Abstract
Introduction:
Maternal circulating 25-hydroxyvitamin D [25(OH)D] has been shown to optimize production of 1,25-dihydroxyvitamin D [1,25(OH)2D] during pregnancy at approximately 100 nmoles/L, which has pronounced effects on fetal health outcomes. Additionally, associations are noted between low maternal 25(OH)D concentrations and vascular pregnancy complications, such as preeclampsia. To further elucidate the effects of vitamin D activity in pregnancy, we investigated the role of maternal 25(OH)D, the nutritional indicator of vitamin D status, in relation to placental maintenance and, specifically, expression of placental gene targets related to angiogenesis and vitamin D metabolism.
Methods:
A focused analysis of placental mRNA expression related to angiogenesis, pregnancy maintenance, and vitamin D metabolism was conducted in placentas from 43 subjects enrolled in a randomized controlled trial supplementing 400 IU or 4,400 IU of vitamin D3 per day during pregnancy. Placental mRNA was isolated from biopsies within one hour of delivery, followed by quantitative PCR. We classified pregnant women with circulating concentrations of <100 nmoles/L as deficient and those with ≥100 nmoles/L as sufficient. The value of each gene’s change in the PCR cycle threshold (ΔCT), which is a relative measure of target concentration, was compared with maternal 25(OH)D concentrations <100 nmoles/L and ≥100 nmoles/L based on a twosample Wilcoxon test.
Results:
Soluble FMS-like tyrosine kinase 1 (sFlt-1) and vascular endothelial growth factor (VEGF) gene expression was significantly downregulated in the maternal subgroup with circulating 25(OH)D ≥100 ng/mL compared to the subgroup <100 ng/mL.
Discussion:
Here, we report a significant association between maternal vitamin D status and the expression of sFlt-1 and VEGF at the mRNA level. Achieving maternal circulating 25(OH)D ≥100 nmoles/L suggests the impact of maternal vitamin D3 supplementation on gene transcription in the placenta, thereby potentially decreasing antiangiogenic factors that may contribute to vascular pregnancy complications.
Keywords: vascular endothelial growth factor, preeclampsia, sFlt-1, vitamin D, placenta
1. Introduction
Pregnancy has been shown to be a critical life stage in which dietary supplementation with vitamin D appears to have a pronounced effect on fetal health outcomes, including a reduction in the risks of premature labor/birth, and additional maternal comorbidities such as gestational diabetes, hypertensive disorders, and infection (1–6). Little is known, however, about the role of maternal vitamin D sufficiency on the fetus and its role in pregnancy protection/maintenance throughout gestation. In a recent NICHD-sponsored, 6-year randomized, double-blind, placebo-controlled trial investigating serum concentrations of active, hormonal vitamin D during pregnancy, circulating maternal 25(OH)D concentrations were found to be optimized at 100 nmol/L (40 ng/mL), which is twice the level normally observed in non-pregnant women (7). In the present study, we aimed to examine the effect of maternal vitamin D status on hormones with vital roles in placental development and maintenance.
The association of lower vitamin D concentrations with non-cardiovascular disease demonstrates a diverse range of pathologies in observational studies, including infectious diseases, obesity, bone health, cancer and multiple sclerosis (8–10). Likewise, there is extensive evidence from laboratory studies to suggest that vitamin D influences the vascular system, supported by observational studies in humans revealing the association of vitamin D insufficiency with increased arterial stiffness and endothelial dysfunction in the conductance and resistance of blood vessels (11). As endothelial dysfunction is a hallmark of pregnancy complications that potentially lead to premature labor and delivery, such as preeclampsia, we hypothesized that maternal circulating vitamin D concentrations may affect the expression of an array of genes linked with angiogenesis and the potential for placental insufficiency secondary to its abnormal vasculature.
Consistent with this hypothesis, multiple studies have shown associations between low maternal 25(OH)D concentrations and the risk of preeclampsia (12–14). Mirzakhani H, et al. (2016) demonstrated in their study of over 800 participants that higher maternal circulating vitamin D concentrations both at the start of the study (first trimester) and in late pregnancy were associated with a lower risk of preeclampsia (15). There is still little known, however, about the effects of maternal circulating vitamin D concentrations on the fetus/fetal tissue; although new and developing studies are attempting to combat the controversy of vitamin D’s relation to pregnancy outcomes. Al-Garawi A, et al. (2016) recently described the gene expression profiles of healthy pregnancy women in the Vitamin D Antenatal Asthma Reduction Trial (VDAART). The conclusions of this study suggest maternal vitamin D levels influence transcriptional profiles and these alterations of the maternal transcriptome may contribute to fetal immune imprinting (16). It is proposed that these transcriptional changes may be related to pregnancy comorbidities, including vascular complications. Women with preeclampsia (a known significant vascular complication of pregnancy) are recognized to be at great risk for adverse pregnancy outcomes with a 20-fold increased risk for maternal mortality and several-fold higher risk for neonatal morbidity and mortality, depending on the gestational age at delivery and the presence of growth restriction in the fetus (17). Therefore, the placenta was chosen, due to its innately high vascularization and as the interface between maternal and fetal tissue, to determine how maternal vitamin D status affects both maternal and fetal tissue on a molecular level.
Our study primarily investigated the role of maternal vitamin D status on placental expression of target genes related to vascular complications of pregnancy; however, a total of three groups of target genes were chosen based on previously reported functions within the placenta. The first group chosen has critical function in the angiogenesis pathway related to pregnancy, and included vascular endothelial growth factor (VEGF), placental growth factor (PGF), and soluble fms-like tyrosine kinase 1 (sFlt-1). Additionally, regulatory genes known for their role in placental (and thus pregnancy) maintenance, which included progesterone receptor B (PRB), estrogen receptor 1 (ESR1), human chorionic gonadotropin β (hCGβ), and human placental lactogen (hPL) were investigated. Finally, genes related to vitamin D metabolism, including vitamin D receptor (VDR), glucocorticoid receptor (GRα, aka NR3C1), 24-hydroxylase (CYP24A1), and CYP27B1 were evaluated. Of note, CYP27B1 is responsible for the production of 1,25(OH)2D from 25(OH)D, while CYP24A1 catalyzes the conversion of 1,25(OH)2D into 24-hydroxlated products, constituting the degradation of the vitamin D molecule (18). We hypothesized that increased mRNA expression of proangiogenic genes and decreased mRNA expression of antiangiogenic factors would be observed in vitamin D sufficient women compared to vitamin D deficient women.
2. Methods
2.1. Study Design
This study was part of a randomized, placebo-controlled clinical trial (NCT 01932788) in which women provided informed consent and were followed from time of enrollment through delivery. The Institutional Review Board at the Medical University of South Carolina approved this study protocol (Pro 00020570). Enrolled mothers were 18–45 years of age who presented at 8–14 weeks’ gestation with a singleton pregnancy. Exclusion criteria included: pre-existing calcium or uncontrolled thyroid/parathyroid disease, requiring chronic diuretic/cardiac medications, sickle cell disease, sarcoidosis, and inflammatory bowel disease. Mothers were randomized to receive placebo or 4000 IU/day vitamin D3 plus the standard prenatal vitamin (containing 400 IU vitamin D3). Mothers were followed monthly through delivery, which coincided with a total of six to seven visits prior to delivery, at which time placental biopsies were obtained. Of note, the treatment group was not known at the time of sample analysis due to study blinding. Table 1 highlights demographic data for the subjects whose placentas were utilized for the following study. Again, these forty-three women and their placentas were studied as a subset of the larger ongoing RCT, in which long term follow-up for the children of these pregnancies continues.
Table 1.
Comparison of maternal and infant characteristics of women classified as 25(OH)D sufficient (≥100 nmoles/L) or deficient (<100 nmoles/L) prior to delivery.
| Maternal 25(OHD <100 nmoles/L | Maternal 25(OH)D ≥100 nmoles/L | Total | p-value* | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Ethnicity | 0.04 | |||
| African American | 5 (11.6) | 6 (13.9) | 11 (25.6) | |
| Hispanic | 6 (13.9) | 11 (25.6) | 17 (39.5) | |
| Caucasian | 1 (2.3) | 13 (20.2) | 14 (32.5) | |
| American Indian | 1 (2.3) | 0 (0.0) | 1 (2.3) | |
| Insurance | 0.10 | |||
| Private | 2 (4.7) | 13 (30.2) | 15 (34.9) | |
| Medicaid | 7 (16.3) | 7 (16.3) | 14 (32.6) | |
| Self-Pay | 4 (9.3) | 10 (23.3) | 14 (32.6) | |
| Marital Status | 0.76 | |||
| Single | 4 (9.3) | 5 (11.6) | 9 (20.9) | |
| Married | 6 (13.9) | 18 (41.9) | 24 (55.8) | |
| Cohabitating | 3 (7.0) | 7 (16.3) | 10 (23.3) | |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Maternal age (years) | 36.9 ± 4.2 | 28.9 ± 4.2 | 28.3 ± 4.3 | 0.31 |
| Maternal BMI | 30.3 ± 8.2 | 28.7 ± 8.1 | 29.2 ± 8.0 | 0.56 |
| Maternal 25(OH)D baseline (ng/mL) | 58.4 ± 22.7 | 71.9 ± 23.7 | 67.9 ± 23.9 | 0.10 |
| Maternal 25(OH)D V6/7 (ng/mL) | 60.9 ± 26.2 | 140.5 ± 31.2 | 116.6 ± 47.2 | |
| Infant gestational age (weeks) | 39.2 ± 1.2 | 38.5 ± 1.8 | 38.7 ± 1.7 | 0.24 |
| Infant birth weight (grams) | 3381 ± 562 | 3363 ± 517 | 3369 ± 524 | 0.64 |
p-values are included to identify the association between maternal vitamin D status and other demographic variables of interest.
Of note, African Americans and Hispanics are more likely to be vitamin D deficient (p=0.043). The type of insurance (p=0.104), maternal marriage status (p=0.759), maternal age (p=0.306), gestational age (p=0.239), baseline 25(OH)D (p=0.096), maternal BMI (p=0.563), birthweight (p=0.644) were not significantly different between the vitamin D sufficient and vitamin D deficient patients.
The maternal serum 25(OH)D concentrations obtained at the visit prior to delivery, either study visit 6 or 7 (abbreviated as V6/7), were separated into two categories: those <100 nmol/L (<40 ng/mL) and those ≥100 nmol/L (≥40 ng/mL). Based on optimized conversion of 25(OH)D to 1,25(OH)2D at ≥100 nmol/L, mothers with total circulating 25(OH)D concentrations <100 nmol/L were defined as “deficient” and those with concentrations ≥100 nmol/L were defined as “sufficient” (1) (7). As is standard in clinical practice, the 25(OH)D concentration was utilized for serum measurements as it provides the best estimate of a patient’s vitamin D status. We chose to study the differences between the two groups based on their 25(OH)D concentrations, rather than their randomized assignment to placebo vs treatment group secondary to the fact that our study participants remain blinded. As this study was a sub-aim of the RCT, the presented information will be released prior to unblinding of the overarching trial.
2.2. Placenta collection
From November 2013 through June 2014, placentae were collected, stored at 4°C, and sampled within 1 hour of delivery (n = 43). A convenience sample of forty three placentas were available from the larger overarching study cohort, due to the processing time limitation at delivery (within 1 hour). Each placenta was sampled at four different sites using a standard biopsy punch (3mm). Biopsy sites were approximately 5cm from the umbilical cord insertion, 2cm from each other, and avoided the placental edge, fibrous knots and large blood vessels. Each biopsy was a full thickness sample taken from the maternal through the fetal side of the placenta. Biopsies were washed briefly in 1X phosphate buffered saline (PBS), immediately placed in RNAlater solution (Thermo Fisher Scientific, Waltham, MA) at 4°C and stored at −20°C until RNA isolation. The protocol was modified from those of Wyatt et al and Pidoux et al (19, 20).
2.3. RNA isolation, reverse transcription and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from each biopsy with TRIzol reagent (Invitrogen Life Technologies, Waltham, MA) and purified with the SV Total RNA Isolation System (Promega, Madison, WI). Total RNA was treated with ribonuclease-free deoxyribonuclease I (DNase I; Qiagen, Venlo, Netherlands) to remove any genomic DNA contamination. Concentrations and quality of RNA samples were evaluated by measuring optical density with a NanoDrop ND-1000 (Thermo Fisher Scientific, Waltham, MA) and by formaldehyde gel electrophoresis. One microgram (μg) of total RNA for the placental biopsy samples passing the RNA quality check were pooled for each subject and reverse transcribed with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Complementary DNA (cDNA) was stored at −20°C until RT-qPCR analysis.
RT-qPCR analysis was performed on biopsy samples. Complementary DNA was analyzed in triplicate by RT-qPCR amplification using an iCycler MyIQ Single Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Each 15-μL DNA amplification reaction contained 10 mM Tris-HCl (pH 7.84), 50 mM KCl, 3 mM MgCl2, 200 μM dNTPs, 0.5% Tween-20, 0.8% glycerol, 2% DMSO, 200-fold dilution of SYBR Green (Invitrogen, Waltham, MA), 0.01 μM Fluorescein Calibration Dye (Bio-Rad, Hercules, CA), 0.2 μM of each primer, 0.01 U/μL AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA) and 2-times diluted cDNA. RT-qPCR amplification conditions included an enzyme activation step of 95°C (5 min) followed by 30–45 cycles of 95°C (15 sec) and a primer specific combined annealing/extension temperature (30 sec). Based on primer optimization runs, 30 cycles were utilized for expression data for the target gene hPL but otherwise 45 cycles of 95°C (15 sec) were utilized for all other target genes. The specificity of amplification was confirmed by the melt-curve analysis.
Triplicate data for each gene were averaged and mRNA expression levels were determined by the comparative CT method (also known as the 2−ΔΔCT method, or for individual data points 2−ΔCT). Primer sequences, annealing/extension temperatures and GenBank accession numbers are reported in Table 2.
Table 2.
Target Gene Data.
| Gene | Forward primer 5’---3’ | Reverse primer 5’---3’ | Annealing/extension temp (°C) | GenBank accession number |
|---|---|---|---|---|
| VEGF | CTTGCTGCTCTACCTCCACCAT | ATGATTCTGCCCTCCTCCTTCT | 62 | AB021221 |
| PGF | CCTACGTGGAGCTGACGTTCT | TCCTTTCCGGCTTCATCTTCT | 60 | S72960 |
| sFlt1 | GGGAAGAAATCCTCCAGAAGAAAGA | GAGATCCGAGAGAAAACAGCCTTT | 64 | AF063657 |
| PRB | TCGGACACCTTGCCTGAAGT | CAGGGCCGAGGGAAGAGTAG | 60 | NM_000926.4 |
| ESR1 | AGATCTTCGACATGCTGCTGGCTA | AGACTTCAGGGTGCTGGACAGAAA | 67.6 | X03635 |
| hCGβ | CTACTGCCCCACCATGACCC | TGGACTCGAAGCGCACATC | 63.2 | J00117.1 |
| hPL | GCATGACTCCCAGACCTCCT | TGCGGAGCAGCTCTAGATTGG | 63.2 | J00118.1 |
| CYP24A1 | CATCATGGCCATCAAAACAAT | GCAGCTCGACTGGAGTGAC | 60 | NM_000782.4 |
| CYP27B1 | CACCCGACACGGAGACCTT | TCAACAGCGTGGACACAAACA | 60 | NM_000785 |
| GRα (NR3C1) | CTTGGATTCTATGCATGAAGTGGTT | TTGGAAGCAATAGTTAAGGAGATTTTC | 61.6 | NM_000176.2 |
| VDR | TGGCTTTCACTTCAATGCTATGA | CGTCGGTTGTCCTTGGTGAT | 62 | NM_000376.2 |
2.4. Statistical analysis
NormFinder software (Aarhus, Denmark) was utilized to identify the normalization gene out of the internal control data (21). GAPDH was identified as having the greatest stability value. Allowing for ease of presentation as well as the ability to present the data as ‘fold change’ in expression (see Equation 1), the aforementioned ΔCT quantification method was used. The ΔCT was identified as the difference between the average cycle threshold of the target gene and the internal control (GAPDH). To be consistent, analyses were based on analyzing Real time PCR data based on ΔCT supported by Yuan et al (22). This equation and its derivation have been previously reported in Applied Biosystems User Bulletin No. 2 (P/N 4303859) (23).
We applied the 2−ΔCT for analysis to correlate the individual data points, rather than compare between two treatment groups, as the ΔΔCT equation is typically utilized. By this method, we were able to compare gene expression in two different samples (vitamin D deficient vs sufficient mothers) and relate this to an internal control gene (GAPDH).
| Equation 1. |
By using the above equation/method for analysis, the data may be interpreted as the expression of the target gene relative to the internal control gene. The data were then separated into the two categories of maternal vitamin D status (i.e. deficient vs sufficient) for comparative purposes. Demographics between the vitamin D sufficient and vitamin D deficient mothers were compared based on two-sample Wilcoxon test and Fisher’s Exact test (see Table 1). The expression of the eleven genes (ΔCT values) were compared between the vitamin D sufficient and vitamin D deficient mothers based on two-sample Wilcoxon test. ΔCT values for the vitamin D sufficient and vitamin D deficient group were summarized using their median and inter quantile range (IQR). The associations between 25(OH)D concentration and gene expression were examined using Spearman’s rank correlation test. Multiple regression models were performed by regressing ΔCT values on 25(OH)D concentration, maternal age, BMI and race/ethnicity for each of the eleven genes. Data were analyzed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Effect of maternal 25(OH)D concentrations on angiogenic factors (VEGF, PGF and sFlt-1)
Thirteen women were defined as vitamin D deficient and thirty as vitamin D sufficient at V6/7 (see Table 1). The results of the two-sample Wilcoxon test revealed the ΔCT values for VEGF and sFlt-1 are significantly different between the vitamin D <100 nmol/L and the vitamin D ≥100 nmol/L group at 0.05 level. For VEGF, the median values are 2.7 (IQR: 2.2, 3.0) and 3.6 (IQR: 2.9, 4.6) in the vitamin D <100 nmol/L group and the vitamin D>100 nmol/L group, respectively. For sFlt-1, the median ΔCT values are −3.7 (IQR: −4.1, −3.6) and −2.7 (IQR: −3.6, −1.9) in the vitamin D <100 nmol/L group and the vitamin D>100 nmol/L group, respectively.
The expression of sFlt-1, PRB, hPL, and GRα were also associated with mother’s baseline vitamin D status. At baseline, thirty-six women were vitamin D deficient and seven women were vitamin D sufficient. A two-sample Wilcoxon test revealed the values for sFlt-1, PRB, hPL and GRα are significantly different between the vitamin D <100 nmol/L and the vitamin D ≥100 nmol/L group at 0.05 level. For sFlt-1, the median ΔCT values are −3.6 (IQR: −4.0, −2.5) and −1.6 (IQR: −2.5,−1.2) in the vitamin D <100 nmol/L group and the vitamin D>100 nmol/L group at V1, respectively. Spearman’s rank correlation tests revealed that higher baseline 25(OH)D concentration is associated with higher ΔCT values of VEGF (r=0.30, p=0.050), PGF (r=0.30, p=0.047), sFlt-1 (r=0.31,p=0.041), PRB (r=0.36, p=0.017) and GRα (r=0.31, p=0.045). Table 3 summarizes each gene’s values in the vitamin D deficient and sufficient group based on their median and interquartile range [(IQR) representing the 25th and 75th percentiles].
Table 3:
The effect of vitamin D deficiency* on placenta gene expression (ΔCT).
| Vitamin D deficient at V1 Median(IQR) | Vitamin D sufficient at V1
Median(IQR) |
p-value | Vitamin D deficient at V6/7 Median(IQR) | Vitamin D sufficient at V6/7 Median(IQR) |
p-value | |
|---|---|---|---|---|---|---|
| VEGF | 3.1(2.2,4.0) | 4.3(3.6,5.0) | 0.068 | 2.7(2.2,3.0) | 3.6(2.9,4.6) | 0.014 |
| PGF | −3.5(−4.0,−2.4) | −2.3(−2.6,−0.5) | 0.056 | −3.4(−4.0,−2.9) | −2.8(−3.9,−1.7) | 0.191 |
| sFlt-1 | −3.6(−4.0,−2.5) | −1.6(−2.5,−1.2) | 0.010 | −3.7(−4.1,−3.6) | −2.7(−3.6,−1.9) | 0.008 |
| PRB | 7.8(6.7,9.0) | 10.0(9.2,10.7) | 0.021 | 7.3(6.5,8.5) | 8.7(7.1,9.5) | 0.113 |
| ESR1 | 7.9(7.4,8.5) | 9.4(8.3,10.0) | 0.069 | 7.6(6.8,8.3) | 8.3(7.5,9.3) | 0.061 |
| hCGb | −6.3(−7.6,−5.5) | −4.5(−5.9,−3.8) | 0.065 | −6.4(−7.2,−6.0) | −5.9(−7.6,−4.6) | 0.355 |
| hPL | −14.4(−14.9,−13.3) | −13.4(−13.8,−12.7) | 0.031 | −13.7(−14.6,−13.4) | −14.2(−14.9,−13.2) | 0.509 |
| CYP24A1 | 5.6(4.5,6.3) | 7.0(5.3,7.5) | 0.237 | 5.6(5.0,6.4) | 5.8(4.7,7.0) | 0.765 |
| CYP27B1 | 7.7(6.8,8.5) | 8.3(8.0,10.0) | 0.097 | 7.6(6.7,8.4) | 8.1(7.0,9.0) | 0.451 |
| GRα | 0.6(−0.5,1.2) | 2.0(1.8,2.2) | 0.022 | 0.2(−1.3,1.1) | 1.0(−0.1,2.0) | 0.128 |
| VDR | 7.4(6.9,8.4) | 8.6(7.3,8.9) | 0.236 | 7.2(7.1,8.2) | 7.7(6.9,8.5) | 0.853 |
25(OH)D<100nmol/L
Additionally, we analyzed the fold change in expression for each of these angiogenic factors. The mean(sd) for PGF of 2−ΔCT (vitamin D deficient group) is 12.5(7.4) and the mean(sd) 2−ΔCT (vitamin D sufficient group) is 11.5(11.5). When compared to the vitamin D deficient group, the mothers who were vitamin D sufficient demonstrated a downregulation in PGF by 1.1 fold. There is no statistically significant difference in PGF expression between the two groups (pval=0.72).
For VEGF, however, the mean(sd) of 2−ΔCT (vitamin D deficient group) is 0.20(0.12) and the mean(sd) of 2−ΔCT (vitamin D sufficient group) is 0.11(0.10). When compared to the vitamin D deficient group, the mothers who were vitamin D sufficient demonstrated a downregulation in VEGF by 1.7 fold. There is a significant difference in VEGF expression between the two groups (pval=0.04) at a significance level of 0.05.
Likewise, the mean(sd) of 2−ΔCT (vitamin D deficient group) is 15.6(8.8) and the mean(sd) of 2−ΔCT (vitamin D sufficient group) is 9.2(8.1) for sFlt-1. When compared to the vitamin D deficient group, the mothers who were vitamin D sufficient demonstrated a downregulation in sFlt-1 by 1.7 fold. There is a significant difference in sFlt-1 expression between the two groups (pval=0.03) at a significance level of 0.05.
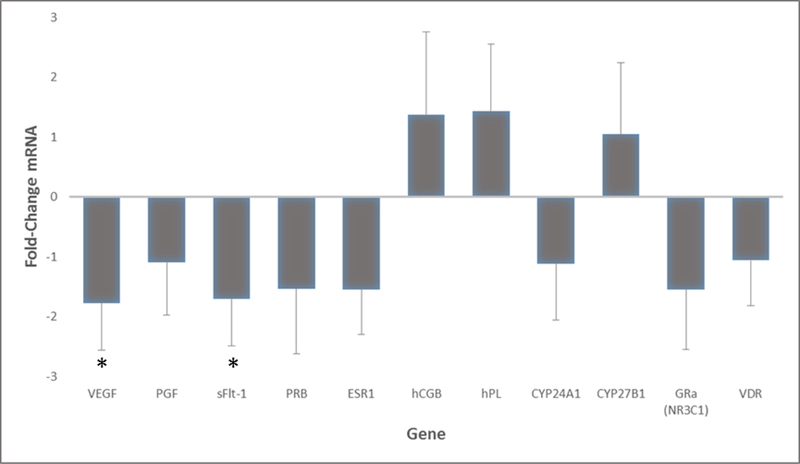
Figure 2 demonstrates the fold change conversion for each target gene as compared with the vitamin D sufficiency. In other words, the mean ΔCT values of each target gene in the vitamin D deficient group were obtained and the mean ΔCT values of each target gene in the vitamin D sufficient group were obtained; the two groups were then compared (based on fold change analysis) to see if a mother’s status (i.e. vitamin D sufficiency) could be correlated with decreased or increased expression of that gene (i.e. up/downregulation).
Figure 2.
Placental target gene expression in vitamin D sufficient cohort as compared to deficient women. Calculated fold change difference between vitamin D deficient vs sufficient groups. Shown negative values indicate downregulation and positive values indicate upregulation in women who were sufficient. Comparatively, vitamin D sufficiency resulted in downregulation of all but three of the genes tested. Results represent the fold change +/− standard deviation of each target gene. Genes with significant results are shown with an asterisk (*).
The effect of baseline 25(OH)D on placental gene expression was examined in multiple regression models adjusting for maternal age, BMI and race/ethnicity. As shown by Table 4, the concentration of baseline 25(OH)D is associated with VEGF expression after considering other variables in the model at a significance level of 0.05. Baseline 25(OH)D is not significantly associated with the expression of other genes we considered after adjusting for maternal age, maternal baseline BMI and race/ethnicity. The concentration of 25(OH)D at V6/7 is not associated with any of the eleven genes we considered at a significance level of 0.05, adjusting the effect of maternal age, maternal baseline BMI and race/ethnicity.
Table 4:
The effect of baseline 25(OH)D concentration on VEGF expression adjusted for maternal age, maternal baseline BMI and maternal race/ethnicity.
| Variables | Estimate | Std. Error | p-value |
|---|---|---|---|
| 25(OH)D at baseline | 0.05 | 0.02 | 0.048 |
| Maternal Age | 0.00 | 0.04 | 0.921 |
| Maternal baseline BMI | 0.01 | 0.03 | 0.584 |
| African American | Reference | ||
| Caucasian | 0.67 | 0.53 | 0.218 |
| Hispanic | 0.94 | 0.47 | 0.055 |
While there is expected to be some baseline level of upregulation in many of these target genes during pregnancy and higher expression of some of these target genes (VEGF and sFlt-1) in vascular disorders of pregnancy, the maternal serum 25(OH)D status (i.e. sufficiency) appears to have a significant effect on downregulating the overall expression of these genes as compared to deficient women. There was a small, although not significant, decrease in PGF expression in the sufficient group.
3.2. Effect of maternal 25(OH)D concentrations on placental maintenance (PRB, ESR1, hCGβ, hPL) and vitamin D metabolism (CYP24A1, CYP27B1, GRα, VDR)
As shown in Table 3, the results of the analysis did reveal a significant association between baseline (V1) 25(OH)D concentrations and certain genes related to placental maintenance, to include PRB (pval=0.02) and hPL (pval=0.03), as well as GRα (pval=0.02), whose role is known in vitamin D metabolism. However, the analysis did not reveal a significant difference for the mRNA expression of ESR1 or hCGβ between the group with circulating 25(OH)D <100 nmol/L vs. ≥100 nmol/L group. Likewise, no significant difference was found in other specific genes involved in vitamin D metabolism, including CYP24A1, CYP27B1, or VDR.
4. Discussion
The major finding of our study was a statistically significant association between maternal vitamin D sufficiency (as defined by a total circulating 25(OH)D concentration >40 ng/mL) and decreased expression (downregulation) of VEGF and sFlt-1 as compared to the expression in deficient mothers, which is the first report of this association. Notably, maternal vitamin D sufficiency at baseline and at the last visit prior to delivery (V6/7) were both associated with decreased expression of sFlt-1 at 0.05 level. Maternal vitamin D sufficiency at baseline and at last visit were also associated with decreased expression of VEGF. The expression of PRB, hPL and GRα were also significantly associated with vitamin D deficiency at baseline. We also observed an association between baseline maternal 25(OH)D concentrations and the expression of VEGF, PGF, sFlt-1, PRB and GRα at a significance level of 0.05.
Due to our limited sample size, none of these associations were significant after the Bonferroni adjustment for multiple testing, however, our results can still provide insights regarding the role of vitamin D in placenta gene regulation. Also, the Bonferroni adjustment is known to be conservative especially when the tests are correlated. In our paper, we found significant correlations among the expression of the eleven genes we considered. For example, the expression of sFlt-1 was associated with GRα (r=0.77, p<0.001) and the expression of VEGF was associated with PRB (r=0.80, p<0.001). Therefore, the Bonferroni approach will lead to overly conservative results for our study.
We can reason from this data set that if our mothers were vitamin D sufficient (≥100 nmol/L), their expression of sFlt-1 was lower than the expression of sFlt-1 in those mothers who were vitamin D deficient. This reduction in expression, with maternal vitamin D sufficiency, suggests the ability to potentially decrease sFlt-1 expression during pregnancy. Reduction in sFlt-1 demonstrates the potential for vitamin D3’s role in decreasing antiangiogenic factors that may contribute to vascular pregnancy complications, such as preeclampsia.
The association between high levels of sFlt-1 and maternal development of preeclampsia has been well demonstrated in both laboratory and clinical studies (24–29). As an antiangiogenic factor, sFlt-1 is known to cause vasoconstriction and endothelial damage in the placenta, thereby inducing a cascade of placental insufficiency, intrauterine fetal growth restriction and vascular comorbidities of pregnancy, such as preeclampsia. Likewise, PGF is often decreased in these disease processes, leading to the well cited relationship and diagnostic predictive value of the sFlt-1/PGF ratio. A higher ratio of sFlt-1 to PGF has been associated with an increased risk of preeclampsia (30–33).
Despite a small and insignificant decrease in the overall expression of PGF in women who were vitamin D sufficient compared to those who were deficient, the results of our study yielded evidence to support a relatively high expression in PGF in this cohort. More importantly, our study revealed the potential at sufficient concentrations of circulating 25(OH)D ≥100 nmol/L to reduce gene expression in sFlt-1, which may provide important clinical data to warrant maternal 25(OH)D monitoring/optimization throughout pregnancy—especially in women predisposed to vascular pregnancy complications. Our results revealed decreased expression of VEGF in women who were considered vitamin D sufficient, which has previously not been described. This may reflect a lower need for new vasculature toward the end of pregnancy/third trimester, as increased vascularization and expression of VEGF is most important in early gestation (34).
This was a candidate gene study, based on existing evidence of the roles of the selected genes in the biological pathways of vitamin D regulation, placental maintenance and vascular complications of pregnancy. Of course, there are limitations to the subjective nature of the selection of these genes secondary to our team’s specific interests, as there are a host of additional genes that could have been chosen for analysis. Further investigation of the role of vitamin D supplementation on placental vascular development and regulation throughout pregnancy is warranted in a larger population, as the independent contribution of a single gene, as compared to the influence and contribution of many other genes, is difficult to assess in this small sample size. However, our genes of interest and the results we obtained provide the foundation for future studies to expound on the effects of maternal 25(OH)D concentrations in relation to the development of vascular compromise/complications of pregnancy.
In terms of clinical limitations of this study, patient compliance/variation of vitamin D concentrations are innate to the study protocol. Each mother in the study obtained a pill count at each individual appointment throughout the course of the study. If the study individuals were not at least 75% compliant with their supplement, they were exited from the study. As the study remains blinded, treatment allocation remains unknown; however, maternal circulating 25(OH)D concentrations were measured on a monthly basis for safety reasons and are the best indicator of vitamin D status (35).
Additionally, placental biopsy sampling yields a possible laboratory limitation. Pidoux et al. describes the intra- and inter-placental variability that may occur as a result of full thickness placental biopsies (20). This was a key reason for the specifications of biopsy sampling sites among our population; however, the limits of processing biopsies that encompass both maternal and fetal tissue remain. As each placental sample was frozen, stored and homogenized using the same method, it is unlikely that this added to the potential discrepancy of mRNA expression variability, especially amongst those genes for which mRNA expression was not statistically significant between the two maternal groups. It is unclear whether no (or little) change in mRNA expression could indicate an increase on the maternal tissue but a decrease on the fetal side, or vice versa. While there are reports of histological separation of placental tissue at the fetal and maternal interface, to our knowledge technology does not yet exist for gross sample separation.
While this study revealed an early molecular basis in favor of vitamin D sufficiency during pregnancy, there is a great deal of work to be done for clinical relevancy. For example, protein analysis is needed to corroborate the mRNA results. Additionally, we expect the results of the overarching clinical study to provide a wealth of knowledge as to the maternal and neonatal outcomes of the supplemented mothers. Two of the women, out of the total 43 we observed, experienced preeclampsia with resultant preterm deliveries at 35 weeks and 36+3 weeks, respectively. While the sample size of this study is large enough to detect placental gene expression changes, it was not large enough to detect associative gene expression changes with clinical outcome.
4.1. Conclusions
Our data may serve to demonstrate the impact of maternal vitamin D3 supplementation on gene transcription in the placenta. The results are suggestive of the possibility to modify placental gene expression to improve placental health for those women at risk for suspected vascular disorders of pregnancy. This is the first step to further elucidate the role of maternal supplementation with vitamin D3 in factors relating to placental health.
Highlights.
Definition of maternal 25(OH)D sufficiency during pregnancy is reviewed.
Placental changes in vascular disorders of pregnancy are discussed.
Sufficient 25(OH)D concentrations in relation to placental expression is presented.
Vitamin D3 supplementation impact on placental gene transcription is highlighted.
Improving outcomes of suspected vascular disorders of pregnancy is offered.
Acknowledgments
Thank you to study coordinators Judy Shary and Nina Forestieri. Additional gratitude is extended to the laboratory mentorship by Drs. John Baatz, Martin Hewison and Bruce Hollis, as well as senior mentorship in biostatistics by Dr. Elizabeth Garrett-Mayer.
Funding
This work was supported by the W.K. Kellogg Foundation and National Institutes of Health (grant UL1TR0000062), The South Carolina Clinical & Translational Research Institute (SCTR) at the Medical University of South Carolina [which is funding by the National Institutes of Health (NIH), National Center for Advancing Translational Science (NCATS Grant UL1TR001450)], and the Guillette Lab within the National Institute of Standards and Technology’s Hollings Marine Laboratory. None of the above funding sources had involvement in the study design; collection, analysis or interpretation of data; in writing of the report; and the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollis BW, Wagner CL. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int. 2013;92(2):128–39. doi: 10.1007/s00223-012-9607-4 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4(3):208–30. doi: 10.3390/nu4030208 PubMed PMID: ; PubMed Central PMCID: PMC3347028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner CL, Baggerly C, McDonnell S, Baggerly KA, French CB, Baggerly L, et al. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009–2011 rates. The Journal of steroid biochemistry and molecular biology. 2016;155(Pt B):245–51. doi: 10.1016/j.jsbmb.2015.10.022 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar LM, Platt RW, Simhan HN. Early-Pregnancy Vitamin D Deficiency and Risk of Preterm Birth Subtypes. Obstetrics and gynecology. 2015. doi: 10.1097/AOG.0000000000000621 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. The Journal of clinical endocrinology and metabolism. 2007;92(9):3517–22. Epub 2007/05/31 doi: 10.1210/jc.2007-0718 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients. 2016;8(5). doi: 10.3390/nu8050301 PubMed PMID: ; PubMed Central PMCID: PMCPMC4882713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2341–57. Epub 2011/06/28 doi: 10.1002/jbmr.463 PubMed PMID: ; PubMed Central PMCID: PMCPMC3183324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflammation research : official journal of the European Histamine Research Society [et al. ]. 2014;63(10):803–19. Epub 2014/07/23 doi: 10.1007/s00011-014-0755-z PubMed PMID: ; PubMed Central PMCID: PMCPMC4160567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutrition research (New York, NY). 2011;31(1):48–54. Epub 2011/02/12 doi: 10.1016/j.nutres.2010.12.001 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Shining light on the vitamin D: Cancer connection IARC report. Dermato-endocrinology. 2009;1(1):4–6. Epub 2010/01/05 PubMed PMID: ; PubMed Central PMCID: PMCPMC2715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. Journal of the American College of Cardiology. 2011;58(2):18692 Epub 2011/07/02 doi: 10.1016/j.jacc.2011.02.051 PubMed PMID: ; PubMed Central PMCID: PMCPMC3896949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. The Journal of clinical endocrinology and metabolism. 2007;92(9):3517–22. Epub 2007/05/31 doi: 10.1210/jc.2007-0718 PubMed PMID: ; PubMed Central PMCID: PMCPMC4288954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology (Cambridge, Mass). 2009;20(5):720–6. Epub 2009/05/20 doi: 10.1097/EDE.0b013e3181a70f08 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. American journal of obstetrics and gynecology. 2010;203(4):366 e1–6. Epub 2010/08/10 doi: 10.1016/j.ajog.2010.06.036 PubMed PMID: ; PubMed Central PMCID: PMCPMC3192365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirzakhani H, Litonjua AA, McElrath TF, O’Connor G, Lee-Parritz A, Iverson R, et al. Early pregnancy vitamin D status and risk of preeclampsia. The Journal of clinical investigation. 2016;126(12):4702–15. Epub 2016/11/15 doi: 10.1172/jci89031 PubMed PMID: ; PubMed Central PMCID: PMCPMC5127689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Garawi A, Carey VJ, Chhabra D, Mirzakhani H, Morrow J, Lasky-Su J, et al. The Role of Vitamin D in the Transcriptional Program of Human Pregnancy. PloS one. 2016;11(10):e0163832 Epub 2016/10/07 doi: 10.1371/journal.pone.0163832 PubMed PMID: ; PubMed Central PMCID: PMCPMC5053446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Association of maternal vitamin D and placenta growth factor with the diagnosis of early onset severe preeclampsia. American journal of perinatology. 2013;30(3):167–72. Epub 2012/08/10 doi: 10.1055/s-0032-1322514 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Archives of biochemistry and biophysics. 2012;523(1):9–18. Epub 2011/11/22 doi: 10.1016/j.abb.2011.11.003 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson DM, Sadovsky Y. The correlation between sampling site and gene expression in the term human placenta. Placenta. 2005;26(5):372–9. Epub 2005/04/27 doi: 10.1016/j.placenta.2004.07.003 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Pidoux G, Gerbaud P, Laurendeau I, Guibourdenche J, Bertin G, Vidaud M, et al. Large variability of trophoblast gene expression within and between human normal term placentae. Placenta. 2004;25(5):469–73. Epub 2004/04/15 doi: 10.1016/j.placenta.2003.10.016 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 21.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer research. 2004;64(15):5245–50. Epub 2004/08/04 doi: 10.1158/0008-5472.can-04-0496 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Yuan JS, Reed A, Chen F, Stewart CN Jr. Statistical analysis of real-time PCR data. BMC bioinformatics. 2006;7:85 Epub 2006/03/01 doi: 10.1186/1471-2105-7-85 PubMed PMID: ; PubMed Central PMCID: PMCPMC1395339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–8. Epub 2002/02/16 doi: 10.1006/meth.2001.1262 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. American journal of obstetrics and gynecology. 2007;196(4):396 e1–7; discussion e7 Epub 2007/04/04 doi: 10.1016/j.ajog.2006.12.024 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Moore AG, Young H, Keller JM, Ojo LR, Yan J, Simas TA, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(12):2651–7. Epub 2012/08/07 doi: 10.3109/14767058.2012.713055 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney international. 2007;71(10):959–61. Epub 2007/05/15 doi: 10.1038/sj.ki.5002281 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England journal of medicine. 2006;355(10):992–1005. Epub 2006/09/08 doi: 10.1056/NEJMoa055352 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350(7):672–83. Epub 2004/02/07 doi: 10.1056/NEJMoa031884 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 29.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–9. Epub 2012/01/21 doi: 10.1161/circulationaha.111.054361 PubMed PMID: ; PubMed Central PMCID: PMCPMC3319742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. American journal of obstetrics and gynecology. 2007;196(3):239 e1–6. Epub 2007/03/10 doi: 10.1016/j.ajog.2006.10.909 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Engels T, Pape J, Schoofs K, Henrich W, Verlohren S. Automated measurement of sFlt1, PlGF and sFlt1/PlGF ratio in differential diagnosis of hypertensive pregnancy disorders. Hypertension in pregnancy. 2013;32(4):459–73. Epub 2013/08/21 doi: 10.3109/10641955.2013.827205 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Stepan H, Hund M, Gencay M, Denk B, Dinkel C, Kaminski WE, et al. A comparison of the diagnostic utility of the sFlt-1/PlGF ratio versus PlGF alone for the detection of preeclampsia/HELLP syndrome. Hypertension in pregnancy. 2016:1–11. Epub 2016/03/31 doi: 10.3109/10641955.2016.1141214 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. The New England journal of medicine. 2016;374(1):13–22. Epub 2016/01/07 doi: 10.1056/NEJMoa1414838 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Gram A, Hoffmann B, Boos A, Kowalewski MP. Expression and localization of vascular endothelial growth factor A (VEGFA) and its two receptors (VEGFR1/FLT1 and VEGFR2/FLK1/KDR) in the canine corpus luteum and uteroplacental compartments during pregnancy and at normal and induced parturition. General and comparative endocrinology. 2015;223:54–65. Epub 2015/09/29 doi: 10.1016/j.ygcen.2015.09.020 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Zerwekh JE. Blood biomarkers of vitamin D status. The American journal of clinical nutrition. 2008;87(4):1087S–91S. Epub 2008/04/11 PubMed PMID: . [DOI] [PubMed] [Google Scholar]