Abstract
Focal adhesion kinase (FAK) plays a vital role in tumor cell proliferation, survival and migration. Altered metabolic pathways fuel rapid tumor growth by accelerating glucose, lipid and glutamine processing. Besides the mitogenic effects of FAK, evidence is accumulating supporting the association between hyper-activated FAK and aberrant metabolism in tumorigenesis. FAK can promote glucose consumption, lipogenesis, and glutamine dependency to promote cancer cell proliferation, motility, and survival. Clinical studies demonstrate that FAK-related alterations of tumor metabolism are associated with increased risk of developing solid tumors. Since FAK contributes to the malignant phenotype, small molecule inhibition of FAK-stimulated bioenergetic and biosynthetic processes can provide a novel approach for therapeutic intervention in tumor growth and invasion.
Keywords: Focal adhesion kinase, glucose, lipogenesis, glutamine, solid tumor, proliferation, motility, molecular targeting, small molecule inhibition
1. Introduction
Cell attachment to the extracellular matrix (ECM) is involved in fundamental activities from embryogenesis to tumorigenesis. Actin filaments are fastened to focal adhesions with the help of a multi-protein complex that “adheres” actin microfibers to the ECM proteins. Numerous proteins located in the focal adhesions or actin filament termini-ECM joint regions have been identified such as α-actinin, filamin, vinculin, fibronectin, integrin, talin, paxillin, and focal adhesion kinase (FAK). Focal adhesions are dynamic structures that constantly change or reorganize in response to microenvironmental cues such as ECM alterations, growth factors, and nutrient availability (Fletcher & Mullins, 2010). For example, integrin engagement or growth factor stimulation promotes FAK interaction with Src, leading to activation of downstream pathways such as Ras/MAPK signaling (Guan & Shalloway, 1992; Schlaepfer, Hanks, Hunter, & van der Geer, 1994). FAK interactions with integrins and growth factor receptors contribute to cell anchorage dependency, motility, and invasive growth, which are associated with malignant overgrowth and pro-survival.
Human FAK, encoded by the protein tyrosine kinase 2 gene, consists of N-terminal FERM, central catalytic, and c-terminal focal adhesion targeting (FAT) domains. The N- and C-terminal domains of FAK form an autoinhibitory structure (Lietha, et al., 2007). FAK interactions with membrane receptors including integrins and IGF1R are believed to induce a conformational switch of FAK to expose phosphorylation sites such as Y397. Phosphorylation of FAK at Y397 can promote FAK binding and phosphorylation of acceptor proteins including Src. FAK activation of Src can stimulate several signal transduction pathways such as PI3K-Akt, RAF/JNK, and Rho/Rac/PAK (J. Zhang, et al., 2013). Activation of those cascades modulates cell motility and survival. For example, Y397 phosphorylation of FAK plays a critical role in cell migration (Ritt, Guan, & Sivaramakrishnan, 2013). Casapse cleavage of FAK into two fragments results in removal of autoinhibition and release of the FAT domain (Gervais, Thornberry, Ruffolo, Nicholson, & Roy, 1998). The FAT fragment inhibits FAK activity through dephosphorylation and induces cell death.
Overexpression and hyperphosphorylation of FAK are associated with many types of solid tumors. Lethality of FAK knock out indicates an essential role of FAK in fetal development. In adult tissues, FAK levels are relatively low, but can be elevated during wound healing and transformation processes (Gates, King, Hanks, & Nanney, 1994; Moissoglu & Gelman, 2003; Nagaharu, et al., 2011; Tamura, et al., 2003). Hyperactivity of FAK can promote survival and motility, contributing to tumorigenicity and metastasis. For example, the levels of FAK expression were low in the normal colon or benign breast epithelium but high in the biopsies derived from patients with colon and metastatic breast cancer (Cance, et al., 2000; Lark, et al., 2005). Furthermore, the levels of FAK mRNA in normal tissues were very low, but were overexpressed in varied primary and metastatic tumors (Golubovskaya, Kweh, & Cance, 2009). FAK overexpression and phosphorylation were associated with Barrett’s associated esophageal adenocarcinoma, prostate-carcinoma, gastric cancer recurrence, squamous cell carcinoma, progression of hepatocellular carcinoma, thyroid cancer, small-cell lung carcinoma, and oral tumor cell invasion (Aprikian, Tremblay, Han, & Chevalier, 1997; Aronsohn, Brown, Hauptman, & Kornberg, 2003; Itoh, et al., 2004; S. J. Kim, et al., 2004; Lai, et al., 2010; Ocak, Chen, Callison, Gonzalez, & Massion, 2012; Rovin, Frierson, Ledinh, Parsons, & Adams, 2002; Schneider, et al., 2002; Watanabe, et al., 2008; Yuan, et al., 2010).
The mitogenic effects of FAK on cell proliferation and survival in normal and neoplastic tissues have been well accepted and reviewed (Hauck, Hsia, & Schlaepfer, 2002; Lietha, et al., 2007; G. Liu, et al., 2008; Schlaepfer, et al., 1994; Ucar & Hochwald, 2010; Zachary & Rozengurt, 1992). The role of FAK in metabolism under normal and disease conditions such as cancer has not been critically evaluated or summarized. Increasing evidence indicates a role for FAK modulation of glucose, lipid and glutamine metabolism that is likely essential for tumor cell rapid growth, survival and invasion.
2. FAK-promoted glucose consumption in neoplastic proliferation
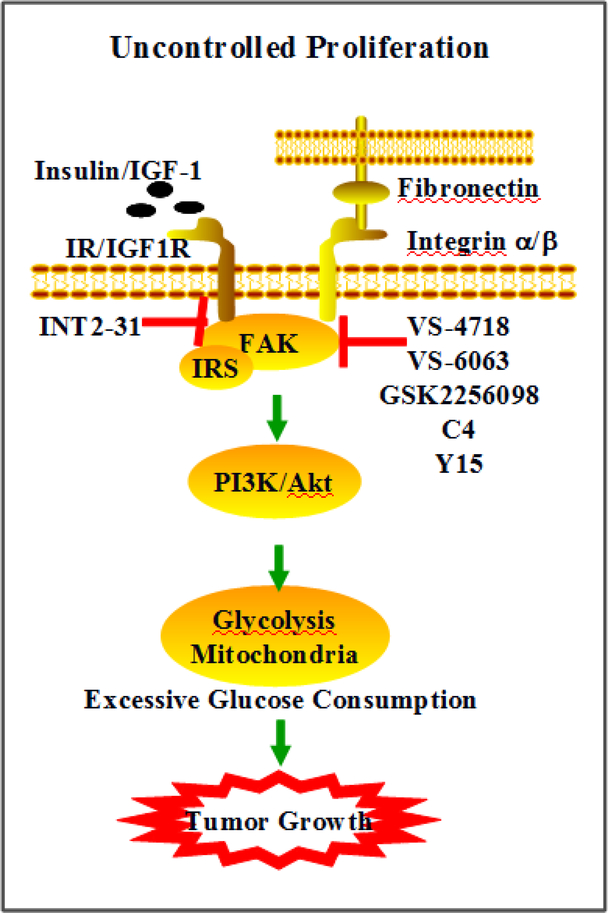
Growth factors such as insulin/IGF-1 and anchorage are primary extracellular cues that stimulate cell proliferation. FAK interactions with IGF-1R and integrins transmit these growth signals by activating effectors such as PI3K/Akt, promoting glucose consumption to fuel rapid growth in tumor cells (Fig 1).
Fig 1. FAK modulation of cancer cell glucose consumption and proliferation.
Growth factors, insulin/IGF1R, and/or anchorage-activated integrin trigger FAK activation. Downstream factors, IRS and PI3K/Akt induce alteration of glycolysis and mitochondrial respiration. Excessive glucose consumption provides energy and precursors to rapidly growing cells. Inhibitors targeting FAK or FAK-IGF1R interactions can prevent malignant cell glucose consumption and growth.
i). FAK modulation of insulin-stimulated glucose uptake
The direct binding of FAK with insulin receptor substrate (IRS) proteins and the role of FAK in controlling expression of insulin receptor substrate-1 (IRS-1) has been previously shown (Lebrun, Baron, Hauck, Schlaepfer, & Van Obberghen, 2000). Insulin binds to its receptors, triggering IRS-FAK activation and intracellular signal cascades that mediate a number of cellular processes including an increase in glucose transport (Baron, Calleja, Ferrari, Alengrin, & Van Obberghen, 1998; El Annabi, Gautier, & Baron, 2001; Goel & Dey, 2002; Huang, Cheung, Parsons, & Bryer-Ash, 2002; Knight, Yamauchi, & Pessin, 1995; Lebrun, et al., 2000; Ouwens, et al., 1996; Pillay, Sasaoka, & Olefsky, 1995). FAK activation reorganizes actin filaments to form a mesh harboring the glucose transporter. Translocation and activation of transporter 4 promotes glucose uptake through PI3K/Akt activation in skeletal muscle cells (Bisht & Dey, 2008). Furthermore, fibronectin activation of FAK through integrin-ECM interaction stimulates glucose uptake in endothelial cells (Paik, Ko, Jung, & Lee, 2009). In hepatocytes, FAK modulates glycogen synthesis by stimulating Akt/GSK-3β signaling (Huang, et al., 2002).
Oncogenes alter metabolic pathways, contributing to increased glucose uptake and dependency for cancer cell viability. This unique feature of malignant cells has been applied to tumor detection using positron emission tomography imaging with the radiolabeled glucose analog 18F-fluorodeoxyglucose. High levels of glucose increase integrin-ECM stimulation of FAK activity and enhances stem cell proliferation (Kroder, et al., 1996). On the other hand, glucose withdrawal induces aberrant tyrosine phosphorylation of focal adhesion protein in glucosedependent cell lines such as glioblastoma, sarcoma, and melanoma (Graham, et al., 2012). FAK modulation of insulin signaling is likely to be cell type specific since FAK activation is negatively correlated with glucose uptake in neuronal cells (Gupta, Bisht, & Dey, 2012).
Glucose is one of the major cellular sources of energy and building materials that are required for proliferation. FAK stimulation of glucose uptake is expected to be correlated with increased proliferation. Indeed, FAK overexpression or hyperactivation is common in solid tumors. In addition, tyrosine kinases including FAK modulate the levels of glucose transporters (Anichini, et al., 1997; Bisht & Dey, 2008) and the proliferation index (T. J. Liu, et al., 2007; Serrels, et al., 2012; Zhelev, et al., 2004) in muscle and tumor cells.
ii). FAK-IGF1R signaling in tumor glucose metabolism
IGF-1/IGF1R signaling has potent effects on cell survival through promoting proliferation and suppressing apoptosis. The metabolic effects of the IGF-1/IGF1R axis on glucose metabolism are less known. Several lines of observations support the role of the IGF-1/IGF1R cascades in glucose processing. First, IGF1R often forms complexes with insulin receptors. Thus, IGF1R can have influence on insulin stimulation of glucose consumption via the insulin receptor/IGF1R complex. Secondly, IGF-1 can directly bind with insulin receptors, indicating IGF signaling in modulation of glucose metabolism. Thirdly, IGF-1/IGF1R stimulation of rapid proliferation needs sustained energy and cellular biosynthesis. IGF1R stimulated glucose uptake can meet this high demand (Kuemmerle, 2012).
Activation of IGF-1R signaling is correlated with primary and metastatic malignancies such as prostate, breast, pancreatic, and lung cancer (Moser, et al., 2008; Putz, et al., 1999; Resnik, Reichart, Huey, Webster, & Seely, 1998; Warshamana-Greene, et al., 2005). The anti-apoptosis property of IGF1R-triggered kinase cascades contributes to cancer cell resistance to cytotoxicity and vascularization. When EGFR pathways are blocked by inhibitors such as Erlotinib, IGF1R can resume EGFR-related signaling and promote breast cancer survival. IGF1R-enhanced angiogenesis is involved in tumor invasion and metastasis (Ackermann, Morse, Delventhal, Carvajal, & Konerding, 2012; Kucab & Dunn, 2003; Menu, et al., 2007).
The Hochwald laboratory and others have demonstrated FAK interaction with and stabilization of IGF1R (Andersson, D’Arcy, Larsson, & Sehat, 2009; W. Liu, et al., 2008). The N-terminal FERM domain of FAK directly binds to IGF1R, leading to PI3K/Akt activation and survival. Small molecule inhibition of FAK-IGF1R interactions induces apoptosis and prevents tumor growth. Antibody-mediated inhibition of IGF1R signaling results in decreased Akt activity and glucose uptake (Shang, et al., 2008). Impaired kinase-independent biological functions of IGF1R leads to decreased intracellular glucose levels and viability of cells derived from human embryonic kidney and metastatic breast cancer (Janku, Huang, Angelo, & Kurzrock, 2013).
iii). Clinical associations of increased glucose levels and cancer risk
Increased glucose uptake and associated metabolism are hallmarks of malignancy. Abnormal IGF1R signaling can contribute to tumor metabolic pathways since increased IGF1R mass and/or activity may enhance insulin receptor-induced glucose utilization. IGF1R signaling may promote the shift of cellular balance favoring biosynthesis to support neoplastic proliferation. Several studies show that high glucose levels are correlated with increased cancer risk (Chocarro-Calvo, Garcia-Martinez, Ardila-Gonzalez, De la Vieja, & Garcia-Jimenez, 2013; Kabat, et al., 2012; Wulaningsih, et al., 2013). A large clinical study (540,309 participants) demonstrated that high serum glucose levels were linked with increased risks of development of colon cancer (Wulaningsih, et al., 2012). Quartile analysis indicated a positive association between glucose levels and risks of kidney cancer (Van Hemelrijck, et al., 2012). These observations demonstrate the possible need for monitoring aberrant glucose levels or metabolism to identify individuals at high risk of developing certain types of malignancies such as colon cancer (Aleksandrova, et al., 2011).
3. Lipid-FAK interactions in tumorigenesis
i). The role of lipids in FAK-promoted tumor motility and invasive growth
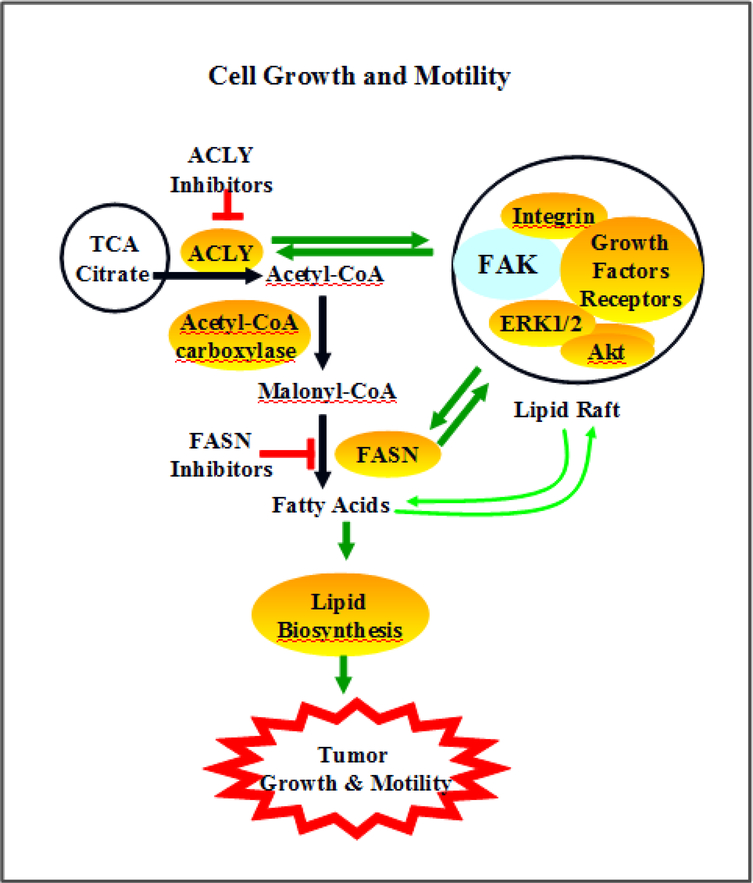
Lipid metabolism can affect the scaffolding and kinase functions of FAK (Fig 2). Lipids in the cell membrane provide the necessary microenvironment for FAK interactions with receptors such as integrins and IGF1R. In addition, lipid rafts can facilitate the translocation of FAK-associated complexes in FAK-promoted signal transduction. For example, lipid rafts are associated with FAK stimulation of ERK1/2 and neurite outgrowth (Niethammer, et al., 2002). Lipid rafts and FAK interactions contribute to cell adhesion signaling (Shima, Nada, & Okada, 2003).
Fig 2. The roles of FAK in lipid-mediated tumor growth and invasion.
FAK interactions with receptors such as IR/IGF1R/integrin and effectors such as PI3K/Akt/ERK are associated with lipid rafts. Formation and translocation of FAK-associated lipid complexes contributes to ACLY and FASN activation, channeling TCA-processed glucose to promote lipogenesis. Excessive lipid biosynthesis can induce cell growth and lipid turnover-mediated motility. Inhibition of ACLY and FASN leads to decreased lipid biosynthesis and tumor growth/invasion.
FAK activation and association with Src family members can transmit growth cues by forming complexes with membrane-bound receptors such as integrins, IGF1R, and EGFR in lipid rafts. Indeed, translocation of the neuronal cell adhesion molecule from FGFR complexes into FAK-associated lipid rafts promotes focal adhesion assembly, cell motility and invasive growth (Lehembre, et al., 2008). Disrupting the lipid rafts attenuates EMT and cell spreading (Lehembre, et al., 2008). A synthetic lipid analog promotes invasiveness of colon cancer cells through upregulation of integrin-FAK phosphorylation/activation, interactions, and scaffolds (Van Slambrouck, et al., 2007).
ii). FAK and lipid metabolism in neoplastic growth
FAK can affect lipid metabolism by controlling the supply of precursors and enzyme activity of proteins involved in lipid synthesis. Citrate is exported from mitochondria into the cytosol and converted to fatty acid (Fig 2). Growth factor and anchorage-dependent activation of FAK enhances glucose uptake. This can maintain the carbon supply for increased lipid synthesis in proliferative cells.
FAK and fatty acid biosynthesis:
Citrate conversion to acetyl-CoA, malonyl-CoA, long chain fatty acid, and unsaturated fatty acid oleate involves ACLY, acetyl-CoA carboxylase, fatty acid synthetase, and stearoyl-CoA desaturase (Fig 2). Aberrant enzyme activity and de novo lipogenesis are associated with many types of solid tumors such as lung, gastric, and breast cancers (Migita, et al., 2008; Varis, et al., 2002; Yancy, et al., 2007). SREBP-1 activation of PI3K/Akt signaling and Myc-regulated glutaminolysis to lipid metabolism are linked to metabolic reprogramming in cancer cells (Guo, Bell, Mischel, & Chakravarti, 2013). Inhibition of key lipogenic enzymes, ACLY and fatty acid synthetase, decreases FAK, Akt, and paxillin activity and cell motility (Zaytseva, et al., 2012). Insulin activation of ACLY involves FAK-induced phosphorylation/translocation of insulin receptors (Brownsey, Edgell, Hopkirk, & Denton, 1984). Depletion of raft cholesterol impairs chemokine and growth factor stimulated FAK recruitment and adhesion, thus, contributing to anoikis like apoptosis (J. H. Jeon, et al., 2010; Le, Honczarenko, Glodek, Ho, & Silberstein, 2005; E. K. Park, et al., 2009; Ramprasad, et al., 2007).
iii). Lipids and cancer risk
Cancer and proliferating cells have enhanced biosynthesis of fatty acids by channeling glucose and/or glutamine into the TCA cycle and upregulation of lipid biosynthetic enzymes (Ridgway, 2013). The levels of certain lipid components and lipogenic enzymes are associated with the risks of kidney and breast cancer (Van Hemelrijck, et al., 2012; Wang, et al., 2013). High fat diets stimulate bile acid secretion into the gastrointestinal tract. Bile acids are correlated with colon cancer, and lipid-lowering drugs may reduce the risk of colorectal tumor (Cai, Dupertuis, & Pichard, 2012; McMichael & Potter, 1985; Simon, et al., 2012; van Duijnhoven, et al., 2011). At high physiological levels, the bile acid deoxycholic acid, induced colonic tumor formation in mice (Bernstein, et al., 2011). Deoxycholic acids decreased phosphorylation of FAK at tyrosine-576/577 (Tyr-576/577) and Tyr-925, promoted Src binding with FAK, and triggered inside-out signaling in colon cancer cells (Khare, Holgren, & Samarel, 2006). FAK interactions with Src can induce downstream cascades including PI3K/Akt. Indeed, bile acid-induced colon cancer is likely associated with PI3K/Akt signaling-increased survival and proliferation (Raufman, Shant, Guo, Roy, & Cheng, 2008).
4. FAK-associated deregulation of glutamine metabolism in cancer cell survival and proliferation
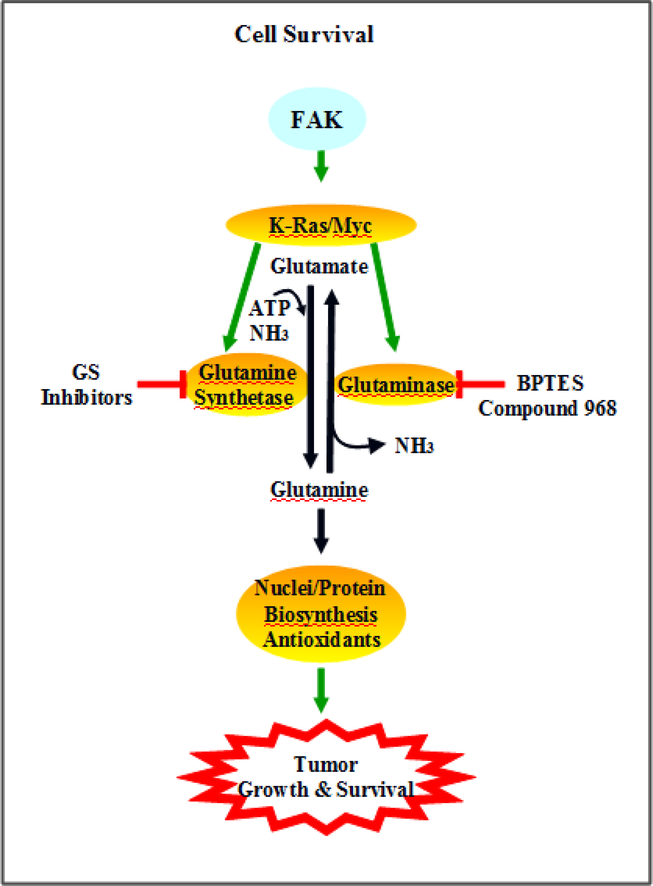
Many cancer cells such as pancreatic ductal adenocarcinoma rely on glutamine for their survival and biosynthetic needs (Wilson, Erickson, Antonyak, & Cerione, 2013). Although the direct link of FAK activation and glutamine deregulation warrants further investigation, current data suggests that the scaffold and kinase functions of FAK can contribute to cell sensing microenvironmental cues and modulation of amino acid and protein metabolism. For example, FAK activation is associated with K-Ras and H-Ras induced transformation of NIH3T3 cells and rat fibroblasts (J. Jeon, et al., 2007). In addition, aberrant activation of oncogenes such as Myc and K-Ras mediate reprograming of glutamine metabolism (Son, et al., 2013; Wise & Thompson, 2010) (Fig 3). FAK hyper-activation is associated with uncontrolled cancer survival and proliferation. Targeting malignancy-specific glutamine consumption provides an unique approach to attack solid tumors.
Fig 3. FAK activation and cancer dependency on glutamine.
FAK activation of oncogenes, K-Ras and Myc, alters the activities of glutamine synthetase and glutaminase. Increased glutamine flux provides precursors for nuclei acid and protein synthesis that are essential for cell proliferation. Furthermore, cancer cells rely on glutamine consumption to generate antioxidants, that neutralize rapid growth-accelerated ROS production, for their survival.
i). FAK and glutamine dependency for tumor growth and invasion
Proliferating cells consume glutamine to fuel the tricarboxylic acid cycle and provide nitrogen for nucleotide, nonessential amino acid and hexosamine biosynthesis (Fig 3). Cancer cells often develop dependency on specific amino acids (Fu, et al., 2003). For example, deprivation of tyrosine and phenylalanine in the medium induces apoptosis of melanoma cells through FAK-related signaling pathways (Fu, Yu, Pelayo, Ferrans, & Meadows, 1999). Glutamine metabolism is dramatically increased in Her2-type breast cancer (S. Kim, Kim do, Jung, & Koo, 2013). Oncogene K-Ras modulation of glutamine metabolism is essential for pancreatic cancer cell survival and growth (Son, et al., 2013). FAK interactions with Her2 promote tumorigenesis (Lark, et al., 2005; Vartanian, Goodearl, Lefebvre, Park, & Fischbach, 2000). Furthermore, micrometastatic cells express activated/phosphorylated FAK, Her2 and PI3K, suggesting the roles of Her2-FAK/Src-PI3K activation in malignant and invasive growth (Kallergi, Mavroudis, Georgoulias, & Stournaras, 2007; Vadlamudi, Sahin, Adam, Wang, & Kumar, 2003).
ii). The association of FAK activation and glutamine-modulated autophagy in cancer cell survival
Autophagy is a key strategy for cell survival under stress conditions such as nutrient deficiency. Degradation of non-essential and/or redundant cellular compartments leads to release of building blocks to fuel key energy and biosynthetic processes. Cancer cells can capture this machinery to retain their pro-survival and proliferative states. For instance, pancreatic cancer cells have elevated levels of autophagy, and suppression of autophagy prevents proliferation and tumor growth. FAK stimulates PI3K/Akt signaling; whereas PI3K/Akt activation increases the levels of glutamine and its synthetase (van der Vos, et al., 2012). Increased glutamine production promotes autophagy, survival and proliferation (Ko, et al., 2011; W. M. Liu, et al., 2013; Nicklin, et al., 2009; Sakiyama, Musch, Ropeleski, Tsubouchi, & Chang, 2009). These observations support the notion that growth factor stimulation of FAK-PI3K-Akt signaling contributes to cell survival and proliferation through upregulation of glutamine synthetase and autophagy.
iii). FAK modulation of glutamine metabolism in stress-resistant neoplastic cells
Rapid cell proliferation demands energy and building blocks. High levels of energy generation and biosynthesis are correlated with production of byproducts such as oxidants. In order to cope with excessive oxidants, FAK-overexpressing solid tumors must upregulate signaling pathways that promote antioxidant production. Growth factor-FAK-PI3K/Akt signaling resulting in increased glutamine synthetase and glutamine levels can serve as anti-oxidative machinery. Son et al., observed that glutamine deprivation suppresses pancreatic ductal tumor cell growth through a non-canonical pathway of glutamine consumption that is involved in serial conversion of glutamine-oxaloacetate-malate-pyruvate (Son, et al., 2013). This process leads to increased NADPH/NADP+ ratios, which has anti-oxidative activity. Antioxidants, GSH and N-acetylcysteine, abolish glutamine deficiency-suppressed cancer cell growth (Son, et al., 2013), suggesting that cancer cells use glutamine metabolism to maintain cellular redox balance. Glutamine has been reported to modulate cell protection against oxidative stress in intestinal epithelial cells (Musch, Hayden, Sugi, Straus, & Chang, 1998). Glutamine supplements attenuate 2,4,6-trinitrobenzene sulfonic acid-induced oxidative stress in a rat model of colitis (Crespo, et al., 2012). Increased flux of glutamine toward glutathione synthesis reduces oxidative stress in flies and human cell lines (Nicolay, et al., 2013). A direct link of FAK modulation of glutamine metabolism in neoplastic cells has not been shown at this time.
iv). The link between aberrant glutamine metabolism and tumorigenesis
Excessive glutamine consumption contributes to malignant survival, genomic instability, and unscheduled proliferation (Fernandez-Marcos & Serrano, 2013; Jeong, et al., 2013). Use of small molecules targeting glutamine metabolism allows linkage of the Rho GTPases and NF-κB to mitochondrial glutaminase hyper-activity in cancer cells (Wilson, et al., 2013). Abnormalities in glutamine metabolism has been linked to pancreatic, lung, and breast cancer, which is associated with oncogenes such as K-Ras, solute-linked carrier family A1 member 5 and Myc (Dang, 2013; Hassanein, et al., 2013; S. Kim, et al., 2013; Son, et al., 2013).
The role of FAK in glutamine deregulation-associated tumorigenesis include 1) activating glutamine metabolism-related oncogenes such as K-Ras, 2) relaying signals from oncogenes to glutamine metabolic enzymes, and 3) direct regulation of glutamine metabolism. FAK levels are often elevated in solid tumors. siRNA inhibition of FAK expression attenuates EGF and fibronectin-stimulated overexpression of oncogenes and cell cycle regulatory proteins, resulting in decreased cell migration and proliferation (J. H. Park & Han, 2009; J. H. Park, Ryu, & Han, 2011). Phosphorylation of FAK at tyrosine 407 negatively regulates FAK activity. Decreased Y407 phosphorylation is correlated with Ras transformation of fibroblasts, indicating that FAK activation stimulates Ras signaling (J. Jeon, et al., 2007; Wade, Brimer, Lyons, & Vande Pol, 2011). On the other hand, R-Ras promotes FAK signaling, and synergizes with alpha2beta1 integrin stimulation of FAK activation (Kwong, Wozniak, Collins, Wilson, & Keely, 2003). Furthermore, Myc activates FAK in neuroblastoma cells (Beierle, et al., 2007). Glutamine restriction inhibits FAK activity and impairs melanoma attachment and spreading, suggesting the involvement of FAK in glutamine metabolism-modulated malignant cell anchorage and motility (Fu, et al., 2004).
5. Targeting FAK-mediated metabolic signaling pathways
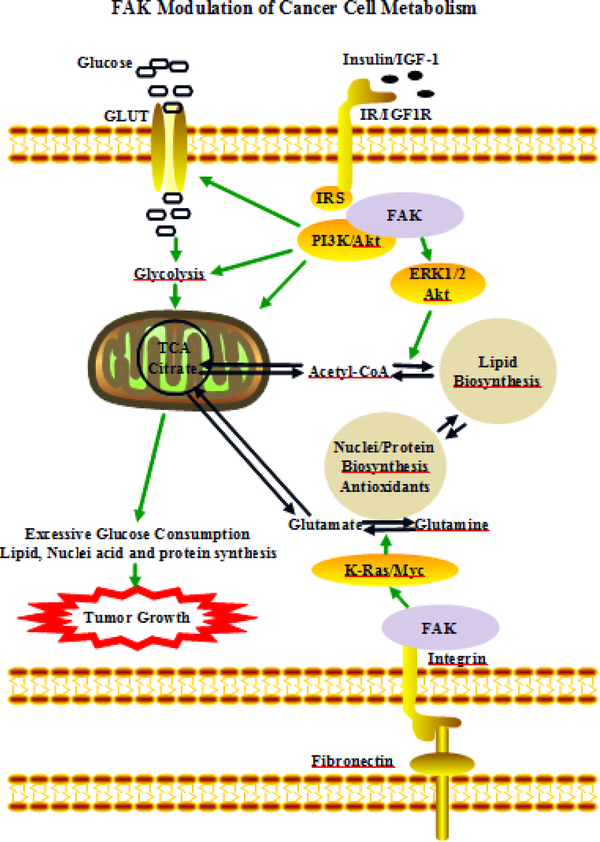
The interplay among glycolytic/mitochondrial glucose processes, lipid biosynthesis, and nucleotide/protein/antioxidant metabolism are dynamically balanced in response to a constantly changing microenvironment. FAK overexpression and hyperactivation can promote glucose metabolism to fuel cell proliferation in cancer cells (Fig 4). Targeting FAK activation and its interactions with proteins that are involved in glucose, fatty acid, and glutamine metabolism can be an attractive approach to combat tumor growth and metastasis. Inhibitors targeting FAK function, FAK binding with IGF1R, FAK-activated key lipogenic enzymes and glutamine synthetase have been developed (Vander Heiden, 2011). The NIH clinical trial database (clinicalTrial.gov) and PubMed have been searched to identify inhibitors targeting FAK activity and its metabolic pathways.
Fig 4. FAK modulation of cancer cell metabolism.
Insulin/IGF1 stimulates FAK-PI3K-Akt signaling through IRS. This modulates glucose metabolism via activation of glucose transporters, glycolytic and mitochondrial enzymes. Citrate can leave the TCA cycle in mitochondria and is converted to lipids. FAK activation of ERK/Akt can promote this conversion and channel glucose to lipids for the biosynthetic needs of rapidly growing cells. Anchorage-dependent stimulation of FAK activity contributes to K-Ras/Myc signaling-related glutamine metabolism.
i). FAK inhibitors
VS-6063 and VS-4718 are drug candidates, developed by Verastem, for FAK inhibition targeting cancer stem cells. A phase I/Ib clinical trial has been conducted with Paclitaxel in combination with the company’s first FAK inhibitor, defactinib or VS-6063, in subjects with advanced ovarian cancer. Verastem reports that VS-6063 was granted orphan drug status by the US FDA and European regulators for mesothelioma, an asbestos-related rare lung cancer with limited treatment options. Verastem also reports that VS-6063 was well tolerated at the dose of 400 mg BID in combination with weekly Paclitaxel. Previously, pre-clinical studies indicated that VS-6063 (formerly PF-04554878) reduced cancer stem cells, primary tumor mass and metastasis (Schultze & Fiedler, 2011). A recently completed Phase I clinical study shows that the FAK inhibitor, PF-00562271, is tolerated at a dose of 125 mg BID in patients with pancreatic, head and neck, prostatic neoplasms (Infante, et al., 2012).
VS-4718 is the second compound, developed by Verastem, currently under study in a Phase I clinical trial evaluating patients with metastatic non-hematologic malignancies.
GSK2256098 is a small molecule inhibitor of FAK developed by GlaxoSmithKline. A phase I clinical trial dose escalation study in subjects with solid tumors known to express FAK has been performed. Preliminary findings from the first trial of GSK2256098 in subjects with mesothelioma suggested that it had some effects on disease spread in patients lacking an active tumor suppressor gene, NF2 ((ECCO), 2012).
Numerous new FAK inhibitors have been designed, produced, and tested, including diarylamino-1,3,5-triazine, 1,3,4-oxadiazole, pyrazolo[4,3-c][2,1]benzothiazines derivatives, cFAK-C4 and Y15 (Dao, et al., 2013; Dunn, Heffler, & Golubovskaya, 2010; Ma, 2011; Schultze & Fiedler, 2010; Tomita, et al., 2013; S. Zhang, et al., 2013).
ii). Inhibitors targeting FAK activation of IR/IGF1R
Insulin and IGF-1 can stimulate FAK activity (Baron, et al., 1998); and FAK binds with and stabilizes IGF-1R. Therefore, FAK hyperactivity can promote insulin/IGF-1 signaling-mediated glucose metabolism in cancer cells. Blocking basic insulin signaling can cause serious metabolic disorders. Inhibitors targeting IGF1R have been developed in the attempt to attenuate tumor growth.
IGF1R inhibitors:
Several companies are currently testing small molecule kinase inhibitors targeting the IGF1R tyrosine kinase and many have had limited utility (Hewish, Chau, & Cunningham, 2009; Pollak, 2008). Over 50 clinical trials of IGF1R inhibitors in patients with many types of tumors have been registered with NIH (Clinicaltrials.gov, August 2013). Inhibitor names, tumor types, trial stages and statuses are summarized in Table 1. Approaches targeting the ATP competitive binding site have limitations due to lack of specificity for IGF1R. This is due to sequence homology and identity, particularly in the kinase domain, and structural similarity of IGF1R to other receptor tyrosine kinases such as the insulin receptor. A similar argument can be made for kinase inhibitors of FAK. In addition, it appears that disruption of the kinase domain is not sufficient to specifically interfere with the downstream signaling of IGF1R (or FAK) and it is unclear whether the kinase function or the scaffolding function of these proteins is more important (Golubovskaya, et al., 2012; Su, et al., 2013).
Table 1. Clinical trials of IGF1R inhibitors in subjects with cancer.
The NIH ClinicalTrials.gov database has been searched to identify studies on inhibitors targeting IGF1R signaling in solid tumors. Inhibitor names, tumor types, trial stages and their status/conclusions are summarized (clinicalTrial.gov)(Lacy, et al., 2008; Molife, et al., 2010; Scagliotti & Novello, 2012).
| Name | Tumor | Trial phase/NIH number | Status/conclusion |
|---|---|---|---|
| PL225B | Advanced Refractory Solid Tumors | Phase 1/NCT01779336 | Recruiting |
| OSI-906 | Breast Cancer | Phase 2/NCT01013506 | Withdrawn/toxicities and progressed |
| Multiple Myeloma | Pahse 1 & 2/NCT01672736 | Recruiting | |
| Lung Cancer | Phase 2/NCT01387386 | Recruiting | |
| AXL1717 | Non Small Cell Lung Cancer | Phase 1/NCT01466647 | Completed/No results posted |
| Glioblastoma, Gliosarcoma | Phases 1 & 2/NCT01721577 | Recruting | |
| Anaplastic Astrocytoma | |||
| Anaplastic | |||
| Oligodendroglioma | |||
| Anaplastic Oligoastrocytoma | |||
| Anaplastic Ependymoma | |||
| Non-small-cell Lung Cancer | Phase 2/NCT01561456 | Recruiting | |
| Squamous Cell Carcinoma | |||
| Adenocarcinoma of the Lung | |||
| Solid Tumors Hematological Malignancies | Phase 1/NCT01062620 | Completed/No results posted | |
| CP-751,871 | Carcinoma, Squamous Cell; | Phase 3/NCT00673049 | Terminated/progressed |
| Carcinoma, | |||
| Adenosquamous; | |||
| Carcinoma, Large Cell; | |||
| Carcinoma, Non-Small-Cell | |||
| Lung | |||
| Multiple Myeloma | Phase 1/NCT01536145 | Completed/tolerated | |
| Solid Tumors | Phase 1/NCT00474760 | Completed/tolerated | |
| RAD001 AMG479 | + Neoplasm Metastases | Phase 1/NCT01122199 | Recruiting |
| AVE1642 | Liver Carcinoma | Phases 1 & 2/NCT00791544 | Withdrawn/discontinued |
| MK-0646 | Non Small Cell Lung Cancer | Phase 2/NCT00799240 | On-going |
| Pancreatic Cancer | Phases 1 & 2/NCT00769483 | Recruiting | |
| R1507 | Neoplasms | Phase 1/NCT00400361 | Completed/tolerated |
| XL228 | Cancer, Lymphoma | Phase 1/NCT00526838 | Terminated/discontinued |
Targeting FAK-IGF1R interaction represents a novel approach to inhibit abnormal hyperactivation of survival signaling. Both FAK and IGF-1R are upregulated and promote the malignant phenotype making them appropriate targets for developmental therapeutics. We have shown that dual inhibition of FAK and IGF1R leads to a synergistic increase in cell detachment and apoptosis (Hochwald, et al., 2009; W. Liu, et al., 2008; Zheng, et al., 2010). However, selective dual small molecule inhibitors of both proteins are not available or demonstrate toxicity (Golubovskaya, et al., 2008; Kurenova, et al., 2009; Watanabe, et al., 2008). Inhibitors targeting FAK protein binding with IGF1R offer significant promise to inhibit the function of both FAK and IGF-1R proteins and be more efficacious than existing IGF-1R inhibitors that have failed testing in clinical trials. These FAK inhibitors may be associated with low rates of side effects for the following reasons: 1) FAK-IGF1R signaling can primarily stimulate abnormal survival signaling. Specific inhibition of FAK FERM domain binding with IGF1R should have minimal effects on FAK binding with other partners, indicating a limited impact on basic signaling. 2) FAK levels decrease after fetal development to very low levels in adult normal tissues; therefore, these inhibitors can have less impact on normal tissues.
Small organic molecules are particularly attractive as inhibitors of intracellular protein– protein interactions because of the ability to modify their structures to achieve optimal target binding, and because of their ease of delivery in in vivo systems. Several investigators have developed an approach for therapeutic intervention by targeting FAK-IGF1R protein-protein binding. Molecular docking, cell-based screening and xenograft mouse models have been used for the identification of lead compounds such as INT2–31 that inhibit FAK-IGF1R binding and tumor growth (Ucar, et al., 2012; Ucar, et al., 2013).
iii). Targeting ACLY and fatty acid synthetase
ACLY hyperactivation is a common feature of many tumors and is correlated with FAK overexpression. Inhibition of ACLY activity induces the arrest of cancer cell cycle progression in vitro and in vivo (Migita, et al., 2013; Zaidi, Swinnen, & Smans, 2012). ACLY deficiency exerts an anticancer effect via increased ROS and p-AMPK (Migita, et al., 2013). These observations support the notion that FAK-ACLY signaling contributes to increased lipogenesis in cancer cells, but potent and specific antagonists to inhibit abnormal constitutive activation of these proteins are lacking.
Normal cells rely on dietary fatty acids. Therefore, depletion of fatty acid synthetase has limited impact on normal cells. FAK and fatty acid synthetase are often highly upregulated in solid tumors. Furthermore, fatty acid synthetase is essential for cancer cell survival. Inhibition of fatty acid synthetase induces apoptosis. Inhibitors targeting fatty acid synthetase prevent cell proliferation and growth of prostate cancer (Chen, Chang, Chuang, Tai, & Hwang, 2012). Several potent inhibitors of fatty acid synthetase have been patented and are commercially available (Pandey, Liu, Xing, Fukuda, & Watabe, 2012). Since the levels of FAK and fatty acid synthetase in normal tissues are low or undetectable, clinical trials of fatty acid synthetase inhibitors in patients with tumors overexpressing FAK and/or fatty acid synthetase may result in the discovery of potent anti-cancer drugs with minimal side-effects. No trials evaluating fatty acid synthetase inhibitors in malignancy have been reported to date.
iv). Inhibition of aberrant glutamine metabolism
FAK can enhance the activities of oncogenes such as K-Ras and Myc; while their activation has been linked to increased glutamine synthetase activity. Derivatives of methionine sulfoximine, phosphorus containing analogues of glutamic acid, bisphosphonates and miscellaneous inhibitors targeting glutamine synthetase have been developed (Berlicki, 2008). A glutaminolytic drug, L-Asparaginase, and the glutamine synthetase inhibitor, methionine sulfoximine, depleted the glutamine pool, arrested cell cycle progression, and induced caspase-3 mediated apoptosis in human hepatocellular carcinoma cells (Tardito, et al., 2011). Oncogenic Myc promotes glutaminase expression and cancer cell addiction to glutamine (Wise, et al., 2008). Inhibition of glutaminase activity using siRNA or small molecule, BPTES [bis-2-(5 phenylacetamido-1, 2, 4-thiadiazol-2-yl) ethyl sulfide], prevented growth and tumorigenesis (Lobo, et al., 2000; Thangavelu, et al., 2012). Glutaminase inhibitors attenuate cancer-related gene expression through histone and epigenome modification (Simpson, Tryndyak, Pogribna, Beland, & Pogribny, 2012). A cell-permeable benzophenanthridinone compound 968 inhibits mitochondria glutaminase, resulting in repressed growth and invasive activity in NIH3T3 cells expressing Dbl, Cdc42-F28L, Rac-F28L or RhoC-F30L mutants, in SKBR3 and in MDA-MB231 cancer cells. The observations suggest that the balance of glutamine and glutamate plays a vital role in tumor cell survival. Depletion of either glutamine or glutamate pools in the tumor cells can lead to oxidant production and apoptosis. Although preclinical results have shown the anticancer effects of targeting glutamine metabolisms, there is no clinical trial that is registered in the NIH clinical trial data base as of August, 2013.
6. Summary and prospective
FAK plays a key role in tumor metabolism that is characterized by excessive consumption of and addictive dependency on glucose, lipids and glutamine. Constitutive FAK binding with and stabilization of IR/IGF1R promotes effectors such as IRS and PI3K/Akt cascades, promoting glucose consumption to fuel rapid cell division and enhance survival.
Lipids are major components of cell membranes that are essential for excessive cell proliferation. The role of FAK in tumor lipid metabolism is less well studied. However, experimental evidence supports the notion that FAK can contribute to the malignant cell deregulation of lipid bioprocesses. First, FAK interactions with membrane receptors such as integrins, EGFR, and IGF1R to form complexes are critical for initiation of lipid biosynthesis. Second, FAK activation is correlated with overexpression of lipid enzymes. Finally, FAK-promoted glucose consumption can provide carbon sources for lipid synthesis.
Oncogene-induced glutamine consumption can fuel rapid cell growth and maintain redox balance in cancer cells. Constitutive FAK activation stimulates oncogenes such as K-Ras and Myc, which promotes the activities of glutamine metabolic enzymes including glutamine synthetase and glutaminase. Increased levels of cellular glutamine pools boost TCA-mediated metabolic pathways and antioxidant production, which is essential for rapid cell proliferation.
Defining and establishing the link between FAK-related pathways and abnormal metabolism can promote the design and development of new drugs for the treatment of cancer. For example, inhibitors targeting FAK modulation of IGF1R, ACLY, and glutaminase have been reported to attenuate abnormal glucose, fatty acid and glutamine consumption as well as tumorigenesis. However, clinical trials of those potent inhibitors in patients with tumors are lacking. It is expected that the development of new potent small molecules and further clinical studies of the known inhibitors targeting FAK and/or its downstream metabolic effectors can lead to the identification of novel compounds to kill cancer cells with minimal side-effects on normal tissues.
Abbreviations
- FAK
focal adhesion kinase
- FASN
Fatty Acid Synthase
- IGF1R
insulin-like growth factor 1 receptor
- ACLY
ATP citrate lyase
- ECM
extracellular matrix
- IRS
insulin receptor substrate
- IR
insulin receptor
- PBTES
Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- GS
glutamine synthetase
- GLUT
glucose transporter
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jianliang Zhang, Department of Surgical Oncology, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263.
Steven N. Hochwald, Department of Surgical Oncology, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263.
Reference
- (ECCO), T. E. C. O. (2012). Mesothelioma drug slows disease progression in patients with an inactive NF2 gene. In ScienceDaily. [Google Scholar]
- Ackermann M, Morse BA, Delventhal V, Carvajal IM, & Konerding MA (2012). Anti-VEGFR2 and anti-IGF-1R-Adnectins inhibit Ewing’s sarcoma A673-xenograft growth and normalize tumor vascular architecture. Angiogenesis, 15, 685–695. [DOI] [PubMed] [Google Scholar]
- Aleksandrova K, Boeing H, Jenab M, Bas Bueno-de-Mesquita H, Jansen E, van Duijnhoven FJ, Fedirko V, Rinaldi S, Romieu I, Riboli E, Romaguera D, Overvad K, Ostergaard JN, Olsen A, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Masala G, Agnoli C, Panico S, Tumino R, Vineis P, Kaaks R, Lukanova A, Trichopoulou A, Naska A, Bamia C, Peeters PH, Rodriguez L, Buckland G, Sanchez MJ, Dorronsoro M, Huerta JM, Barricarte A, Hallmans G, Palmqvist R, Khaw KT, Wareham N, Allen NE, Tsilidis KK, & Pischon T (2011). Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila), 4, 1873–1883. [DOI] [PubMed] [Google Scholar]
- Andersson S, D’Arcy P, Larsson O, & Sehat B (2009). Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem Biophys Res Commun, 387, 36–41. [DOI] [PubMed] [Google Scholar]
- Anichini E, Zamperini A, Chevanne M, Caldini R, Pucci M, Fibbi G, & Del Rosso M (1997). Interaction of urokinase-type plasminogen activator with its receptor rapidly induces activation of glucose transporters. Biochemistry, 36, 3076–3083. [DOI] [PubMed] [Google Scholar]
- Aprikian AG, Tremblay L, Han K, & Chevalier S (1997). Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int J Cancer, 72, 498–504. [DOI] [PubMed] [Google Scholar]
- Aronsohn MS, Brown HM, Hauptman G, & Kornberg LJ (2003). Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope, 113, 1944–1948. [DOI] [PubMed] [Google Scholar]
- Baron V, Calleja V, Ferrari P, Alengrin F, & Van Obberghen E (1998). p125Fak focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tyrosine kinase receptors. J Biol Chem, 273, 7162–7168. [DOI] [PubMed] [Google Scholar]
- Beierle EA, Trujillo A, Nagaram A, Kurenova EV, Finch R, Ma X, Vella J, Cance WG, & Golubovskaya VM (2007). N-MYC regulates focal adhesion kinase expression in human neuroblastoma. J Biol Chem, 282, 12503–12516. [DOI] [PubMed] [Google Scholar]
- Berlicki L (2008). Inhibitors of glutamine synthetase and their potential application in medicine. Mini Rev Med Chem, 8, 869–878. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, & Bernstein H (2011). Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol, 85, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht B, & Dey CS (2008). Focal Adhesion Kinase contributes to insulin-induced actin reorganization into a mesh harboring Glucose transporter-4 in insulin resistant skeletal muscle cells. BMC Cell Biol, 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey RW, Edgell NJ, Hopkirk TJ, & Denton RM (1984). Studies on insulin-stimulated phosphorylation of acetyl-CoA carboxylase, ATP citrate lyase and other proteins in rat epididymal adipose tissue. Evidence for activation of a cyclic AMPindependent protein kinase. Biochem J, 218, 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F, Dupertuis YM, & Pichard C (2012). Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr Opin Clin Nutr Metab Care, 15, 99–106. [DOI] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, & Xu L (2000). Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res, 6, 2417–2423. [PubMed] [Google Scholar]
- Chen HW, Chang YF, Chuang HY, Tai WT, & Hwang JJ (2012). Targeted therapy with fatty acid synthase inhibitors in a human prostate carcinoma LNCaP/tk-lucbearing animal model. Prostate Cancer Prostatic Dis, 15, 260–264. [DOI] [PubMed] [Google Scholar]
- Chocarro-Calvo A, Garcia-Martinez JM, Ardila-Gonzalez S, De la Vieja A, & Garcia-Jimenez C (2013). Glucose-induced beta-catenin acetylation enhances Wnt signaling in cancer. Mol Cell, 49, 474–486. [DOI] [PubMed] [Google Scholar]
- Crespo I, San-Miguel B, Prause C, Marroni N, Cuevas MJ, Gonzalez-Gallego J, & Tunon MJ (2012). Glutamine treatment attenuates endoplasmic reticulum stress and apoptosis in TNBS-induced colitis. PLoS One, 7, e50407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV (2013). MYC, Metabolism, Cell Growth, and Tumorigenesis. Cold Spring Harb Perspect Med, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao P, Jarray R, Le Coq J, Lietha D, Loukaci A, Lepelletier Y, Hadj-Slimane R, Garbay C, Raynaud F, & Chen H (2013). Synthesis of novel diarylamino-1,3,5-triazine derivatives as FAK inhibitors with anti-angiogenic activity. Bioorg Med Chem Lett, 23, 4552–4556. [DOI] [PubMed] [Google Scholar]
- Dunn KB, Heffler M, & Golubovskaya VM (2010). Evolving therapies and FAK inhibitors for the treatment of cancer. Anticancer Agents Med Chem, 10, 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Annabi S, Gautier N, & Baron V (2001). Focal adhesion kinase and Src mediate integrin regulation of insulin receptor phosphorylation. FEBS Lett, 507, 247–252. [DOI] [PubMed] [Google Scholar]
- Fernandez-Marcos PJ, & Serrano M (2013). Sirt4: the glutamine gatekeeper. Cancer Cell, 23, 427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher DA, & Mullins RD (2010). Cell mechanics and the cytoskeleton. Nature, 463, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YM, Yu ZX, Li YQ, Ge X, Sanchez PJ, Fu X, & Meadows GG (2003). Specific amino acid dependency regulates invasiveness and viability of androgen-independent prostate cancer cells. Nutr Cancer, 45, 60–73. [DOI] [PubMed] [Google Scholar]
- Fu YM, Yu ZX, Pelayo BA, Ferrans VJ, & Meadows GG (1999). Focal adhesion kinase-dependent apoptosis of melanoma induced by tyrosine and phenylalanine deficiency. Cancer Res, 59, 758–765. [PubMed] [Google Scholar]
- Fu YM, Zhang H, Ding M, Li YQ, Fu X, Yu ZX, & Meadows GG (2004). Specific amino acid restriction inhibits attachment and spreading of human melanoma via modulation of the integrin/focal adhesion kinase pathway and actin cytoskeleton remodeling. Clin Exp Metastasis, 21, 587–598. [DOI] [PubMed] [Google Scholar]
- Gates RE, King LE Jr., Hanks SK, & Nanney LB (1994). Potential role for focal adhesion kinase in migrating and proliferating keratinocytes near epidermal wounds and in culture. Cell Growth Differ, 5, 891–899. [PubMed] [Google Scholar]
- Gervais FG, Thornberry NA, Ruffolo SC, Nicholson DW, & Roy S (1998). Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J Biol Chem, 273, 17102–17108. [DOI] [PubMed] [Google Scholar]
- Goel HL, & Dey CS (2002). Focal adhesion kinase tyrosine phosphorylation is associated with myogenesis and modulated by insulin. Cell Prolif, 35, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Figel S, Ho BT, Johnson CP, Yemma M, Huang G, Zheng M, Nyberg C, Magis A, Ostrov DA, Gelman IH, & Cance WG (2012). A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo. Carcinogenesis, 33, 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Kweh FA, & Cance WG (2009). Focal adhesion kinase and cancer. Histol Histopathol, 24, 503–510. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, & Cance WG (2008). A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem, 51, 7405–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NA, Tahmasian M, Kohli B, Komisopoulou E, Zhu M, Vivanco I, Teitell MA, Wu H, Ribas A, Lo RS, Mellinghoff IK, Mischel PS, & Graeber TG (2012). Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol Syst Biol, 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, & Shalloway D (1992). Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature, 358, 690692. [DOI] [PubMed] [Google Scholar]
- Guo D, Bell EH, Mischel P, & Chakravarti A (2013). Targeting SREBP-1-driven Lipid Metabolism to Treat Cancer. Curr Pharm Des. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Bisht B, & Dey CS (2012). Focal adhesion kinase negatively regulates neuronal insulin resistance. Biochim Biophys Acta, 1822, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, Clark JE, Alborn WE, Eisenberg R, & Massion PP (2013). SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res, 19, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, & Schlaepfer DD (2002). The focal adhesion kinase--a regulator of cell migration and invasion. IUBMB Life, 53, 115–119. [DOI] [PubMed] [Google Scholar]
- Hewish M, Chau I, & Cunningham D (2009). Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov, 4, 54–72. [DOI] [PubMed] [Google Scholar]
- Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, Magis A, Ostrov D, Cance WG, & Golubovskaya VM (2009). A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle, 8, 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Cheung AT, Parsons JT, & Bryer-Ash M (2002). Focal adhesion kinase (FAK) regulates insulin-stimulated glycogen synthesis in hepatocytes. J Biol Chem, 277, 18151–18160. [DOI] [PubMed] [Google Scholar]
- Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG, Shreeve SM, Burris HA, & Siu LL (2012). Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol, 30, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, & Maehara Y (2004). Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res, 10, 2812–2817. [DOI] [PubMed] [Google Scholar]
- Janku F, Huang HJ, Angelo LS, & Kurzrock R (2013). A kinase-independent biological activity for insulin growth factor-1 receptor (IGF-1R) : implications for inhibition of the IGF-1R signal. Oncotarget, 4, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Lee H, Park H, Lee JH, Choi S, Hwang J, Han IO, & Oh ES (2007). Phosphorylation of focal adhesion kinase at Tyrosine 407 negatively regulates Ras transformation of fibroblasts. Biochem Biophys Res Commun, 364, 1062–1066. [DOI] [PubMed] [Google Scholar]
- Jeon JH, Kim SK, Kim HJ, Chang J, Ahn CM, & Chang YS (2010). Lipid raft modulation inhibits NSCLC cell migration through delocalization of the focal adhesion complex. Lung Cancer, 69, 165–171. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, Xu X, Li C, Wang RH, Lee J, Csibi A, Cerione R, Blenis J, Clish CB, Kimmelman A, Deng CX, & Haigis MC (2013). SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell, 23, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat GC, Kim MY, Strickler HD, Shikany JM, Lane D, Luo J, Ning Y, Gunter MJ, & Rohan TE (2012). A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. Br J Cancer, 106, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallergi G, Mavroudis D, Georgoulias V, & Stournaras C (2007). Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol Med, 13, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Holgren C, & Samarel AM (2006). Deoxycholic acid differentially regulates focal adhesion kinase phosphorylation: role of tyrosine phosphatase ShP2. Am J Physiol Gastrointest Liver Physiol, 291, G1100–1112. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim do H, Jung WH, & Koo JS (2013). Expression of glutamine metabolismrelated proteins according to molecular subtype of breast cancer. Endocr Relat Cancer, 20, 339–348. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park JW, Yoon JS, Mok JO, Kim YJ, Park HK, Kim CH, Byun DW, Lee YJ, Jin SY, Suh KI, & Yoo MH (2004). Increased expression of focal adhesion kinase in thyroid cancer: immunohistochemical study. J Korean Med Sci, 19, 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JB, Yamauchi K, & Pessin JE (1995). Divergent insulin and platelet-derived growth factor regulation of focal adhesion kinase (pp125FAK) tyrosine phosphorylation, and rearrangement of actin stress fibers. J Biol Chem, 270, 1019910203. [DOI] [PubMed] [Google Scholar]
- Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, & Martinez-Outschoorn UE (2011). Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol Ther, 12, 10851097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroder G, Bossenmaier B, Kellerer M, Capp E, Stoyanov B, Muhlhofer A, Berti L, Horikoshi H, Ullrich A, & Haring H (1996). Tumor necrosis factor-alpha- and hyperglycemia-induced insulin resistance. Evidence for different mechanisms and different effects on insulin signaling. J Clin Invest, 97, 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucab JE, & Dunn SE (2003). Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis, 17, 41–47. [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF (2012). Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol Metab Clin North Am, 41, 409–423, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurenova EV, Hunt DL, He D, Magis AT, Ostrov DA, & Cance WG (2009). Small molecule chloropyramine hydrochloride (C4) targets the binding site of focal adhesion kinase and vascular endothelial growth factor receptor 3 and suppresses breast cancer growth in vivo. J Med Chem, 52, 4716–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong L, Wozniak MA, Collins AS, Wilson SD, & Keely PJ (2003). R-Ras promotes focal adhesion formation through focal adhesion kinase and p130(Cas) by a novel mechanism that differs from integrins. Mol Cell Biol, 23, 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, Sharma A, Enriquez Sarano M, Pollak M, Jagannath S, Richardson P, & Gualberto A (2008). Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol, 26, 3196–3203. [DOI] [PubMed] [Google Scholar]
- Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ, Lee JC, & Shen TL (2010). Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol, 177, 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, & Cance W (2005). High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol, 18, 1289–1294. [DOI] [PubMed] [Google Scholar]
- Le Y, Honczarenko M, Glodek AM, Ho DK, & Silberstein LE (2005). CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in pro-B cells. J Immunol, 174, 2582–2590. [DOI] [PubMed] [Google Scholar]
- Lebrun P, Baron V, Hauck CR, Schlaepfer DD, & Van Obberghen E (2000). Cell adhesion and focal adhesion kinase regulate insulin receptor substrate-1 expression. J Biol Chem, 275, 38371–38377. [DOI] [PubMed] [Google Scholar]
- Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, Jonkers J, & Christofori G (2008). NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J, 27, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, & Eck MJ (2007). Structural basis for the autoinhibition of focal adhesion kinase. Cell, 129, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Meng X, Jin Y, Bai J, Zhao Y, Cui X, Chen F, & Fu S (2008). Inhibitory role of focal adhesion kinase on anoikis in the lung cancer cell A549. Cell Biol Int, 32, 663670. [DOI] [PubMed] [Google Scholar]
- Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, Jackson D, de Groot J, & Yung WK (2007). Inhibition of both focal adhesion kinase and insulinlike growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther, 6, 1357–1367. [DOI] [PubMed] [Google Scholar]
- Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, & Hochwald SN (2008). FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis, 29, 1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WM, Huang P, Kar N, Burgett M, Muller-Greven G, Nowacki AS, Distelhorst CW, Lathia JD, Rich JN, Kappes JC, & Gladson CL (2013). Lyn Facilitates Glioblastoma Cell Survival under Conditions of Nutrient Deprivation by Promoting Autophagy. PLoS One, 8, e70804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo C, Ruiz-Bellido MA, Aledo JC, Marquez J, Nunez De Castro I, & Alonso FJ (2000). Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem J, 348 Pt 2, 257–261. [PMC free article] [PubMed] [Google Scholar]
- Ma WW (2011). Development of focal adhesion kinase inhibitors in cancer therapy. Anticancer Agents Med Chem, 11, 638–642. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, & Potter JD (1985). Host factors in carcinogenesis: certain bile-acid metabolic profiles that selectively increase the risk of proximal colon cancer. J Natl Cancer Inst, 75, 185–191. [PubMed] [Google Scholar]
- Menu E, Jernberg-Wiklund H, De Raeve H, De Leenheer E, Coulton L, Gallagher O, Van Valckenborgh E, Larsson O, Axelson M, Nilsson K, Van Camp B, Croucher P, & Vanderkerken K (2007). Targeting the IGF-1R using picropodophyllin in the therapeutical 5T2MM mouse model of multiple myeloma: beneficial effects on tumor growth, angiogenesis, bone disease and survival. Int J Cancer, 121, 1857–1861. [DOI] [PubMed] [Google Scholar]
- Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, Okumura S, Nakagawa K, & Ishikawa Y (2008). ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res, 68, 8547–8554. [DOI] [PubMed] [Google Scholar]
- Migita T, Okabe S, Ikeda K, Igarashi S, Sugawara S, Tomida A, Taguchi R, Soga T, & Seimiya H (2013). Inhibition of ATP citrate lyase induces an anticancer effect via reactive oxygen species: AMPK as a predictive biomarker for therapeutic impact. Am J Pathol, 182, 1800–1810. [DOI] [PubMed] [Google Scholar]
- Moissoglu K, & Gelman IH (2003). v-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J Biol Chem, 278, 47946–47959. [DOI] [PubMed] [Google Scholar]
- Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D, Spicer J, Postel-Vinay S, Yin D, Lipton A, Demers L, Leitzel K, Gualberto A, & de Bono JS (2010). The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, openlabel study. Br J Cancer, 103, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J, Schlitt HJ, Geissler EK, & Stoeltzing O (2008). Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur J Cancer, 44, 1577–1586. [DOI] [PubMed] [Google Scholar]
- Musch MW, Hayden D, Sugi K, Straus D, & Chang EB (1998). Cell-specific induction of hsp72-mediated protection by glutamine against oxidant injury in IEC18 cells. Proc Assoc Am Physicians, 110, 136–139. [PubMed] [Google Scholar]
- Nagaharu K, Zhang X, Yoshida T, Katoh D, Hanamura N, Kozuka Y, Ogawa T, Shiraishi T, & Imanaka-Yoshida K (2011). Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am J Pathol, 178, 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, & Murphy LO (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell, 136, 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay BN, Gameiro PA, Tschop K, Korenjak M, Heilmann AM, Asara JM, Stephanopoulos G, Iliopoulos O, & Dyson NJ (2013). Loss of RBF1 changes glutamine catabolism. Genes Dev, 27, 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, & Schachner M (2002). Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J Cell Biol, 157, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocak S, Chen H, Callison C, Gonzalez AL, & Massion PP (2012). Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer, 118, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens DM, Mikkers HM, van der Zon GC, Stein-Gerlach M, Ullrich A, & Maassen JA (1996). Insulin-induced tyrosine dephosphorylation of paxillin and focal adhesion kinase requires active phosphotyrosine phosphatase 1D. Biochem J, 318 ( Pt 2), 609614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JY, Ko BH, Jung KH, & Lee KH (2009). Fibronectin stimulates endothelial cell 18F-FDG uptake through focal adhesion kinase-mediated phosphatidylinositol 3kinase/Akt signaling. J Nucl Med, 50, 618–624. [DOI] [PubMed] [Google Scholar]
- Pandey PR, Liu W, Xing F, Fukuda K, & Watabe K (2012). Anti-cancer drugs targeting fatty acid synthase (FAS). Recent Pat Anticancer Drug Discov, 7, 185–197. [DOI] [PubMed] [Google Scholar]
- Park EK, Park MJ, Lee SH, Li YC, Kim J, Lee JS, Lee JW, Ye SK, Park JW, Kim CW, Park BK, & Kim YN (2009). Cholesterol depletion induces anoikis-like apoptosis via FAK down-regulation and caveolae internalization. J Pathol, 218, 337349. [DOI] [PubMed] [Google Scholar]
- Park JH, & Han HJ (2009). Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol, 297, C935–944. [DOI] [PubMed] [Google Scholar]
- Park JH, Ryu JM, & Han HJ (2011). Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol, 226, 267–275. [DOI] [PubMed] [Google Scholar]
- Pillay TS, Sasaoka T, & Olefsky JM (1995). Insulin stimulates the tyrosine dephosphorylation of pp125 focal adhesion kinase. J Biol Chem, 270, 991–994. [DOI] [PubMed] [Google Scholar]
- Pollak M (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer, 8, 915–928. [DOI] [PubMed] [Google Scholar]
- Putz T, Culig Z, Eder IE, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, & Klocker H (1999). Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res, 59, 227–233. [PubMed] [Google Scholar]
- Ramprasad OG, Srinivas G, Rao KS, Joshi P, Thiery JP, Dufour S, & Pande G (2007). Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cell Motil Cytoskeleton, 64, 199–216. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Shant J, Guo CY, Roy S, & Cheng K (2008). Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol, 215, 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik JL, Reichart DB, Huey K, Webster NJ, & Seely BL (1998). Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res, 58, 1159–1164. [PubMed] [Google Scholar]
- Ridgway ND (2013). The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol, 48, 20–38. [DOI] [PubMed] [Google Scholar]
- Ritt M, Guan JL, & Sivaramakrishnan S (2013). Visualizing and manipulating focal adhesion kinase regulation in live cells. J Biol Chem, 288, 8875–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin JD, Frierson HF Jr., Ledinh W, Parsons JT, & Adams RB (2002). Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate, 53, 124–132. [DOI] [PubMed] [Google Scholar]
- Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, & Chang EB (2009). Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology, 136, 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti GV, & Novello S (2012). The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev, 38, 292–302. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, & van der Geer P (1994). Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature, 372, 786–791. [DOI] [PubMed] [Google Scholar]
- Schneider GB, Kurago Z, Zaharias R, Gruman LM, Schaller MD, & Hendrix MJ (2002). Elevated focal adhesion kinase expression facilitates oral tumor cell invasion. Cancer, 95, 2508–2515. [DOI] [PubMed] [Google Scholar]
- Schultze A, & Fiedler W (2010). Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin Investig Drugs, 19, 777–788. [DOI] [PubMed] [Google Scholar]
- Schultze A, & Fiedler W (2011). Clinical importance and potential use of small molecule inhibitors of focal adhesion kinase. Anticancer Agents Med Chem, 11, 593–599. [DOI] [PubMed] [Google Scholar]
- Serrels A, McLeod K, Canel M, Kinnaird A, Graham K, Frame MC, & Brunton VG (2012). The role of focal adhesion kinase catalytic activity on the proliferation and migration of squamous cell carcinoma cells. Int J Cancer, 131, 287–297. [DOI] [PubMed] [Google Scholar]
- Shang Y, Mao Y, Batson J, Scales SJ, Phillips G, Lackner MR, Totpal K, Williams S, Yang J, Tang Z, Modrusan Z, Tan C, Liang WC, Tsai SP, Vanderbilt A, Kozuka K, Hoeflich K, Tien J, Ross S, Li C, Lee SH, Song A, Wu Y, Stephan JP, Ashkenazi A, & Zha J (2008). Anti-xenograft tumor activity of a humanized antiinsulin-like growth factor-I receptor monoclonal antibody is associated with decreased AKT activation and glucose uptake. Mol Cancer Ther, 7, 2599–2608. [DOI] [PubMed] [Google Scholar]
- Shima T, Nada S, & Okada M (2003). Transmembrane phosphoprotein Cbp senses cell adhesion signaling mediated by Src family kinase in lipid rafts. Proc Natl Acad Sci U S A, 100, 14897–14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MS, Rosenberg CA, Rodabough RJ, Greenland P, Ockene I, Roy HK, Lane DS, Cauley JA, & Khandekar J (2012). Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk. Ann Epidemiol, 22, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson NE, Tryndyak VP, Pogribna M, Beland FA, & Pogribny IP (2012). Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics, 7, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, & Kimmelman AC (2013). Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature, 496, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Gao L, Meng F, Guo LW, Rothschild J, & Gelman IH (2013). Adhesion-mediated cytoskeletal remodeling is controlled by the direct scaffolding of Src from FAK complexes to lipid rafts by SSeCKS/AKAP12. Oncogene, 32, 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Osajima A, Nakayamada S, Anai H, Kabashima N, Kanegae K, Ota T, Tanaka Y, & Nakashima Y (2003). High glucose levels inhibit focal adhesion kinase-mediated wound healing of rat peritoneal mesothelial cells. Kidney Int, 63, 722–731. [DOI] [PubMed] [Google Scholar]
- Tardito S, Chiu M, Uggeri J, Zerbini A, Da Ros F, Dall’Asta V, Missale G, & Bussolati O (2011). L-Asparaginase and inhibitors of glutamine synthetase disclose glutamine addiction of beta-catenin-mutated human hepatocellular carcinoma cells. Curr Cancer Drug Targets, 11, 929–943. [DOI] [PubMed] [Google Scholar]
- Thangavelu K, Pan CQ, Karlberg T, Balaji G, Uttamchandani M, Suresh V, Schuler H, Low BC, & Sivaraman J (2012). Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc Natl Acad Sci U S A, 109, 7705–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita N, Hayashi Y, Suzuki S, Oomori Y, Aramaki Y, Matsushita Y, Iwatani M, Iwata H, Okabe A, Awazu Y, Isono O, Skene RJ, Hosfield DJ, Miki H, Kawamoto T, Hori A, & Baba A (2013). Structure-based discovery of cellular-active allosteric inhibitors of FAK. Bioorg Med Chem Lett, 23, 1779–1785. [DOI] [PubMed] [Google Scholar]
- Ucar DA, & Hochwald SN (2010). FAK and interacting proteins as therapeutic targets in pancreatic cancer. Anticancer Agents Med Chem, 10, 742–746. [DOI] [PubMed] [Google Scholar]
- Ucar DA, Kurenova E, Garrett TJ, Cance WG, Nyberg C, Cox A, Massoll N, Ostrov DA, Lawrence N, Sebti SM, Zajac-Kaye M, & Hochwald SN (2012). Disruption of the protein interaction between FAK and IGF-1R inhibits melanoma tumor growth. Cell Cycle, 11, 3250–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar DA, Magis AT, He DH, Lawrence NJ, Sebti SM, Kurenova E, Zajac-Kaye M, Zhang J, & Hochwald SN (2013). Inhibiting the Interaction of cMET and IGF-1R with FAK Effectively Reduces Growth of Pancreatic Cancer Cells in vitro and in vivo. Anticancer Agents Med Chem, 13, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Sahin AA, Adam L, Wang RA, & Kumar R (2003). Heregulin and HER2 signaling selectively activates c-Src phosphorylation at tyrosine 215. FEBS Lett, 543, 76–80. [DOI] [PubMed] [Google Scholar]
- van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, Verhagen LP, Groot Koerkamp MJ, Braat AK, Dansen TB, Holstege FC, Gebhardt R, Burgering BM, & Coffer PJ (2012). Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nat Cell Biol, 14, 829–837. [DOI] [PubMed] [Google Scholar]
- van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A, Overvad K, Toft-Petersen AP, Tjonneland A, Hansen L, Boutron-Ruault MC, Clavel-Chapelon F, Cottet V, Palli D, Tagliabue G, Panico S, Tumino R, Vineis P, Kaaks R, Teucher B, Boeing H, Drogan D, Trichopoulou A, Lagiou P, Dilis V, Peeters PH, Siersema PD, Rodriguez L, Gonzalez CA, Molina-Montes E, Dorronsoro M, Tormo MJ, Barricarte A, Palmqvist R, Hallmans G, Khaw KT, Tsilidis KK, Crowe FL, Chajes V, Fedirko V, Rinaldi S, Norat T, & Riboli E (2011). Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut, 60, 1094–1102. [DOI] [PubMed] [Google Scholar]
- Van Hemelrijck M, Garmo H, Hammar N, Jungner I, Walldius G, Lambe M, & Holmberg L (2012). The interplay between lipid profiles, glucose, BMI and risk of kidney cancer in the Swedish AMORIS study. Int J Cancer, 130, 2118–2128. [DOI] [PubMed] [Google Scholar]
- Van Slambrouck S, Grijelmo C, De Wever O, Bruyneel E, Emami S, Gespach C, & Steelant WF (2007). Activation of the FAK-src molecular scaffolds and p130CasJNK signaling cascades by alpha1-integrins during colon cancer cell invasion. Int J Oncol, 31, 1501–1508. [PubMed] [Google Scholar]
- Vander Heiden MG (2011). Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov, 10, 671–684. [DOI] [PubMed] [Google Scholar]
- Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, Frierson H Jr., Powell SM, Knuutila S, Kallioniemi A, & El-Rifai W (2002). Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res, 62, 2625–2629. [PubMed] [Google Scholar]
- Vartanian T, Goodearl A, Lefebvre S, Park SK, & Fischbach G (2000). Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in schwann cells. Biochem Biophys Res Commun, 271, 414–417. [DOI] [PubMed] [Google Scholar]
- Wade R, Brimer N, Lyons C, & Vande Pol S (2011). Paxillin enables attachmentindependent tyrosine phosphorylation of focal adhesion kinase and transformation by RAS. J Biol Chem, 286, 37932–37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Scholtens D, Holko M, Ivancic D, Lee O, Hu H, Chatterton RT Jr., Sullivan ME, Hansen N, Bethke K, Zalles CM, & Khan SA (2013). Lipid metabolism genes in contralateral unaffected breast and estrogen receptor status of breast cancer. Cancer Prev Res (Phila), 6, 321–330. [DOI] [PubMed] [Google Scholar]
- Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, & Krystal GW (2005). The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res, 11, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Takaoka M, Sakurama K, Tomono Y, Hatakeyama S, Ohmori O, Motoki T, Shirakawa Y, Yamatsuji T, Haisa M, Matsuoka J, Beer DG, Nagatsuka H, Tanaka N, & Naomoto Y (2008). Dual tyrosine kinase inhibitor for focal adhesion kinase and insulin-like growth factor-I receptor exhibits anticancer effect in esophageal adenocarcinoma in vitro and in vivo. Clin Cancer Res, 14, 4631–4639. [DOI] [PubMed] [Google Scholar]
- Wilson KF, Erickson JW, Antonyak MA, & Cerione RA (2013). Rho GTPases and their roles in cancer metabolism. Trends Mol Med, 19, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, & Thompson CB (2008). Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A, 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, & Thompson CB (2010). Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci, 35, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, Walldius G, & Van Hemelrijck M (2012). Serum Lipids and the Risk of Gastrointestinal Malignancies in the Swedish AMORIS Study. J Cancer Epidemiol, 2012, 792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulaningsih W, Holmberg L, Garmo H, Zethelius B, Wigertz A, Carroll P, Lambe M, Hammar N, Walldius G, Jungner I, & Van Hemelrijck M (2013). Serum glucose and fructosamine in relation to risk of cancer. PLoS One, 8, e54944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy HF, Mason JA, Peters S, Thompson CE 3rd, Littleton GK, Jett M, & Day AA (2007). Metastatic progression and gene expression between breast cancer cell lines from African American and Caucasian women. J Carcinog, 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zheng Q, Fan J, Ai KX, Chen J, & Huang XY (2010). Expression and prognostic significance of focal adhesion kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol, 136, 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I, & Rozengurt E (1992). Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell, 71, 891894. [DOI] [PubMed] [Google Scholar]
- Zaidi N, Swinnen JV, & Smans K (2012). ATP-citrate lyase: a key player in cancer metabolism. Cancer Res, 72, 3709–3714. [DOI] [PubMed] [Google Scholar]
- Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O’Connor K, Morris AJ, Sunkara M, Weiss HL, Lee EY, & Evers BM (2012). Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res, 72, 1504–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Francois R, Iyer R, Seshadri M, Zajac-Kaye M, & Hochwald SN (2013). Current understanding of the molecular biology of pancreatic neuroendocrine tumors. J Natl Cancer Inst, 105, 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Luo Y, He LQ, Liu ZJ, Jiang AQ, Yang YH, & Zhu HL (2013). Synthesis, biological evaluation, and molecular docking studies of novel 1,3,4-oxadiazole derivatives possessing benzotriazole moiety as FAK inhibitors with anticancer activity. Bioorg Med Chem, 21, 3723–3729. [DOI] [PubMed] [Google Scholar]
- Zhelev Z, Bakalova R, Ohba H, Ewis A, Ishikawa M, Shinohara Y, & Baba Y (2004). Suppression of bcr-abl synthesis by siRNAs or tyrosine kinase activity by Glivec alters different oncogenes, apoptotic/antiapoptotic genes and cell proliferation factors (microarray study). FEBS Lett, 570, 195–204. [DOI] [PubMed] [Google Scholar]
- Zheng D, Golubovskaya V, Kurenova E, Wood C, Massoll NA, Ostrov D, Cance WG, & Hochwald SN (2010). A novel strategy to inhibit FAK and IGF-1R decreases growth of pancreatic cancer xenografts. Mol Carcinog, 49, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]