Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are well established as effective treatments for patients with type 2 diabetes. GLP-1 RAs augment insulin secretion and suppress glucagon release via the stimulation of GLP-1 receptors. Although all GLP-1 RAs share the same underlying mechanism of action, they differ in terms of formulations, administration, injection devices and dosages. With six GLP-1 RAs currently available in Europe (namely, immediate-release exenatide, lixisenatide, liraglutide; prolonged-release exenatide, dulaglutide and semaglutide), each with its own characteristics and administration requirements, physicians caring for patients in their routine practice face the challenge of being cognizant of all this information so they are able to select the agent that is most suitable for their patient and use it in an efficient and optimal way. The objective of this review is to bring together practical information on the use of these GLP-1 RAs that reflects their approved use.
Funding: Eli Lilly and Company.
Plain Language Summary: Plain language summary available for this article.
Keywords: Administration, Clinical practice, Devices, Diabetes, European Prescribing Information, GLP-1 RAs, Warnings
PLain Language Summary
Type 2 diabetes (T2D) is a common condition characterized by insulin resistance and dysfunction of insulin-producing beta cells. T2D can be treated using glucagon-like peptide-1 receptor agonists (GLP-1 RAs). These well-established and effective treatments lower blood sugar levels and reduce body weight. Six GLP-1 RAs are currently available in Europe: the immediate-release formulations exenatide, lixisenatide and liraglutide and the prolonged-release formulations exenatide, dulaglutide and semaglutide. These drugs all have the same mechanism of action, but they differ in a number of important features, including (1) treatment frequency (e.g. twice daily vs. once weekly); (2) whether they need to be taken at a particular time in relation to meals; (3) suitability for use in particular patient populations (e.g. elderly patients and those with kidney or liver problems); and (4) characteristics of the injection device (e.g. whether it is ‘ready to use’ by the patient or requires mixing of different components, and whether the needle is pre-attached and hidden or needs to be attached by the patient). The GLP-1 RAs also show some differences in their effects on cardiovascular events and in the incidence of side effects such as nausea and vomiting. First opinion physicians need to be aware of all this information so that they can select the agent that is most suitable for each patient. The aim of this review is to bring together practical information on the use of the GLP-1 RAs.
Introduction
The first step in the management of type 2 diabetes (T2D) is modification of diet and lifestyle, often with the addition of the oral antidiabetic drug metformin, which nowadays is the drug of first choice for monotherapy. As the disease progresses, treatment of T2D becomes more complex. When initial approaches are no longer sufficient to maintain the glycated hemoglobin (HbA1c) target, even with the highest approved dosage of metformin, guidelines recommend that additional antidiabetic drugs be added to the treatment regimen [1]. Comorbidities further complicate the management of T2D, as they need to be taken into account when considering the most appropriate drug and doses if treatment intensification is needed. Indeed, in studies conducted both in Europe and the USA, over 90% of patients with T2D exhibited two or more chronic conditions, the most common being hypertension, overweight/obesity, hyperlipidemia, chronic kidney disease and/or cardiovascular (CV) disease [2]. Thus, although glucose control remains the major focus in the management of patients with T2D, treatment should always be considered in the context of a comprehensive approach to include comorbid conditions [1].
In addition to metformin, available glucose-lowering agents include sulfonylureas, thiazolidinediones, glinides, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP4) inhibitors, sodium–glucose cotransporter 2 (SGLT2) inhibitors, GLP-1 RAs and insulin.
Drugs belonging in the GLP-1 RA class, the focus of this review, reduce glucose levels by augmenting insulin secretion and suppressing glucagon release in a glucose-dependent manner; GLP-1 RAs also delay gastric emptying and increase satiety [3]. GLP-1 RAs reduce fasting and postprandial glucose levels by stimulating GLP-1 receptors [4]. The physiological effects of GLP-1 RAs are summarized in Table 1 [5–9]. Endogenous GLP-1 has a half-life of 2–3 min due to degradation by DPP-4. In contrast, GLP-1 RAs are resistant to degradation by DPP-4, resulting in prolongation of their half-life and facilitating their clinical use [3].
Table 1.
The physiological effects of glucagon-like peptide-1 receptor agonists
| Location | Increased | Decreased |
|---|---|---|
| Brain | Neuroprotection (preclinical) | Appetite |
| Cardiovascular system | Regional and global LV function | Blood pressure |
| Heart rate (Clinical) | Endothelial dysfunction (Preclinical) | |
| – | Ischemia-induced myocardial damage | |
| Muscle | Glucose uptakea | – |
| Adipose tissue | Glucose uptake | – |
| Lipolysis | – | |
| Liver | – | Glucose productiona |
| Lipid profile | ||
| Stomach | – | Gastric emptying (Clinical) |
| Kidney | Natriuresis | – |
| Pancreas | Glucose-dependent insulin secretion (Clinical) | Glucose-dependent glucagon secretion (Clinical) |
| Beta cell proliferationb | Beta cell apoptosisb |
GLP-1 RAs have been available in Europe and the USA for more than a decade. The first available GLP-1 RA, immediate-release, twice-daily exenatide, was approved in Europe in 2006. Additional agents subsequently gained regulatory approval and are currently being marketed, including once-daily lixisenatide and liraglutide, and once-weekly prolonged-release exenatide, dulaglutide and semaglutide. Albiglutide, another once-weekly GLP-1 RA, is not included in this review as its manufacture and sale were discontinued in July 2018 [10].
GLP-1 RAs share the same underlying mechanism of action, but they differ in terms of formulations, administration, injection devices and dosages. Although all of the known GLP-1 RAs produce clinically significant reductions in HbA1c levels and body weight, some differences between them have been reported in head-to-head studies [11], including their impact on CV risk factors [12] and gastrointestinal tolerability [13].
With six GLP-1 RAs currently available in Europe, each with its own characteristics and administration requirements, physicians caring for patients in their routine practice face the challenge of being cognizant of all this information in order to be able to select the agent most suitable for their patient and use it in an efficient and optimal way. In fact, some surveys indicate that there is a need for more education and knowledge on the use of these agents in primary care settings [14]. The objective of this review is to bring together practical information based on European Union (EU) labeling on the use of available GLP-1 RAs, focusing on posology, modes of administration/devices, clinical efficacy, use in special populations and safety and precautions. In this review we focus on EU labels; as such, an in-depth review of available clinical data on these GLP-1 RAs is beyond the scope of the article. Therefore, information from observational or pragmatic studies or information from prescribing information approved in other regions is not included, with the exception of certain safety information. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. The aim of the paper is to gather very practical information to facilitate the use of this class of drugs.
Posology and Mode of Administration
Initial recommended dosages and administration requirements with regard to meals for each of the GLP-1 RAs discussed in this review are presented in Table 2. Of note, GLP-1 RAs administered daily require dose titration, whereas this is not necessary for the once-weekly drugs dulaglutide and exenatide. The recommended dose of dulaglutide when used in combination with other glucose-lowering agents, including insulin, is 1.5 mg once weekly; when used as monotherapy, the recommended dose of dulaglutide is 0.75 mg once weekly [15]. The recommended dose of prolonged-release exenatide is 2 mg once weekly [16]. The starting dose of once-weekly semaglutide is 0.25 mg; this should be increased to 0.5 mg after 4 weeks and can be further increased to 1 mg if required after at least 4 weeks at 0.5 mg once weekly [17].
Table 2.
Dosage and administration requirements for glucagon-like peptide-1 receptor agonists based on European Union summary of product characteristics of each agent
| Drug | Titration | Initial dosage | Recommended dosage | Administration in relation to meals | Missed dose |
|---|---|---|---|---|---|
| Once-daily | |||||
| Exenatide | Yes | 5 mcg BID for at least 1 month | 5–10 mcg BIDb | Should be administered within 60 min before main meals | Continue with the next scheduled dose |
| Liraglutide | Yes | 0.6 mg OD for at least 1 week | 1.2–1.8 mg ODc | At any time, without regard to meals |
≤ 12 h: administer the dose as soon as possible > 12 h: skip the dose |
| Lixisenatide | Yes | 10 mcg OD for 14 days | 20 mcg OD | Should be administered within 60 min before any meal | Administer the dose within 1 h before the next meal |
| Once-weekly | |||||
| Exenatide | No | Not applicable | 2 mg once weekly | At any time, without regard to meals | Administer the next dose as soon as practical. Only one injection should be administered in a 24-h period |
| Dulaglutide | Noa | Not applicable |
Monotherapy: 0.75 mg once weekly Add-on therapy: 1.5 mg once weekly |
At any time, without regard to meals |
≥ 3 days until the next scheduled dose: administer the dose as soon as possible < 3 days: skip the dose, wait and administer their next regularly scheduled weekly dose |
| Semaglutide | Yes | 0.25 mg once weekly for 4 weeks | 0.5–1.0 mg once weekly (dose increase after 4 weeks if required) | At any time, without regard to meals |
≥ 5 days until the next scheduled dose: administer the dose as soon as possible < 5 days: skip the dose, wait and administer their next regularly scheduled weekly dose |
Data were extracted from references [15–20, 30]
BID Twice a day, OD once daily
aIn elderly patients, a lower dose of 0.75 mg once weekly can be considered
bImmediate-release exenatide should be initiated at 5 mcg per dose administered BID for at least 1 month to improve tolerability; the dose can then be increased to 10 mcg BID to further improve glycemic control
cLiraglutide should be initiated at a dose of 0.6 mg daily to improve gastrointestinal tolerability; after at least 1 week, the dose should be increased to 1.2 mg and a further increase to 1.8 mg may be required to further improve glycemic control
GLP-1 RAs differ in their requirements for the timing of administration with regard to meals. Immediate-release exenatide and lixisenatide should be administered before meals because they are associated with a greater delay in gastric emptying than once-weekly exenatide and dulaglutide [11]. Thus, immediate-release exenatide should be administered at any time within the 60-min period before the morning and evening meal (or two main meals of the day, approximately ≥ 6 h apart) [18]. Immediate-release exenatide should not be administered after a meal [18]. Once-daily lixisenatide also should be administered within 1 h before any meal of the day, preferably the same meal every day [19]. By contrast, prolonged-release exenatide [16], once-daily liraglutide and once-weekly dulaglutide and semaglutide [15, 17, 20] can be administered without taking meals into account.
Indications
All GLP-1 RAs are indicated in combination with other glucose-lowering medication(s), including insulin, when these—together with diet and exercise—do not provide adequate glycemic control [15–20]. Liraglutide, dulaglutide and semaglutide are also indicated as monotherapy when there is inadequate glycemic control with diet/exercise and when metformin is considered to be inappropriate due to intolerance or contraindications [15, 17, 20].
Administration in Combination with Oral Antidiabetic Drugs
Recommendations for using GLP-1 RAs in combination with other antihyperglycemic agents vary across the agents. All GLP-1 RAs have been studied in combination with metformin, with or without sulfonylureas [21]. When a GLP-1 RA is administered in combination with a sulfonylurea, a reduction in the dose of the sulfonylurea should be considered, to reduce the risk of hypoglycemia [15–20]. GLP-1 RAs in combination with DPP-4 inhibitors do not provide additive glucose-lowering effects and therefore should be avoided [22].
Immediate- and prolonged-release exenatide, liraglutide, dulaglutide and semaglutide can be administered with pioglitazone—a thiazolidinedione—without the need to change the dose of pioglitazone [15–18, 20]. No information on dose adjustments of pioglitazone appears in the summary of product characteristics (SPC) of lixisenatide [19]. The use of the combination of GLP-1 RAs with SGLT2 inhibitors in patients with T2D has only been studied in randomized clinical trials with prolonged-release exenatide [23, 24] and dulaglutide [25], and the results are summarized in their EU SPCs [15, 16].
Administration in Combination with Insulin
In general, when a GLP-1 RA (e.g. immediate-release exenatide, liraglutide, lixisenatide, dulaglutide or semaglutide) is administered in combination with basal insulin, it is recommended that a reduction in the dose of basal insulin should be considered to reduce the risk of hypoglycemia [15, 18–20]. It has been reported that when prolonged-release exenatide was added to basal insulin, no initial dose adjustment of insulin was required [16]. There are no specific recommendations in the SPCs on how to reduce the dose of insulin. In some studies, the dose of insulin was reduced by 20% at randomization in all patients [26] or in those with an HbA1c of < 8% [27, 28].
The addition of dulaglutide to a therapeutic regimen of prandial insulin lispro has also been evaluated in a randomized clinical trial in patients with T2D who did not achieve the target glycemic control with a basal–bolus regimen [29]. In this study, dulaglutide in combination with insulin lispro produced significantly greater reductions in HbA1c levels compared to insulin glargine combined with insulin lispro. However, there are limited data to support the general use of GLP-1 RA in combination with prandial insulin.
Precautions for the Use of GLP-1 RAs in Special Populations
According to EU prescribing information, GLP-1 RAs should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis.
Based on EU labels, there is no contraindication of the use of GLP-1 RAs in patients with personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome [15–20, 30]. However, based on prescribing information issued by the U.S. Food and Drug Administration the use of dulaglutide, liraglutide, semaglutide and exenatide once weekly is contraindicated in these patients [31–34].
Gender and ethnicity have no clinically meaningful effect on the pharmacokinetics of GLP-1 RAs [15–20, 30]; therefore, dose adjustment based on these characteristics is not required. GLP-1 RAs do not require dose adjustments based on the body weight or the body mass index.
Most GLP-1 RAs do not require dose adjustment in elderly patients. However, a lower starting dose (0.75 mg once weekly) of dulaglutide can be considered in patients aged ≥ 75 years [15]. Also, immediate-release exenatide should be used with caution, and dose escalation from 5 to 10 mcg should proceed conservatively in patients aged > 70 years [16].
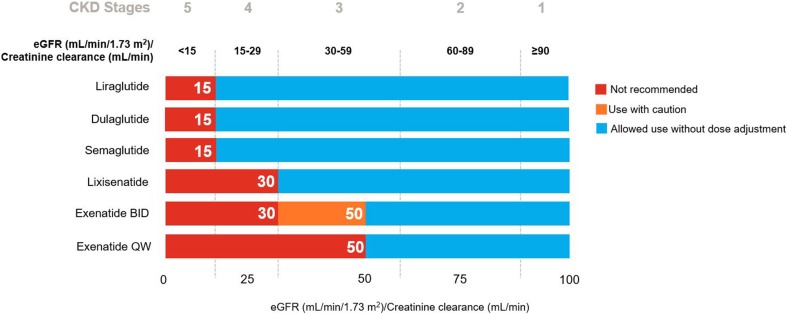
With the exception of exenatide, all GLP-1 RAs can be used without dose adjustments in patients with mild (estimated glomerular filtration rate [eGFR] 50–80 mL/min) or moderate (eGFR 30–50 mL/min) renal impairment [15–20] (Fig. 1). In patients with moderate renal impairment (creatinine clearance 30–50 mL/min), immediate-release exenatide requires dose escalation [18], and exenatide once-weekly is not recommended [16]. Liraglutide [20], dulaglutide [15] and semaglutide [17] can be used in patients with severe renal impairment (eGFR ≥ 15 to < 30 mL/min) without dose adjustment; however, the remaining GLP-1 RAs are not recommended in this population [16, 18, 19]. GLP-1 RAs are not recommended in patients with end-stage renal disease (eGFR < 15 mL/min) [15–20].
Fig. 1.
Recommendations for the use of glucagon-like peptide-1 receptor agonists in patients with renal impairment. Based on the EU summary of product characteristic of each agent [15–20]. BID twice daily, CKD Chronic Kidney Disease, eGFR estimated glomerular filtration rate, QW once weekly
With the exception of liraglutide, all of the GLP-1 RAs can be used without dose adjustment in patients with hepatic impairment, regardless of severity [15–20]. The pharmacokinetics of liraglutide may be altered in patients with hepatic impairment due to the binding of liraglutide to serum albumin and concomitant hypoalbuminemia [20]. Systemic exposure to liraglutide has been found to be significantly lower (44%) in patients with severe hepatic impairment (Child–Pugh score > 9); therefore, this drug is not recommended in this population [20].
Safety Issues, General Warnings and Precautions for the Use of GLP-1 RAs
As a class, GLP-1 RAs are generally well tolerated [35]. The most common adverse events are nausea and vomiting, both of which are usually transient and of mild or moderate severity, and patients can develop tolerance to these adverse effects over time [35]. The frequency of nausea among patients ranges from 13% with dulaglutide (0.75 mg), approximately 20% with liraglutide, prolonged-release exenatide, dulaglutide (1.5 mg) and semaglutide, 26% with lixisenatide and 36% with one-daily exenatide. The rate of discontinuation due to gastrointestinal adverse events ranges from about 1% with prolonged-release exenatide to 5% with semaglutide and once-daily exenatide [15–20].
Experience is limited on the use of the GLP-1 RAs in patients with severe gastrointestinal diseases, including gastroparesis. Therefore, these drugs are not recommended in these patients [15–20, 30].
Acute pancreatitis has been observed with the use of GLP-1 RAs [36]. According to the SPCs of all GLP-1 RAs, patients should be informed of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain [15–20, 30]. If pancreatitis is suspected, these drugs should be discontinued; if acute pancreatitis is confirmed, they should not be restarted [15–20, 30]. Of note, two recent meta-analyses found no evidence that treatment with GLP-1 RAs increases the risk of acute pancreatitis or pancreatic cancer [37, 38]. Large, randomized CV outcome trials with GLP-1 RAs have not found any increased risk of pancreatitis or pancreatic cancers [37, 39–41].
Injection-site reactions have been described with GLP-1 RAs; however, it is difficult to compare the incidence between the GLP-1 RAs as data are limited. In one head-to-head comparison, injection-site nodules were less frequent with liraglutide than with once-weekly exenatide (1% vs. 10%) [42], while in another trial dulaglutide and liraglutide were associated with a similar frequency of injection-site reactions (< 1%) [43]. In one randomized, open-label trial, the frequency of injection-site reactions was reported in 1% of patients with dulaglutide at a dose of 0.75 mg and semaglutide at a dose of 0.5 mg; at a higher dose of dulaglutide (1.5 mg) and semaglutide (1.0 mg), 3% and 2% of patients reported injection-site reactions, respectively [44]. Patients moving from twice-daily exenatide to once-weekly exenatide reported more injection-site reactions (5.4%) than did patients on continuous once-weekly treatment (0%) [45]. In another trial, injection-site reactions were more frequent with once-weekly exenatide than twice-daily exenatide (5.4% vs. 2.4%, respectively) [46].
There has been some concern—based on results from animal studies—regarding a potential association between the use of GLP-1 RAs and the occurrence of medullary thyroid tumors [47, 48]. However, a meta-analysis of 26 randomized controlled trials (RCTs) of once-weekly GLP-1 RAs showed that, compared to other antidiabetic drugs, once-weekly GLP-1 RAs did not increase the risk of any tumor [49].
Dehydration, sometimes leading to renal impairment and acute renal failure, has been reported in patients treated with GLP-1 RAs [47]. Therefore, patients treated with these drugs should be advised of the potential risk of dehydration [15–20], which usually occurs in association with gastrointestinal adverse effects but may occur without these adverse effects, and should take precautions to avoid fluid depletion [47]. In patients with diabetic retinopathy, an increased risk of developing diabetic retinopathy complications when treated with insulin and semaglutide has been observed. Therefore, caution should be exercised when prescribing semaglutide in combination with insulin to patients with diabetic retinopathy [17].
For a detailed description of other tolerability or safety issues of specific GLP-1 RAs, practicing physicians are referred to the corresponding SPCs [15–20].
Clinical Efficacy
Effects on Glycemic Control
Overall, as shown by a meta-analysis of 57 randomized and non-randomized studies, GLP-1 RAs are effective in improving glycemic outcomes in patients with T2D, with an overall lower risk of hypoglycemia than insulin or sulfonylureas [50]. In a meta-analysis of 82 RCTs, the mean HbA1c decrease from baseline was approximately 1% versus placebo [51]. Another meta-analysis of 11 RCTs showed that prolonged-release exenatide and dulaglutide are associated with greater reductions in HbA1c level than basal insulin, whereas immediate-release exenatide and liraglutide were found not to differ in this regard from the comparator [52]. In a meta-analysis of 19 RCTs, GLP-1 RAs added to basal insulin therapy led to a greater reduction of HbA1c level and body weight than therapy with basal insulin with or without a rapid-acting insulin [53]. A meta-analysis of 13 RCTs reported that GLP-1 RAs were superior in terms of HbA1c level and weight reductions compared to DPP4 inhibitors, without increasing the incidence of hypoglycemia [54].
Predictors of response to GLP-1 RAs may differ across agents, while body weight and disease duration do not appear to affect the efficacy of these medications [55]. However, a higher HbA1c level at treatment initiation is associated with a greater efficacy of GLP-1 RAs [56].
Other Effects
From the clinical perspective, GLP-1 RAs represent a unique approach for the treatment of diabetes, with benefits extending beyond glucose control, including beneficial effects on body weight, blood pressure and beta-cell function [57, 58].
Weight
The effect of GLP-1 RAs on body weight is often included as a secondary outcome in many GLP-1 RA clinical trials [59]. The association between weight loss and GLP-1 RAs is well documented [59, 60]. In a meta-analysis of 51 randomized controlled and uncontrolled trials, weight loss with GLP-1 RAs ranged from − 3.31 to − 1.22 kg compared to placebo, insulin or other oral antidiabetic drugs [60].
Cardiovascular Events and Mortality
Regulatory authorities recommend demonstrating CV safety for new antidiabetic agents [61, 62]. Recent systematic reviews have demonstrated that, as a class, GLP-1 RAs are associated with a reduction in the risks of CV events [59, 63]. For individual agents, however, this has only been demonstrated for liraglutide and semaglutide [39, 40, 49, 63]. Lixisenatide and prolonged-release exenatide have demonstrated a neutral effect on CV events [41, 64]. The impact of dulaglutide on CV events is currently being investigated in the REWIND RCT [65]. Further evidence to support the CV safety of GLP-1 RAs comes from a systematic review of 21 RCTs and four observational studies with GLP-1 RAs which showed no evidence of an increased risk of heart failure or hospitalization for heart failure with these medications [66].
Of the four GLP-1 RAs involved in these meta-analyses (lixisenatide, liraglutide, semaglutide and prolonged-release exenatide), only liraglutide and semaglutide have been shown in RCTs to reduce CV events (any major adverse CV events: CV death, non-fatal myocardial infarction or non-fatal stroke) in patients with T2D who are at high CV risk [39, 40]. Liraglutide also significantly reduced all-cause death. Based on the American Diabetes Association (ADA) 2018 guidelines, consideration should be given to adding an agent with evidence of CV risk reduction to the therapeutic regimen of patients with diabetes and atherosclerotic CV disease [22].
Characteristics of the Different GLP-1 RA Devices
The complexity of dose regimens and patients’ perceptions of medications, including perceived difficulty/ease of administration and fear of injections, are among the important factors affecting acceptance of the initiation of an injectable treatment and/or medication adherence in patients with T2D [67, 68]. Therefore, full knowledge of the characteristics and features of the different GLP-1 RA devices and their administration requirements (Table 3) could help physicians select the most appropriate agent for their patients.
Table 3.
Mode of administration and characteristics of glucagon-like peptide-1 receptor agonist pre-filled pen devices based on European Union summary of product characteristics
| Drug | Reconstitution or mixing required | Automatic dose administration | Need to prime device before use | Needle attachment required | Dose selection required | Single use |
|---|---|---|---|---|---|---|
| Daily | ||||||
| Exenatide | No | No | Yes | Yes. Needles are not included | Yes | No |
| Liraglutide | No | No | Yes | Yes. Needles are not included | Yes | No |
| Lixisenatide | No | No | Yes | Yes. Needles are not included | Yes | No |
| Once-weekly | ||||||
| Exenatide | Yes | No | No | Yes. Needles are included | No | Yes |
| Exenatide BCISE (pre-filled pen) | Yes | Yes | No | No. Pre-attached hidden needle | No | Yes |
| Dulaglutide | No | Yes | No | No. Pre-attached hidden needle | No | Yes |
| Semaglutide | No | No | Yes | Yes. Needles are included | Yes | No |
All devices for daily formulations of GLP-1 RAs require needle attachment; however, needles are not provided by the manufacturer in the product’s container for any daily GLP-1 RA. Devices for daily formulations and semaglutide require selection of the dose in the injection device and removal/disposal of the needle after each injection. Weekly formulations of dulaglutide and exenatide do not require selection of the dose and are single-use devices (Table 3) [15–20]. Prolonged-release exenatide requires reconstitution [16]. The pre-filled pen has two chambers containing exenatide powder and solvent. The powder in one chamber must be mixed with the solvent in the other chamber of the pre-filled pen. The solvent should be visually inspected before use and should only be used if it is clear and free of particulate matter. After suspension, the mixture should only be used if it is white to off-white and cloudy [16]. Prolonged-release exenatide has also recently become available as a single-use, fixed-dose, pre-filled pen that features a pre-attached hidden needle and automatic dose administration that requires mixing of the medicine by shaking the device for 15 s before injection (Table 3) [16].
Dulaglutide includes a pre-attached hidden needle, and the dose is administered automatically by pressing a button (Table 3) [15]. The device is ready to use by the patient, since no dose selection, needle attachment, reconstitution or mixing procedure is required. The ease of use of dulaglutide was evaluated in an uncontrolled study conducted in 214 patients with T2D [69]. In this study, 99% of patients considered the device to be easy or very easy to use [69]. In a study comparing patient perceptions of the injection devices used with liraglutide and dulaglutide, the dulaglutide device was associated with slightly higher scores for ease of use and convenience compared to the liraglutide device [70]. In a study conducted in 382 patients with T2D via an internet survey, patient preferences between liraglutide and twice-daily exenatide were evaluated using four attributes: efficacy as evaluated by HbA1c; incidence of nausea; incidence of hypoglycemia; and dosing frequency [36]. In this study, 96% of respondents preferred the profile of liraglutide over that of exenatide. In an open-label task and interview-based pilot study, the liraglutide and lixisenatide pen devices were associated with higher user satisfaction than the immediate-release exenatide pen [71].
Place of GLP-1 RAs in the Treatment of T2D
The GLP-1 RAs are well established as effective treatments for patients with T2D for whom lifestyle management (e.g. weight control, increased exercise) and antihyperglycemic monotherapy are insufficient to achieve glycemic targets. Clinical guidelines consider GLP-1 RAs as second-line treatment after metformin (dual therapy), as well as triple therapy and in combination with insulin [22]. Notably, the GLP-1 RAs can be considered to be an option when treatment intensification is required [1, 72, 73], both as monotherapy (liraglutide, dulaglutide and semaglutide have been approved for use in this setting [15, 17, 20]) and combination therapy. However, cost-effectiveness is an important consideration as the high cost of GLP-1 RAs may act as a barrier for some patients [22].
Clinical evidence from many RCTs has shown that the GLP-1 RAs produce clinically meaningful improvements in glycemic control, including significant reductions in HbA1c and fasting plasma glucose levels, both in patients with T2D of recent onset and in those with disease of long duration [74].
When used as components of dual or triple therapy regimens, GLP-1 RAs offer the double beneficial effect of a consistent glycemic control and a moderate weight loss with a low rate of hypoglycemia. A GLP-1 RA could be a preferred option when weight control [75–77] or avoidance of hypoglycemia is particularly important [76]. They also may be used as an alternative to basal insulin for certain patients, since they provide at least similar efficacy but without weight gain and, in some cases, a lower risk of hypoglycemia [4]. Most GLP-1 RAs do not require dose adjustment in elderly patients or in patients with mild to moderate renal impairment; therefore, they represent a useful treatment option in these patient populations.
In addition, GLP-1 RAs appear to have a favorable profile regarding CV risk factors. In fact, some agents are associated with a risk reduction of CV events [40] while others have a neutral effect in this regard [41]. Thus, GLP-1 RAs also offer potential advantages in patients at higher risk of CV events [76].
As a class, the GLP-1 RAs are generally well tolerated, with nausea and vomiting being the most common adverse events. It is important to inform patients initiating GLP-1 RA therapy that gastrointestinal related adverse effects may occur initially but that these effects are transient and are typically mild to moderate in nature [78]. If nausea is bothersome, the patient may be advised to take smaller meals, avoid spicy foods and make healthier food choices, since nausea is commonly reported after consuming a large or high-fat meal [78]. Also, returning the patient to a lower GLP-1 RA dose for ≥ 1 week before repeating the incremental dosing steps can often prove successful in managing gastrointestinal adverse effects associated with this class of agents [78].
Conclusions
The GLP-1 RAs are a unique class of effective antihyperglycemic agents that enhance the actions of the naturally occurring peptide GLP-1. The mechanism of action of GLP-1 RAs is glucose dependent; as such, there is a low risk of hypoglycemia when not used in combination with sulfonylureas or insulin. Although, GLP-1 RAs share the same general mechanism of action, they differ in terms of their formulations, indications (monotherapy and/or combined therapy), injection devices, dosages, precautions and use in special patient populations. These agents also differ in terms of their effects on CV risk factors and gastrointestinal tolerability.
In accordance with clinical guidelines, GLP-1 RAs are not currently considered first-line therapy for T2D. They are, however, considered as second-line therapy in combination with oral antidiabetic drugs or insulin.
In this review, we have summarized and evaluated information relating to all of these issues, with a particular focus on practical considerations. This information has been compiled to help physicians select the most appropriate GLP-1 RA and to optimize the use of these agents, thereby improving the management of patients with T2D and enhancing health outcomes.
Acknowledgements
Funding
This work and associated article processing charges were supported by Eli Lilly and Company. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
The authors would like to thank Fernando Rico-Villademoros (COCIENTE S.L., Madrid, Spain) and Caroline Perry (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this article. Support for this assistance was funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Irene Romera is an employee of Eli Lilly and Company. Ana Cebrián-Cuenca has taken part in advisory boards for Sanofi and AstraZeneca Pharmaceuticals LP, and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb Company, Eli Lilly and AstraZeneca Pharmaceuticals LP. Fernando Álvarez Guisasola declares that he has no conflict of interest. Fernando Gomez Peralta has received a research grant from Sanofi, Novo Nordisk, Boehringer Ingelheim and Eli Lilly; has taken part in advisory boards for Sanofi, Novo Nordisk, and AstraZeneca Pharmaceuticals LP; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb Company, Eli Lilly and AstraZeneca Pharmaceuticals LP. Jesús Reviriego is an employee and stock shareholder in Eli Lilly and Company.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7308866.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 2.Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 4.Lovshin JA. Glucagon-like peptide-1 receptor agonists: a class update for treating type 2 diabetes. Can J Diabetes. 2017;41(5):524–535. doi: 10.1016/j.jcjd.2017.08.242. [DOI] [PubMed] [Google Scholar]
- 5.Smilowitz NR, Donnino R, Schwartzbard A. Glucagon-like peptide-1 receptor agonists for diabetes mellitus: a role in cardiovascular disease. Circulation. 2014;129(22):2305–2312. doi: 10.1161/CIRCULATIONAHA.113.006985. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V. Glucagon-like peptide-1 analogues: an overview. Indian J Endocrinol Metab. 2013;17(3):413–421. doi: 10.4103/2230-8210.111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra S, Baruah MP, Sahay RK, Unnikrishnan AG, Uppal S, Adetunji O. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: past, present, and future. Indian J Endocrinol Metab. 2016;20(2):254–267. doi: 10.4103/2230-8210.176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109(8):962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 9.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54(1):146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 10.GlaxoSmithKline. TANZEUM (albiglutide) discontinuation—Q&A. 2017. https://www.tanzeum.com/pdfs/consumer-faq.pdf. Accessed 21 Jun 2018.
- 11.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317–332. doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalsgaard NB, Vilsboll T, Knop FK. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk factors: a narrative review of head-to-head comparisons. Diabetes Obes Metab. 2018;20(3):508–519. doi: 10.1111/dom.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336–347. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 14.Matza LS, Curtis SE, Jordan JB, Adetunji O, Martin SA, Boye KS. Physician perceptions of GLP-1 receptor agonists in the UK. Curr Med Res Opin. 2016;32(5):857–864. doi: 10.1185/03007995.2016.1147025. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency. Trulicity; summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002825/WC500179470.pdf. Accessed 29 May 2018.
- 16.European Medicines Agency. Bydureon; summary of product characteristics. 2018. http://ec.europa.eu/health/documents/community-register/2018/20180827141897/anx_141897_en.pdf. Accessed 03 Sept 2018.
- 17.European Medicines Agency. Ozempic; summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004174/WC500244163.pdf. Accessed 03 Sept 2018.
- 18.European Medicines Agency. Byetta; summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. Accessed 29 May 2018.
- 19.European Medicines Agency. Lyxumia; summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed 29 May 2018.
- 20.European Medicines Agency. Victoza; summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed 29 May 2018.
- 21.St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79–89. doi: 10.1515/jtim-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2018. Diabetes Care. 2018;41(Suppl 1):S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour SA, Frias JP, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20:1515–1519. doi: 10.1111/dom.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 25.Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16(7):636–644. doi: 10.1111/dom.12262. [DOI] [PubMed] [Google Scholar]
- 27.Pozzilli P, Norwood P, Jodar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9) Diabetes Obes Metab. 2017;19(7):1024–1031. doi: 10.1111/dom.12937. [DOI] [PubMed] [Google Scholar]
- 28.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 29.Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Eperzan. Summary of product characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Accessed 29 May 2018.
- 31.Food and Drug Administration. Trulicity; highlights of prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s011s013lbl.pdf. Accessed 30 Aug 2018.
- 32.Food and Drug Administration. Victoza; highlights of prescribing information. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf. Accessed 30 Aug 2018.
- 33.Food and Drug Administration. Bydureon; highlights of prescribing information. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf. Accessed 30 Aug 2018.
- 34.Food and Drug Administration. Ozempic; highlights of prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed 03 Sept 2018.
- 35.Raccah D. Safety and tolerability of glucagon-like peptide-1 receptor agonists: unresolved and emerging issues. Expert Opin Drug Saf. 2017;16(2):227–236. doi: 10.1080/14740338.2017.1268598. [DOI] [PubMed] [Google Scholar]
- 36.Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–661. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- 37.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19(9):1233–1241. doi: 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- 38.Storgaard H, Cold F, Gluud LL, Vilsboll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906–908. doi: 10.1111/dom.12885. [DOI] [PubMed] [Google Scholar]
- 39.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 40.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381(9861):117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 43.Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 44.Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 45.Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33(6):1255–1261. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–1310. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 47.Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11(3–4):202–230. doi: 10.1900/RDS.2014.11.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thrasher J. Pharmacologic management of type 2 diabetes mellitus: available therapies. Am J Med. 2017;130(Suppl 6):S4–S17. doi: 10.1016/j.amjmed.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 50.Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123–139. doi: 10.2147/DMSO.S130834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldrop G, Zhong J, Peters M, et al. Incretin-based therapy in type 2 diabetes: an evidence based systematic review and meta-analysis. J Diabetes Complications. 2018;32(1):113–122. doi: 10.1016/j.jdiacomp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Singh S, Wright EE, Jr, Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wysham CH, Lin J, Kuritzky L. Safety and efficacy of a glucagon-like peptide-1 receptor agonist added to basal insulin therapy versus basal insulin with or without a rapid-acting insulin in patients with type 2 diabetes: results of a meta-analysis. Postgrad Med. 2017;129(4):436–445. doi: 10.1080/00325481.2017.1297669. [DOI] [PubMed] [Google Scholar]
- 54.Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(Suppl 1):S68–S76. doi: 10.1111/dom.13137. [DOI] [PubMed] [Google Scholar]
- 55.Monami M, Dicembrini I, Nreu B, Andreozzi F, Sesti G, Mannucci E. Predictors of response to glucagon-like peptide-1 receptor agonists: a meta-analysis and systematic review of randomized controlled trials. Acta Diabetol. 2017;54(12):1101–1114. doi: 10.1007/s00592-017-1054-2. [DOI] [PubMed] [Google Scholar]
- 56.Bihan H, Ng WL, Magliano DJ, Shaw JE. Predictors of efficacy of GLP-1 agonists and DPP-4 inhibitors: a systematic review. Diabetes Res Clin Pract. 2016;121:27–34. doi: 10.1016/j.diabres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. doi: 10.7573/dic.212283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nauck MA, Meier JJ, Cavender MA. Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 59.Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones (Athens). 2018;17(3):333–350. doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- 60.Sun F, Chai S, Li L, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015:157201. doi: 10.1155/2015/157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith RJ, Goldfine AB, Hiatt WR. Evaluating the cardiovascular safety of new medications for type 2 diabetes: Time to reassess? Diabetes Care. 2016;39(5):738–742. doi: 10.2337/dc15-2237. [DOI] [PubMed] [Google Scholar]
- 62.European Medicines Agency. Assessment report for GLP-1 based therapies. 2013. https://www.ema.europa.eu/documents/report/assessment-report-article-53-procedure-glp-1-based-therapies_en.pdf. Accessed 23 Oct 2018.
- 63.Jia X, Alam M, Ye Y, Bajaj M, Birnbaum Y. GLP-1 receptor agonists and cardiovascular disease: a meta-analysis of recent cardiac outcome trials. Cardiovasc Drugs Ther. 2018;32(1):65–72. doi: 10.1007/s10557-018-6773-2. [DOI] [PubMed] [Google Scholar]
- 64.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 65.ClinicalTrials.gov. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND). 2018. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed 10 Sept 2018.
- 66.Li L, Li S, Liu J, et al. Glucagon-like peptide-1 receptor agonists and heart failure in type 2 diabetes: systematic review and meta-analysis of randomized and observational studies. BMC Cardiovasc Disord. 2016;16:91. doi: 10.1186/s12872-016-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rex J, Jensen KH, Lawton SA. A review of 20 years’ experience with the NovoPen family of insulin injection devices. Clin Drug Investig. 2006;26(7):367–401. doi: 10.2165/00044011-200626070-00001. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Perez LE, Alvarez M, Dilla T, Gil-Guillen V, Orozco-Beltran D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matfin G, Brunt KV, Zimmermann AG, Threlkeld R, Ignaut DA. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naïve patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071–1079. doi: 10.1177/1932296815583059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34:1457–64. doi: 10.1080/03007995.2018.1465903. [DOI] [PubMed] [Google Scholar]
- 71.Stauder U, Enginee D, Elton H, Penfornis A, Edelman S. Comparative assessment of lixisenatide, exenatide, and liraglutide pen devices: a pilot user-based study. J Diabetes Sci Technol. 2014;8(1):123–131. doi: 10.1177/1932296813511733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23(2):207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 73.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2018 executive summary. Endocr Pract. 2018;24(1):91–120. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- 74.Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409–418. doi: 10.1111/dom.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Artola Menéndez S. Actualización del algoritmo de hiperglucemia 2017. Diabetes Práctica. 2017;08(02):57–60. [Google Scholar]
- 76.Kim HJ, Park SO, Ko SH, et al. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus: a position statement of the Korean Diabetes Association. Diabetes Metab J. 2017;41(6):423–429. doi: 10.4093/dmj.2017.41.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.American Diabetes Association. 7. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41[Suppl 1]:S65–72. [DOI] [PubMed]
- 78.Reid TS. Practical use of glucagon-like peptide-1 receptor agonist therapy in primary care. Clin Diabetes. 2013;31(4):148–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.