Abstract
Acute myeloid leukemia (AML) is an aggressive hematological malignancy with limited treatment options. Inflammation is often a contributing factor to the development and progression of AML, and related diseases, and can potentiate therapy failure. Previously, we had identified anti-inflammatory roles and anti-AML efficacy for blueberry extracts. The present study extended these observations to determine that the polyphenol quercetin inhibited neutral sphingomyelinase (N-SMase) activity and exerted anti-AML efficacy. Moreover, quercetin was shown to exert combinatorial anti-AML efficacy with nanoliposomal ceramide. Overall, this demonstrated that quercetin could block the pro-inflammatory actions of N-SMase and augment the efficacy of anti-AML therapeutics, including ceramide-based therapeutics.
Keywords: acute myeloid leukemia, blueberry, quercetin, neutral sphingomyelinase, nanoliposomal ceramide
Introduction
Inflammation has been linked to the development and progression of myelodysplastic syndrome (MDS) and AML [1–5], including the emergence of drug resistance [6]. Shin et al. demonstrated that AML1-ETO leukemogenesis could be mediated in part by loss of TLE4, which results in an upregulation of a Wnt-mediated pro-inflammatory phenotype [7]. In their study, inhibition of pro-inflammatory cyclooxygenase activity reversed the pro-leukemogenic phenotype triggered by TLE4 knockdown. This highlighted a key role for inflammation in the development of AML arising from the t(8;21) chromosomal translocation. Moreover, the Shanghai Health Study recently indicated that immune-mediated inflammation was linked to the development of aplastic anemia, MDS and AML following exposure to benzene [8]. In another study, Ye et al. showed that adipose tissue-resident chronic myeloid leukemia stem cells exhibited a pro-inflammatory phenotype and could evade chemotherapy [9]. Likewise, it was recently shown that a deregulated pro-inflammatory cytokine environment exists in patients with MDS and that this can be augmented upon treatment with hypomethylating therapies [10]. Altogether, these studies highlight the importance of inflammation in the pathogenesis of malignant myeloid hematological diseases and further indicate that inflammation may contribute to therapy failure.
Recently, we demonstrated that blueberry extracts could exert anti-AML therapeutic efficacy utilizing cell lines, primary patient samples, and multiple in vivo models [11]. Blueberries are rich sources for polyphenols, and both blueberries and polyphenols have been well-recognized for their health benefits [12,13]. Earlier, we had used models of neuroinflammation to show that blueberry extracts could inhibit neutral sphingomyelinase (N-SMase) and NADPH oxidase (NOX) activity [14,15]. N-SMase is an enzyme that liberates ceramide from sphingomyelin at the plasma membrane [14,16]. Its activity can be triggered by inflammatory mediators, growth factors, as well as chemotherapeutics. Ceramide is a bioactive sphingolipid that is widely recognized to induce cellular stress and apoptosis and its generation has long been noted in response to chemotherapy [17–19]. Importantly, ceramide can play a role in growth factor signaling as an integral part of lipid microdomains, also known as rafts, which are necessary to bring together components of these signaling pathways [17,18]. The ability for N-SMase-generated ceramide to promote inflammation presents a therapeutic conundrum as there is a growing interest in the use of ceramide-based therapeutics for the treatment of cancer and leukemia [17]. However, ceramide may exert differential effects depending on its subcellular localization and its metabolism to the profoundly pro-inflammatory bioactive sphingolipid ceramide-1-phosphate [20].
In the present study, we identified the polyphenol quercetin as a component of blueberry extracts that inhibits N-SMase activity. We then evaluated anti-AML efficacy for the combination of quercetin and nanoliposomal ceramide (Lip-C6). Altogether, this reveals that the anti-AML therapeutic efficacy of ceramide-based therapeutics such as Lip-C6 can be augmented by co-treatment with an anti-inflammatory/N-SMase compound.
Materials and Methods
Cell Culture
Murine C1498 and 32D-FLT3-ITD cells, and human U937 cells were maintained at 37°C, and 5% CO2, in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Human KG-1 cells were likewise maintained, in IMDM supplemented with 20% FBS and 1% penicillin/streptomycin.
Nanoliposome formulation
Nanoliposomes were prepared by the Penn State College of Medicine Drug Discovery Core following previously established methods. All lipids were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Ghost nanoliposomes (Lip-Ghost) and Lip-C6 were prepared as previously described [19,21]. Briefly, lipids dissolved in chloroform, or other organic solvents, were combined in specific molar ratios, dried to a film under a stream of nitrogen, and then hydrated by addition of 0.9% NaCl. Solutions were sealed, heated at 60°C (60 min), and subjected to vortex mixing and sonicated until light no longer diffracted through the suspension. The lipid vesicle-containing solution was quickly extruded at 60°C by passing the solution 10 times through 100 nm polycarbonate filters in an Avanti Mini-Extruder. Nanoliposomal size and integrity was determined using a Malvern Zetasizer Nano ZS at 25°C. Nanoliposome formulations were stored at room temperature until use.
In Vitro Assays
Cellular viability assays were performed as previously described using a Cell Titer 96 AQueous Non-Radioactive Cell Proliferation Assay according to the manufacturer’s instructions (Promega, Madison, WI) [11,19,21]. N-SMase assays were performed as previously described using a Sphingomyelinase Inhibitor Screening kit from Cayman Chemical (Ann Arbor, MI) according to the manufacturer’s instructions [14]. An ELISA assay was performed using previously established methods adapted using a specific cysteine sulfenic acid monoclonal antibody from Millipore (Billerica, MA) [15].
Blueberry Extraction and Fractionation
Solvents and reagents were obtained from VWR (Radnor, PA) and Sigma (St. Louis, MO). Vaccinium uliginosum was harvested in the interior of Alaska for extraction as previously described [14,15]. Briefly, whole berries were lyophilized, crushed to powder, and a crude extract was prepared by extracting with aqueous acetone (70/30 acetone/water), and dried by rotory-evaporation and lyophilization. For fractionation, crude extracts were separated by silica gel chromatography. Fractions were collected by elution with 80/20 dichloromethane/methanol, assessed individually by thin layer chromatography (TLC), and dried by rotary evaporation. Fraction 1 was further separated by silica gel flash column chromatography, eluted using 92/8 dichloromethane/methanol followed by pure methanol, and TLC was used to assess fractions (Figure 1A-B). The N-SMase assay was used through the fractionation process to define inhibitory bioactive fractions using an inflammation-stimulated SH-SY5Y neuroblastoma cell model (unpublished). Fraction 1,28 underwent a final clean-up purification by silica gel flash column chromatography, where elution with 85/15 dichloromethane/methanol yielded a relatively pure compound that was characterized by LC-MS (ESI, Q-TOF, in positive ion mode) and 1H-NMR and identified as quercetin-3-O-arabinoside (Figure 1C).
Figure 1.
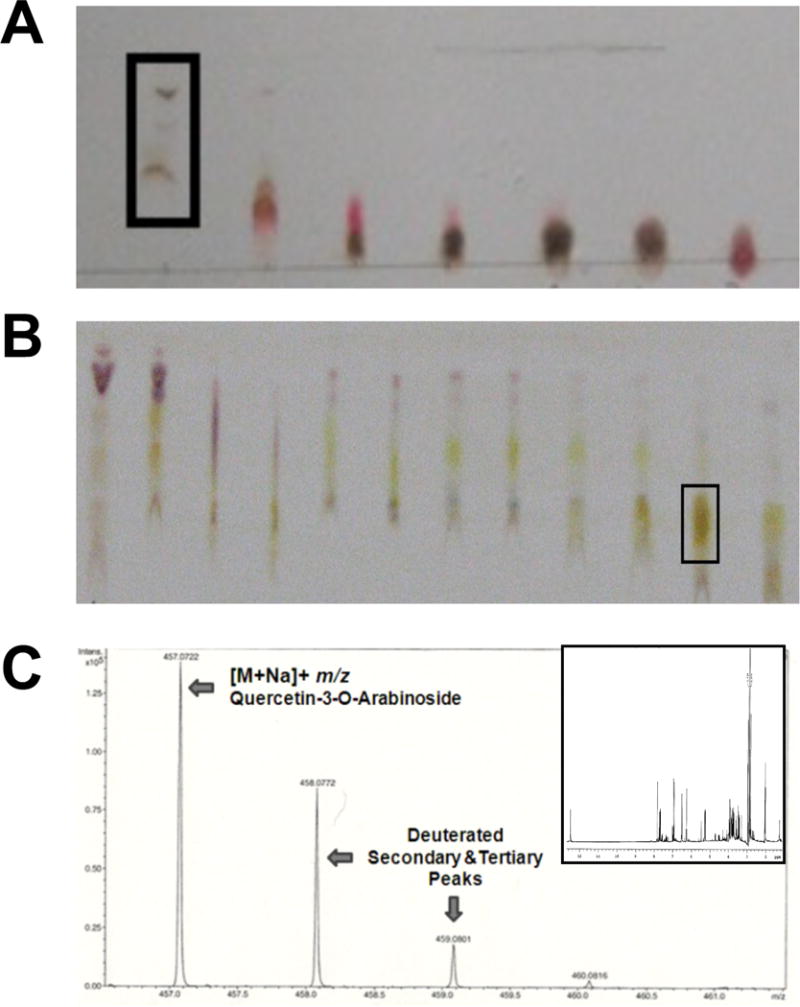
Crude blueberry extract was separated by repeated silica column fractionation and a N-SMase assay was used to determine fractions that inhibited enzyme activity. (a) Representative thin layer chromatography of the initial fractionation using dichloromethane/methanol as the solvent (92/8) with fraction #1 selected for further separation. (b) Representative thin layer chromatography of the second fractionation using dichloromethane/methanol as the solvent (85/15) with fraction #28 selected for further separation. (c) Following a clean-up fractionation, quercetin-3-O-arabinoside was identified by LC-MS (ESI, Q-TOF, in positive ion mode) and 1H-NMR (inset) as the primary component of fraction 1,28.
Data Analysis
CalcuSyn Software (Biosoft, Cambridge, UK) was used to determine combinatorial effects of treatments [21]. Cellular viability data was used for this analysis, and a Combination Index (CI) less than or equal to 0.9 was considered synergistic. CI values greater than or equal to 1.1 were considered antagonistic, whereas CI values between 0.9 and 1.1 were considered additive.
Results and Discussion
This study sought to identify a specific blueberry component with anti-AML activity. Given a potential role for N-SMase in inflammatory responses [14,16], a bioassay-directed approach was used to fractionate crude blueberry extracts to identify quercetin-3-O-arabinoside as a specific N-SMase-inhibiting compound (Figure 1). Inhibition of N-SMase by the parental compound quercetin was verified in AML cell lines (Figure 2A), and coincided with inhibition in cysteine oxidation (Figure 2B). Quercetin may reduce cysteine oxidation either through an antioxidant effect, or by limiting N-SMase-dependent oxidative effects such as those mediated by NOX [15,16,20]. Importantly, the combination of quercetin with Lip-C6 did not alter its ability to inhibit N-SMase or block cysteine oxidation. The ability of quercetin to inhibit N-SMase may prevent paradoxical pro-inflammatory/leukemogenic effects associated with ceramide generation. Therefore, therapies that stimulate or delivery ceramide to malignant cells may be more effective because that ceramide can more effectively exert its classical apoptotic program.
Figure 2.
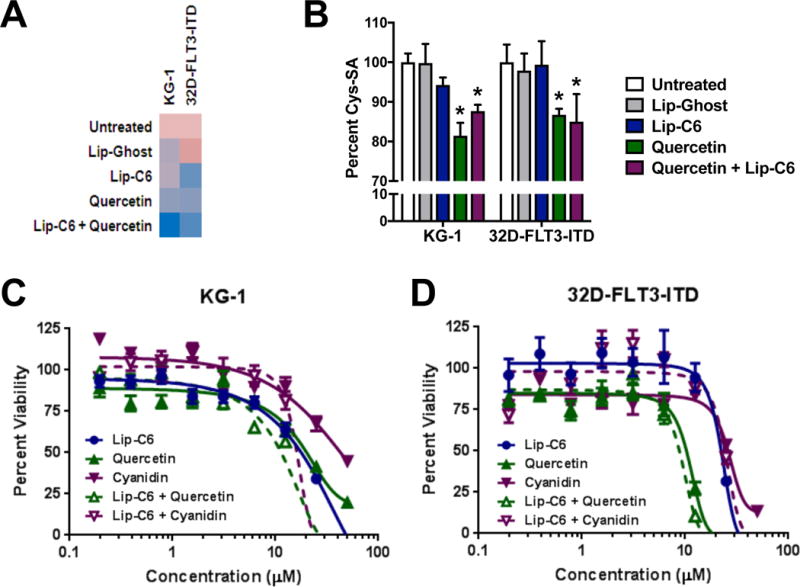
Quercetin inhibits N-SMase activity and exerts combinatorial anti-AML efficacy with Lip-C6. (a) N-SMase inhibition by quercetin was confirmed by measuring N-SMase activity in AML cell lines (KG-1, 32D-FLT3-ITD) following 4-hour exposure to respective controls, 10 μM liposomal C6-ceramide (Lip-C6), 10 μM quercetin, or the combination of both. Activity (fluorescence) per total protein content was normalized to the untreated controls, and a heat map was generated (pink: high activity, blue low activity). (b) Cellular cysteine sulfenic acid content (oxidation) was determined by ELISA. Following 4-hour exposure of KG-1 and 32D-FLT3-ITD cells to respective controls (white bar: untreated, gray bar: empty/ghost liposomes), 10 μM Lip-C6 (blue bar), 10 μM quercetin (green bar), or the combination of both (purple bar) (1-way ANOVA, *p<0.05 compared with both controls, n=4)). (c) Cellular viability was determined by MTS assay following 48-hour exposure of KG-1 cells to quercetin (green line), Lip-C6 (blue line), or the combination of both (dashed green line). Cyanidin (purple line), or a combination of cyaniding and Lip-C6 (dashed purple line) was used to compare a distinct polyphenol also found in the blueberry. (d) In a similar manner, cellular viability was assessed using the 32D-FLT3-ITD cell line.
Next, the anti-AML activity of quercetin was confirmed using AML cell lines (Figure 2C-D). Another polyphenol, cyanidin, was not as effective as an anti-AML agent as quercetin was. This may further suggest that quercetin is a polyphenol partially responsible for the anti-AML efficacy of blueberry extracts [11]. Notably, the combination of quercetin and Lip-C6 exerted a more profound anti-AML effect than either treatment alone or than the combination of Lip-C6 and cyanidin (Figure 2C-D). Finally, we conducted a combinatorial index analysis that showed the combination of quercetin and Lip-C6 was synergistic in KG-1 cells, but that the combination of cyanidin and Lip-C6 was not (Table 1). Overall, these results demonstrated anti-AML efficacy for the polyphenol quercetin, which may act synergistically with the ceramide-based therapeutic Lip-C6.
Table 1.
Combinatorial index analysis.
| Lip-C6 : Quercetin | Lip-C6 : Cyanidin | ||||||
|---|---|---|---|---|---|---|---|
| CI Value | S.D. | μM (1:1 ratio) | CI Value | S.D. | μM (1:1 ratio) | ||
| U937 | ED50 | 1.551 | 0.22 | 18.2 | 4.797 | 1.579 | 34.2 |
| ED75 | 2.051 | 0.364 | 31.6 | 2.943 | 1.117 | 41.5 | |
| ED90 | 2.739 | 0.693 | 55 | 2.419 | 1.124 | 50.2 | |
| C1498 | ED50 | 1.72 | 0.203 | 5.5 | 1.685 | 0.022 | 4.8 |
| ED75 | 1.979 | 0.289 | 9.7 | 1.862 | 0.024 | 8.1 | |
| ED90 | 2.279 | 0.542 | 17.3 | 2.067 | 0.042 | 13.5 | |
| KG-1 | ED50 | 0.772 | 0.3 | 6.4 | 1.073 | 0.267 | 11.9 |
| ED75 | 0.706 | 0.254 | 8.7 | 0.975 | 0.223 | 14.4 | |
| ED90 | 0.685 | 0.263 | 11.8 | 0.913 | 0.202 | 17.5 | |
| 32D-FLT3-ITD | ED50 | 1.073 | 0.098 | 6.9 | 1.675 | 0.313 | 19.2 |
| ED75 | 0.996 | 0.087 | 8.7 | 1.456 | 0.251 | 22.8 | |
| ED90 | 0.928 | 0.084 | 10.9 | 1.282 | 0.222 | 27.1 | |
Our prior studies highlighted the anti-AML, N-SMase-inhibiting, and NOX-inhibiting utility of blueberry extracts [11,14,15]. These studies noted that identification of the specific bioactive components from blueberry extracts responsible for these effects may be of significant interest to the development of minimally toxic therapies. This was thought to be due in part to the non-toxic consumption of these fruits. Moreover, compounds with specific anti-inflammatory bioactivity may be especially useful due to the specific role of inflammation in the pathogenesis of AML and related malignant hematological disorders [1–10]. Hence, the anti-inflammatory compounds from blueberry extracts may be useful because they can block inflammatory pathways that would otherwise negate the effects of cytotoxic therapies. In the case of quercetin, the blockade of N-SMase serves to diminish a potentially pro-inflammatory axis further mediated by ceramide kinase and NOX [15,20]. Consequently, therapies that stimulate ceramide generation, or deliver exogenous ceramide, may trigger the classical ceramide-mediated apoptotic pathways. Therefore, quercetin can augment and focus the anti-AML efficacy of Lip-C6 and other ceramide-based therapeutics.
Acknowledgments
The authors would like to thank Drs. Thomas P. Clauson and Thomas B. Kuhn of the University of Alaska-Fairbanks for their helpful insight during the blueberry extraction and fractionation process. We would like to thank Dr. Hubert Serve of the University of Münster for generously providing the 32D-FLT3-ITD cells. This study was funded in part by the National Institute for General Medical Sciences of the National Institutes for Health under award number P20-GM103395 (K.L.D.), the National Cancer Institute of the National Institutes for Health under award number K22-CA190674 (B.M.B.) and P01-CA171983 (M.K. and D.F.C.), as well as the Penn State University Kiesendahl Family Endowed Leukemia Research Fund (D.F.C.), the Kenneth Noel Memorial Fund (D.F.C.), and PA Tobacco Settlement funds (M.K. and D.F.C.). The Penn State Research Foundation has licensed ceramide-based delivery systems and nanotechnologies to Keystone Nano Inc. (State College, PA). M.K. serves as the C.M.O. of Keystone Nano, Inc.
References
- 1.Gañán-Gómez I, Wei Y, Starczynowski DT, Colla S, Yang H, Cabrero-Calvo M, Bohannan ZS, Verma A, Steidl U, Garcia-Manero G. Deregulation of innate immune inflammatory signaling in myelodysplastic syndromes. Leukemia. 2015 Jul;29(7):1458–69. doi: 10.1038/leu.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rankin EB, Narla A, Park JK, Lin S, Sakamoto KM. Biology of the bone marrow microenvironment and myelodysplastic syndromes. Mol Genet Metab. 2015 Sep-Oct;116(1-2):24–8. doi: 10.1016/j.ymgme.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes MR, Pereira JK, de Melo Campos P, Machado-Neto JA, Traina F, Saad ST, Favaro P. De novo AML exhibits greater microenvironment dysregulation compared to AML with myelodysplasia-related changes. Sci Rep. 2017 Jan;13(7):40707. doi: 10.1038/srep40707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Sejas DP, Zhang X, Qiu Y, Nattamai KJ, Rani R, Rathbun KR, Geiger H, Williams DA, Bagby GC, Pang Q. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007 Nov;117(11):3283–95. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickmann M, Macke L, Sundarasetty BS, Stamer K, Figueiredo C, Blasczyk R, Heuser M, Krauter J, Ganser A, Stripecke R. Monitoring dendritic cell and cytokine biomarkers during remission prior to relapse in patients with FLT3-ITD acute myeloid leukemia. Ann Hematol. 2013 Aug;92(8):1079–90. doi: 10.1007/s00277-013-1744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakker E, Qattan M, Mutti L, Demonacos C, Krstic-Demonacos M. The role of microenvironment and immunity in drug response in leukemia. Biochim Biophys Acta. 2016 Mar;1863(3):414–26. doi: 10.1016/j.bbamcr.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Shin TH, Brynczka C, Dayyani F, Rivera MN, Sweetser DA. TLE4 regulation of wnt-mediated inflammation underlies its role as a tumor suppressor in myeloid leukemia. Leuk Res. 2016 Sep;48:46–56. doi: 10.1016/j.leukres.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross SA, Paustenbach DJ. Shanghai Health Study (2001-2009): What was learned about benzene health effects? Crit Rev Toxicol. 2017 Dec;15:1–35. doi: 10.1080/10408444.2017.1401581. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, Stevens B, Pei S, Balys M, Ashton JM, Klemm DJ, Woolthuis CM, Stranahan AW, Park CY, Jordan CT. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016 Jul;19(1):7. 23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moudra A, Hubackova S, Machalova V, Vancurova M, Bartek J, Reinis M, Hodny Z, Jonasova A. Dynamic alterations of bone marrow cytokine landscape of myelodysplastic syndromes patients treated with 5-azacytidine. Oncoimmunology. 2016 May 13;5(10):e1183860. doi: 10.1080/2162402X.2016.1183860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGill CM, Brown TJ, Cheng YY, Fisher LN, Shanmugavelandy SS, Gustafson SJ, Dunlap KL, Lila MA, Kester M, Toran PT, Claxton DF, Barth BM. Therapeutic effect of blueberry extracts for acute myeloid leukemia. Int J Biopharm Sci. 2017 Oct 10;1:102. [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlap KL, Reynolds AJ, Duffy LK. Total antioxidant power in sled dogs supplemented with blueberries and the comparison of blood parameters associated with exercise. Comp Biochem Physiol A Mol Integr Physiol. 2006 Apr;143(4):429–34. doi: 10.1016/j.cbpa.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Kellogg J, Wang J, Flint C, Ribnicky D, Kuhn P, De Mejia EG, Raskin I, Lila MA. Alaskan wild berry resources and human health under the cloud of climate change. J Agric Food Chem. 2010 Apr;58(7):14. 3884–900. doi: 10.1021/jf902693r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson SJ, Barth BM, McGill CM, Clauson TP, Kuhn TB. Wild Alaskan blueberry extracts inhibit a magnesium-dependent neutral sphingomyelinase activity in neurons exposed to TNFa. Curr Top Nutraceutical Res. 2007;5(4):183–8. [Google Scholar]
- 15.Gustafson SJ, Dunlap KL, McGill CM, Kuhn TB. A nonpolar blueberry fraction blunts NADPH oxidase activation in neuronal cells exposed to tumor necrosis factor-alpha. Oxid Med Cell Longev. 2012;2012:768101. doi: 10.1155/2012/768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barth BM, Gustafson SJ, Kuhn TB. Neutral sphingomyelinase activation precedes NADPH oxidase-dependent damage in neurons exposed to the proinflammatory cytokine tumor necrosis factor-alpha. J Neurosci Res. 2012 Jan;90(1):229–42. doi: 10.1002/jnr.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barth BM, Cabot MC, Kester M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem. 2011 Nov;11(9):911–9. doi: 10.2174/187152011797655177. [DOI] [PubMed] [Google Scholar]
- 18.Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013 Jan;13(1):51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 19.Brown TJ, Garcia A, Kissinger LN, Shanmugavelandy SS, Wang X, Cabot MC, Kester M, Claxton DF, Barth BM. Therapeutic combination of nanoliposomal safingol with nanoliposomal ceramide for acute myeloid leukemia. J Leuk (Los Angel) 2013 Apr;1:26. 110. [Google Scholar]
- 20.Barth BM, Gustafson SJ, Hankins JL, Kaiser JM, Haakenson JK, Kester M, Kuhn TB. Ceramide kinase regulates TNFα-stimulated NADPH oxidase activity and eicosanoid biosynthesis in neuroblastoma cells. Cell Signal. 2012 Jun;24(6):1126–33. doi: 10.1016/j.cellsig.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth BM, Brown TJ, Adams MT, Garcia AM, Fisher LN, Fritz JL, Beck AJ, McGill CM, Kester M, Tran MA, Claxton DF. Combinatorial Efficacy of Nanoliposomal Ceramide and the Antioxidant 7,8-Benzoflavone for Acute Myeloid Leukemia. J Leuk (Los Angel) 2014 Sep;2:23. 152. [Google Scholar]


