Correction to: Diabetes Ther (2018) 9:37–47 10.1007/s13300-017-0330-z
In the original publication, the text in Table 2 stated ‘Hypersensitivity to the active substance, to Ferinject, or to any of its excipients’. The authors would like to make changes to the text and have replaced it to ‘Hypersensitivity to ferric carboxymaltose or to any of the excipients of the study medication’. The authors have also made alterations to Fig. 1. Corrected Fig. 1 and Table 2 are given below:
Table 2.
Key inclusion and exclusion criteria
| Key inclusion criteria | Men and women older than 18 years Diagnosis of type 2 diabetes and iron deficiency defined as follows: HbA1c ≥ 48 mmol/mol (6.5%) and < 69 mmol/mol (8.5%) Serum ferritin < 150 ng/mL or transferrin saturation < 25% if hemoglobin < 14 g/dL Serum ferritin < 100 ng/mL or transferrin saturation < 20% if hemoglobin ≥ 14 g/dL and ≤ 15 g/dL |
| Key exclusion criteria | Continuous subcutaneous insulin infusion Thalassemia Hemoglobin > 15 g/dL (≥ 9.31 mmol/L) C-reactive protein > 15 mg/L Change in HbA1c of more than ± 0.3% within the last 3 months Hypersensitivity to ferric carboxymaltose or to any of the excipients of the study medication Known serious hypersensitivity to other parenteral iron products History of acquired iron overload History of erythropoietin-stimulating agent, IV or high-dose oral iron therapy or blood transfusion < 12 weeks prior to randomization Body weight ≤ 40 kg Chronic or active liver disease Vitamin B12 and/or serum folate deficiency Current malignancy under treatment Renal function GFR < 30 mL/min/1.73 m2 Significant major cardiovascular disease ongoing or in the past 3 months Polyneuropathy without ischemia Pregnant or nursing (lactating) women Any person not willing to use adequate contraceptive precautions during the study and for up to 5 days after the last scheduled dose of study medication |
Fig. 1.
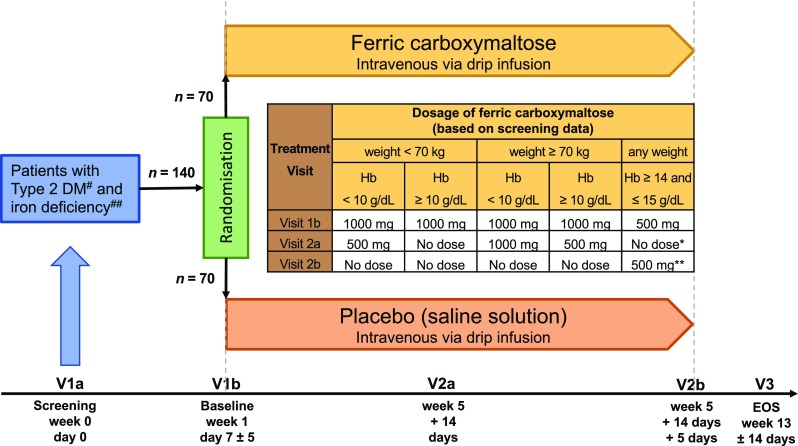
CLEVER study design. #Defined as HbA1c ≥ 48 mmol/mol (6.5%) and < 69 mmol/mol (8.5%). ##Defined as serum ferritin < 150 ng/mL or transferrin saturation < 25% if hemoglobin < 14 g/dL or serum ferritin < 100 ng/mL or transferrin saturation < 20% if hemoglobin ≥ 14 g/dL and ≤ 15 g/dL. *Control parameter: ferritin and transferrin saturation. **If still iron deficient at V2a [serum ferritin < 150 ng/mL or transferrin saturation < 25%], an additional dose of 500 mg ferric carboxymaltose is given at V2b, otherwise it is not
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


