Abstract
The maximum yield of xylanase from Aureobasidium melanogenum PBUAP46 was 5.19 ± 0.08 U ml−1 when cultured in a production medium containing 3.89% (w/v) rice straw and 0.75% (w/v) NaNO3 as carbon and nitrogen sources, respectively, for 72 h. This enzyme catalyzed well and was relatively stable at pH 7.0 and room temperature (28 ± 2 °C). The produced xylanase was used to hydrolyze xylans from four tropical weeds, whereupon it was found that the highest amounts of reducing sugars in the xylan hydrolysates of cogon grass (Imperata cylindrical), Napier grass (Pennisetum purpureum), and vetiver grass (Vetiveria zizanioides) were at 20.44 ± 0.84, 17.50 ± 0.29, and 19.44 ± 0.40 mg 100 mg xylan−1, respectively, but it was not detectable in water hyacinth (Eichhornia crassipes) hydrolysate. The highest combined amount of xylobiose and xylotriose was obtained from vetiver grass; thus, it was selected for further optimization. After optimization, xylanase digestion of vetiver grass xylan at 27.94 U g xylan−1 for 92 h 19 min gave the highest amount of reducing sugars (23.65 ± 1.34 mg 100 mg xylan−1), which were principally xylobiose and xylotriose. The enriched XOs exhibited a prebiotic property, significantly stimulating the growth of Lactobacillus brevis and L. casei by a factor of up to 3.5- and 6.5-fold, respectively, compared to glucose.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1586-y) contains supplementary material, which is available to authorized users.
Keywords: Tropical weed, Xylan, Xylanase, Xylooligosaccharide, Prebiotic
Introduction
Xylooligosaccharides (XOs) are sugar oligomers containing two-to-ten xylose molecules linked by β-1, 4-glycosidic linkages (Carvalho et al. 2013). They are sweet tasting and edible, but non-digestible and have been used in many pharmaceutical, feed, and food industrial applications as a low-calorie sweetener (Vazquez et al. 2000; Carvalho et al. 2013). Moreover, XOs reportedly exhibit a prebiotic activity by stimulating the growth of beneficial bacteria in the intestine, such as bifidobacteria and lactobacilli, which are common probiotic bacteria (Crittenden et al. 2002; Chapla et al. 2012). Due to their prebiotic property, XOs have been commercialized as functional foods or additives in food and drinks (Vazquez et al. 2000). However, the prebiotic activity of XOs depends mainly on their molecular weight which, in turn, is related to their degree of polymerization (DP). XOs with a low DP exhibit more prebiotic potential than those with a higher DP (Moura et al. 2007). Therefore, most commercial XOs contain mainly xylobiose and xylotriose with a DP of two and three, respectively (Gullón et al. 2008).
Although XOs can be produced by the partial hydrolysis of xylan, which is one of the major plant cell-wall components and can be extracted from any plant lignocellulosic biomass, the commercially available purified xylans, mostly from hardwoods, are relatively expensive. Thus, cheaper crude xylans are considerably more cost-effective for XO production. Some fast-growing, invasive tropical weeds, such as grasses and aquatic weeds, are of interest as alternative sources of xylans due to their abundance, low economic value and need of eradication. The hydrolysis of xylan can be achieved using xylanase enzymes produced from several microorganisms, including black yeast (Aureobasidium) and, especially, its color-variant strains that reportedly produce a relatively high activity of thermostable xylanase (Leathers 1986). In Thailand, a number of color-variant isolates of Aureobasidium with high xylanase activity have been obtained from various habitats (Punnapayak et al. 2003; Prasongsuk et al. 2005, 2018; Lotrakul et al. 2009; Manitchotpisit et al. 2009; Yanwisetpakdee et al. 2016), and it is of interest to investigate their potential in the production of xylanase that could be used for XO production. To further reduce the XO processing cost, xylanases that catalyze well under mild conditions and that are stable at room temperature are particularly desired, since this would lower the heating cost and simplify the processes of substrate preparation and product analysis. Therefore, in this study, XO production from four selected tropical weed xylans, namely, cogon grass (Imperata cylindrical), Napier grass (Pennisetum purpureum), vetiver grass (Vetiveria zizanioides), and water hyacinth (Eichhornia crassipes), by enzymatic hydrolysis using xylanase from Aureobasidium was investigated. Twenty-six Aureobasidium strains were screened for the production of xylanase that functions well at pH 7 and room temperature, followed by the optimization of xylanase production from the selected strain (the one that gave the highest XOs yield) using low-cost substrates. Finally, the prebiotic property of the produced XOs was determined.
Materials and methods
Microorganisms and culture conditions
Twenty-six strains of Aureobasidium previously isolated in Thailand (Manitchotpisit et al. 2009; Yanwisetpakdee et al. 2016) were obtained from the culture collection of the Plant Biomass Utilization Research Unit, Department of Botany, Faculty of Science, Chulalongkorn University, Bangkok, Thailand. The reference strain, A. pullulans NRRL Y2311-1, was obtained from the ARS Culture Collection, USDA, Peoria, IL, USA. All the strains were cultured in yeast malt (YM) broth medium at room temperature (28 ± 2 °C) with 150-rpm agitation for 3 days. For long-term storage, the cells were lyophilized and stored at 4 °C. The cultures were also maintained in YM agar at 4 °C for short-term storage. Lactobacillus brevis TISTR 868 and L. casei TISTR 390 were obtained from the Thailand Institute of Scientific and Technological Research, and maintained on de Man Rogosa Sharpe (MRS) agar at 4 °C (de Man et al. 1960).
Screening of xylanase production
All the Thai Aureobasidium strains and A. pullulans NRRL Y2311-1 were tested for their ability to produce xylanase in liquid medium using corncob as the sole carbon source (Bankeeree et al. 2014). The cultures were incubated at room temperature (28 ± 2 °C) with agitation at 150 rpm for 3 days. After centrifugation at 18,000×g for 10 min, the supernatants were used as the crude enzyme for xylanase and β-xylosidase activity determination. Xylanase activity was assayed using 1% (w/v) beechwood xylan (Sigma-Aldrich, Germany) as the substrate in 50 mM sodium phosphate buffer (pH 7.0) at room temperature for 10 min. Reducing sugars released from the reaction were measured by the dinitrosalicylic acid (DNS) method (Miller 1959). β-Xylosidase activity was assayed as described by Bankeeree et al. (2018) using p-nitrophenyl-β-d-xylopyranoside (pNPX) as the substrate. One unit (U) of xylanase or β-xylosidase activity was defined as the amount of enzyme that liberated 1 µmol of xylose equivalents or p-nitrophenol per min, respectively. The crude enzyme from the strain of Aureobasidium producing the highest xylanase activity was incubated in 50 mM sodium phosphate buffer (pH 7) at room temperature to determine the enzyme stability. The activity was determined at 1-day intervals for 7 days. The residual activity (%) was calculated in comparison with that prior to incubation.
Optimization of xylanase production
The highest xylanase-producing Aureobasidium strain was selected for optimization of xylanase production. Different carbon and nitrogen sources were added in the xylanase production medium in the various combinations using a factorial experimental design. The carbon sources evaluated at 1% (w/v) were the common Thai agricultural residues such as corncob, sugarcane bagasse, and rice straw, while the nitrogen sources evaluated at 0.67% (w/v) were l-asparagine, peptone, sodium nitrate, and ammonium sulfate. The combination of carbon and nitrogen sources providing the highest xylanase activity was selected for optimal concentration determination by response surface methodology (RSM) using a central composite design (CCD) with two variables. The central values (zero level) for carbon (X1) and nitrogen sources (X2) were 4.00% and 0.80% (w/v), respectively.
Determination of the biomass composition
The contents of cellulose, hemicellulose, and lignin in the plant biomass substrates were analyzed by forage fiber analysis according to the method of Goering and Van Soest (1970) which enabled the differentiation of fiber fractions using specific detergents. In brief, a neutral detergent was used to determine the neutral detergent fiber (NDF) or total cell-wall components, including hemicelluloses, cellulose, lignin, and fiber-bound proteins. Concentrated sulfuric acid was used to solubilize the acid detergent fiber (ADF) which consisted of cellulose and lignin. Cellulose was then determined by calculating the difference between ADF and the acid detergent lignin (ADL), and hemicelluloses as the difference between ADF and NDF.
Xylan extraction
Cogon grass, Napier grass, vetiver grass, and water hyacinth collected from Nonthaburi province, Thailand, were oven-dried at 60 °C, ground, and then filtered through a 20-mesh screen to obtain 1-mm particles. Xylan was extracted from the plant sample using 12% (w/v) NaOH solution containing 1% (w/v) NaBH4 at a solid-to-liquid ratio of 1:25 [modified from Akpinar et al. (2009) and Samanta et al. (2012)]. The reactions were incubated at room temperature with 150-rpm agitation for 16 h. Supernatants were collected, neutralized to pH 7 by glacial acetic acid, and precipitated by 95% ethanol. The xylan pellets were oven-dried (60 °C) until a constant weight was obtained. The contents of cellulose, hemicellulose, and lignin of all the samples were analyzed as described above.
Enzymatic hydrolysis of xylan
Extracted xylans and commercial beechwood xylan were separately suspended to a final concentration of 4% (w/v) in 50 mM sodium phosphate buffer (pH 7.0) and used as substrates for xylan hydrolysis. The crude enzyme at 10 U g substrate−1 was added to the substrate solution (5 ml) with adjusting the total volume to 10 ml with the same buffer prior to incubation at room temperature with 150-rpm agitation for 96 h. The hydrolysis reaction was terminated by boiling the mixture for 15 min. The total reducing sugar in the hydrolysate was measured by the DNS method, whereas the contents of xylose, xylobiose, and xylotriose were analyzed by HPLC (Akpinar et al. 2009). The hydrolysis of weed xylan providing the highest combined xylobiose and xylotriose level was selected to investigate the optimal enzyme dosage and incubation time by RSM using CCD with two variables. The central values for enzyme dosage (X1) and incubation time (X2) were 26 U g xylan−1 and 96 h, respectively.
XOs enrichment and determination of prebiotic property
The XOs produced in the reaction mixture were concentrated by lyophilization and resuspended in distilled water at a final concentration of 2% (w/v). The enrichment of XOs was performed by adding activated carbon powder at 20% (w/v), and shaken at 200 rpm for 30 min before the mixture was suctioned through a 50-ml porous glass filter number 3 and washed with distilled water. The XOs were eluted in 1500 ml of 15% (v/v) ethanol and concentrated by evaporation prior to lyophilization to yield the partially purified XOs as a white solid (Yang et al. 2007). The molecular weights of the crude and enriched XOs were analyzed by electro-spray ionization-positive mass spectrometry (ESI-MS; Micromass Quattro Micro), while the prebiotic property of the enriched XOs was determined by their effect on the growth of L. brevis (TISTR 868) and L. casei (TISTR 390). Each bacterial strain was inoculated into MRS broth for seed culture preparation and grown at room temperature, with 150 rpm agitation, for 16 h. The OD600 of the seed culture was then adjusted to 0.1 by sterile medium prior to adding 1% (v/v) into the fresh MRS broth supplemented with 2 mg carbon ml−1 of one of glucose, the enriched XOs or commercial yeast β-glucan (Pronova Lab., Thailand), or with no addition of a carbon source as the negative control. The cultures were incubated at 37 °C under a static condition in an anaerobic jar flooded with carbon dioxide (CO2) for 48 h. The growth of bacteria was assessed using a standard plate count technique under anaerobic conditions as described above.
Statistical analysis
The results are expressed as the mean ± one standard deviation (SD) derived from three replications. One-way analysis of variance (ANOVA) and Duncan’s multiple range tests (DMRT) at the level of 5% were used to determine significant differences among the means when applicable using the SPSS software, version 16.0 (SPSS Inc., US).
Results and discussion
Screening of xylanase production
Among 26 Thai Aureobasidium strains, A. melanogenum PBUAP46 produced the highest xylanase activity (2.70 ± 0.01 U ml−1) when assayed at pH 7 and room temperature (supplementary data I). The xylanase yield of this strain was comparable to that of the reference strain A. pullulans NRRL Y-2311-1. The fact that this xylanase catalyzes well at neutral pH and room temperature would contribute to a simple operating process without the cost of heating and related equipment, which is quite different from the general optimal condition for Aureobasidium xylanases that require pH 3–5 and a temperature of 35–55 °C (Ohta et al. 2001; Tanaka et al. 2005; Leite et al. 2007; Verjans et al. 2010; Yegin 2017). To obtain the maximum yield of XOs, the xylanase used in the hydrolysis reaction must be free of β-xylosidase activity, and therefore, it was noted that β-xylosidase activity was not detectable from the culture supernatant of A. melanogenum PBUAP46 (data not shown), suggesting that it was suitable for XO production.
The crude xylanase from A. melanogenum PBUAP46 exhibited a relatively high stability at pH 7 and room temperature. The remaining activity was greater than 80% after 3 days and gradually decreased to 40% after 7-day incubation (supplementary data II). A. melanogenum xylanase with a comparable stability at pH 7 and 30 °C was reported by Ohta et al. (2001). However, they reported residual activity only after 1-day incubation; thus, the stability of the xylanase from this strain after a longer storage time is not known.
Optimization of xylanase production
To replace the commercially available but expensive purified xylanase, the crude enzyme was produced by the selected A. melanogenum PBUAP46 and used in XO production. Corncob, rice straw, and sugarcane bagasse are common agricultural wastes available in Thailand and have been used for enhancing xylanase production in various microorganisms (Chapla et al. 2010; Bankeeree et al. 2014; Kaushik et al. 2014) and so were used here as carbon sources in this study. The optimum pH and temperature were determined using a one-factor-at-a-time approach in the ranges of pH 5–7 and at temperature of 25–30 °C, respectively. Since xylanase yield was not significantly affected by these factors (data not shown), the optimization of xylanase production was performed by culturing A. melanogenum PBUAP46 in the production medium in which paired combinations of carbon and nitrogen sources had been combined under the same condition of pH 5.0 and temperature at 28 ± 2 °C. The combination of rice straw and sodium nitrate provided the highest xylanase activity at 4.10 ± 0.28 U ml−1 (Fig. 1). This likely reflected the lowest lignin content of rice straw, which allowed the hemicellulose to be more readily accessible and digestible to the xylanase (Table 1) (Guo et al. 2009). Once the small amount of constitutively produced xylanase liberated short xylan fragments from the exposed hemicelluloses, they subsequently stimulated the production of the inducible xylanase to a greater extent (Lubomr and Peter 1998; Kulkarni et al. 1999). In addition to carbon, a nitrogen source is also essential for the growth of organisms and universally affects enzyme production. It has been reported that different Aureobasidium strains prefer different forms of nitrogen, ranging from peptone to ammonium and nitrate (Nasr et al. 2013; Yu and Gu 2013), and that this property is strain-dependent.
Fig. 1.
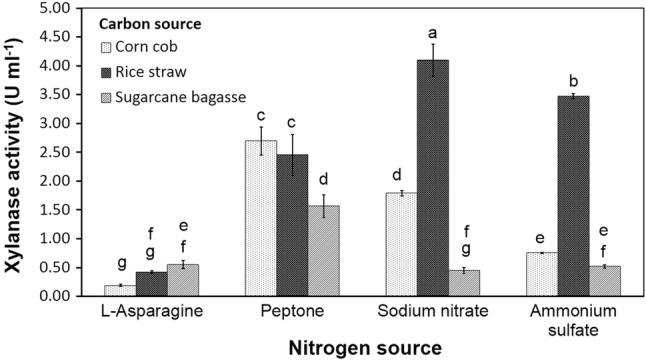
Effects of carbon and nitrogen sources on xylanase production by A. melanogenum PBUAP46. Xylanase activity is shown as the mean ± SD, derived from three replicates. Different letters above each bar indicate a significantly different value (ANOVA and DMRT, P ≤ 0.05)
Table 1.
Contents of cellulose, hemicellulose, and lignin in the selected carbon substrates for xylanase production and selected plant biomasses
| Agricultural waste/plant | Cellulose (%)a | Hemicellulose (%)a | Lignin (%)a |
|---|---|---|---|
| Corn cob | 28.79 ± 0.51 | 41.60 ± 0.59 | 6.29 ± 0.38 |
| Rice straw | 34.05 ± 0.50 | 30.94 ± 0.26 | 3.79 ± 0.28 |
| Sugarcane bagasse | 36.77 ± 0.23 | 27.86 ± 1.15 | 9.62 ± 0.50 |
| Cogon grass | 34.20 ± 1.23 | 33.64 ± 0.67 | 5.59 ± 0.79 |
| Napier grass | 30.67 ± 0.70 | 32.98 ± 0.54 | 3.48 ± 0.27 |
| Vetiver grass | 30.74 ± 0.34 | 36.15 ± 0.53 | 5.03 ± 0.44 |
| Water hyacinth | 24.88 ± 0.65 | 21.82 ± 0.50 | 2.90 ± 0.30 |
aMean ± SD derived from three replicates
To find the optimal concentrations of rice straw and sodium nitrate, RSM using CCD with two variables was employed. The levels of factors applied were based on preliminary experiments (data not shown) and the response values, i.e., xylanase activity, are presented in Table 2. The response surface plot is shown in Fig. 2. The response Y (xylanase activity) was expressed by the following equation:
Table 2.
CCD-RSM used for medium optimization for A. melanogenum PBUAP46 xylanase production
| Run | Code level | Actual level | Xylanase activity (U ml−1) | ||
|---|---|---|---|---|---|
| X 1 | X 2 | Rice straw (%) | NaNO3 (%) | ||
| 1 | +1 | +1 | 5.00 | 1.20 | 4.09 |
| 2 | +1.414 | 0 | 5.41 | 0.80 | 4.25 |
| 3 | +1 | −1 | 5.00 | 0.40 | 3.85 |
| 4 | 0 | −1.414 | 4.00 | 0.23 | 4.20 |
| 5 | −1 | −1 | 3.00 | 0.40 | 4.25 |
| 6 | −1.414 | 0 | 2.59 | 0.80 | 4.44 |
| 7 | −1 | +1 | 3.00 | 1.20 | 3.81 |
| 8 | 0 | +1.414 | 4.00 | 1.37 | 3.79 |
| 9 | 0 | 0 | 4.00 | 0.80 | 4.95 |
| 10 | 0 | 0 | 4.00 | 0.80 | 4.81 |
| 11 | 0 | 0 | 4.00 | 0.80 | 4.96 |
| 12 | 0 | 0 | 4.00 | 0.80 | 4.77 |
Fig. 2.
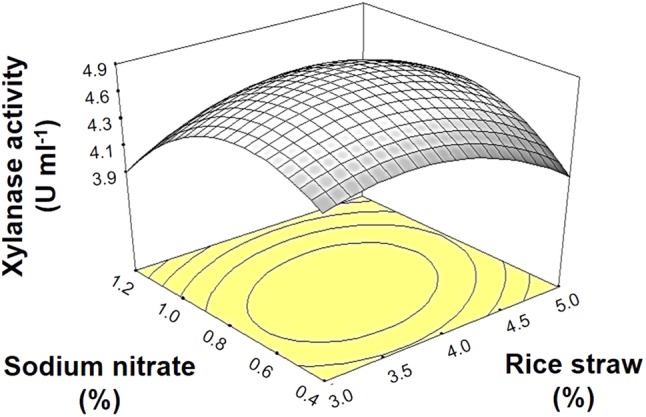
Three-dimensional response surface plot showing the effect of rice straw and sodium nitrate concentrations on the xylanase activity produced by A. melanogenum PBUAP46
where Y is the xylanase activity (U ml−1), X1 is the rice straw concentration (%), and X2 is the sodium nitrate concentration (%).
Based on the R2 value (0.95), the evaluated variables accounted for 95% of the observed response, thus validating the RSM plot. The RSM analysis revealed that the optimum condition could be achieved when adjusting the concentrations of rice straw and sodium nitrate to 3.89% and 0.75% (w/v), respectively, with the highest xylanase activity then predicted to be 4.88 U ml−1. Xylanase production was then performed under the suggested concentrations to confirm the prediction. It was found that the production yielded 5.19 ± 0.13 U ml−1 of xylanase, which was not significantly different from the predicted value, suggesting that the equation was accurate. Using this optimized medium, the production yield was 1.27-fold higher than that with the unoptimized medium.
Xylan extraction
The amounts of cellulose, hemicellulose, and lignin in the different grasses and water hyacinth as potential xylan sources were investigated (Table 1). The cogon grass, Napier grass, and vetiver grass biomasses comprised about 30–34% cellulose and 33–36% hemicellulose, while the water hyacinth biomass had lower contents of these cell-wall components. The lignin contents in these non-woody species ranged from 3 to 6%, which was much lower than those of perennial trees (15–40%) (Marques et al. 2010; Neutelings 2011). The three grass species presented a broadly similar composition of these three components, which was quite different to that of water hyacinth. In general, the quantity and types of polysaccharides in hemicellulose vary between different plants. The previous analysis of the monosaccharides derived from hemicellulose revealed that around 60% of grass hemicellulose was xylose with a high variation (around 10–25%) between species (Schädel et al. 2010a, b). This indicated the high amount of xylan as a substrate required for XOs production. In this study, three grass species (cogon, Napier, and vetiver grasses) plus the water hyacinth were selected as alternative xylan sources, because they are fast-growing species with rapid biomass production and are so abundantly available that localized eradication is often required. Moreover, these three grass species have a high hemicellulose and low lignin contents, and therefore, lignin pretreatment could be omitted. Water hyacinth was also selected, because it infests many aquatic systems resulting in environmental pollution and economic losses (Xia et al. 2013).
The plant biomasses were extracted with alkaline solution to obtain the crude xylans. The treatment enhanced the cleaving of the linkages between hemicellulose and lignin resulting in the consequential dissolution of hemicellulose polysaccharides (Peng et al. 2009). After extraction and precipitation, the yields of xylan obtained from water hyacinth, vetiver grass, cogon grass, and Napier grass were 48.12 ± 2.29, 58.09 ± 4.99, 68.87 ± 8.19, and 85.91 ± 10.09% (w/w) of the hemicellulose content, respectively. The extraction efficiency from the grasses was much higher than that from water hyacinth, corresponding with their hemicellulose and lignin contents. Among the three grasses, Napier grass contained the lowest lignin content leading to its highest xylan yield. The amount of xylan obtained from Napier grass was similar to that obtained from natural grass (Sehima nervosum) xylan (89.39% of the hemicellulose content) using the same approach (Samanta et al. 2012).
Enzymatic hydrolysis of xylan
The extracted xylans were converted to XOs, in comparison with the commercial beechwood xylan, by enzymatic hydrolysis using the crude xylanase from A. melanogenum PBUAP46. The incubation time was fixed at 96 h based on the preliminary time course experiments (data not shown). From the four extracted xylans, cogon grass xylan yielded the highest amount of reducing sugars (20.44 ± 0.84 mg 100 mg xylan−1), but this was not significantly different from that of vetiver grass (19.44 ± 0.40 mg 100 mg xylan−1). The reducing sugar yield from Napier grass xylan was significantly lower (17.50 ± 0.29 mg 100 mg xylan−1), while water hyacinth xylan could not be hydrolyzed and yielded only 0.23 ± 0.12 mg XOs 100 mg xylan−1. As expected, hydrolysis of the commercial purified xylan resulted in the highest yield of the reducing sugars (29.58 ± 0.98 mg 100 mg xylan−1), although this was only 1.45-times higher than that of cogon grass. The amount of reducing sugars obtained from the reaction implied the digestibility of each substrate. The differences could be contributed by the xylan structure and enzyme specificity, both of which affected the DP of the XOs’ products (Akpinar et al. 2009). Moreover, the purity of xylan also played a crucial role in its digestibility, as evidenced by the high yield of reducing sugars obtained from the 90% pure beechwood xylan. Note that the reducing sugar yields obtained from hydrolysis of grass xylans in this study were higher than those previously reported of 11.4 ± 0.6 mg 100 mg from wheat straw xylan−1 and 5.5 ± 0.2 mg 100 mg from rice straw xylan−1 (Chapla et al. 2013). However, comparison among different reports must be interpreted with caution, as different conditions were employed.
Both xylobiose and xylotriose have been reported to be major components in commercial XOs (Gullón et al. 2008), and therefore, their relative yields in the xylanase hydrolysis were used as the criterion for selection. Results from the HPLC analysis showed that the contents of xylobiose and xylotriose in all species except cogon grass corresponded well with the reducing sugar contents detected by DNS (Fig. 3). Among the three grass species, the highest amount of xylobiose (5.65 ± 0.18 mg 100 mg xylan−1) and xylotriose (3.02 ± 0.18 mg 100 mg xylan−1) were obtained from vetiver grass. The water hyacinth biomass was not hydrolyzed by the crude xylanase, as no reducing sugar, xylobiose, or xylotriose was detected. Based on the contents of reducing sugars and XOs, the hydrolysis of all grass xylans was significantly less efficient than that of the commercial xylan. Nevertheless, grass remained an attractive raw material for XO production due to its abundance and approximately 5.7-fold lower cost of the extracted xylan ($700 kg−1) compared to the commercial xylan ($4000 kg−1 from Sigma-Aldrich).
Fig. 3.
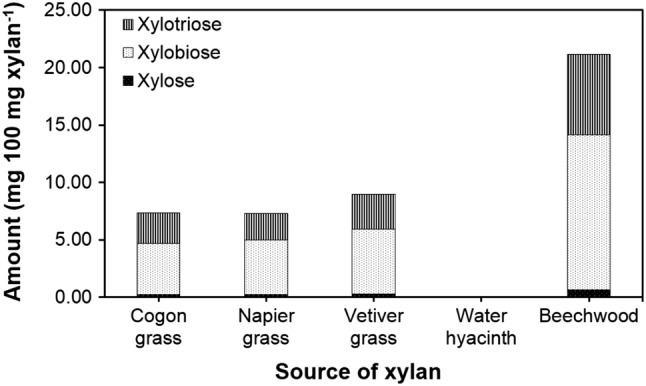
Amounts of xylose, xylobiose, and xylotriose obtained from the hydrolysis of the xylans from the three grasses and water hyacinth by the crude xylanase from A. melanogenum PBUAP46 in comparison with that from the commercial beechwood xylan, as determined by HPLC analysis. Data are presented as the mean, derived from three replicates
Based on the obtained XO contents, the vetiver grass xylan was then selected for optimization of the xylanase reaction. To find the optimal enzyme concentration and incubation time, RSM using CCD with two variables was employed. The levels of factors applied were based on preliminary experiments (data not shown) and the response values (reducing sugar) are presented in Table 3. The response surface plot is shown in Fig. 4. The response Y (reducing sugar) could be expressed by the equation:
Table 3.
CCD-RSM used to optimize vetiver grass xylan hydrolysis
| Run | Code level | Actual level | Reducing sugar (mg 100 mg xylan−1) | ||
|---|---|---|---|---|---|
| X 1 | X 2 | Enzyme content (U g xylan−1) | Incubation time (h) | ||
| 1 | +1 | +1 | 34 | 120 | 21.15 |
| 2 | +1.414 | 0 | 37.31 | 96 | 21.52 |
| 3 | +1 | −1 | 34 | 72 | 23.26 |
| 4 | 0 | −1.414 | 26 | 62.06 | 21.06 |
| 5 | −1 | −1 | 18 | 72 | 21.03 |
| 6 | −1.414 | 0 | 14.69 | 96 | 20.13 |
| 7 | −1 | +1 | 18 | 120 | 21.35 |
| 8 | 0 | +1.414 | 26 | 129.94 | 21.38 |
| 9 | 0 | 0 | 26 | 96 | 23.38 |
| 10 | 0 | 0 | 26 | 96 | 23.78 |
| 11 | 0 | 0 | 26 | 96 | 23.44 |
| 12 | 0 | 0 | 26 | 96 | 23.87 |
Fig. 4.
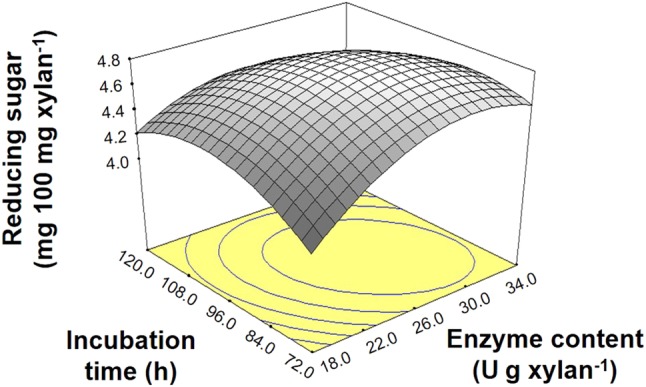
Three-dimensional response surface plot showing the effect of the xylanase enzyme concentration and incubation time on the reducing sugar yield obtained after vetiver xylan hydrolysis
where Y is the reducing sugar (mg 100 mg xylan−1), X1 is the enzyme content (U g xylan−1), and X2 is the incubation time (h).
Based on the R2 value (0.91), the evaluated variables accounted for 91% of the observed response, and therefore, the response surface plot analysis appeared valid. The RSM analysis predicted that the highest amount of reducing sugar (23.70 mg 100 mg xylan−1) could be achieved when incubating the substrate with 27.94 U g xylan−1 for 92 h 19 min. When the hydrolysis was performed under these conditions, the level of obtained reducing sugars (23.65 ± 1.34 mg 100 mg xylan−1) was not significantly different to the predicted level, suggesting that the equation was accurate. In addition, the xylose, xylobiose, and xylotriose yields were 0.47 ± 0.36, 10.58 ± 0.77, and 2.69 ± 0.20 mg 100 mg xylan−1, respectively. The total amount of xylobiose and xylotriose was 1.53 times higher than at the original condition, while the reaction time was shorten by 3 h.
Determination of the structure and prebiotic activity
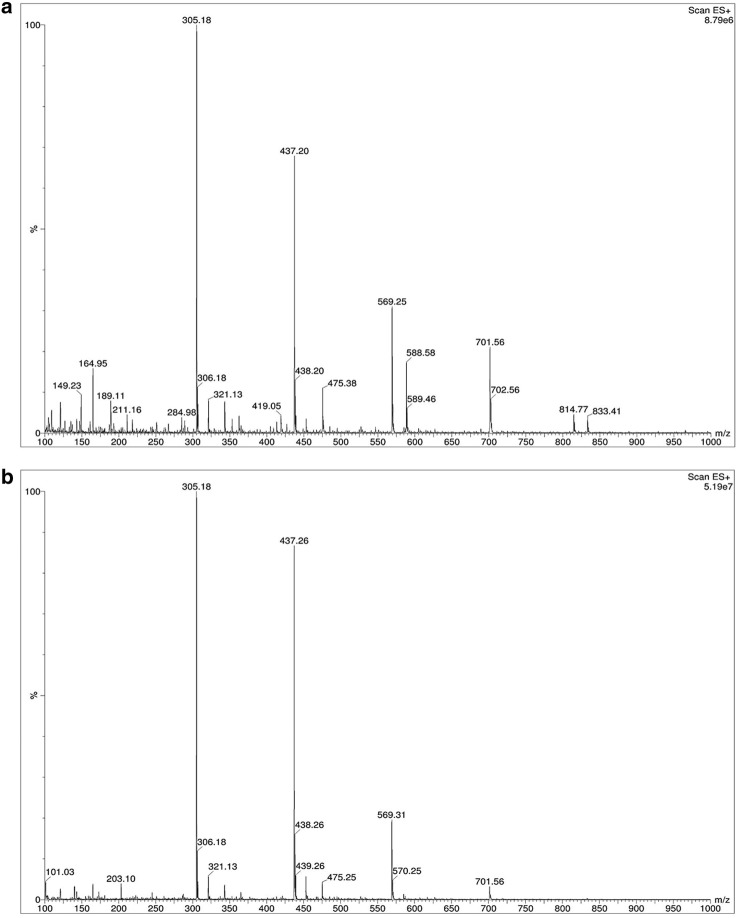
The crude and enriched XOs obtained from the xylan hydrolysis under the optimum conditions were analyzed by ESI-MS to identify all different DP of XOs constitute in crude and enriched xylan hydrolysate. HPLC analysis would require standard samples of higher molecular weight XOs; thus, ESI-MS was a method of choice for characterization. Xylobiose and xylotriose were revealed to be the major components of the vetiver grass xylan hydrolysate, as seen by the main peaks at m/z 305 (M + Na)+ and 437 (M + Na)+, respectively. However, the peaks at m/z 569 (M + Na)+, 701 (M + Na)+, and 833 (M + Na)+ indicated the presence of other higher molecular weight oligosaccharides (Fig. 5a). Xylose was also detected as a peak at m/z 189 (M + K)+ as well as other impurities represented by small peaks. After partial purification by activated carbon adsorption, xylobiose and xylotriose remained the major components, while impurities, xylose, and other oligosaccharides were removed as indicated by the reduced signals and disappearance of peaks (Fig. 5b).
Fig. 5.
Representative mass spectra of the a crude and b enriched vetiver XOs
The effect of enriched vetiver XOs on the growth of probiotic bacteria, namely, L. brevis and L. casei, in MRS medium was investigated in this study when different supplements were added at a final concentration of 2 mg carbon ml−1 to the MRS medium. The enriched XOs clearly promoted the growth of L. brevis, attaining 1.69 ± 0.36 × 108 colony forming units (CFU) ml−1, compared to the lower growth with glucose and yeast β-glucan at 4.78 ± 1.17 × 107 and 5.00 ± 1.45 × 107 CFU ml−1, respectively. This could have resulted from the presence of xylobiose in the XOs, since this is the only type of XO utilized by L. brevis (Immerzeel et al. 2014; Li et al. 2015; Singh et al. 2015). In the case of L. casei, its growth was also enhanced by the enriched vetiver XOs with the highest growth at 6.11 ± 1.64 × 108 CFU ml−1, which was not significantly different from that with commercial yeast β-glucan (Table 4). From these results, it could be defined that the enriched Vetiver XOs have excellent potential as a prebiotic supplement in foods, especially at a commercial level due to their economic worthiness.
Table 4.
Growth of L. brevis and L. casei in MRS medium supplemented with enriched vetiver XOs and other compounds at 2 mg carbon ml−1 medium
| Supplement | Bacterial growth (CFU ml− 1)* | |
|---|---|---|
| L. brevis | L. casei | |
| None | 5.00 ± 1.33 × 107b | 3.44 ± 0.51 × 108B |
| Glucose | 4.78 ± 1.17 × 107b | 9.44 ± 1.02 × 107C |
| Enriched vetiver XOs | 1.69 ± 0.36 × 108a | 6.11 ± 1.64 × 108A |
| Yeast β-glucan | 5.00 ± 1.45 × 107b | 5.67 ± 1.67 × 108AB |
*Data are shown as the mean ± SD, derived from three replicates. Different letters in the same column indicate a significantly different value (ANOVA and DMRT, P ≤ 0.05)
Conclusion
The present study established the potential of A. melanogenum PBUAP46 for production of the xylanase with its ability to produce XOs from some tropical weeds. Moreover, the produced XOs exhibited prebiotic property by stimulating the growth of some probiotic Lactobacilli. Thus, the results obtained from this study prove that prebiotic XOs could be produced by enzymatic hydrolysis with A. melanogenum PBUAP46 xylanase using vetiver grass xylan as a substrate. Further research on the larger scale production of XOs and the exploitation of XOs in food or other related applications should be explored.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This study was financially supported by the Development and Promotion of Science and Technology talents project (DPST), and the Asia Research Center, Chulalongkorn University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Tanutcha Patipong, Phone: +662-218-5477, Email: younggirl26@hotmail.com.
Pongtharin Lotrakul, Phone: +662-218-5497, Email: pongtharin.l@chula.ac.th.
Panuwat Padungros, Phone: +662-218-7582, Email: panuwat.p@chula.ac.th.
Hunsa Punnapayak, Phone: +662-218-5468, Email: phunsa@chula.ac.th.
Wichanee Bankeeree, Phone: +662-218-5493, Email: wichanee.b@chula.ac.th.
Sehanat Prasongsuk, Phone: +662-218-5491, Email: sehanat.p@chula.ac.th.
References
- Akpinar O, Erdogan K, Bostanci S. Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr Res. 2009;344:660–666. doi: 10.1016/j.carres.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Bankeeree W, Lotrakul P, Prasongsuk S, Chaiareekij S, Eveleigh DE, Kim SW, Punnapayak H. Effect of polyols on thermostability of xylanase from a tropical isolate of Aureobasidium pullulans and its application in prebleaching of rice straw pulp. Springerplus. 2014;3:37. doi: 10.1186/2193-1801-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankeeree W, Akada R, Lotrakul P, Punnapayak H, Prasongsuk S. Enzymatic hydrolysis of black liquor xylan by a novel xylose-tolerant, thermostable β-xylosidase from a tropical strain of Aureobasidium pullulans CBS 135684. Appl Biochem Biotechnol. 2018;184:919–934. doi: 10.1007/s12010-017-2598-x. [DOI] [PubMed] [Google Scholar]
- Carvalho AFA, Neto PDO, da Silva DF, Pastore GM. Xylooligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int. 2013;51:75–85. doi: 10.1016/j.foodres.2012.11.021. [DOI] [Google Scholar]
- Chapla D, Divecha J, Madamwar D, Shah A. Utilization of agroindustrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochem Eng J. 2010;49:361–369. doi: 10.1016/j.bej.2010.01.012. [DOI] [Google Scholar]
- Chapla D, Pandit P, Shah A. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol. 2012;115:215–221. doi: 10.1016/j.biortech.2011.10.083. [DOI] [PubMed] [Google Scholar]
- Chapla D, Dholakiya S, Madamwar D, Shah A. Characterization of purified fungal endoxylanase and its application for production of value added food ingredient from agroresidues. Food Bioprod Proc. 2013;91:682–692. doi: 10.1016/j.fbp.2013.08.005. [DOI] [Google Scholar]
- Crittenden R, Karppinen S, Ojanen S, Tenkanen M, Fagerstrom R, Matto J, Saarela M, Mattila-Sandholm T, Poutanen K. In-vitro fermentation of cereal dietary fiber carbohydrates by probiotic and intestinal bacteria. J Sci Food Agric. 2002;82:781–789. doi: 10.1002/jsfa.1095. [DOI] [Google Scholar]
- De Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- Goering HK, Van Soest PJ. Agriculture handbook No.379. Washington: Agriculture Research Service USDA; 1970. Forage fiber analysis (apparatus, reagent, producers and some applications) [Google Scholar]
- Gullón P, Moura P, Esteves M, Girio FM, Domínguez H, Parajó JC. Assessment on the fermentability of xylooligosaccharides from rice husks by probiotic bacteria. J Agric Food Chem. 2008;56:7482–7487. doi: 10.1021/jf800715b. [DOI] [PubMed] [Google Scholar]
- Guo GL, Hsu DC, Chen WH, Chen WH, Hwang WS. Characterization of enzymatic saccharification for acid-pretreated lignocellulosic materials with different lignin composition. Enzym Microbiol Technol. 2009;45:80–87. doi: 10.1016/j.enzmictec.2009.05.012. [DOI] [Google Scholar]
- Immerzeel P, Falck P, Galbe M, Adlercreutz P, Karlsson EN, Stålbrand H. Extraction of water-soluble xylan from wheat bran and utilization of enzymatically produced xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp. LWT Food Sci Technol. 2014;56:321–327. doi: 10.1016/j.lwt.2013.12.013. [DOI] [Google Scholar]
- Kaushik P, Mishra A, Malik A. Dual application of agricultural residues for xylanase production and dye removal through solid state fermentation. Int Biodeterior Biodegrad. 2014;96:1–8. doi: 10.1016/j.ibiod.2014.08.006. [DOI] [Google Scholar]
- Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Leathers TD. Color variants of Aureobasidium pullulans overproduce xylanase with extremely high specific activity. Appl Environ Microbiol. 1986;52:1026–1030. doi: 10.1128/aem.52.5.1026-1030.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite RSR, Bocchini DA, Martins EDS, Silva D, Gomes E, Da Silva R. Production of cellulolytic and hemicellulolytic enzymes from Aureobasidium pulluans on solid state fermentation. Appl Biochem Biotechnol. 2007;137:281–288. doi: 10.1007/s12010-007-9058-y. [DOI] [PubMed] [Google Scholar]
- Li Z, Summanen PH, Komoriya T, Finegold SM. In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp. Int J Food Sci Nutr. 2015;66:919–922. doi: 10.3109/09637486.2015.1064869. [DOI] [PubMed] [Google Scholar]
- Lotrakul P, Deenarn P, Prasongsuk S, Punnapayak H. Isolation of Aureobasidium pullulans from bathroom surfaces and their antifungal activity against some Aspergilli. Afr J Microbiol Res. 2009;3:253–257. [Google Scholar]
- Lubomr K, Peter B. Disaccharides permeases: constituents of xylanolytic and mannanolytic systems of Aureobasidium pullulans. Biochim Biophys Acta. 1998;1425:560–566. doi: 10.1016/S0304-4165(98)00112-3. [DOI] [PubMed] [Google Scholar]
- Manitchotpisit P, Leathers TD, Peterson SW, Kurtzman CP, Li XL, Eveleigh DE, Lotrakul P, Prasongsuk S, Dunlap CA, Vermillion KE, Punnapayak H. Multilocus phylogenetic analyses, pullulan production and xylanase activity of tropical isolates of Aureobasidium pullulans. Mycol Res. 2009;113:1107–1120. doi: 10.1016/j.mycres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Marques G, Rencoret J, Gutiérrez A, del Río JC. Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric J. 2010;4:93–101. doi: 10.2174/1874331501004010093. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Moura P, Barata R, Carvalheiro F, Girio F, Loureiro-Dias MC, Esteves MP. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT Food Sci Technol. 2007;40:963–972. doi: 10.1016/j.lwt.2006.07.013. [DOI] [Google Scholar]
- Nasr S, Soudi MR, Salmanian AH, Ghadam P. Partial optimization of endo-1,4-beta-xylanase production by Aureobasidium pullulans using agro-industrial residues. Iran J Basic Med Sci. 2013;16:1245–1253. doi: 10.22038/IJBMS.2013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutelings G. Lignin variability in plant cell walls: contribution of new models. Plant Sci. 2011;181:379–386. doi: 10.1016/j.plantsci.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Ohta K, Moriyama S, Tanaka H, Shige T, Akimoto H. Purification and characterization of an acidophilic xylanase from Aureobasidium pullulans var. melanigenum and sequence analysis of the encoding gene. J Biosci Bioeng. 2001;92:262–270. doi: 10.1016/S1389-1723(01)80260-7. [DOI] [PubMed] [Google Scholar]
- Peng F, Ren JL, Xu F, Bian J, Peng P, Sun RC. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J Agric Food Chem. 2009;57:6305–6317. doi: 10.1021/jf900986b. [DOI] [PubMed] [Google Scholar]
- Prasongsuk S, Sullivan R, Kuhirun M, Eveleigh DE, Punnapayak H. Thailand habitats as sources of pullulan-producing strains of Aureobasidium pullulans. World J Microbiol Biotechnol. 2005;21:393–398. doi: 10.1007/s11274-004-2237-x. [DOI] [Google Scholar]
- Prasongsuk S, Lotrakul P, Ali I, Bankeeree W, Punnapayak H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018;63:129–140. doi: 10.1007/s12223-017-0561-4. [DOI] [PubMed] [Google Scholar]
- Punnapayak H, Sudhadham M, Prasongsuk S, Pichayangkura S. Characterization of Aureobasidium pullulans isolated from airborne spores in Thailand. J Ind Microbiol Biotechnol. 2003;30:89–94. doi: 10.1007/s10295-002-0016-y. [DOI] [PubMed] [Google Scholar]
- Samanta AK, Jayapal N, Kolte AP, Senani S, Sridhar M, Suresh KP, Sampath KT. Enzymatic production of xylooligosaccharides from alkali solubilized xylan of natural grass (Sehima nervosum) Bioresour Technol. 2012;112:199–205. doi: 10.1016/j.biortech.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Schädel C, Blöchl A, Richter A, Hoch G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol Biochem. 2010;48:1–8. doi: 10.1016/j.plaphy.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Schädel C, Blöchl A, Richter A, Hoch G. Hemicellulose concentration and composition in plant cell walls under extreme carbon source-sink imbalances. Physiol Plant. 2010;139:241–255. doi: 10.1111/j.1399-3054.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- Singh RD, Banerjee J, Arora A. Prebiotic potential of oligosaccharides: a focus on xylan derived oligosaccharides. Bioact Carbohydr Diet Fibre. 2015;5:19–30. doi: 10.1016/j.bcdf.2014.11.003. [DOI] [Google Scholar]
- Tanaka H, Muguruma M, Ohta K. Purification and properties of a family-10 xylanase from Aureobasidium pullulans ATCC 20524 and characterization of the encoding gene. Appl Microbiol Biotechnol. 2005;70:202–211. doi: 10.1007/s00253-005-0045-3. [DOI] [PubMed] [Google Scholar]
- Vazquez MJ, Alonso JL, Dominguez H, Parajo JC. Xylooligosaccharides manufacture and applications. Trends Food Sci Technol. 2000;11:387–393. doi: 10.1016/S0924-2244(01)00031-0. [DOI] [Google Scholar]
- Verjans P, Dornez E, Delcour JA, Courtin CM. Selectivity for water unextractable arabinoxylan and inhibition sensitivity govern the strong bread improving potential of an acidophilic GH11 Aureobasidium pullulans xylanase. Food Chem. 2010;123:331–337. doi: 10.1016/j.foodchem.2010.04.039. [DOI] [Google Scholar]
- Xia A, Cheng J, Song W, Yu C, Zhou J, Cen K. Enhancing enzymatic saccharification of water hyacinth through microwave heating with dilute acid pretreatment for biomass energy utilization. Energy. 2013;61:158–166. doi: 10.1016/j.energy.2013.09.019. [DOI] [Google Scholar]
- Yang CH, Yang SF, Liu WH. Production of xylooligosaccharides from xylans by extracellular xylanases from Thermobifida fusca. J Agric Food Chem. 2007;55:3955–3959. doi: 10.1021/jf0635964. [DOI] [PubMed] [Google Scholar]
- Yanwisetpakdee B, Lotrakul P, Prasongsuk S, Seelanan T, White JF, Eveleigh DE, Kim SW, Punnapayak H. Associations among halotolerance, osmotolerance and exopolysaccharide production of Aureobasidium melanogenum strains from habitats under salt stress. Pak J Bot. 2016;48:1229–1239. [Google Scholar]
- Yegin S. Single-step purification and characterization of an extreme halophilic, ethanol tolerant and acidophilic xylanase from Aureobasidium pullulans NRRL Y-2311-1 with application potential in the food industry. Food Chem. 2017;221:67–75. doi: 10.1016/j.foodchem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Yu X, Gu Z. Optimization of nutrition constituents for feruloyl oligosaccharides production by a new isolate of Aureobasium pullulans 2012 under fermentation on wheat bran. Bioresources. 2013;8:6434–6447. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.