. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):37–50. [DOI] [PubMed] [Google Scholar]
- 33. Hata A, Chen YG. TGF‐beta signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8(9):a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu P, Lin X, Feng XH. Posttranslational regulation of Smads. Cold Spring Harb Perspect Biol. 2016;8(12):a022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Budi EH, Duan D, Derynck R. Transforming growth factor‐beta receptors and smads: regulatory complexity and functional versatility. Trends Cell Biol. 2017;27(9):658–672. [DOI] [PubMed] [Google Scholar]
- 36. Miyazawa K, Miyazono K. Regulation of TGF‐beta family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol. 2017;9(3):a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hill CS. Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol. 2016;8(10):a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massague J. TGFbeta signalling in context. Nat Rev. 2012;13(10):616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan X, Zhang J, Sun Q, et al. p21‐Activated kinase 2 (PAK2) inhibits TGF‐beta signaling in Madin‐Darby canine kidney (MDCK) epithelial cells by interfering with the receptor‐Smad interaction. J Biol Chem. 2012;287(17):13705–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L, Zhou F, ten Dijke P. Signaling interplay between transforming growth factor‐beta receptor and PI3K/AKT pathways in cancer. Trends Biochem Sci. 2013;38(12):612–620. [DOI] [PubMed] [Google Scholar]
- 41. Zhang YE. Non‐Smad signaling pathways of the TGF‐beta family. Cold Spring Harb Perspect Biol. 2017;9(2):a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morikawa M, Koinuma D, Miyazono K, Heldin CH. Genome‐wide mechanisms of Smad binding. Oncogene. 2013;32(13):1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lonn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGFbeta receptors and Smads. Cell Res. 2009;19(1):21–35. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen LK, Kholodenko BN. Feedback regulation in cell signalling: lessons for cancer therapeutics. Semin Cell Dev Biol. 2016;50:85–94. [DOI] [PubMed] [Google Scholar]
- 45. Yan X, Chen YG. Smad7: not only a regulator, but also a cross‐talk mediator of TGF‐beta signalling. Biochem J. 2011;434(1):1–10. [DOI] [PubMed] [Google Scholar]
- 46. Yan X, Liao H, Cheng M, et al. Smad7 protein interacts with receptor‐regulated Smads (R‐Smads) to inhibit transforming growth factor‐beta (TGF‐beta)/Smad signaling. J Biol Chem. 2016;291(1):382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Z, Fan Y, Xie F, et al. Breast cancer metastasis suppressor OTUD1 deubiquitinates SMAD7. Nat Commun. 2017;8(1):2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yan X, Pan J, Xiong W, et al. Yin Yang 1 (YY1) synergizes with Smad7 to inhibit TGF‐beta signaling in the nucleus. Sci China Life Sci. 2014;57(1):128–136. [DOI] [PubMed] [Google Scholar]
- 49. Wang X, Liao P, Fan X, et al. CXXC5 associates with Smads to mediate TNF‐alpha induced apoptosis. Curr Mol Med. 2013;13(8):1385–1396. [DOI] [PubMed] [Google Scholar]
- 50. Peng X, Li G, Wang Y, et al. CXXC5 is required for cardiac looping relating to TGFbeta signaling pathway in zebrafish. Int J Cardiol. 2016;214:246–253. [DOI] [PubMed] [Google Scholar]
- 51. Clevers H, Nusse R. Wnt/beta‐catenin signaling and disease. Cell. 2012;149(6):1192–1205. [DOI] [PubMed] [Google Scholar]
- 52. Wiese KE, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Development. 2018;145(12):dev165902. [DOI] [PubMed] [Google Scholar]
- 53. Majidinia M, Aghazadeh J, Jahanban‐Esfahlani R, Yousefi B. The roles of Wnt/beta‐catenin pathway in tissue development and regenerative medicine. J Cell Physiol. 2018;233(8):5598–5612. [DOI] [PubMed] [Google Scholar]
- 54. Song X, Wang S, Li L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell. 2014;5(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gammons M, Bienz M. Multiprotein complexes governing Wnt signal transduction. Curr Opin Cell Biol. 2018;51:42–49. [DOI] [PubMed] [Google Scholar]
- 56. Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22(5):717–727. [DOI] [PubMed] [Google Scholar]
- 57. van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. 2012;4(10):a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tortelote GG, Reis RR, de Almeida MF, Abreu JG. Complexity of the Wnt/betacatenin pathway: searching for an activation model. Cell Signal. 2017;40:30–43. [DOI] [PubMed] [Google Scholar]
- 59. London TB, Lee HJ, Shao Y, Zheng J. Interaction between the internal motif KTXXXI of Idax and mDvl PDZ domain. Biochem Biophys Res Commun. 2004;322(1):326–332. [DOI] [PubMed] [Google Scholar]
- 60. Michiue T, Fukui A, Yukita A, et al. XIdax, an inhibitor of the canonical Wnt pathway, is required for anterior neural structure formation in Xenopus. Dev Dyn. 2004;230(1):79–90. [DOI] [PubMed] [Google Scholar]
- 61. Kim MS, Yoon SK, Bollig F, et al. A novel Wilms tumor 1 (WT1) target gene negatively regulates the WNT signaling pathway. J Biol Chem. 2010;285(19):14585–14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130(4):597–600. [DOI] [PubMed] [Google Scholar]
- 63. Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–1679. [DOI] [PubMed] [Google Scholar]
- 64. Khanna KK, Keating KE, Kozlov S, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20(4):398–400. [DOI] [PubMed] [Google Scholar]
- 65. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)‐mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non‐genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–559. [DOI] [PubMed] [Google Scholar]
- 66. Marshall PA, Hernandez Z, Kaneko I, et al. Discovery of novel vitamin D receptor interacting proteins that modulate 1,25‐dihydroxyvitamin D3 signaling. J Steroid Biochem. 2012;132(1–2):147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Palmer HG, Gonzalez‐Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E‐cadherin and the inhibition of beta‐catenin signaling. J Cell Biol. 2001;154(2):369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Egan JB, Thompson PA, Vitanov MV, et al. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta‐catenin activity in colon cancer cells. Mol Carcinogen. 2010;49(4):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci USA. 2007;104(9):3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. L'Hote D, Georges A, Todeschini AL, et al. Discovery of novel protein partners of the transcription factor FOXL2 provides insights into its physiopathological roles. Hum Mol Genet. 2012;21(14):3264–3274. [DOI] [PubMed] [Google Scholar]
- 71. Kuhnl A, Valk PJ, Sanders MA, et al. Downregulation of the Wnt inhibitor CXXC5 predicts a better prognosis in acute myeloid leukemia. Blood. 2015;125(19):2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Treppendahl MB, Mollgard L, Hellstrom‐Lindberg E, Cloos P, Gronbaek K. Downregulation but lack of promoter hypermethylation or somatic mutations of the potential tumor suppressor CXXC5 in MDS and AML with deletion 5q. Eur J Haematol. 2013;90(3):259–260. [DOI] [PubMed] [Google Scholar]
- 73. Guo X, Wang XF. Signaling cross‐talk between TGF‐beta/BMP and other pathways. Cell Res. 2009;19(1):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Attisano L, Wrana JL. Signal integration in TGF‐beta, WNT, and Hippo pathways. F1000Prime Reports. 2013;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Greif PA, Dufour A, Konstandin NP, et al. GATA2 zinc finger 1 mutations associated with biallelic CEBPA mutations define a unique genetic entity of acute myeloid leukemia. Blood. 2012;120(2):395–403. [DOI] [PubMed] [Google Scholar]
- 77. Devaney JM, Wang S, Funda S, et al. Identification of novel DNA‐methylated genes that correlate with human prostate cancer and high‐grade prostatic intraepithelial neoplasia. Prostate Cancer Prostatic Dis. 2013;16(4):292–300. [DOI] [PubMed] [Google Scholar]
- 78. Zhang G, Hoersch S, Amsterdam A, et al. Comparative oncogenomic analysis of copy number alterations in human and zebrafish tumors enables cancer driver discovery. PLoS Genet. 2013;9(8):e1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cui Z, Shen Y, Chen KH, Mittal SK, Yang JY, Zhang G. KANK1 inhibits cell growth by inducing apoptosis though regulating CXXC5 in human malignant peripheral nerve sheath tumors. Sci Rep. 2017;7:40325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–2515. [DOI] [PubMed] [Google Scholar]
- 81. Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yasar P, Ayaz G, Muyan M. Estradiol‐Estrogen Receptor alpha mediates the expression of the CXXC5 gene through the estrogen response element‐dependent signaling pathway. Sci Rep. 2016;6:37808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan S, Li H, Zhang W, et al. NUDT21 negatively regulates PSMB2 and CXXC5 by alternative polyadenylation and contributes to hepatocellular carcinoma suppression. Oncogene. 2018;37(35):4887–4900. [DOI] [PubMed] [Google Scholar]
- 84. Knappskog S, Myklebust LM, Busch C, et al. RINF (CXXC5) is overexpressed in solid tumors and is an unfavorable prognostic factor in breast cancer. Ann Oncol. 2011;22(10):2208–2215. [DOI] [PubMed] [Google Scholar]
- 85. Lee SH, Seo SH, Lee DH, Pi LQ, Lee WS, Choi KY. Targeting of CXXC5 by a competing peptide stimulates hair regrowth and wound‐induced hair neogenesis. J Invest Dermatol. 2017;137(11):2260–2269. [DOI] [PubMed] [Google Scholar]
- 86. Kim HY, Choi S, Yoon JH, et al. Small molecule inhibitors of the Dishevelled‐CXXC5 interaction are new drug candidates for bone anabolic osteoporosis therapy. EMBO Mol Med. 2016;8(4):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ma S, Choi J, Jin X, et al. Discovery of a small‐molecule inhibitor of Dvl‐CXXC5 interaction by computational approaches. J Comput Aided Mol Des. 2018;32(5):643–655. [DOI] [PubMed] [Google Scholar]


