Abstract
In this paper we examine spending by privately insured patients with four conditions often treated with specialty drugs: cancer, kidney disease, rheumatoid arthritis, and multiple sclerosis. Despite having employer-sponsored health insurance, these patients face substantial risk for high out-of-pocket spending. In contrast to traditional pharmaceuticals, we find that specialty drug use is largely insensitive to cost sharing, with price elasticities ranging from 0.01 to 0.21. Given the expense of many specialty drugs, care management should focus on making sure that patients who will most benefit receive them. Once such patients are identified, it makes little economic sense to limit coverage.
Introduction
The adoption of multi-tiered formularies and other cost-control mechanisms such as prior authorization requirements, mandatory generic substitution, and mail-order pharmacies have helped slow the growth in outpatient prescription drug spending, from 16 percent in 2000 to 8 percent in 2004.1 While demand is still increasing, employers and health plan sponsors are much less concerned about runaway spending on oral medications as they were just several years earlier.
By contrast, the demand for specialty drugs—which include most injectibles and biologic agents-continues to accelerate. Biotechnology-derived agents target a gene or protein and typically are injected or infused. They are often used to treat complex, chronic conditions such as anemia, cancer, growth hormone deficiency, and multiple sclerosis. Many of these agents provide highly sophisticated treatment for which there are few other viable treatment options, but at prices that can be substantially higher than traditional medications. Since only a small percentage of health plan members are afflicted with these conditions, the total population of specialty drug users is quite small, ranging from 1 to 5 percent of members in a typical health plan.2 However, costs of specialty drugs are expected to increase substantially in the near future as new drugs enter the market for the treatment of diabetes, osteoporosis, and rheumatoid arthritis – diseases that affect much larger populations.
Given the growth in both the number of products available and their expense, many insurers are contemplating a variety of payment and distribution strategies to control their use and costs. At the same time, the high cost of specialty drugs, usually combined with other expenses that are associated with treating a chronic condition, means many users are at financial risk for high out-of-pocket expenses. Thus the challenge lies in how to best manage the use of these drugs to ensure appropriate and affordable access.
In this paper, we use data from more than 50 health plans to document the variability in coverage of specialty pharmaceuticals and the consequences for plan spending and patients’ out-of-pocket expenses. Our analyses focus on four diseases where treatment with specialty products is common—cancer, kidney disease, rheumatoid arthritis, and multiple sclerosis. We examine how responsive specialty use is to changes in benefit design, and contrast this demand curve to traditional oral agents.
Methods
First, we aggregated spending on specialty drugs covered under the medical and pharmacy benefit. Second, we computed an index of plan generosity and examined the relationship between cost-sharing and spending. The salient details are discussed below. Additional detail on our methods and results are contained in a technical appendix, published as an online supplement to this paper.3
Data
We assembled pharmacy and medical claims from 55 health plans offered by 15 large employers in 2003 and 2004.4 The data cover 1,471,574 beneficiaries (n=2,344,127 person-years) continuously enrolled in a plan for an entire year. For this study, we restricted our attention to patients with at least two primary diagnoses for cancer, kidney disease, rheumatoid arthritis, or multiple sclerosis as indicated by ICD codes. These four conditions were selected because they are chronic diseases that are commonly treated with specialty drugs. For this study, kidney disease was defined as having chronic renal insufficiency, anemia, or end-stage renal disease.5 For cancer patients, we included spending on renal-related agents as well as chemotherapeutic agents to account for the relatively large fraction of patients taking specialty products for anemia. The claims captured all health care claims and encounters, including prescription drugs, inpatient, emergency, and ambulatory services. Most drug claims include information on the type of drug, drug name, national drug code, dosage, days supplied, and place of purchase (retail or mail-order). The medical claims included the date of service, diagnosis and procedure codes, type of facility and provider.
Use of specialty drugs.
Historically, injectable medications have been administered by a physician or nurse in a clinical setting and covered under the medical benefit. As such, medical benefit plan designs were intended to compensate physicians for professional services related to the administration of injectable medications, as well as to reimburse them for the cost of those medications. Specific medication costs are not identified and, for the patient, coverage typically involves a single copayment for each physician office visit. However, many newer injectibles can be administered by the patient at home and accessed though physicians, community pharmacists, or mail-order pharmacies. In addition, specialty drugs paid through the major medical benefit are 20% to 30% more expensive on average than those paid through the pharmacy benefit.6 As a result, more specialty drugs are moving under the pharmacy benefit and traditional cost-control measures are being applied, such as bulk purchasing for best product price, copayments, closer scrutiny of utilization and outcomes.
We used medical claims to identify use of specialty products from physicians’ offices, home care agencies, and outpatient facilities such as outpatient hospital clinics. All claim records were scanned to flag whether any prescription drug was administered; we then used the Healthcare Common Procedure Coding System or the Current Procedural Terminology code to identify the biologic agents. (For example, a code of J0880 refers to an injection of darbepoetin alfa.) To identify biologic agents distributed through retail and mail order pharmacies, we constructed lists of all the products associated with any HCPCS code, and then searched for pharmacy claims using the drug names and national drug codes.
Plan generosity towards specialty drugs
Our main interest was to estimate how use of specialty drugs responds to cost-sharing. But one cannot infer how generously a plan will cover specialty drugs—or any drug for that matter—merely by looking at its stated medical or pharmacy benefits. Multi-tier formularies are now the standard, and they offer discounts for purchases through mail-order or in-network pharmacies. Deductibles, out-of-pocket maximums, and benefit caps also complicate these calculations. These added complexities mean that the price a consumer will pay for a given drug depends not only on its tier, but also on where it is dispensed and at what time of year. In the case of biologic agents, this issue is further confounded because many of the products are administered by a nurse or physician and paid as part of medical services.
As a consequence, we measure plan generosity as the ratio of total out-of-pocket payments for certain categories of specialty drugs relative to total payments. So, for example, when we examine use of rheumatoid arthritis (RA), we compute the ratio of total out-of-pocket payments for RA-related specialty drugs divided by their total cost. Plans with higher cost-sharing are less generous by construction.7 Since use of some of the specialty drugs is rare, the estimated cost-sharing rates can be quite variable across plans and (rarely) include 0% and 100%. We conducted additional analysis based on two cutoffs for plan size; that is, we ran models restricting our attention to plans with at least 10 and then 100 members who used each class of specialty products in that year. The results in general were not sensitive to this exclusion restriction, nor were they sensitive to models that weighted by the number of patients in the plan with the condition.
Other factors affecting specialty drug use
Our models included controls for patient characteristics available in claims data: age, gender, and work status of the sponsor (active or retired), and status (primary beneficiary or dependent). Because the information in claims data are limited, we included socioeconomic measures that are likely to influence the demand and supply of specialty drugs such as urban residence and median household income in the zip code of residence. We controlled for the most common comorbid conditions based on the presence of ICD-9 diagnostic codes in the medical claims: hypertension, chronic heart failure, diabetes, asthma, lipid disorder, depression, arthritis, migraine, and gastric acid disorder.
Statistical analysis
Our analyses used a two-part model for each of the four conditions (cancer, kidney disease, multiple sclerosis, and rheumatoid arthritis). The first part of the model, including all patients with the sentinel conditions, used probit regression to estimate the probability that a member used any specialty drug. The second part of the model used a generalized linear model with a logarithmic link function and normally distributed errors to estimate the level of drug spending among members with at least some use. We chose the generalized linear model because it predicted specialty drug expenditures better than the standard two-part model, but our conclusions are insensitive to this choice.
For each disease, we used the results from the two-part model to estimate a price elasticity of use, as well as an overall elasticity on spending. We used estimates from the first part of the model to predict the probability of specialty use for each person with the condition at the first and third quartiles of plan generosity. We used the second part of the model to predict spending conditional upon having at least one claim. Total spending was predicted using the product of the two. The predictions were then averaged over all individuals in that disease group and an (arc) elasticity was computed.8
Results
Exhibit 1 shows the most commonly used specialty drugs. They include treatments for cancer, rheumatoid arthritis (RA), anemia, psoriasis, multiple sclerosis (MS) and other disorders. The expense of some of them is apparent. For example, total spending in 2004 for etanercept (Enbrel®), a treatment for rheumatoid arthritis and psoriasis, was $16 million or about $10,000 per user. Spending on leuprolide acetate (Lupron®) for prostate cancer totaled $6.3 million for 1,943 users, or about $3,200 annually per user. The 17 hemophiliacs taking Recombinant Factor VIII spent more than $1.7 million on the drug for an average of more than $100,000 per user. This extreme example highlights two defining characteristics of specialty pharmaceuticals; they are used less frequently but are more expensive than typical pharmaceutical treatments.
Exhibit 1.
Most common specialty products used in 2004
| Product | Total Spending | No. of users | Primary Use |
|---|---|---|---|
| Etanercept injection | 16,212,909 | 1,532 | a tumor-necrosis factor (TNF) inhibitor for reducing inflammation in rheumatoid arthritis |
| Darbepoetin alfa injection | 9,079,720 | 1,275 | an erythropoiesis-stimulating protein used to treat anemia in patients with kidney disease or undergoing chemotherapy |
| Interferon beta-1a | 6,815,551 | 634 | an immunomodulator for relapse-remitting MS to decrease the frequency of clinical exacerbations and delay the accumulation of physical disability |
| Rituximab | 6,556,596 | 260 | a monoclonal antibody therapy used to target cancer cells in non-Hodgkins lymphoma patients; also used for RA |
| Infliximab | 6,513,118 | 449 | a TNF inhibitor used in conjunction with first-line drugs to reduce inflammation in rheumatoid arthritis; also used to treat Crohn’s disease |
| pegfilgrastim | 6,412,737 | 604 | a colony stimulating factor that stimulates production of white blood cells in patients receiving anti-cancer drugs |
| Leuprolide acetate suspension | 6,287,387 | 1,943 | a gonadotropin-releasing (LH-RH) hormone analog to stop production of testosterone/estrogen to stop growth of diseased cells involved in prostate cancer and endometriosis |
| IV immune globulin | 5,516,199 | 160 | an immunizing agent to prevent or reduce the severity of certain infections in patients at an increased risk from infection |
| Paclitaxel | 3,549,327 | 396 | an antineoplastic used to target cancer cells in patients with metastatic breast/ovarian cancer and some lung cancers |
| Mycophenolate mofetil oral | 3,276,388 | 825 | an immunosuppressant used in combination with other medications to keep bodies from attacking and rejecting transplanted organs |
| Docetaxel | 2,914,423 | 279 | an antineoplastic used to target cancer cells in patients with breast cancer, prostate cancer or non-small cell lung cancer |
| Oxaliplatin | 2,835,209 | 130 | an antineoplastic used to target cancer cells in patients with cancer of the colon or rectum |
| Interferon beta-1b | 2,831,988 | 249 | an immunomodulator for relapse-remitting MS to decrease the frequency of clinical exacerbations and delay the accumulation of physical disability |
| Tacrolimus | 2,692,113 | 541 | an immunosuppressive agent used to prevent the body from rejecting a transplanted organ |
| Carboplatin injection | 2,455,462 | 375 | an alkylating agent that targets cancer cells for the treatment of ovarian cancer |
| Filgrastim | 2,422,763 | 405 | a growth factor used decrease the chance and the duration of problems due to low white blood cell counts |
| Zoledronic acid | 1,838,856 | 298 | a bisphosphonate used to slow bone breakdown and decrease the amount of calcium released from the bones into the blood in patients with cancer-related hypercalcemia, multiple myeloma, or certain types of bone metastases |
| Factor viii recombinant | 1,723,558 | 17 | a synthetic protein that activates substances in blood to form clots and decrease bleeding episodes in patients with haemophilia A |
| Gemcitabine HCl | 1,686,354 | 187 | a pyrimidine analog that slows or stops the growth of cancer cells for patients with adenocarcinoma of the pancreas |
| Trastuzumab | 1,595,758 | 56 | a monoclonal antibody therapy used to target cancer cells in patients with metastatic breast cancer whose tumors overexpress the HER2 protein |
| Goserelin acetate implant | 1,557,985 | 614 | a 3-month implant of a synthetic hormone that decreases the amount of estrogen/estradiol and testosterone in the blood in patients with prostate cancer, endometriosis, and certain breast cancers in pre- and perimenopausal women |
| Irinotecan injection | 1,444,407 | 92 | an antineoplastic used to target cancer cells in patients with colon or rectal cancer |
| Methotrexate sodium | 1,136,904 | 4,835 | an antimetabolite used to slow the growth of certain cells in patients with cancer, rheumatoid arthritis, and psoriasis |
| Granisetron HCl injection | 1,061,438 | 733 | a 5-HT3 medication given for prevention of chemotherapy-induced nausea and for pre- and post-surgical nausea and vomiting |
| Doxorubic HCl | 1,028,738 | 386 | an antineoplastic used to target cancer cells for patients with a variety of cancers |
| Dolasetron mesylate | 979,597 | 661 | A serotonin antagonist to prevent nausea and vomiting that may be caused by cancer chemotherapy or anesthesia |
| Pamidronate disodium | 910,244 | 186 | a second-generation bisphosphonate used to inhibit bone resorption for the treatment of tumor-related hypercalcemia, bone metastases and Paget’s disease |
| Methylprednisolone | 872,137 | 54,413 | A corticosteroid to relieve inflammation from certain forms of arthritis; skin, blood, kidney, eye, thyroid, and intestinal disorders; and multiple sclerosis |
| Sirolimus | 793,487 | 160 | An immunosuppressive agent used to prevent the body from rejecting a transplanted organ |
| Epoetin alfa, ESRD use | 635,236 | 46 | An erythropoiesis stimulating protein that causes bone marrow to make more red blood cells for the treatment of anemia associated with kidney failure |
Source: Authors’ calculations from claims data, 2004.
Exhibit 2 shows the characteristics of the patient populations in 2004 compared with the general covered population. Patients with cancer are much older (average=68 years), as one would expect given the prevalence profile. Patients with multiple sclerosis tend to be younger than the other conditions. Only 14% of these patients are over the age 65, and half of them are currently working. Patients with RA and kidney disease tend to fall somewhere between these extremes. The patients with cancer, kidney disease, and rheumatoid arthritis have more co-morbid conditions than those with multiple sclerosis or the population-at-large. For example, these patients have higher rates of heart disease, diabetes, lipid disorders, and hypertension. Patients with multiple sclerosis most resemble those in the general population in terms of their co-morbidity profile, with the notable exception that they are more likely to have migraines and depression.
Exhibit 2.
Sample characteristics, 2004 only
| Kidney | Rheumatoid | Multiple | |||
|---|---|---|---|---|---|
| Characteristic | All | Cancer | Disease | Arthritis | Sclerosis |
| No. of patients | 1,219,078 | 42,997 | 45,068 | 9,066 | 2,537 |
| Demographics | |||||
| Age | 46.72 | 67.68 | 61.88 | 61.17 | 52.20 |
| Aged 65 or older | 0.25 | 0.63 | 0.49 | 0.42 | 0.14 |
| Male | 0.48 | 0.50 | 0.41 | 0.28 | 0.24 |
| Income ($ thousands)** | 31.52 | 31.07 | 30.87 | 30.64 | 31.61 |
| Married | 0.55 | 0.69 | 0.61 | 0.67 | 0.69 |
| Currently working | 0.58 | 0.18 | 0.31 | 0.31 | 0.50 |
| Primary beneficiary | 0.51 | 0.70 | 0.66 | 0.60 | 0.59 |
| Health conditions | |||||
| Cancer | 0.035 | 1.000 | 0.125 | 0.066 | 0.038 |
| Chronic renal insufficiency | 0.010 | 0.031 | 0.282 | 0.026 | 0.013 |
| Anemia | 0.029 | 0.110 | 0.778 | 0.104 | 0.062 |
| End-stage renal disease | 0.001 | 0.003 | 0.031 | 0.001 | 0.001 |
| Rheumatoid arthritis | 0.007 | 0.014 | 0.024 | 1.000 | 0.013 |
| Multiple sclerosis | 0.002 | 0.002 | 0.004 | 0.004 | 1.000 |
| Hypertension | 0.139 | 0.314 | 0.352 | 0.274 | 0.154 |
| Heart disease | 0.182 | 0.418 | 0.484 | 0.356 | 0.196 |
| Diabetes | 0.056 | 0.121 | 0.209 | 0.091 | 0.052 |
| Asthma | 0.020 | 0.024 | 0.034 | 0.041 | 0.022 |
| Lipid disorder | 0.054 | 0.110 | 0.098 | 0.090 | 0.057 |
| Depression | 0.030 | 0.035 | 0.056 | 0.050 | 0.091 |
| Arthritis | 0.039 | 0.092 | 0.115 | 0.175 | 0.041 |
| Gastric disorder | 0.025 | 0.053 | 0.076 | 0.060 | 0.040 |
| Migraine | 0.007 | 0.006 | 0.011 | 0.013 | 0.028 |
| Lung disease | 0.008 | 0.025 | 0.028 | 0.022 | 0.007 |
| Total spending | |||||
| Medical | 4,578 | 23,041 | 25,925 | 13,529 | 10,784 |
| Drug | 1,460 | 5,200 | 5,293 | 5,793 | 9,783 |
| Total | 6,038 | 28,241 | 31,218 | 19,321 | 20,567 |
| Out-of-pocket spending | |||||
| Medical | 1,371 | 7,241 | 7,756 | 3,919 | 2,408 |
| Drug | 316 | 1,170 | 1,122 | 892 | 893 |
| Total | 1,687 | 8,411 | 8,878 | 4,811 | 3,301 |
Subsequent analyses use combined 2003 and 2004 samples.
Median household income in three-digit zip code of residence from the 1990 census
Source: Authors’ calculations from claims data, 2004.
Patients with the four sentinel conditions are clearly expensive. Average annual medical spending for these patients ranges from $19,321 (RA) to $31,218 (kidney disease) compared with costs of only $6,038 for the average beneficiary. Patients also face a financial burden averaging between $3,301 (MS) and $8,878 (kidney disease) in out-of-pocket expenses.
Exhibit 3 takes a closer look at the financial burden faced by these patients. To get a better estimate of the tails of the distribution, spending in 2003 is included in the distribution. (Spending figures for 2003 are not adjusted for inflation, but such an adjustment would not materially affect the results.) All of these patients are privately-insured through large employers, and so one would expect the coverage to be generous. Despite this fact, it is clear that patients with these diseases are still at-risk for substantial spending. More than 10% of patients with cancer have out-of-pocket costs that exceed $18,585 in a year; and 5% have costs that exceed $35,660. A similar pattern holds for patients with kidney disease, and, to a lesser extent, patients with RA. Patients with MS are at less risk with a 95th percentile of $9,000.
Exhibit 3.
Distribution of out-of-pocket spending for all beneficiaries and those with selected conditions, 2003–2004
| Percentiles | |||||
|---|---|---|---|---|---|
| Disease | Median | 75th | 90th | 95th | 99th |
| All Out-of-Pocket Spending | |||||
| Multiple Sclerosis | 1,185 | 2,465 | 5,116 | 9,092 | 42,830 |
| Rheumatoid Arthritis | 1,208 | 2,874 | 8,777 | 17,450 | 52,343 |
| Cancer | 1,509 | 5,097 | 18,585 | 35,660 | 91,381 |
| Kidney Disease | 1,313 | 4,385 | 18,324 | 36,603 | 100,303 |
| Medical Services Only | |||||
| Multiple Sclerosis | 587 | 1,327 | 3,496 | 7,319 | 38,211 |
| Rheumatoid Arthritis | 628 | 1,772 | 7,122 | 15,417 | 49,556 |
| Cancer | 989 | 4,081 | 16,385 | 32,532 | 84,643 |
| Kidney Disease | 769 | 3,205 | 16,450 | 33,760 | 95,068 |
| Drugs Only | |||||
| Multiple Sclerosis | 436 | 852 | 1,749 | 2,778 | 5,284 |
| Rheumatoid Arthritis | 446 | 816 | 1,586 | 2,542 | 6,407 |
| Cancer | 336 | 677 | 1,441 | 2,576 | 12,416 |
| Kidney Disease | 386 | 763 | 1,533 | 2,551 | 9,995 |
Source: Authors’ calculations from claims data, 2003–2004.
Given the high cost of specialty products, it is worth considering to what extent the financial risk for these conditions is generated by drug spending. The lower panels of Exhibit 3 show the distributions by medical and drug spending separately. A close inspection shows that the risks associated with medical spending is much higher than for drugs. The 95th percentile for out-of-pocket drug spending for the four conditions is around $2,500, whereas out-of-pocket medical spending can be as high as $33,760 for kidney disease.
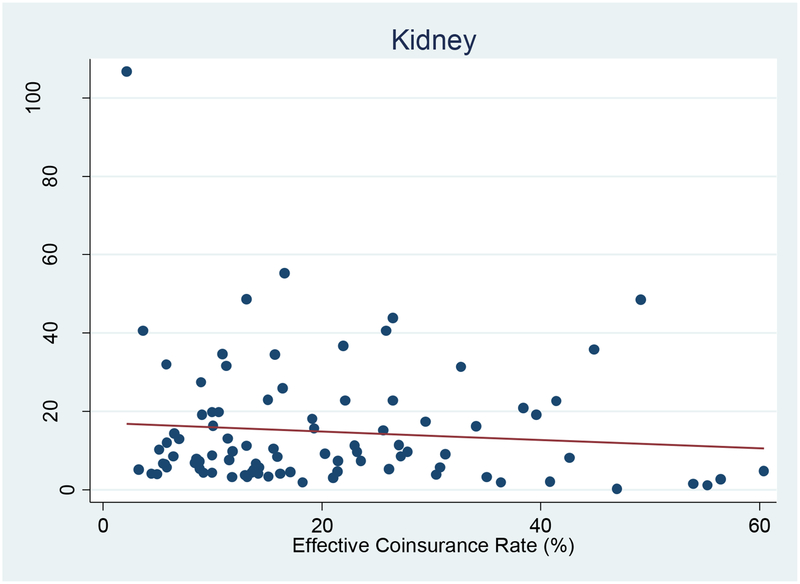
The long tails in out-of-pocket spending suggest that these patients face substantial cost-sharing for some of their service use. This raises a question of whether cost-sharing discourages use of specialty products. Our analysis uses variation in coverage generosity across health plans and over time (2003–2004) to identify how cost-sharing affects specialty drug use for each patient population. Exhibit 4 provides a useful heuristic for our analysis. Each point on the plot shows the relationship between plan generosity and spending on kidney-related specialty products. Our measure of plan generosity is the effective coinsurance rate for kidney-related specialty products, as described earlier. As shown by the regression line, each one percentage point increase in the effective coinsurance rate for kidney-related specialty drugs leads to an insignificant $0.11 reduction in per patient spending (p=0.39), or $0.25 (p=0.09) when weighted by the number of users in the plan. Thus, there does not appear to be a strong relationship between plan generosity and use of specialty drugs by kidney disease patients. (There is one influential outlier with a low coinsurance rate and very high spending; excluding this point would only serve to “flatten the regression line” and make the relationship even less strong.) Of course, other factors could bias this finding; for example, if patients in the more generous plans had fewer co-morbid conditions. Thus, we ran multivariate models of individual use that control for other observable factors.
Exhibit 4. Effective coinsurance rate for kidney-related products and spending, 2003–2004 (n=90 plans).
Source: Authors’ calculations from claims data, 2003–2004.
Exhibit 5 summarizes the findings from our multivariate models. Because our measure of plan generosity is an average coinsurance rate, we report the effects of plan generosity as an elasticity which can be interpreted as the percentage change in spending (or use) associated with a 1 percent increase in effective coinsurance rates. (The actual parameter estimates are available upon request.) So, for example, if a plan were to double cost-sharing for RA-related specialty drugs, our models indicate that overall spending on RA drugs would fall by 21% (p<.05). For cancer drugs, however, spending would be reduced by only 1%. Using our two part model, we can also compute the elasticity of whether patients use any drugs at all and the amount of conditional spending. In fact, we find that coinsurance does not significantly affect the level of spending at all once a patient initiates specialty drug use. What is most striking about these results is how inelastic demand is—that is, how insensitive patients are to price—in comparison to traditional pharmaceuticals where it is not uncommon to observe responses of 30% to 50% when copayments double.
Exhibit 5.
Relationship between price changes and use of pharmaceuticals
| Condition | Overall elasticity of speciality drug spending | Elasticity of any specialty drug use | Elasticity of conditional spending | ||
|---|---|---|---|---|---|
| Rheumatoid arthritis | −0.21 | ** | −0.05 | *** | −0.16 |
| Kidney disease | −0.11 | −0.06 | −0.03 | ||
| Multiple sclerosis | −0.07 | ** | −0.03 | *** | −0.05 |
| Cancer | −0.01 | −0.10 | * | 0.11 |
p<0.10;
p<.05;
p<.01
Source: Authors’ calculations from claims data, 2003–2004.
Sensitivity Analysis
One possible explanation why we observe inelastic responses is that our principal measure of plan generosity is measured with error, biasing our estimates towards zero. To test this, we instrumented for the effective coinsurance rate for specialty drugs with an identically constructed rate for non-specialty drugs. The estimated price elasticities generally moved towards zero when we used this instrument (for example, the conditional elasticity goes from −0.16 to −0.04 for rheumtatoid arthritis). This suggests that the inelastic responses we observed in the data were not driven by measurement error in the key independent variable.
We also examined the sensitivity of our findings to alternative specifications. As shown in the Technical Appendix, excluding binary indicators for comorbid conditions or plan type (HMO, PPO, POS, FFS) had little impact on the estimated elasticities. Similarly, the use of medical plan characteristics (deductibles and copayments) instead of an index of average medical generosity did not change our conclusion that demand for these products is inelastic.
Discussion
As spending on specialty drugs increases, benefit managers’ interest in monitoring and containing their utilization has intensified. Plans that cover physician-administered injectibles under their medical benefit are starting to move them to their pharmacy benefit, where they can be more easily subjected to the same utilization management as tablets and capsules. Further, health plans that cover these drugs under their pharmacy plan are increasingly requiring consumers to share the costs of high-cost drugs via coinsurance rather than copayments. For example, some plans may require beneficiaries to pay 25% coinsurance for high-cost drugs, with a maximum out-of-pocket expense of $1,000 per year. While existing specialty drugs treat diseases of relatively low prevalence, newer biologic products are aimed at much larger patient populations such as diabetics and asthmatics. Demand for these products may not be as inelastic as what we observed in this study.
Insurance markets work best when there is the chance of substantial loss, when that loss is sufficiently rare and uncertain, and the presence of coverage will not alter behavior much.9 Viewed this way, specialty drugs would appear to warrant greater, not less coverage than traditional pharmaceutical agents.10 It is worth considering each of these principles separately.
Use is rare and uncertain
Risk pools function best when many people contribute premiums to fund the occasional loss. Fire insurance is a useful example. In contrast, traditional oral agents fail this test. In our employer-based database with over 1.2 million covered lives, more than 70% of members filled at least one prescription in 2004. Thus, the use of pharmaceuticals is the rule rather than the exception. Furthermore, many of the most common classes of medications—including treatments for cholesterol, high blood pressure, and diabetes—are chronic medications that are taken in known quantities over long periods and perhaps a lifetime. There is little uncertainty inherent in their use. People purchase the drugs at known intervals in a 30-day or 90-day supply and the price is (or at least could be) known without much upside fluctuation. As we have documented here, though, use of specialty products is much lower—around 1 or 2% of the insured population.11 It is also the case that many of these products are taken for short periods of time, and only when a chronic disease invokes extreme symptoms. A clear manifestation of the uncertainty is that it is very difficult to predict who will use biologic agents and with what level of compliance.
Specialty drugs involve substantial losses.
Insurance has some costs associated with it, so people do not value insurance against small losses. The real value arises when the risk is catastrophic. While traditional oral agents can be expensive, most of them will not result in catastrophic spending. Whereas 17% of all beneficiaries had medical spending that exceeded $5,000 in 2004, only 7% had pharmaceutical costs above that limit, and when they did it was often because they used biologic agents. On the other hand, our results demonstrate that patients using specialty drugs can face extreme financial burden not just for their biologic products but across the entire constellation of health care services.
Demand is relatively inelastic
One of the fundamental problems with insurance is that it can induce people to either behave in a risky manner or to consume care of little value. Conversely, if one can identify medical services where people use the same amount, irrespective of price, then this type of care is a good candidate for coverage. The RAND Health Insurance Experiment (HIE) randomly enrolled over 2,700 families into health insurance plans that ranged from free care to 95% coinsurance. The results definitively demonstrated that when people have to pay for more of their care out of their own pockets, they will use fewer medical services. But the type of service matters. Demand for inpatient and outpatient care was the least elastic, whereas use of dental and mental health services were most responsive to changes in the copayment.12 This finding goes a long way towards explaining why virtually every health insurer covers hospital and ambulatory care, but not necessarily these other services. More evidence has convincingly shown that demand for prescription drugs is elastic as well. Our own work suggests that doubling copayments in the most common plans will reduce spending by about 33%. But this result does not carry over to specialty drugs. Our findings suggest much less elastic price responses of between 1% and 21%. These results imply that changes in demand have small effects on use of these services, a point highlighted by Exhibit 4.
Welfare Effects.
With some health care services, such as physician services, the high prices induced by insurance can be viewed as waste in the sense that they transfer money from insurance beneficiaries to health care providers (although doctors might object to calling it “waste”). Pharmaceuticals are different, however, in two key ways. First, they typically are inexpensive to produce—i.e., involve low marginal costs—so excess consumption is not an economic problem (although it may be a clinical worry). The fact that someone takes another pill will not cost society much in the way of resources, whereas an extra bypass surgery does. Second, the high prices of pharmaceuticals reflect a necessary reward to pharmaceutical innovation. Without monopoly pricing, society would have to find some other way to ensure future innovation—perhaps through processes like patent buyouts or direct government investment in drug development.13 In fact, while pharmaceutical prices appear high relative to marginal cost, most of the benefits from treatment accrue to patients. For example, Philipson and Jena (2005) find that despite the perceived high prices of antiretroviral therapy for human immunodeficiency virus, only 5% of the more than $1 trillion in value generated by these drugs went to manufacturers.14
Ultimately, it is still an open question whether insurance provides too little or too generous an incentive to pharmaceutical innovation.15 What is clear from this literature, however, is that when patients derive great benefit from a specialty drug—even one with high production costs—and their demand is inelastic, high cost-sharing is undesirable in both a static and dynamic sense. Given the high cost of these specialty drugs, insurers would be better off finding ways to manage utilization so only patients who would benefit will get access to them, rather than pursuing high copayment policies designed to deter use by all patients regardless of clinical need.
Concluding remarks
Increased cost sharing for specialty products will not reduce use of these products dramatically, but will only serve to transfer a much larger financial burden from the health plan to the patient. It also will do little to reduce overall health care spending. Management of these drugs may rightly focus on making sure only patients who will most benefit receive them, but once such patients are identified, it makes little sense to limit coverage.
Supplementary Material
Acknowledgement
This research was supported by Amgen, Inc. with additional funding from United Healthcare and the National Institute on Aging through its support of the RAND Roybal Center for Health Policy Simulation and the Claude D. Pepper Center at University of California, Los Angeles. The authors are solely responsible for the manuscript’s content. By prior contractual arrangement, neither Amgen, United Healthcare, or the National Institute on Aging had any authority over the design and conduct of the study; the collection, analysis, preparation, and interpretation of the data; and preparation of the manuscript.
Contributor Information
Dana P. Goldman, RAND Corporation and the National Bureau of Economic Research, 1776 Main Street, Santa Monica, CA 90401, Tel: 310.451.7017, Fax: 310.260.8155.
Geoffrey F. Joyce, RAND Corporation, 1776 Main Street, Santa Monica, CA 90401, Tel: 310.393.0411, Fax: 310.260.8156.
Grant Lawless, Amgen Corporation, 1 Amgen Center Drive, MS36-2-A, Thousand Oaks, CA 91320, Tel: 805.447.8024, Fax: 805.376.8550.
William H. Crown, i3 Innovus, 1 Newton Executive Park, Newton, MA 02462, Tel: (617) 552-5265, Fax: (617) 244 9669.
Vincent Willey, HealthCore, 800 Delaware Ave. 5th Floor, Wilmington, DE 19801-1366, Tel: 302-230-2000, Fax: 302-230-2020.
Citations and Notes
- 1.Smith C, Cowan C, Heffler S, Catlin A, NHAT, “National Health Spending In 2004: Recent Slowdown Led By Prescription Drug Spending,” Health Affairs, 2006, Vol. 25, No. 1, pp. 186–196. [DOI] [PubMed] [Google Scholar]
- 2.Specialty Pharmacy News, January 2004.
- 3.Technical Appendix.
- 4.Not every health plan was available in both years; in total there were 91 plan-years.
- 5.The cause of anemia cannot be ascertained in claims data so all patients with anemia are included in this category. This aggregation is consistent given that specialty drugs used to treat anemia also do not vary with underlying disease. Sensitivity analysis demonstrated that the subsequent elasticities are similar when anemia patients are excluded.
- 6.Pinsonault P, “Understanding Formularies: Formulary Strategies Evolve in Response to New Trends and Issues,” Pharmaceutical Representative, March 1, 2004. [Google Scholar]
- 7.This index is similar in spirit to the market basket approach employed by Goldman et al. (2004) in the Journal of the American Medical Association. A true market basket could not be constructed for biologics since the quantity of drug supplied is not recorded in medical claims. (Goldman D, Joyce G, Escarce J, Pace J, Solomon M, Laouri M, Landsman P and Teutsch S, “Pharmacy Benefits and the Use of Drugs by the Chronically-Ill,” Journal of the American Medical Association, 2004, Vol. 291, No. 19, pp. 2344–50). [DOI] [PubMed] [Google Scholar]
-
8.Let A and B be the 25th and 75th
quartiles of our plan generosity measure. For example, the cost-sharing
levels were 13% (A) and 26% (B) for kidney disease. Using the probit (first)
equation, we predicted pi (A)
and pi (B) for everyone in the
sample. The arc elasticity was then computed as:
The terms and refer to the average predictions across the entire sample with the condition. To get estimates for the elasticity of conditional use, the predictions from the second stage (the GLM model) are computed as Condi (A) and Condi (B), and substituted into the arc elasticity equation. To derive the elasticity of overall spending, we used Yi (A) = Pi (A) · Condi (A) and Yi (B) = Pi (B) · Condi (B). - 9.Chernew ME, Encinosa WE and Hirth RA, “Optimal Health Insurance: The Case of Observable, Severe Illness,” Journal of Health Economics, 2000, Vol. 19, No. 5, pp. 585–609. [DOI] [PubMed] [Google Scholar]
- 10.Fendrick AM, Smith DG, Chernew ME and Shah SN, “A Benefit-Based Copay For Prescription Drugs: Patient Contribution Based On Total Benefits, Not Drug Acquisition Cost,” American Journal of Managed Care, 2001, Vol. 7, No. 9, pp. 861–867. [PubMed] [Google Scholar]
- 11.While the fraction of users is low, that number is expected to increase substantially in the near future as new drugs enter the market for the treatment of diabetes, osteoporosis, and other diseases that affect much larger populations.
- 12.Newhouse JP, “Free for All?: Lessons from the Rand Health Insurance Experiment,” Boston: Harvard University Press, 1994, See Table 4.18. [Google Scholar]
- 13.Kremer M, “Patent Buyouts: A mechanism for Encouraging Innovation,” Quarterly Journal of Economics, 1998, Vol. 113, No. 4, pp. 1137–1167. [Google Scholar]
- 14.Philipson Tomas J. and Jena Anupam B., “Who Benefits from New Medical Technologies? Estimates of Consumer and Producer Surpluses for HIV/AIDS Drugs,” Forum for Health Economics & Policy, 2005, Forum: Biomedical Research and the Economy, Article 3, http://www.bepress.com/fhep/biomedical_research/3 [Google Scholar]
- 15.As long as insurance markets are competitive and production costs are low, then lower patient cost-sharing will improve welfare in a static setting (Gaynor Martin, Haas-Wilson Deborah and Vogt William B., “Are Invisible Hands Good Hands? Moral Hazard, Competition, and the Second-Best in Health Care Markets,” Journal of Political Economy, University of Chicago Press, 2000, Vol. 108, No. 5, pp. 992–1005. See Alan M. Garber, Charles I. Jones, and Paul Romer, “Insurance and Incentives for Medical Innovation,” Forum for Health Economics & Policy, Forum: Biomedical Research and the Economy, 2006, Article 4, http://www.bepress.com/fhep/biomedical_research/4, for a different approach to this issue which might justify limits on monopoly pricing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.