Abstract
Objective:
Recent data suggest that visit-to-visit variability of cholesterol is associated with cardiovascular events. We evaluated the role of lipid variability as a determinant of end-stage renal disease (ESRD).
Approach and Results:
Using nationally representative data from the Korean National Health Insurance System, 8,493,277 subjects who were free of ESRD and who underwent three or more health examinations during 2005–2010 were followed to the end of 2015. Total cholesterol (TC) variability was measured using the coefficient of variation (CV), standard deviation (SD), and the variability independent of the mean (VIM). The primary outcome was the development of ESRD, defined as a combination of the relevant disease code and the initiation of renal replacement therapy. There were 11,247 cases of ESRD during a median follow-up of 6.1 years. There was a graded association between a higher TC variability and incident ESRD. In the multivariable adjusted model, the hazard ratios and 95% confidence intervals (CI) comparing the highest versus lowest quartiles of CV of TC were 2.66 (95% CI, 2.52–2.82). The results were consistent when the variability of TC was modeled using SD and VIM, and were independent of preexisting chronic kidney disease
Conclusions:
Increasing TC variability was associated with an increasing incidence of ESRD.
INTRODUCTION
Chronic kidney disease (CKD) is a growing public health problem. More than 10% of the adult population in the U.S. is estimated to have stage 1–4 CKD.1 The estimated prevalence in Korea of CKD including both reduced estimated glomerular filtration rate (eGFR) and albuminuria is 8.2%,2 and its prevalence is increasing worldwide with the growing prevalence of obesity and metabolic diseases.3 CKD has substantial importance because it is considered a strong risk factor for cardiovascular morbidity and mortality.
Targeting modifiable factors has been frequently recommended as a first-line strategy for reducing the risks of kidney disease progression and cardiovascular disease (CVD) in patients with CKD. Previous studies have suggested that lipid abnormalities may contribute to the progression of kidney disease.4–6 In a study of nondiabetic patients with primary chronic renal disease, elevated plasma concentrations of apolipoprotein B and low-density lipoprotein (LDL) cholesterol, but not reduced concentrations of high-density lipoprotein cholesterol, were correlated with faster progression of renal insufficiency, even after controlling for proteinuria.4 Previous meta-analyses of randomized trials have indicated that lowering LDL cholesterol might reduce the rate of loss of glomerular filtration by approximately 1 mL/min per year.5 An intensive treatment strategy with 80 mg atorvastatin reduced the risk of kidney failure by 31% compared with treatment with 10 mg atorvastatin.6,7
Recently, interest in the intra-individual variability of various physiological measures has led to the recognition of their role as a risk factor for health-related outcomes. For example, increased blood-pressure variability and lowered heart-rate variability (HRV) have been repeatedly linked to adverse outcomes such as vascular events, impaired cognition, and mortality.8–11 In addition, visit-to-visit variability in blood pressure has been shown to predict the risk of end-stage renal disease (ESRD), independent of the achieved systolic blood pressure (SBP).8 Intra-individual lipid variability was also associated with an increased risk of cardiovascular events in subjects with coronary artery disease.12–14 However, the role of lipid variability as a determinant of incident ESRD has not been evaluated. Moreover, previous studies on lipid variability have important limitations, such as being restricted to very specific or high-risk populations, having a small sample size, or assessing only selected outcomes.12–14
To understand better the association of lipid variability with adverse renal outcomes in the broader and general population, we analyzed nationally representative data from the Korean National Health Insurance System (NHIS).
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Baseline characteristics of study population
The characteristics of participants classified by quartiles of coefficient of variation (CV) of total cholesterol (TC) are described in Table 1. Subjects in higher quartiles of TC variability were older, more likely to be women, had a higher prevalence of comorbid conditions, and more frequently used lipid-lowering agents. The mean TC levels in all four groups were approximately 193 mg/dL. The CV values of TC in the Q1–Q4 groups were 3.88 ± 1.35%, 7.30 ± 0.85%, 10.56 ± 1.10%, and 17.85 ± 5.15%, respectively. Baseline levels of eGFR were comparable between groups. The proportion of subjects with proteinuria increased gradually from the subjects with the lowest to the highest TC variability. Similar patterns of baseline characteristics were noted by quartiles of standard deviation (SD) and variability independent of the mean (VIM) (Supplemental Table I, II). P-values for the trend were < 0.0001 for all variables because of the large size of the study population.
Table 1.
Baseline characteristics of subjects according to the total cholesterol variability measured as coefficient of variation
| Q1 (n = 2123320) | Q2 (n = 2123348) | Q3 (n = 2123290) | Q4 (n = 2123319) | |
|---|---|---|---|---|
| Age (years) | 47.5 ± 13.7 | 47.1 ± 13.4 | 47.9 ± 13.6 | 51.3 ± 14.0 |
| Sex (male) | 1269424 (59.8) | 1304130 (61.4) | 1259575 (59.3) | 1124192 (53.0) |
| Body mass index (kg/m2) | 23.7 ± 3.1 | 23.7 ± 3.1 | 23.7 ± 3.1 | 24.0 ± 3.1 |
| Systolic BP (mmHg) | 122.0 ± 14.5 | 122.1 ± 14.4 | 122.4 ± 14.5 | 123.6 ± 15.0 |
| Diastolic BP (mmHg) | 76.2 ± 9.8 | 76.3 ± 9.7 | 76.5 ± 9.8 | 76.8 ± 9.9 |
| Fasting glucose (mg/dL) | 95.9 ± 19.9 | 96.1 ± 20.4 | 96.8 ± 21.5 | 99.7 ± 25.3 |
| eGFR (ml/min/1.73 m2) | 87.0 ± 42.5 | 86.8 ± 42.0 | 86.9 ± 40.0 | 86.6 ± 38.0 |
| eGFR<60ml/min/1.73m2 | 130210 (6.13) | 132972 (6.26) | 134639 (6.34) | 166277 (7.83) |
| Proteinuriaa | 40608 (1.91) | 41417 (1.95) | 44862 (2.11) | 61451 (2.89) |
| BaselineTC (mg/dL) | 194.5 ± 31.5 | 195.5 ± 32.9 | 196.9 ± 35.4 | 196.6 ± 43.9 |
| Mean TC (mg/dL) | 193.8 ± 30.7 | 193.3 ± 30.4 | 193.1 ± 30.5 | 194.9 ± 31.1 |
| TC variability | ||||
| CV (%) | 3.88 ± 1.35 | 7.30 ± 0.85 | 10.56 ± 1.10 | 17.85 ± 5.15 |
| SD (mg/dL) | 7.51 ± 2.89 | 14.11 ± 2.78 | 20.40 ± 3.88 | 34.88 ± 11.89 |
| VIM (%) | 7.52 ± 2.62 | 14.17 ± 1.67 | 20.50 ± 2.16 | 34.62 ± 9.97 |
| Current smoker | 544239 (25.6) | 568227 (26.8) | 550010 (25.9) | 467522 (22.0) |
| Alcohol drinking | 1069579 (50.4) | 1093253 (51.5) | 1060687 (50.0) | 925146 (43.6) |
| Regular exercise | 415338 (19.6) | 417455 (19.7) | 416895 (19.6) | 421675 (19.9) |
| Income (lower 10%) | 152692 (7.2) | 153483 (7.2) | 163715 (7.7) | 185020 (8.7) |
| Hypertension | 495329 (23.3) | 494317 (23.3) | 534054 (25.2) | 735539 (34.6) |
| Diabetes mellitus | 141734 (6.7) | 146742 (6.9) | 167901 (7.9) | 288407 (13.6) |
| Dyslipidemia | 257094 (12.1) | 292737 (13.8) | 372603 (17.6) | 725471 (34.2) |
| On lipid-lowering agent | 178173 (8.4) | 189822 (8.9) | 244829 (11.5) | 583840 (27.5) |
| Cardiovascular diseases | 32967 (1.55) | 32570 (1.53) | 38569 (1.82) | 77579 (3.65) |
| Any malignancy | 20941 (1.0) | 20771 (1.0) | 23182 (1.1) | 32460 (1.5) |
Data are expressed as the means ± SD, or n (%). P-values for the trend were < 0.0001 for all variables because of the large size of the study population.
BP, blood pressure; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; SD, standard deviation; TC, total cholesterol; VIM, variability independent of the mean
Proteinuria was defined as having urinary protein ≥ 1+ on dipstick testing in fasting morning urine.
The baseline CV, SD, and VIM of TC were significantly higher in subjects with incident ESRD than in those without ESRD (Table 2), although the baseline and mean TC levels did not differ greatly according to the occurrence of ESRD.
Table 2.
Baseline characteristics of subjects according to the incident end-stage renal disease
| No ESRD (n = 8482030) | ESRD (n = 11247) | |
|---|---|---|
| Age (years) | 48.4 ± 13.8 | 61.6 ± 12.8 |
| Sex (male) | 4949517 (58.4) | 7804 (69.4) |
| Body mass index (kg/m2) | 23.8 ± 3.1 | 24.1 ± 3.3 |
| Systolic BP (mmHg) | 122.5 ± 14.6 | 133.3 ± 18.0 |
| Diastolic BP (mmHg) | 76.4 ± 9.8 | 79.9 ± 11.0 |
| Fasting glucose (mg/dL) | 97.1 ± 21.9 | 119.7 ± 52.1 |
| eGFR (ml/min/1.73 m2) | 86.9 ± 40.7 | 51.1 ± 35.4 |
| eGFR<60ml/min/1.73m2 | 556715 (6.56) | 7383 (65.64) |
| Proteinuriaa | 183153 (2.12) | 5185 (46.5) |
| Baseline TC (mg/dL) | 195.9 ± 36.2 | 192.7 ± 45.8 |
| Mean TC (mg/dL) | 193.8 ± 30.7 | 196.5 ± 35.5 |
| TC variability | ||
| CV (%) | 9.89 ± 5.85 | 13.97 ± 8.36 |
| SD (mg/dL) | 19.21 ± 12.05 | 27.59 ± 17.75 |
| VIM (%) | 19.19 ± 11.33 | 27.07 ± 16.18 |
| Current smoker | 2127491 (25.1) | 2507 (22.3) |
| Alcohol drinking | 4145065 (48.9) | 3600 (32.0) |
| Regular exercise | 1669116 (19.7) | 2247 (20.0) |
| Income (lower 10%) | 653720 (7.7) | 1190 (10.6) |
| Hypertension | 2250017 (26.5) | 9222 (82.0) |
| Diabetes mellitus | 739482 (8.7) | 5302 (47.1) |
| Dyslipidemia | 1642680 (19.4) | 5225 (46.5) |
| On lipid-lowering agent | 1191358 (14.1) | 5306 (47.2) |
| Cardiovascular diseases | 180411 (2.13) | 1274 (11.33) |
| Any malignancy | 97030 (1.1) | 324 (2.9) |
Data are expressed as the means ± SD, or n (%). P-values were < 0.0001 for all variables because of the large size of the study population.
BP, blood pressure; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; SD, standard deviation; TC, total cholesterol; VIM, variability independent of the mean.
Proteinuria was defined as having urinary protein ≥ 1+ on dipstick testing in fasting morning urine.
TC variability and the risk of ESRD
Over a median (5–95%) 6.1 (5.0–6.8) years of follow-up after the TC variability assessment period, 11,247 (0.13%; 0.22/1000 person-years) participants developed ESRD. The incidence of ESRD increased progressively with higher levels of TC variability in both subjects with baseline eGFR ≥ 60 and eGFR < 60 ml/min/1.73m2 (Figure 1, Supplemental Figure II). The incidence rate was approximately 200–330% higher in the CV Q4 group compared with the other groups (Table 3). An incrementally higher risk of ESRD was observed for higher CV quartiles compared with the lowest quartile group in all models. After adjusting for age, sex, body mass index (BMI), alcohol drinking, smoking, regular exercise and income, the hazard ratios (HR) for incident ESRD were 1.15 (95% confidence interval [95% CI], 1.08–1.23), 1.39 (1.31–1.48), and 2.66 (2.52–2.82) for the second, third, and fourth quartiles versus the first quartile of CV of TC, respectively (Table 3, model 1). After further controlling for other confounding factors, including baseline eGFR and proteinuria, the association between cholesterol variability and incident ESRD remained significant (HR [95% CI]: Q2, 1.09 [1.02–1.16]; Q3, 1.22 [1.15–1.30]; Q4, 1.60 [1.51–1.70]; Table 3, model 4). TC variability as measured by SD or VIM was also an independent predictor of ESRD, even after full multivariable adjustment.
Figure 1.
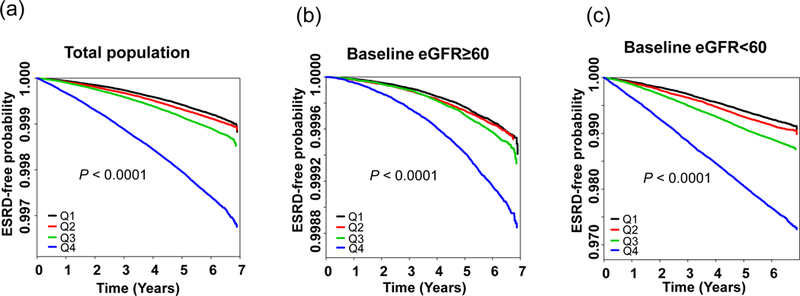
Kaplan-Meier estimates of end-stage renal disease (ESRD)-free probability by quartiles of total cholesterol variability measured as coefficient of variation in the total population (a), baseline eGFR ≥ 60 (b), and baseline eGFR < 60 population (c)
Table 3.
Hazard ratios and 95% confidence intervals of end-stage renal disease by quartiles of total cholesterol variability
| Events (n) | Follow-up duration (person-years) | Incidence rate (per 1000 person-years) | Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|---|---|---|
| CV | ||||||||
| Q1 | 1649 | 12552854 | 0.13 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1859 | 12669787 | 0.15 | 1.15 (1.08–1.23) | 1.12 (1.05–1.20) | 1.11 (1.04–1.19) | 1.09 (1.02–1.16) | |
| Q3 | 2338 | 12638900 | 0.19 | 1.39 (1.31–1.48) | 1.29 (1.21–1.38) | 1.25 (1.17–1.33) | 1.22 (1.15–1.30) | |
| Q4 | 5401 | 12480864 | 0.43 | 2.66 (2.52–2.82) | 2.00 (1.90–2.12) | 1.73 (1.63–1.83) | 1.60 (1.51–1.70) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| SD | ||||||||
| Q1 | 1675 | 12546345 | 0.13 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1838 | 12692068 | 0.15 | 1.09 (1.02–1.16) | 1.07 (1.00–1.14) | 1.06 (0.99–1.13) | 1.05 (0.98–1.12) | |
| Q3 | 2339 | 12600566 | 0.19 | 1.31 (1.23–1.40) | 1.22 (1.15–1.30) | 1.19 (1.12–1.27) | 1.18 (1.11–1.26) | |
| Q4 | 5395 | 12503426 | 0.43 | 2.50 (2.37–2.64) | 1.92 (1.81–2.03) | 1.66 (1.57–1.76) | 1.55 (1.46–1.65) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| VIM | ||||||||
| Q1 | 1657 | 12552700 | 0.13 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1857 | 12669222 | 0.15 | 1.15 (1.07–1.23) | 1.12 (1.05–1.20) | 1.11 (1.04–1.18) | 1.08 (1.01–1.16) | |
| Q3 | 2333 | 12639539 | 0.19 | 1.39 (1.30–1.48) | 1.29 (1.21–1.37) | 1.24 (1.17–1.33) | 1.21 (1.14–1.29) | |
| Q4 | 5400 | 12480944 | 0.43 | 2.66 (2.52–2.82) | 2.00 (1.89–2.12) | 1.73 (1.64–1.83) | 1.60 (1.51–1.70) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
Model 1: adjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise and income
Model 2: adjusted for model 1 plus diabetes mellitus and hypertension
Model 3: adjusted for model 2 plus mean total cholesterol level and use of lipid-lowering agent
Model 4: adjusted for model 3 plus estimated glomerular filtration rate and proteinuria
The risk of ESRD according to TC variability and BMI groups
The overall association between BMI and the risk of ESRD showed an inverted J curve (Figure 2). Higher adjusted HRs of ESRD were observed in the highest TC variability (Q4) group, regardless of BMI category. When analyzed by multivariate Cox regression analysis, compared with normal BMI (18.5 ≤ BMI < 23.0) and the lowest three quartiles of TC variability, the subgroup with Q4 TC variability who were underweight had the highest risk of ESRD (HR 2.49, 95% CI: 2.11–2.92). The lowest risk was observed among individuals with class II obesity (30 ≤ BMI < 35) and TC variability Q1–3 (HR 0.56, 95% CI: 0.47–0.66). Results were consistent when the variability of TC was determined using SD and VIM (Supplemental Figure III).
Figure 2.
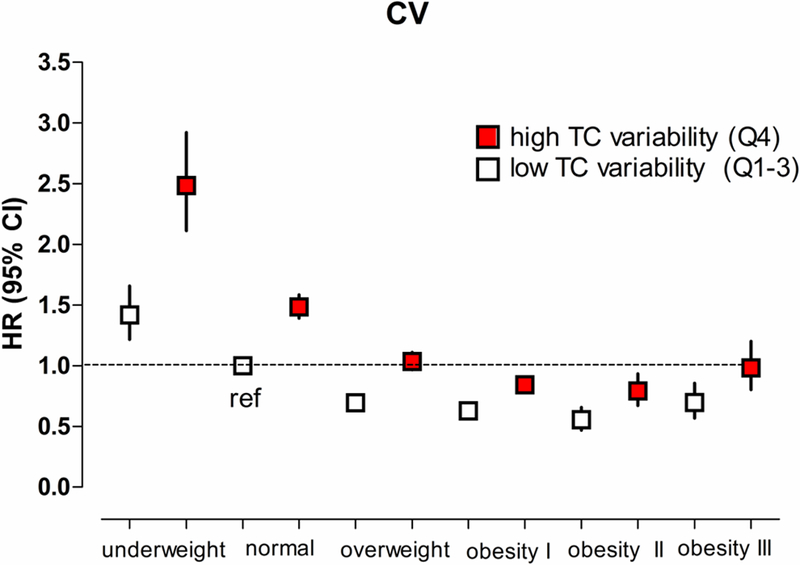
Hazard ratios and 95% confidence intervals of end-stage renal disease (ESRD) according to the BMI categories and TC variability measured as coefficient of variation. High TC variability was defined as the highest quartile (Q4) of TC variability. Subjects were classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), obesity class I (25–29.9 kg/m2) obesity class II (30–35 kg/m2) or obesity class III (BMI ≥ 35 kg/m2). The group with low TC variability (Q1–3 of TC variability) and normal weight (BMI 18.5–22.9 kg/m2) was used as the reference group.
Subgroup analyses
Analyses stratified by age, sex, the presence or absence of obesity, diabetes mellitus, hypertension, dyslipidemia, any malignancy, impaired renal function, CVD and use of lipid-lowering agents were performed (Figure 3). The Q4 group of TC variability (Q4) remained predictive of ESRD in all studied subgroups compared with the Q1–3 groups. Higher adjusted HRs of incident ESRD were observed in the subgroup that was younger (< 55 years), male, not obese, without diabetes mellitus, dyslipidemia, or CVD, and not taking any lipid-lowering agents. These findings were consistent when using other indices of variability, including SD and VIM (Supplemental Figures IV, V). To account for the possible influence of previous renal function on incident ESRD, we performed a subgroup analysis based on the presence of low GFR defined as baseline eGFR < 60 mL/min/1.73 m2. The associations between TC variability and ESRD were consistent in subjects with or without low GFR.
Figure 3.

Hazard ratios and 95% confidence intervals of end-stage renal disease (ESRD) in the highest quartile vs. lower three quartiles of total cholesterol variability (coefficient of variation) in subgroups. Adjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise, income, diabetes mellitus, hypertension, mean total cholesterol level, use of lipid lowering-agent, glomerular filtration rate and proteinuria.
Sensitivity analyses
When subjects with ESRD that occurred within 2 years of follow-up were excluded, the hazard ratios were slightly attenuated, but the overall pattern of higher risk of developing ESRD at higher TC variability remained significant (Table 4). When the baseline TC level was used instead of the mean TC level in the Cox proportional hazards model 3, the results were nearly identical (Supplemental Table III). When the systolic blood pressure (SBP) and fasting blood glucose (FBG) were used instead of hypertension and diabetes mellitus, we also found similar results (Supplemental Table IV). The number of TC measurements may influence the variability. In order to overcome this limitation, we performed sensitivity analyses with subjects who had 5 measurements of TC (Supplemental Table V) or 3 measurements of TC (Supplemental Table VI). An incrementally higher risk of ESRD was observed with higher variability quartile groups compared to the lowest quartile group in all models. Sensitivity analysis on subjects with 5 measurements of TC showed even higher hazard ratios compared to the original analysis.
Table 4.
Hazard ratios and 95% confidence intervals of end-stage renal disease by quartiles of total cholesterol variability: Sensitivity analysis excluding ESRD within 2 years of follow-up
| Events (n) | Follow-up duration (person-years) | Incidence rate (per 1000 person-years) | Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|---|---|---|
| CV | ||||||||
| Q1 | 1334 | 12552506 | 0.11 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1459 | 12669346 | 0.12 | 1.12 (1.04–1.20) | 1.09 (1.01–1.17) | 1.08 (1.00–1.17) | 1.06 (0.99–1.15) | |
| Q3 | 1830 | 12638321 | 0.15 | 1.35 (1.25–1.44) | 1.25 (1.17–1.34) | 1.21 (1.13–1.30) | 1.19 (1.11–1.28) | |
| Q4 | 3928 | 12479308 | 0.32 | 2.38 (2.24–2.54) | 1.81 (1.70–1.93) | 1.58 (1.48–1.68) | 1.48 (1.39–1.58) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| SD | ||||||||
| Q1 | 1348 | 12545981 | 0.11 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1424 | 12691606 | 0.11 | 1.05 (0.97–1.13) | 1.02 (0.95–1.10) | 1.02 (0.94–1.10) | 1.01 (0.94–1.09) | |
| Q3 | 1807 | 12599982 | 0.14 | 1.25 (1.17–1.34) | 1.17 (1.09–1.26) | 1.14 (1.06–1.22) | 1.14 (1.06–1.22) | |
| Q4 | 3972 | 12501912 | 0.32 | 2.27 (2.13–2.41) | 1.75 (1.65–1.87) | 1.53 (1.43–1.63) | 1.44 (1.35–1.54) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| VIM | ||||||||
| Q1 | 1342 | 12552348 | 0.11 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Q2 | 1453 | 12668779 | 0.12 | 1.11 (1.03–1.19) | 1.08 (1.00–1.16) | 1.07 (0.99–1.15) | 1.06 (0.98–1.14) | |
| Q3 | 1832 | 12638972 | 0.15 | 1.34 (1.25–1.44) | 1.25 (1.16–1.34) | 1.21 (1.13–1.30) | 1.19 (1.11–1.28) | |
| Q4 | 3924 | 12479381 | 0.31 | 2.38 (2.24–2.53) | 1.81 (1.70–1.92) | 1.58 (1.48–1.68) | 1.48 (1.39–1.58) | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
Model 1: adjusted for age, sex, body mass index, alcohol drinking, smoking, regular exercise and income
Model 2: adjusted for model 1 plus diabetes mellitus and hypertension
Model 3: adjusted for model 2 plus mean total cholesterol level and use of lipid-lowering agent
Model 4: adjusted for model 3 plus estimated glomerular filtration rate and proteinuria
DISCUSSION
In this nationwide population-based cohort study, we demonstrated that TC variability was associated with a higher risk for ESRD development during a 6.1 year follow-up period. A dose-response relationship was noted between higher TC variability and the primary outcome. The association persisted after multivariable adjustment for important potential confounders, including baseline eGFR, proteinuria, and underlying diseases. The results obtained using different indices of lipid variability were largely consistent. These data add to the evidence that TC variability is a risk factor for ESRD as well as for myocardial infarction, stroke, and all-cause mortality.12–14 Our study also demonstrated a greater impact of lipid variability in underweight subjects relative to that in normal weight subjects.
A significant relationship was observed between TC variability and progression to ESRD. This is relevant because the glomerulus is adversely affected by the same pathophysiological factors that lead to atherosclerosis, including dyslipidemia, hypertension, endothelial dysfunction, insulin resistance, inflammation, and oxidative stress.15 Glomerular lipid deposits and the appearance of foam cells accompany renal injury in models of hyperlipidemia.15,16 High fluctuations in blood cholesterol could directly contribute to the progression of ESRD by leading to instability of the vascular wall owing to fluctuations in the composition of lipid deposits.
Even though the prospective association between TC variability and incidence of ESRD was significant and independent of other risk factors, the potential for reverse causality is of concern. It is possible that lipid variability accelerates renal disease progression and/or that renal disease increases lipid variability. Two of the significant factors that determine the levels of plasma cholesterol-rich lipoproteins are deterioration in renal function and the degree of proteinuria.17 Patients with impaired renal function exhibit increased concentrations of triglycerides, even when their serum creatinine levels are within normal limits. Patients with nephrotic-range proteinuria exhibit an acquired LDL-receptor deficiency.17 People with more significant renal disease might have greater TC variability because of changes in their lipid metabolism. In our study, subjects in the higher TC variability group (Q4) were more likely to have albuminuria than those with lower TC variability (Q1–3). However, after controlling for baseline GFR and proteinuria as confounders, we found a consistent association between TC variability and incident ESRD. Moreover, a sensitivity analysis that excluded subjects with outcomes occurring in the first 2 years of follow-up revealed similar results.
The rate of progression to ESRD in the underweight group (BMI < 18.5 kg/m2) with high TC variability (Q4) was higher than in the remaining groups. Underweight was previously associated with an increased risk of progression of impaired renal function.18 Additional protein wasting caused by chronic disease progression might be less well tolerated in the underweight group because of their low protein reserves. In an Australian population with stage 3–4 CKD,19 a BMI in the overweight (25–29.9 kg/m2) or obesity class I/II (30–39.9 kg/m2) range was associated with a reduced risk of progression of renal disease and mortality, although the results were no longer significant when the BMI exceeded 40 kg/m2. Although BMI cutoff values for obesity differ according to the ethnic group,20,21 subjects with a BMI of 25–34.9 kg/m2 tend to have the lowest risk of progression of ESRD, regardless of ethnicity. A slightly elevated BMI potentially represents better overall nutrition, a high muscle mass, and an increased ability to adapt to the protein-energy wasting state commonly observed in kidney disease.18 Therefore, lipid variability may render subjects more susceptible to a poor outcome when their BMI is low.
In contrast, excessive BMI has also been reported as a risk factor for the incidence of ESRD in several studies of CKD,22–24 and focal segmental glomerulosclerosis and glomerulomegaly are often observed in obese people. Excess adiposity may be associated with ectopic lipid accumulation in the kidney, which in turn may be associated with structural and functional changes that mediate obesity-related renal disease.22 The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study data showed that metabolic health modified the association of BMI with the risk of incident ESRD, in that a higher BMI was associated with lower ESRD risk in those without, but not in those with, metabolic syndrome.23 It is also possible that the improved nutritional status, elevated adiponectin levels, and increased energy reserves associated with an elevated BMI may not only negate but also dominate the deleterious pro-inflammatory and atherogenic effects of the increased fat mass.23, 24
High sympathetic and low parasympathetic tone is associated with cardiovascular disease. Low HRV, a marker for sympathetic activation, is related to the progression of renal dysfunction.25 Low HRV may directly contribute to adverse renal outcomes by increasing atherosclerosis, vasoconstriction, arrhythmia, sodium retention, renin release, and blood pressure. Increased SBP variability has also been linked to adverse renal outcomes.26 Increased BP variability causes greater stress on blood vessels and leads to endothelial dysfunction, thereby promoting early target-organ damage.27 Although the mechanisms are different, these fluctuations in physiological measures such as BP, HR, glucose, and lipid levels could cause adverse health outcomes and/or be predictors of worse outcomes. Patients with higher variability in both LDL cholesterol and BP had higher incidence of cardiovascular events compared with lower variability in both parameters,28 suggesting an additive effect of different variability parameters on the risk of CVD. Increased variabilities in multiple parameters could be caused by variable compliance to healthy diet or drug treatment. However, in a previous study, the correlation between cholesterol variability and BP variability was poor.28 In our study, the correlations between TC variability and systolic BP variability (r = 0.058) or glucose variability (r = 0.107) were also not strong.
There are limitations to our study. First, this was an observational study, and therefore, the association found between TC variability and renal end-points may not be causal. As mentioned above, reverse causality is plausible because people with more significant renal disease (or proteinuria) might have greater TC variability. However, to minimize the possible effects of reverse causality, subjects with preexisting ESRD were excluded. A sensitivity analysis that also excluded subjects with ESRD occurring in the first 2 years of follow-up also showed similar results. Moreover, we found that the associations between TC variability and ESRD were consistent in subjects with and without low GFR. Second, the causes of renal disease were not identifiable in our study. Third, we defined proteinuria by dipstick testing and therefore did not quantitate the proteinuria. However, measuring proteinuria by dipstick testing was useful in identifying the development of ESRD.29 Fourth, the study population consisted of Korean men and women; therefore, it is uncertain whether these findings can be generalized to other ethnic groups. Lastly, there is no consensus on the ideal statistical measure of visit-to-visit TC variability. The degree of TC variability is in general roughly proportional to mean TC levels, and this dependence of TC variability on mean TC has led to the use of methods that assess TC variability in normalized units and are aimed at accounting for the mean TC level. This can be done by computing CV or VIM. In our study, the results were similar and consistent across all indices of TC variability.
The current study has several distinguishing points. To our knowledge, it is the first study of the relationship between TC variability and ESRD development in a large general population, using a well-established and validated longitudinal national database over 6 years. Adiposity is an important risk factor for ESRD and may be partially responsible for the association between lipid variability and ESRD. However, we found a more significant association in underweight subjects: underweight subjects with high TC variability had the highest risk of ESRD. Our study demonstrated a greater impact of lipid variability on low-risk groups, such as younger age, subjects without diabetes, dyslipidemia or CVD, and not on lipid-lowering agents. We could assume that not the medication or underlying diseases, but the fluctuation of TC level per se could have affected the outcome. Although the precise mechanism is unclear, a more uniform and less variable lipid levels might be important for preventing progression to ESRD, especially in the low-risk subjects. Future studies should examine whether reducing the variability in lipid parameters decreases the risk of progression to ESRD.
Supplementary Material
Highlights.
Increasing TC variability was associated with an increasing incidence of ESRD.
Underweight subjects with high TC variability had the highest risk of ESRD.
Our study demonstrated a greater impact of lipid variability on low-risk subjects, such as younger age, non-DM, or not on dyslipidemic medications groups.
A more uniform and less variable lipid level might be important for preventing progression to ESRD.
ACKNOWLEDGEMENT
The authors thank Prof. Gunseog Kang (Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea) for statistical assistance. This study was performed using the database from National Health Insurance System (NHIS-2016-1-175), and the results do not necessarily represent the opinion of National Health Insurance Corporation.
SOURCES OF FUNDING
This work was supported in part by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2016R1C1B1009972).
Nonstandard Abbreviations and Acronyms
- TC
total cholesterol
- BMI
body mass index
- BP
blood pressure
- CKD
chronic kidney disease
- CV
coefficient of variation
- ESRD
end-stage renal disease
- HR
heart rate
- SBP
systolic blood pressure
- SD
standard deviation
- VIM
variability independent of the mean
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 2.Park JI, Baek H, Jung HH. Prevalence of Chronic Kidney Disease in Korea: the Korean National Health and Nutritional Examination Survey 2011–2013. J Korean Med Sci 2016; 31: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon MJ, Hyun YY, Lee KB, Kim H, Jung HS, Yun KE, Ahn J, Rampal S, Zhao D, Suh BS, Chung EC, Shin H, Pastor-Barriuso R, Guallar E. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann Intern Med 2016; 164: 305–312. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson O, Mulec H, Knight-Gibson C, Attman PO, Kron B, Larsson R, Weiss L, Wedel H, Alaupovic P. Lipoprotein abnormalities are associated with increased rate of progression of human chronic renal insufficiency. Nephrol Dial Transplant 1997; 12: 1908–1915. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17: 2006–2016. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 7.Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, Zhang H. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am J Kidney Dis 2016; 67: 881–892. [DOI] [PubMed] [Google Scholar]
- 8.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol 2016; 68: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57: 160–166. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996; 94: 2850–2855. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J Am Geriatr Soc 2006; 54: 1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit RA, Trompet S, Sabayan B, le Cessie S, van der Grond J, van Buchem MA, de Craen AJ, Jukema JW. Higher Visit-to-Visit Low-Density Lipoprotein Cholesterol Variability Is Associated With Lower Cognitive Performance, Lower Cerebral Blood Flow, and Greater White Matter Hyperintensity Load in Older Subjects. Circulation 2016; 134: 212–221. [DOI] [PubMed] [Google Scholar]
- 13.Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH; TNT Steering Committee and Investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol 2015; 65: 1539–1548. [DOI] [PubMed] [Google Scholar]
- 14.Boey E, Gay GM, Poh KK, Yeo TC, Tan HC, Lee CH. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis 2016; 244: 86–92. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int 2000; 58: 293–301. [DOI] [PubMed] [Google Scholar]
- 16.Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int 1988; 33: 917–924. [DOI] [PubMed] [Google Scholar]
- 17.Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J 2011; 5: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP, Kalantar-Zadeh K. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 2014; 56: 415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis E, Campbell K, Gobe G, Hawley C, Isbel N, Johnson DW. Association of anthropometric measures with kidney disease progression and mortality: a retrospective cohort study of pre-dialysis chronic kidney disease patients referred to a specialist renal service. BMC Nephrol 2016; 17: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, Kim EM, Suh SH, Shin HJ, Lee KR, Lee KY, Lee SY, Lee SY, Lee SK, Lee CB, Chung S, Jeong IK, Hur KY, Kim SS, Woo JT; Committee of Clinical Practice Guidelines; Korean Society for the Study of Obesity. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) 2014; 29: 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization, Regional Office for the Western Pacific (WPRO), International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific Perspective: Redefining obesity and its treatment Sydney, Health Communications Australia Pty Ltd, 2000. [Google Scholar]
- 22.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28. [DOI] [PubMed] [Google Scholar]
- 23.Panwar B, Hanks LJ, Tanner RM, Muntner P, Kramer H, McClellan WM, Warnock DG, Judd SE, Gutiérrez OM. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int 2015; 87: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2007; 2: 992–998. [DOI] [PubMed] [Google Scholar]
- 25.Brotman DJ, Bash LD, Qayyum R, Crews D, Whitsel EA, Astor BC, Coresh J. Heart rate variability predicts ESRD and CKD-related hospitalization. J Am Soc Nephrol 2010; 21: 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drawz PE, Babineau DC, Brecklin C, He J, Kallem RR, Soliman EZ, Xie D, Appleby D, Anderson AH, Rahman M; CRIC Study Investigators. Heart rate variability is a predictor of mortality in chronic kidney disease: a report from the CRIC Study. Am J Nephrol 2013; 38: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T, Sakabe K, Ushigome E, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis 2012; 220: 155–159. [DOI] [PubMed] [Google Scholar]
- 28.Bangalore S, Fayyad R, Messerli FH, Laskey R, DeMicco DA, Kastelein JJ, Waters DD. Relation of Variability of Low-Density Lipoprotein Cholesterol and Blood Pressure to Events in Patients With Previous Myocardial Infarction from the IDEAL Trial. Am J Cardiol 2017; 119: 379–387. [DOI] [PubMed] [Google Scholar]
- 29.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 2006; 17: 1444–1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


