Abstract
Inhibition of fear involves learning and then appropriately responding to safety signals, and has been shown to be impaired in PTSD patients. Response inhibition refers to cognitive control and likely uses the same prefrontal cortex circuits as fear inhibition, and has also been implicated in PTSD. Impaired inhibition can serve as an intermediate phenotype for PTSD and can be measured with neuroimaging and psychophysiological tools. We first review the neurobiological mechanisms of fear and response inhibition. Next, we summarize the functional magnetic resonance imaging (fMRI) and psychophysiological studies using fear and response inhibition paradigms in PTSD patients. Finally, we evaluate the theranostic role of impaired inhibition in PTSD risk and treatment response.
1. Introduction
Posttraumatic stress disorder (PTSD) is a debilitating mental illness that can develop after experiencing a traumatic event. PTSD is a heterogeneous disorder, which presents with different symptom domains, specifically, re-experiencing, avoidance and numbing, negative cognitions, and hyper-arousal symptoms (American Psychiatric Association, 2013). Given this complexity, clinical and research progress in the field can be greatly enhanced by measuring phenotypes that are closer to the neurobiology of the disorder. Such neurobiological intermediate phenotypes can increase our understanding of the etiology of the disorder and provide better theranostic indicators for treatment.
Impaired inhibition of fear can serve as an intermediate phenotype for PTSD and can be measured with neuroimaging and psychophysiological tools. A hallmark feature of PTSD is an exaggerated fear response to trauma reminders despite being in a safe environment (Jovanovic et al., 2012; Jovanovic et al., 2010; Jovanovic et al., 2009). Learning to recognize threat and show the appropriate fear response is a crucial mechanism for survival, because it helps to avoid future danger (Maren, 2001). However, it is just as important to respond appropriately when a stimulus does not predict danger. Inhibition of fear responses involves learning and then appropriately responding to safety signals, i.e. the ability to discriminate between danger and safety cues and suppress fear responses in the presence of safety cues (Jovanovic et al., 2012). Conditioning paradigms can be used to measure safety signal learning in human and non-human models (Christianson et al., 2012; Myers et al., 2009).
Fear responses can be measured translationally in Pavlovian fear conditioning paradigms, such as fear-potentiated startle, which was originally developed in rodent models (Davis, 1992). Animal models have also been used to develop paradigms to measure fear inhibition, such as conditioning discrimination and conditional inhibition (Jovanovic and Norrholm, 2011; Myers et al., 2009). In humans, fear-potentiated startle can be used to measure both expression and suppression of fear. This suppression of the fear response, i.e., fear inhibition, is impaired in PTSD patients (Jovanovic et al., 2012; Jovanovic and Ressler, 2010). Inhibition also takes place at a cognitive level. When something unexpected happens, the human brain has the ability to inhibit the initial response and to adjust the behavioral response accordingly (van Gaal et al., 2010). This cognitive control function is often defined as response inhibition and is an essential component of human behavior (Albert et al., 2010). The goal of this review is to describe impaired inhibition, as a potential intermediate phenotype for PTSD. We first review the neurobiological mechanisms of fear and response inhibition. Next, we summarize the functional magnetic resonance imaging (fMRI) and psychophysiological studies using fear conditioning and extinction, and response inhibition paradigms in PTSD patients. Finally, we evaluate the theranostic role of impaired inhibition in PTSD risk and treatment response.
1.1. Neurobiological Mechanisms of Fear Inhibition
As noted above, fear inhibition reflects the ability to differentiate danger and safety signals (Jovanovic and Ressler, 2010). Fear inhibition can only take place when the fear is initially learned by means of fear conditioning, i.e. learning an association between an aversive stimulus (unconditioned stimulus; US,) and a neutral stimulus (conditioned stimulus; CS), resulting in a fear response to the neutral stimulus (LeDoux, 2000). A safety signal is typically a second CS which is not paired with the aversive US, and should therefore elicit no fear response. Fear inhibition can then be measured as the degree of fear to this second CS. Fear inhibition is also relevant for extinction, a learning process in which the danger signal is repeatedly presented without the US, so that the CS no longer predicts the US (Milad et al., 2008). Extinction processes and relevance to PTSD is covered in detail by Zuj and Norrholm (this issue), therefore, we will focus primarily on fear inhibition to safety signals. Contextual information can also play an important role in fear inhibition processes.
The neurobiological model of fear inhibition in PTSD suggests that responses to fear-evoking stimuli can elevate amygdala activity to the point at which cortical inputs cannot suppress this activity during presentations of threatening stimuli (Liberzon et al., 1999; Milad et al., 2009; Rauch et al., 2000; Stevens et al., 2013; Stevens et al., 2017). Figure 1 presents a schematic representation of neurobiological mechanisms underlying the stress response, fear conditioning, and fear inhibition. During fear conditioning, the aversive US elicits a stress response and this information is sent via the locus coeruleus (LC) to the basolateral nucleus of the amygdala (BLA). Within the BLA the information about the US becomes associated with the perception of the CS and contextual information is relayed by the hippocampus (Kim and Jung, 2006; Poulos et al., 2010). Attention to the fear-relevant stimuli is mediated by the dorsal anterior cingulate cortex (dACC; Milad et al., 2007). The BLA activates the central nucleus of the amygdala (CeA), which is the key output region of the amygdala and activates the fear response (Kim and Jung, 2006; LeDoux et al., 1988). Inhibition of this fear response involves the hippocampus and the ventromedial prefrontal cortex (vmPFC; Jovanovic and Ressler, 2010; Milad et al., 2008; Quirk and Mueller, 2007). When presented with the safety signal, the hippocampus activates the vmPFC, which in turn inhibits neurons in the amygdala (Corcoran and Quirk, 2007). The output from the CeA is consequently reduced, leading to an inhibition of the fear response (Jovanovic and Ressler, 2010).
Figure 1.
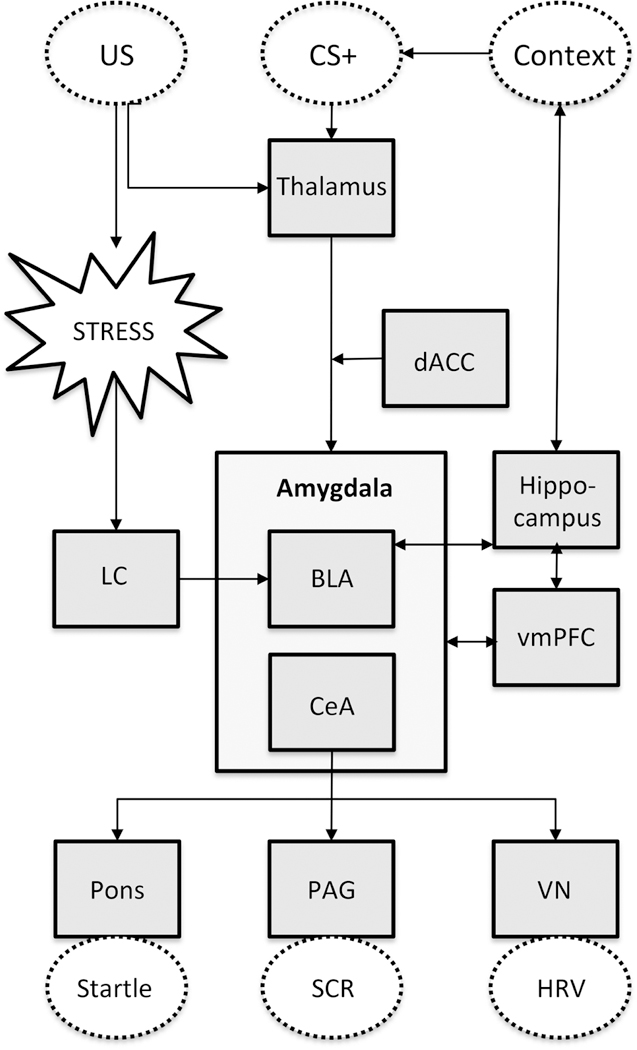
A simplified schematic overview of the neurobiological mechanisms underlying the stress response, fear conditioning and fear inhibition. The squared boxes indicate brain areas and the dotted lines indicate observable features and the HPA axis. Abbreviations: US = unconditioned stimulus, CS = conditioned stimulus, LC = locus coeruleus, VN = vagus nerve, dACC = dorsal anterior cingulate cortex, BLA = basolateral amygdala, CeA = central amygdala, vmPFC = ventromedial prefrontal cortex, PAG = periaquaductal gray, VN = vagal nucleus
1.2. Response Inhibition
Response inhibition is defined as the ability to suppress irrelevant or inappropriate actions in response to a novel information (Albert et al., 2010; Hedden and Gabrieli, 2010). A distinction can be made between reactive and proactive response inhibition. Reactive inhibition is defined as stopping an already initiated response when presented with a stop signal, whereas proactive inhibition is the anticipation of stopping based on cues or context signaling the likelihood of a stop (Aron, 2011; Zandbelt and Vink, 2010). The Go/NoGo task is most commonly used experimental paradigm to test response inhibition (Leibenluft et al., 2007). In this paradigm, participants are asked to respond to a Go stimulus, and on a small proportion of the trials, the Go stimulus is followed by a stop signal (No Go) indicating that the participant has to withhold their response (Logan and Cowan, 1984). The stop signal anticipation task (SSAT; Zandbelt and Vink, 2010) is often used to assess proactive inhibition; this paradigm includes a cue that signals a percent likelihood of a stop signal occurring.
A simplified graphic overview of the neurobiological mechanisms of reactive inhibition is presented in Figure 2. The cortico-striatal-cortical motor loop is involved in responding and in response inhibition, because it regulates the continuing of movements. In this loop there is a direct and an indirect pathway that respectively activates or inhibits the motor cortex (Alexander and Crutcher, 1990; Alexander et al., 1986; Pollack, 2001). When the Go signal is observed, the premotor cortex (PMC) activates the dorsal striatum (putamen and caudate nucleus). Once activated, the dorsal striatum inhibits the substantia nigra pars reticulata (SNr)/internal segments of the globus pallidus (GPi). The SNr/GPi reduces its inhibition of the thalamus, which ultimately activates the motor cortex, resulting in the motor response (Alexander and Crutcher, 1990; Alexander et al., 1986). For the response inhibition process, the right inferior frontal gyrus (rIFG) is essential, because of its role in attentional monitoring and detection of the stop signal (Duann et al., 2009; Hampshire et al., 2010) or expectancy violation (Zandbelt et al., 2013). The pre-supplementary motor area (preSMA) is the primary site for the actual motor response inhibition (Duann et al., 2009; Zandbelt et al., 2013), and activates the subthalamic nucleus (STN; (Aron et al., 2007; Boehler et al., 2010). The STN activates the SNr/GPi, which in turn inhibits the thalamus. Finally, this results in decreased activation of the motor cortex and an inhibition of the motor response (Aron et al., 2007; Boehler et al., 2010). In addition, there is an indirect route from the preSMA via the dorsal striatum and external segments of the globus pallidus (GPe), also resulting in an inhibition of the motor response (Aron et al., 2007; Boehler et al., 2010).
Figure 2.
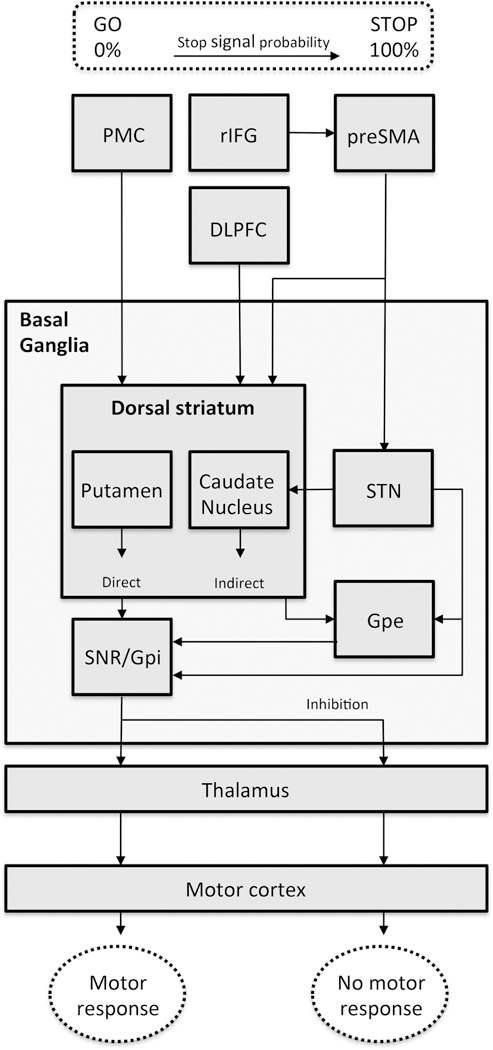
A simplified schematic overview of the neurobiological aspects of reactive and proactive response inhibition. In this model we included the rIFG as attentional monitor and suggest influence of the rIFG on the preSMA during increasing stop signal probability. We propose a main role of the preSMA for both reactive and proactive inhibition. The squared boxes indicate brain areas and the dotted lines indicate observable features and the basal ganglia and striatum. Abbreviations: PMC = premotor cortex, rIFG = right inferior frontal gyrus, DLPFC = dorsolateral prefrontal cortex, preSMA = presupplementary motor area, SNr = substantia nigra pars reticulate, GPi = internal segments of the globus pallidus, GPe = external segments of the globus pallidus, SNc = substantia nigra pars compacta, STN = subthalamic nucleus.
2. Functional MRI Measures of Inhibition in PTSD
Neuroimaging studies have used fear and response inhibition paradigms to assess phenotypes for PTSD diagnosis and treatment outcome. Table 1 is an overview all 22 articles that result from the Pubmed search [TITLE-ABS-KEY] “PTSD” AND “inhibition” OR “extinction” AND “MRI” OR “fMRI” OR “magnetic resonance imaging” OR “neural correlates”. Other articles that resulted from this search but did not use fMRI or did not use an inhibition task were excluded. Most studies were cross-sectional studies comparing PTSD patients with trauma controls and/or healthy controls. A total of 4 longitudinal fMRI studies using an inhibition paradigm have been conducted, 1 predicting future PTSD in recently traumatized civilians (van Rooij et al., 2018) and 3 pre- and post-treatment studies (Falconer et al., 2008; Helpman et al., 2016; van Rooij et al., 2015).
Table 1.
Overview of Neuroimaging Literature on Inhibition
| Publication | Inhibition | Design | Study population | fMRI analyses | Phenotypes for PTSD risk | Phenotypes for PTSD treatment response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Construct | Task | Longitudinal? | Population | PTSD patients (N) | Trauma controls (N) | Healthy controls (N) | ROI or whole brain analyses | fMRI contrast | Increased brain activation PTSD | Decreased brain activation PTSD | Functional connectivity | |
| Carrion | 2008 | Response inhibition | Go/NoGo | – | Youth | 16 | n/a | 14 | whole brain (height, p < .05 and extent, p < .05, corrected for multiple comparisons) | NoGo > Go | medial frontal cortex | middle frontal cortex | – | – |
| Falconer | 2008 | Response inhibition | Go/NoGo | – | Community sample | 23 | 17 | 23 | whole brain (p < .005, uncorrected) | NoGo (PTSD vs HC) | putamen, cerebellum, postcentral cortex, parahippocampus, cuneus | medial PFC, ventrolateral PFC | – | – |
| Milad | 2009 | Fear inhibition | Fear conditioning, extinction, recall | – | Community sample | 16 | 15 | n/a | ROI and whole brain (p < .001, uncorrected) | 1. Extinction learning 2. Extinction recall | 1. ROI: amygdala 1. Whole brain: bilateral superior temporal cortex 2. ROI: dACC 2. Whole brain: right cerebellar cortex, right medial parietal cortex and left occipital cortex | 2. ROI: hippocampus and vmPFC (extinction recall; positive correlation with recall memory) | – | – |
| Rougemont-Bucking | 2011 | Fear inhibition | Context-dependent fear conditioning, extinction, recall | – | Community sample | 18 | 16 | n/a | whole brain (p < .0001 and p < .001 for ROIs) | 1. Late conditioning, 2. Extinction learning 3. Extinction recall | 1.2.3. dACC | 2.3. vmPFC | – | – |
| Bruce | 2013 | Emotional interference processing | Attentional interference task | – | Women with IPV | 32 | n/a | 21 | ROI | Attending fearful > ignoring fearful faces | Insula | – | – | – |
| Sripada | 2013 | Fear inhibition | Multimodal fear conditionin, extinction | – | Veterans | 15 | n/a | n/a | whole brain (p < .05, FWE-corrected and small volume correction for ROIs) | 1. Context presentation (prior to CS during extinction learning) 2. Following CS + E (during extinction learning) | 1. Bilateral hippocampus and left amygdala correlated positively with PTSD sx 2. right amygdala, right hippocampus and right insula correlated positively with PTSD sx | – | – | – |
| Falconer | 2013 | Response inhibition | Go/NoGo | Treatment outcome (6 months) | Patients from traumatic stress clinic | 13 | n/a | n/a | whole brain(p < .005, uncorrected) | NoGo > Go | – | – | – | Greater left frontostriatal inhibition network (left IFC, orbitofrontal cortex and dorsal striatum), anterior medial prefrontal cortex and parahippocampus related to better treatment response |
| Jovanovic | 2013 | Response inhibition | Go/NoGo | – | Women with civilian trauma | 20 | 21 | n/a | ROI and whole brain (p < .05, FWE-corrected) | NoGo > Go | – | vmPFC | – | – |
| Garfinkel | 2014 | Fear inhibition | Fear conditioning, extinction, recall | – | OEF/OIF veterans | 14 | 14 | n/a | whole brain (p < .05, FWE-corrected and small volume correction for ROIs) | 1. Exctinction recall (CS + E > CS-) 2. Fear renewal (to CS+ or CS-) | 1. Insula, postcentral gyrus, left parietal lobe | 1. vmPFC, mPFC and lingual gyrus 2. Amygdala and vmPFC (to CS+) 2. Hippocampus and cerebellum (to CS-) | – | – |
| Shvil | 2014 | Fear inhibition | Fear conditioning, extinction | – | Community sample | 31 | 25 | n/a | ROI | Extinction recall; men vs women | left dACC in men vs women (all PTSD+) | – | – | – |
| van Rooij | 2014 | Response inhibition and context processing | Stop signal anticipation task (SSAT) | – | Male veterans | 28 | 26 | 25 | ROI; no whole brain differences | 1. Response inhibition: Stop > Go2. Context processing: Stop cues > Go cues | 1. left pre/ postcentral gyrus | 2. right Inferior Frontal Gyrus( rIFG) | – | – |
| Steiger | 2015 | Fear inhibition | Differential context and cue conditioning paradigm | – | Outpatient clinic patients/ community sample | 12 | 14 | 11 | whole brain (p < .05, FWE-corrected and small volume correction for ROIs) | Context + > context - | Hippocampal activation (and less differentiation between threat and safety) | – | – | – |
| van Rooij | 2015 | Response inhibition and context processing | Stop signal anticipation task (SSAT) | Treatment outcome (6–8 months) | Male veterans | 41 | 22 | n/a | ROI and whole brain (p < .05, FWE-corrected) | 1. Response inhibition: Stop > Go2. Context processing: Stop cues > Go cues | – | 2. Increased left inferior parietal lobe (IPL) activation (whole brain) predicts better treatment response | ||
| Wicking | 2016 | Fear inhibition | Cued fear conditioning, extinction and renewal | – | Adult trauma | 18 | 18 | 18 | ROI | Extinction recall in novel context | Amygdala, hippocampus | – | – | – |
| Dretch | 2016 | Fear inhibition | Predictable/ unpredictable threat conditioning paradigm | – | Deployment-exposed active and veterans | 38 | 40 | n/a | Whole brain (p < .05, corrected) | Predictable > unpredictable threat | – | amygdala, hippocampus, insula and superior and middle temporal gyrus | – | – |
| Diener | 2016 | Fear inhibition | Differential aversive conditioning paradigm | – | Outpatient clinic patients/ community sample | 14 | 14 | 13 | ROI | CS+ > CS- | – | amygdala | PTSD: negative interaction amygdala and dlPFC | – |
| Helpman | 2016 | Fear inhibition | Fear conditioning, extinction | Treatment outcome (10 weeks) | community sample | 16 | 16 | n/a | ROI | Extinction recall | – | – | – | Decrease in sgACC and hippocampal/ parahippocampal activaton was associated with reduction in PTSD symptoms post-treatment |
| Aupperle | 2016 | Response inhibition | Stop signal task | – | Women with IPV | 10 | n/a | 12 | ROI and whole brain (p < .05, FWE-corrected) | 1. Stop > NoStop 2. Hard > easy trials | 1. dlPFC, anterior insula 2. lateral FC, anterior insula | 1. mPFC, posterior cingulate (DMN) 2. mPFC, posterior cingulate (DMN) | – | – |
| Stevens | 2016 | Response inhibition | Go/NoGo | – | Women with civilian trauma | 37 | 53 | n/a | ROI and whole brain (p < .05, FWE- | NoGo > Go | – | rACC (with increasing levels of childhood trauma) | – | – |
| van Rooij | 2016 | Response inhibition | Go/NoGo | – | Women with civilian trauma *PTSD symptoms as continuous measure | *73 | *73 | n/a | corrected) ROI and whole brain (p < .05, FWE-corrected) | NoGo > Go | – | less hippocampal activation (correlation with more PTSD symptoms) | – | – |
| Clausen | 2017 | Cognitive inhibition | Multisource Interference task | – | Combat veterans *PTSD symptoms as continuous measure | *39 | *39 | n/a | ROI | Incongruent > congruent trials | – | less medial PFC and rACC (correlation with more PTSD symptoms) | PTSD: reduced functional connectivity between medial PFC and rACC with bilateral lateral PFC. | – |
| van Rooij | 2018 | Response inhibition | Go/NoGo | PTSD diagnosis (3 and 6 months post-trauma) | Recently traumatized civilians brought to the Emergency Room *PTSD symptoms as continuous measure | *27 (sample 1)*31 (sample 2) | *27 (sample 1)*31 (sample 2) | n/a | ROI and whole brain (p < .05, FWE-corrected) | NoGo > Go | – | ROI: Less hippocampal activation Whole brain: less middle cingulate cortex and more right middle frontal gyrus activation | – | – |
2.1. fMRI Inhibition Phenotypes for PTSD Diagnosis
A key region for inhibition is the ventromedial prefrontal cortex (vmPFC) or the rostral anterior cingulate cortex (rACC), as this region is thought to regulate emotional and behavioral responses by inhibiting the amygdala (Stevens et al., 2013). Indeed, using a fear inhibition paradigm, reduced vmPFC activation in PTSD patients compared to controls was demonstrated during extinction learning (Milad et al., 2009; Rougemont-Bücking et al., 2011), extinction recall (Garfinkel et al., 2014; Milad et al., 2009; Rougemont-Bücking et al., 2011) and fear renewal to the CS+ (Garfinkel et al., 2014). Moreover, vmPFC activation was positively correlated with recall memory (Milad et al., 2009). Also during response inhibition, several studies showed reduced vmPFC (Jovanovic et al., 2013a) or medial PFC activation (Aupperle et al., 2016; Falconer et al., 2008) in patients compared to controls. Furthermore, in PTSD patients, rostral ACC (or vmPFC) activation was found to correlate with childhood trauma (Stevens et al., 2016). Reduced medial PFC and rACC activation also correlated with more PTSD symptoms in a multisource interference task comparing incongruent and congruent trials. Moreover, reduced functional connectivity between these regions and bilateral lateral PFC was observed in PTSD patients (Clausen et al., 2017). In a study with traumatized youth, increased medial frontal cortex activation was found in the PTSD patients compared to healthy controls, but reduced middle frontal cortex activation was observed (Carrion et al., 2008).
It is postulated that the vmPFC inhibits the overactive amygdala (Stevens et al., 2013). Increased amygdala activation has consistently been demonstrated in PTSD patients during extinction learning (Milad et al., 2009; Sripada et al., 2013), and extinction recall in novel context (Wicking et al., 2016). Decreased amygdala activation was observed during fear conditioning (Diener et al., 2016), during fear renewal in response to the CS+ (Garfinkel et al., 2014), and in response to predictable vs. unpredictable threat (Dretsch et al., 2016). Furthermore, PTSD patients showed a negative interaction between the amygdala and the dlPFC (Diener et al., 2016).
As part of this neurocircuitry, the hippocampus is important for context processing and memory. A positive correlation between hippocampal activation during extinction learning and PTSD symptoms was observed (Sripada et al., 2013). A context > no context contrast also revealed more hippocampal activation in PTSD patients compared to controls even though PTSD patients showed less differentiation between threat and safety (Steiger et al., 2015). However, most studies point to diminished functionality of the hippocampus. Decreased hippocampal activation in PTSD patients versus controls has been observed during extinction recall, and correlated positively recall memory (Milad et al., 2009), during fear renewal in response to the CS-(Garfinkel et al., 2014) and in response to predictable vs. unpredictable threat (Dretsch et al., 2016). Furthermore, reduced hippocampal activation during a response inhibition task correlated with increased PTSD symptoms in a chronically traumatized population (van Rooij et al., 2016), and predicted future PTSD symptoms in recently traumatized civilians (van Rooij et al., 2018).
The dorsal anterior cingulate cortex (dACC) is part of the salience network, and is important for directing attention. PTSD patients showed increased dACC activation during late fear conditioning and extinction learning (Rougemont-Bücking et al., 2011), and during extinction recall (Milad et al., 2009; Rougemont-Bücking et al., 2011). A comparison of male vs. female PTSD patients showed more left dACC activation in men during extinction recall (Shvil et al., 2014).
Another key region of the salience network is the insula, which is thought to be involved in interoceptive awareness. Insula activation during extinction learning was positively correlated with PTSD symptoms (Sripada et al., 2013). Furthermore, PTSD patients compared to controls showed increased insula activation during extinction recall, whereas patients showed reduced activation during predictable vs. unpredictable threat (Dretsch et al., 2016). Using a response inhibition paradigm, increased anterior insula activation was observed in PTSD patients compared to controls for both Stop vs NoStop and Hard vs Easy trials contrasts (Aupperle et al., 2016). Increased insula activation to attending vs. ignoring fearful faces was observed during an attentional interference task (Bruce et al., 2013).
Regions often implicated in response inhibition are the pre/post-central gyrus (motor and sensorimotor cortex), the right inferior frontal gyrus (rIFG) and striatum. Increased postcentral gyrus activation in PTSD patients compared to controls during inhibition has indeed been observed in several studies during extinction recall (Sripada et al., 2013) and during response inhibition (Falconer et al., 2008; van Rooij et al., 2014). This is thought to indicate decreased suppression of the motor cortex, resulting in increased activation of the motor cortex and impaired response inhibition (van Rooij et al., 2014). Decreased rIFG activation has been demonstrated in PTSD patients during context processing (van Rooij et al., 2016), indicating impaired signaling of the contextual cue needed to guide behavior.
Other regions that were differently activated in PTSD patients compared to controls across several studies were the cerebellum and bilateral superior temporal cortex/gyrus. Increased right cerebellar cortex activation was observed during extinction recall (Milad et al., 2009) and response inhibition (Falconer et al., 2008). However, during fear renewal, decreased cerebellum activation was observed in PTSD patients compared to controls (Garfinkel et al., 2014). Increased bilateral superior temporal cortex was observed during extinction learning (Milad et al., 2009), whereas reduced activation of the superior and middle temporal gyrus was observed in response to predictable vs. unpredictable threat (Dretsch et al., 2016).
2.1.1. Summary
It is likely that fear inhibition and response inhibition lhave shared circuitry (Jovanovic et al., 2013a), in that inhibitory circuits regulate both emotion and non-emotion regions. The core inhibition circuits that are impaired in PTSD are the vmPFC and hippocampus, and neuroimaging studies have indeed demonstrated decreased functioning of these regions using both fear inhibition and response inhibition paradigms. The involvement of the salience network (amygdala, dACC, and insula) during response inhibition in PTSD is less clear, although some studies show increased insula activation during both fear and response inhibition. On the other hand, the role of motor reponse regions in PTSD has only been observed primarily using response inhibition paradigms. It can therefore be concluded that there is important shared inhibition neurocircuitry that is implicated in PTSD, and different specific target regions are involved depending on the nature of the inhibition task.
2.2. fMRI Inhibition Phenotypes for PTSD Therapy
Only 3 studies to date have used an inhibition paradigm in a longitudinal pre- and post-treatment study. Helpman and colleagues (Helpman et al., 2016) used the fear conditioning and extinction paradigm before and after 10 weeks of prolonged exposure (PE). Pre- and post-treatment fMRI data was available for 16 PTSD patients and 16 trauma controls. PTSD patients showed greater right rACC activation at baseline compared to follow-up. Furthermore, a decrease in sgACC and hippocampal/parahippocampal activation was associated with reduced PTSD symptoms. In an fMRI study of 13 treatment-seeking PTSD patients prior to 8 weekly cognitive behavioral therapy (CBT) sessions, PTSD severity was measured pre-treatment and 6 months later (Falconer et al., 2013). Greater activation during a Go/NoGo task in the left frontostriatal network, including the left IFC, orbitofrontal cortex and dorsal striatum, anterior medial PFC and parahippocampus was related to lower PTSD severity post-treatment, controlling for pre-treatment severity, but using a lenient statistical threshold (p<0.005, uncorrected). Finally, van Rooij and colleagues collected pre- and post-treatment scans using the stop signal inhibition task to measure both response inhibition and contextual cue processing (van Rooij et al., 2015). Scans were collected from 41 war veterans with PTSD and 22 control veterans with a 6–8 month interval during which the patients received trauma-focused therapy. No pre-to post-treatment differences were observed in the regions of interest, i.e., left motor cortex and right IFG and striatum, however, whole brain analyses (p<0.001, k=47, FWE-corrected) showed that more left inferior parietal lobe (IPL) activation during context processing predicted a greater symptom reduction (van Rooij et al., 2015).
In addition to pre/post-treatment studies using inhibition tasks, inhibition tasks were used in two fMRI studies assessing the effects of potential pharmacotherapies. A placebo-controlled between-subjects (N=14 in each group) fMRI study was used to examine the effects of tetrahydrocannibinol (THC) on vmPFC and hippocampal activation using a fear conditioning and extinction task (Rabinak et al., 2014). Participants who used THC compared to the placebo control group showed heightened vmPFC and hippocampal activation in response to the extinguished CS+ during extinction recall (Rabinak et al., 2014). Finally, Ebrahimi and colleagues (2017) performed a placebo-controlled fMRI study assessing the effects of D-cycloserine (DCS) on appetitive and aversive learning using monetary wins and losses as US. The DCS group showed reduced amygdala activation and enhanced amygdala-vmPFC coupling during extinction recall (Ebrahimi et al., 2017).
2.2.1. Summary
Studies show that functional neuroimaging measures of fear inhibition and response inhibition are related to PTSD treatment outcomes. Specifically, decreased sgACC and hippocampal activation during fear inhibition, and increased pre-treatment left IPL and frontrostriatal activation during response inhibition is related to better outcomes. More studies are needed to substantiate these findings, but these studies underscore the importance of assessing fear and response inhibition as phenotypes of PTSD treatment response.
3. Psychophysiological Measures of Inhibition in PTSD
As discussed above and shown in Figure 1, the amygdala is an integral part of the neural circuit that controls fear responses and the peripheral targets of fear responses, such as those that can be measured using psychophysiological recordings (Davis et al., 1993). Specifically, the amygdala activates several loci of the peripheral nervous system, including the sympathetic nervous system via the PAG which increases sweat gland activity measured by skin conductance response (SCR), and the vagus nerve increasing heart-rate variability (HRV). In addition, the amygdala directly stimulates the pons, a brain region that lies within a circuit mediating fear-potentiated startle (FPS) responses. Therefore, conditioned fear can be observed as increased SC and FPS responses, as compared to safety conditions or baseline, respectively, within specific learning paradigms. On the other hand, during safe conditions, the vmPFC inhibition of the amygdala should decrease SCR and FPS; however, PTSD patients with impaired fear inhibition may continue to display elevated levels of SCR or FPS.
These peripheral psychophysiological measures can be easily and non-invasively captured on the surface of the skin and provide objective metrics associated with PTSD symptoms (Jovanovic et al., 2009). Psychophysiological reactivity to reminders of the traumatic experience has been extensively studied in PTSD over the last 25 years, with most studies showing heightened fear responses in patients compared to controls (Norrholm and Jovanovic, 2018; Orr et al., 1993; Pole, 2007). However, the sensitivity and specificity of psychophysiological measures has been debated (Keane et al., 1998), and likely depends on the task used to capture the fear response. The majority of psychophysiological tasks have captured reactivity to trauma-related stimuli, which has high sensitivity but relatively low specificity (Keane et al., 1998); inhibition of fear may offer more promise in PTSD specificity.
3.1. Psychophysiological Inhibition Phenotypes for PTSD Diagnosis
While heightened fear responses to conditioned safety signals may be a common feature of all anxiety disorders (Duits et al., 2015), safety signal learning has frequently been associated specifically with hyperarousal symptoms of PTSD (Glover et al., 2011; Michopoulos et al., 2015). Further, the inability to transfer learned safety to a novel context may be a specific deficit in PTSD. Transfer of safety can be tested in paradigms designed to examine inhibition of fear when a safety signal is paired with a conditioned danger cue. For example, a conditional discrimination task (termed AX+/BX−) begins by training individuals to discriminate between danger and safety, and then tests fear responses to a compound stimulus which combines both cues (Jovanovic et al., 2005; Myers et al., 2009). In healthy individuals, fear-potentiated startle to the compound cue is reduced relative to the danger cue (Jovanovic et al., 2005). In individuals with current PTSD, the inhibition by the safety signal may be too weak to reduce the fear response (Jovanovic et al., 2009; Sijbrandij et al., 2013). In fact, impaired fear inhibition is associated both with acute and persistent PTSD symptoms (Jovanovic et al., 2013b; Sijbrandij et al., 2013). However, using the same conditional discrimination task did not how impaired inhibition in individuals with high trait anxiety (Kindt and Soeter, 2014), or depression without comorbid PTSD (Jovanovic et al., 2010), suggesting specificity for PTSD.
Fear extinction can also be used to measure fear inhibition (see Zuj and Norrholm, this issue, for in depth review), and has shown that impaired extinction of SCR to danger signals is associated with chronic PTSD (Blechert et al., 2007; Milad et al., 2008; Wessa and Flor, 2007), and predicts future PTSD symptom severity (Guthrie and Bryant, 2006). Elevated heart rate responses to both safety signals during conditioning and sanger signals during extinction have been associated with symptom severity even in soldiers who report sub-threshold PTSD (Costanzo et al., 2016). Inhibition of fear-potentiated startle responses during extinction also show deficits in PTSD populations, including military (Acheson et al., 2015) and civilian (Norrholm et al., 2011) trauma populations.
3.1.1. Summary
Taken together, these studies point to impaired fear inhibition as a robust phenotype for PTSD and psychophysiological assessments of the fear-potentiated startle as a reliable measure for fear inhibition in PTSD.
3.2. Psychophysiological Inhibition Phenotypes for PTSD Therapy
While psychophysiological measures have a rich history of use with PTSD, they have seldom been used in PTSD treatment. There is a small number of studies that have used trauma-evoked startle responses (Robison-Andrew et al., 2014; Rothbaum et al., 2014) or HR (Wangelin and Tuerk, 2015) pre- and post-treatment, with all of these showing positive treatment outcomes. Fear inhibition has only been examined with treatment in two studies. In addition to changes in fMRI, the study by Helpman and colleagues examined SCR during extinction before and after 10 weeks of prolonged exposure therapy in PTSD patients (Helpman et al., 2016). The study found treatment-related improvements in inhibition of SCR during extinction that correlated with change in PTSD symptoms. Finally, a recent case study incorporated these measures as assessment at 1 month follow-up after treatment and found that imaginal exposure therapy immediately after trauma exposure was associated with normal levels of fear inhibition of FPS during conditioning and extinction observed in healthy control participants (Post et al., 2017). However, this study did not measure FPS prior to treatment, so was not able to show treatment-related change.
While there have been very few published studies that have used fear inhibition as a treatment outcome in PTSD, some emerging studies have used pharmacological manipulations of fear inhibition. These studies are useful in determining potential targets for drug discovery for novel therapeutics for PTSD. For example, intranasal oxytocin facilitates inhibition of fear measured with SCR during extinction (Eckstein et al., 2015) and FPS during extinction recall (Acheson et al., 2013). Administration of cannabinoids in the form of THC also facilitates extinction of SCR (Rabinak et al., 2013). While these studies were conducted in healthy participants targeting fear inhibition, one recent study examined FPS in subjects with PTSD and trauma controls, and found that dexamethasone administration the night prior to fear conditioning normalized the impairments in fear inhibition (Michopoulos et al., 2017). These studies represent the first step in using fear inhibition to test the effects of pharmacological agents in PTSD.
3.2.1. Summary
While there is a small number of treatment studies of PTSD that have used psychophysiological measures, these studies show an improvement in fear inhibition after successful treatment, suggesting the importance of including psychophysiological measures in future clinical research.
4. Conclusions
Fear inhibition and response inhibition have significant overlap in circuitry as both depend on prefrontal regulation of diverse processes, and hippocampal information to guide these processes. Neuroimaging measures have been used to show impairments in PTSD for both fear and response inhibition processes, whereas psychophysiological measures have specifically been used to demonstrate fear inhibition deficits in PTSD. Inhibition studies in PTSD have mostly focused on fear inhibition as this is more directly related to hyperarousal symptoms observed in PTSD. However, given the neurobiological overlap between fear and response inhibition processes it would be interesting to focus on response inhibition to establish changes in the fear inhibition domain. For example, targeting non-emotional circuits to increase regulation over emotional or fear processes could be an interesting novel treatment approach. Brain modulation techniques, including transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) could be used to target the brain’s inhibitory neurocircuitry.
Fear conditioning studies that index inhibition of fear, such as safety signal learning and extinction, can be used to objectively measure deficits in PTSD using fMRI, fear-potentiated startle, skin conductance response, and heart rate. Using such methods to assess PTSD diagnosis and symptoms in an emerging literature are showing promising specificity for PTSD; however, it is unclear that these methods will be able to accurately diagnose PTSD in the absence of other clinical measures. It is likely that impaired fear inhibition can be added to a battery of assessment to determine a profile of risk. This battery could also include other mechanisms related to inhibition of fear such as extinction retention and overgeneralization, or other measures assessing response inhibition.
Further, there is a very small number of studies that have examined fear inhibition clinically as a measure of treatment outcome. In fact, the small number of PTSD therapy studies looking at inhibition utilized fMRI, and no studies to date have used psychophysiological measures of fear inhibition. The current state of the science is the translational development of interventions targeting fear inhibition, and several promising pharmacological agents are showing facilitation effects in humans. Future studies will need to build on these results using randomized clinical trials with psychophysiological outcomes. Currently, these measures show a lot of promise as an intermediate phenotype for PTSD treatment, however, the clinical utility of fear inhibition paradigms remains to be seen.
Acknowledgements
Dr. Jovanovic has funding from the National Institutes of Health (MH092576, MH098212, MH100122, MH111682) and Brain and Behavior Research Foundation (NARSAD). Dr. van Rooij has funding from Brain and Behavior Research Foundation (NARSAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson DT, Feifel D, de Wilde S, McKinney R, Lohr JB, Risbrough VB, 2013. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology 229(1), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB, 2015. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology 51, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J, Lupez-Martin S, Carretie L, 2010. Emotional context modulates response inhibition: Neural and behavioral data. Neuroimage 49(914–921). [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, 1990. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences 13, 266–271. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL, 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Aron AR, 2011. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry 69, e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V, 2007. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience 27, 11860–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.P., 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Aupperle RL, Stillman AN, Simmons AN, Flagan T, Allard CB, Thorp SR, Norman SB, Paulus MP, Stein MB, 2016. Intimate Partner Violence PTSD and Neural Correlates of Inhibition. Journal of Traumatic Stress 29(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH, 2007. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 45. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG, 2010. Pinning down response inhibition in the brain - Conjunction analyses of the Stop-signal task. Neuroimage 52, 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce SE, Buchholz KR, Brown WJ, Yan L, Durbin A, Sheline YI, 2013. Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder()(). NeuroImage : Clinical 2, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL, 2008. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety 25(6), 514–526. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, Sangha S, 2012. Inhibition of Fear by Learned Safety Signals: A Mini-Symposium Review. The Journal of Neuroscience 32(41), 14118–14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen AN, Francisco AJ, Thelen J, Bruce J, Martin L, McDowd J, Simmons WK, Aupperle RL, 2017. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depression and anxiety 34(5), 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ, 2007. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectrums 12, 200–206. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Jovanovic T, Norrholm SD, Ndiongue R, Reinhardt B, Roy MJ, 2016. Psychophysiological Investigation of Combat Veterans with Subthreshold Post-traumatic Stress Disorder Symptoms. Military Medicine 181(8), 793–802. [DOI] [PubMed] [Google Scholar]
- Davis M, 1992. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci 13(1), 35–41. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M, 1993. Fear-potentiated startle: A neural and pharmacological analysis. Behavioral Brain Research 58, 175–198. [DOI] [PubMed] [Google Scholar]
- Diener SJ, Nees F, Wessa M, Wirtz G, Frommberger U, Penga T, Ruttorf M, Ruf M, Schmahl C, Flor H, 2016. Reduced amygdala responsivity during conditioning to trauma-related stimuli in posttraumatic stress disorder. Psychophysiology 53(10), 1460–1471. [DOI] [PubMed] [Google Scholar]
- Dretsch MN, Wood KH, Daniel TA, Katz JS, Deshpande G, Goodman AM, Wheelock MD, Wood KB, Denney TS Jr, Traynham S, Knight DC, 2016. Exploring the Neurocircuitry Underpinning Predictability of Threat in Soldiers with PTSD Compared to Deployment Exposed Controls. The Open Neuroimaging Journal 10, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li C.-s., 2009. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience 29, 10171–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, Baas JMP, 2015. Updated Meta-Analysis of Classical Fear Conditionoing in the Anxiety Disorders. Depression and Anxiety 32(4), 239–253. [DOI] [PubMed] [Google Scholar]
- Ebrahimi C, Koch SP, Friedel E, Crespo I, Fydrich T, Ströhle A, Heinz A, Schlagenhauf F, 2017. Combining D-cycloserine with appetitive extinction learning modulates amygdala activity during recall. Neurobiology of Learning and Memory 142, 209–217. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Grinevich V, Kendrick KM, Maier W, Hurlemann R, 2015. Oxytocin Facilitates the Extinction of Conditioned Fear in Humans. Biological Psychiatry 78(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM, 2008. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry & Neuroscience : JPN 33(5), 413–422. [PMC free article] [PubMed] [Google Scholar]
- Falconer EM, Allen A, Felmingham KL, Williams LM, Bryant RA, 2013. Inhibitory Neural Activity Predicts Response to Cognitive-Behavioral Therapy for Posttraumatic Stress Disorder. J Clin Psychiatry 74(9), 895–901. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I, 2014. Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. The Journal of Neuroscience 34(40), 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, 2011. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress Anxiety 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA, 2006. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med 68. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM, 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE, 2010. Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. NeuroImage 2010;51:421–431. Neuroimage 51, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier FR, Neria M, Shvil E, Malaga Aragon M.J., Markowitz JC, Lindquist MA, Wager TD, Milad MR, Neria Y, 2016. Neural Changes in Extinction Recall Following Prolonged Exposure Treatment for PTSD: A Longitudinal fMRI Study. NeuroImage: Clinical 12, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ, 2013a. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex 49(7), 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62(2), 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan E, 2005. Fear Potentiation and Fear Inhibition in a Human Fear-Potentiated Startle Paradigm. Biological Psychiatry 57(12), 1559–1564. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, 2011. Neural Mechanisms of Impaired Fear Inhibition in Posttraumatic Stress Disorder. Frontiers in Behavioral Neuroscience 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ, 2010. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety 27(3), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ, 2009. Posttraumatic Stress Disorder May be Associated with Impaired Fear Inhibition: Relation to Symptom Severity. Psychiatry Research 167(1–2), 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ, 2010. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. The American journal of psychiatry 167(6), 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Sakoman A, Kozarić-Kovačić D, Meštrović A, Duncan EJ, Davis M, Norrholm SD, 2013b. Acute Stress Disorder Versus Chronic Posttraumatic Stress Disorder: Inhibition of Fear as a Function of Time Since Trauma. Depression and anxiety 30(3), 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek D, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW, 1998. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Journal of Consulting and Clinical Psychology 66, 914–923. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW, 2006. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews 30, 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, 2014. Fear Inhibition in High Trait Anxiety. PLoS ONE 9(1), e86462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, 2000. Emotion circuits in the brain, Annual Review of Neuroscience pp. 155–184. [DOI] [PubMed]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ, 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Rich B, Vinton D, Nelson E, Fromm S, Berghorst L, Joshi P, Robb A, Schachar R, Dickstein D, 2007. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry 164(1), 52–60. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM, 1999. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 45, 817–826. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, 1984. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review 91, 291–327. [DOI] [PubMed] [Google Scholar]
- Maren S, 2001. Neurobiology of Pavlovian fear conditioning, Annual Review of Neuroscience pp. 897–931. [DOI] [PubMed]
- Michopoulos V, Norrholm SD, Stevens JS, Glover EM, Rothbaum BO, Gillespie CF, Schwartz AC, Ressler KJ, Jovanovic T, 2017. Dexamethasone Facilitates Fear Extinction and Safety Discrimination in PTSD: A Placebo-Controlled, Double-Blind Study. Psychoneuroendocrinology 83, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ, 2015. CRP genetic variation and CRP levels are associated with increased PTSD symptoms and physiological responses in a highly traumatized civilian population. The American journal of psychiatry 172(4), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK, 2008. Presence and Acquired Origin of Reduced Recall for Fear Extinction in PTSD: Results of a Twin Study. Journal of Psychiatric Research 42(7), 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko N.a.B., Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry 66(12), 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL, 2007. A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biological Psychiatry 62(10), 1191–1194. [DOI] [PubMed] [Google Scholar]
- Myers KM, Toufexis DJ, Winslow JT, Jovanovic T, Norrholm SD, Duncan E, Davis M, 2009. Measurement of fear inhibition in rats, monkeys, and humans with and without posttraumatic stress disorder, using the AX+, BX− paradigm, in: Whalen PJ, Phels EA (Eds.), The Human Amygdala The Guilford Press, New York City. [Google Scholar]
- Norrholm SD, Jovanovic T, 2018. Fear Processing, Psychophysiology, and PTSD. Harvard Review of Psychiatry 26(3), 129–141. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ, 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 69(6), 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR, 1993. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology 102, 152–159. [DOI] [PubMed] [Google Scholar]
- Pole N, 2007. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin 133(5), 725–746. [DOI] [PubMed] [Google Scholar]
- Pollack AE, 2001. Anatomy, physiology, and pharmacology of the basal ganglia. Neurologic Clinics 19, 523–534. [DOI] [PubMed] [Google Scholar]
- Post LM, Michopoulos V, Stevens JS, Reddy R, Maples JL, Morgan JR, Rothbaum AO, Jovanovic T, Ressler KJ, Rothbaum BO, 2017. Psychological and psychobiological responses to immediate early intervention in the emergency department: Case report of one-session exposure therapy for the prevention of PTSD. Practice innovations (Washington, D.C.) 2(2), 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong HW, Fanselow MS, 2010. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proceedings of the National Academy of Sciences of the United States of America 107(14881–14885). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D, 2007. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, Phan KL, 2014. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiology of learning and memory 113, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL, 2013. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 64(1), 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK, 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry 47(9), 769–776. [DOI] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, Echiverri-Cohen A, Giardino N, Defever A, Norrholm SD, Jovanovic T, Rothbaum BO, Liberzon I, Rauch SAM, 2014. Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat Veterans. Journal of Anxiety Disorders 28(4), 358–362. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop BW, Davis M, Bradley B, Duncan EJ, Rizzo A, Ressler KJ, 2014. A Randomized, Double-Blind Evaluation of d-Cycloserine or Alprazolam Combined With Virtual Reality Exposure Therapy for Posttraumatic Stress Disorder in Iraq and Afghanistan War Veterans. American Journal of Psychiatry 171(6), 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR, 2011. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neuroscience & Therapeutics 17(4), 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, Milad MR, Neria Y, 2014. Sex differences in extinction recall in posttraumatic stress disorder: A pilot fMRI study. Neurobiology of Learning and Memory 113, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Engelhard IM, Lommen MJJ, Leer A, Baas JMP, 2013. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). Journal of Psychiatric Research 47(12), 1991–1997. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Garfinkel SN, Liberzon I, 2013. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Frontiers in Human Neuroscience(OCT) [DOI] [PMC free article] [PubMed]
- Steiger F, Nees F, Wicking M, Lang S, Flor H, 2015. Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. International Journal of Psychophysiology 98(3), 584–593. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, Jovanovic T, 2016. Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in PTSD, but not trauma-exposed controls. Depression and Anxiety doi 10.1002/da.22506. [DOI] [PMC free article] [PubMed]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ, 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research 47(10), 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim Y, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, Ressler KJ, 2017. Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biological Psychiatry 81(12), 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VAF, 2010. Unconscious activation of the prefrontal No-Go network. Journal of Neuroscience 30, 4143–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M, 2015. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology 40(3), 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, Geuze E, 2014. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during inhibition. Journal of Psychiatry and Neuroscience 39(4), 130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Fani N, Smith AK, Kerley KA, Lori A, Ressler KJ, Jovanovic T, 2016. Childhood Trauma and COMT Genotype Interact to Increase Hippocampal Activation in Resilient Individuals. Frontiers in Psychiatry 7(156). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, Ogbonmwan YE, Shin J, Nugent NR, Hudak LA, Rothbaum BO, Ressler KJ, Jovanovic T, 2018. The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biological Psychiatry 82(2), 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangelin BC, Tuerk PW, 2015. Taking the pulse of prolonged exposure therapy: Physiological reactivity to trauma imagery as an objective measure of treatment response. Depression and Anxiety 32(12), 927–934. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H, 2007. Failure of Extinction of Fear Responses in Posttraumatic Stress Disorder: Evidence From Second-Order Conditioning. Am J Psychiatry 164(11), 1684–1692. [DOI] [PubMed] [Google Scholar]
- Wicking M, Steiger F, Nees F, Diener SJ, Grimm O, Ruttorf M, Schad LR, Winkelmann T, Wirtz G, Flor H, 2016. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiology of Learning and Memory 136, 116–126. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M, 2013. Expectations and violations: Delineating the neural network of proactive inhibitory control. Human Brain Mapping 34, 2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M, 2010. On the role of the striatum in response inhibition. PLos ONE 5, e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]


