Abstract
Introduction
The effect of glycemic control on wound healing in patients with diabetic foot ulcers (DFUs) is inconsistent among different studies. This study was performed to investigate the association between level of hemoglobin A1c (HbA1c) at baseline as well as during treatment and wound healing and mortality in patients with DFU.
Methods
Hospitalized DFU patients were recruited consecutively with their basic clinical data collected and treated according to clinical practice guidelines. These patients were followed-up for 1 year to observe the outcomes, including ulcer healing and death. The associations between baseline HbA1c level or mean HbA1c level during treatment and wound healing as well as mortality were evaluated in univariate and multivariate logistic regression models.
Results
By the end of the follow-up, 40 (13.4%) patients had died. A total of 168 (65.1%) patients achieved ulcer healing in the remaining 258 living participants. Baseline HbA1c was not associated with ulcer healing in unadjusted or adjusted models (P > 0.05). The wound healing rate was higher (OR 2.01, 95% CI 1.02–3.96, P < 0.05) after adjustment when HbA1c was controlled between 7.0% and 8.0% during treatment compared to HbA1c controlled at less than 7.0%. This probability of ulcer healing increased to 3 (OR = 3.01, 95% CI 1.32–6.86, P = 0.01) after adjustment in the subgroup with baseline HbA1c no more than 8.0%. Neither baseline HbA1c nor mean HbA1c during treatment presented any correlation with 1-year death rate.
Conclusion
A reasonable HbA1c target, a range between 7.0% and 8.0% during treatment, could facilitate ulcer healing without increase of mortality in patients with DFU, especially for those with better glycemic control at admission.
Keywords: Diabetic foot ulcers, HbA1c, Mortality, Wound healing
Introduction
Diabetic foot ulcer (DFU) is one of the most common complications of diabetes with an estimated lifetime incidence of 15–25% in the population with diabetes [1]. Considerable advances regarding the mechanism and management of DFU have been achieved over the past several decades [2–4]; however, the therapeutic efficacy of these medical measures is still unsatisfactory. Moreover, DFU remains a serious challenge for public health because of the high amputation rate and high mortality.
Hyperglycemia and many other related factors, such as micro- and macrovascular complications, were illustrated as possible mechanisms that prevent the ulcers from wound healing [5, 6]. Blood glucose management is therefore considered a basic component of the treatment of wound healing in DFU patients. Only a few studies have focused on the control of blood glucose and the healing of DFU. Previous clinical studies showed no significant association between baseline HbA1c and wound healing [7, 8]. However, recent research detected that, for those patients with baseline HbA1c < 7.5%, a rise in HbA1c during treatment was associated with an increased chance of wound healing [8].
Patients with DFU are often documented to suffer from many concomitant diseases, which heightens the need for careful management based on blood glucose control. Emerging evidence showed that lower or higher levels of blood glucose may be linked to the increase of death [9, 10], in addition to the delay of wound healing. In the present study, we compared wound healing and mortality among our DFU patients according to the baseline HbA1c or mean HbA1c during treatment.
Methods
Study Population
Hospitalized patients with DFU in our department from January 2013 to December 2016 were consecutively recruited into our study. Exclusion criteria included (1) grade 5 wounds according to the Wagner ulcer classification system which required major amputation, (2) patients with persistent severe heart failure (NYHA class IV), (3) chronic diseases at end stage which deteriorated gradually and the condition could not be improved, e.g., uremia, decompensated cirrhosis, terminal malignant tumors, (4) patients with type1 diabetes, (5) non-compliant patients.
All procedures performed in studies involving human participants were in accordance with the institutional review board of Ruijin Hospital affiliated to the Shanghai Jiao Tong University School of Medicine and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from each enrolled patient.
Clinical Characteristics Collection
All the enrolled patients were interviewed on admission for medical history collection, including type and duration of diabetes mellitus, duration of DFU, smoking status, therapeutic regimen for diabetes, previous history of foot ulcer and amputation, presence of comorbidities (hypertension, coronary heart disease, stroke). Fasting venous blood was drawn in 24 h for measurement of white blood cell count, HbA1c, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, serum albumin, and serum creatinine. Value of eGFR was calculated according to the MDRD equation for Chinese based on age, sex, and serum creatinine [11].
The first HbA1c value after admission was defined as baseline HbA1c. HbA1c was measured every 3 months according to standard care of glycemic management, and HbA1c measurement was repeated at the endpoint. Mean HbA1c during treatment was defined as the average of all HbA1c values after baseline HbA1c.
Peripheral arterial disease was diagnosed if any two of the following cases occurred [12]: (1) disappearance of two or more peripheral pulses; (2) significant artery stenoses of a lower limb reported by Doppler ultrasound, the peak systolic velocity ratio at the site of the stenosis and the adjacent normal artery greater than or equal to 2.5 [13]; (3) angiogram showed lower limb artery occlusion or a reduction over 50% of lumen diameter.
In the current study, peripheral neuropathy was diagnosed by a combination of neuropathic symptoms and signs with abnormal result of the 10-g monofilament test or vibration test using a 128-Hz tuning fork [14]. Hypertension was confirmed by blood pressure in excess of 140/90 mmHg or history of antihypertensive medicine use [15]. History of coronary heart disease was ascertained from medical records, including ischemic heart disease accompanied by abnormal electrocardiogram or stress test, myocardial infarction with obvious abnormalities in electrocardiogram and plasma enzyme testing, or any medical procedures for coronary revascularization [16]. Similarly, previous stroke was confirmed from medical records related to ischemic or hemorrhagic stroke, regardless of whether the patient had recovered or not [16].
Wound Severity Assessment and Intervention
All ulcers were assessed on admission. The extent of an ulcer was graded according to Wagner’s classification [17]. Ulcer was characterized as infected when two or more of the following items were met: (1) local swelling or induration; (2) erythema; (3) local tenderness or pain; (4) local warmth; (5) purulent discharge. According to the Infectious Disease Society of America classification system [18], which was easy for clinicians to use owing to clear definitions and relatively fewer categories, infection severity was further classified into mild, moderate, and severe.
For infected ulcers, the antibiotic usage strictly complied with the clinical practice guideline from the Infectious Diseases Society of America [18]. Wound intervention included wound care, debridement, and minor amputation. Other treatment included control of blood glucose, off-loading, local antimicrobial dressings, vacuum-assisted closure, and improvement of main organ function if needed.
Outcomes and Follow-Up
The outcomes were ulcer healing and death. Ulcer healing was defined as complete epithelialization which is maintained with no drainage for at least 2 weeks. The whole observation period lasted for 1 year; follow-up would terminate in advance if the patient died. For patients who continue to receive treatment given by our department’s medical team, follow-up was done twice a week during clinic visits for dressing change. If any signs of moderate to severe infection were observed, readmission would be considered. For patients who continued their treatment at other institution, information about outcome was obtained by contacting patients or their relatives every month by telephone.
Statistical Analysis
The data were analyzed using SAS 9.3 (SAS Institute, Cary, NC) statistical software. Continuous variables were reported as mean ± standard deviation (SD) or median (interquartile ranges) depending on normality while the categorical variables were expressed as frequency (percentage). Variables with a skewed distribution were log10-transformed to achieve a normal distribution. We used linear regression analysis and Cochran–Armitage trend chi-square to test for trends across HbA1c quartiles for continuous variables and categorical variables, respectively. The associations between baseline HbA1c level or mean HbA1c level during treatment and wound healing as well as mortality rate were evaluated in univariate and multivariate logistic regression models. The accumulated healing rates and death rates in different groups were plotted as Kaplan–Meier curves, and we used the log-rank test to make comparisons. All statistical tests were performed two-sided at a significance level of 0.05.
Results
Baseline Characteristics and Follow-Up Outcome
In total, 298 participants were recruited in this study, 69.5% of whom were male. The clinical characteristics of the patients at study inclusion are summarized both overall and stratified by baseline HbA1c level in Table 1. Subjects in the current study had a long duration of diabetes accompanied by unsatisfactory glycemic control and an extremely high prevalence of neuropathy and peripheral artery disease.
Table 1.
Baseline characteristics of study patients stratified by baseline HbA1c
| Characteristics | Total n = 298 |
Baseline HbA1c | P for trend | |||
|---|---|---|---|---|---|---|
| ≤ 7% n = 60 |
7.1–8% n = 45 |
8.1–9% n = 62 |
> 9% n = 131 |
|||
| Male, n (%) | 207 (69.5) | 43 (71.7) | 27 (60.0) | 42 (67.7) | 95 (70.2) | 0.08 |
| Age, year | 68.2 ± 10.7 | 71.2 ± 9.9 | 70.4 ± 9.0 | 67.3 ± 10.1 | 66.6 ± 11.4 | 0.02 |
| BMI, kg/m2 | 23.4 ± 3.3 | 23.4 ± 3.3 | 23.0 ± 3.5 | 23.9 ± 2.7 | 23.3 ± 3.5 | 0.92 |
| Diabetes duration, year | 15 (9, 20) | 11.5 (6, 20) | 17.5 (10, 20) | 14 (7, 20) | 15 (10, 20) | 0.90 |
| Duration of DFU, day | 60 (20, 120) | 96 (30, 270) | 60 (30, 95) | 30 (20, 90) | 30 (15, 90) | 0.01 |
| Current smoker, n (%) | 100 (33.5) | 14 (23.3) | 15 (33.3) | 23 (37.1) | 48 (36.6) | 0.43 |
| Prior ulcer, n (%) | 69 (23.2) | 23 (38.3) | 9 (20.0) | 13 (21.0) | 24 (18.3) | 0.12 |
| Prior amputation, n (%) | 32 (10.7) | 10 (16.7) | 8 (17.8) | 6 (9.7) | 8 (6.1) | 0.12 |
| SBP, mmHg | 135 (130, 150) | 132 (125,150) | 140 (130, 150) | 135 (120, 150) | 132 (130, 150) | 0.67 |
| DBP, mmHg | 75 (70, 80) | 79 (72, 81) | 80 (74, 82) | 74 (70, 80) | 75 (68, 80) | 0.74 |
| Hypertension, n (%) | 201 (67.4) | 46 (76.7) | 33 (73.3) | 42 (67.7) | 80 (61.1) | 0.21 |
| CHD, n (%) | 77 (25.8) | 15 (25.0) | 12 (26.7) | 16 (25.8) | 34 (26.0) | 0.99 |
| Stroke, n (%) | 65 (21.8) | 19 (31.7) | 9 (20.0) | 7 (11.3) | 30 (22.9) | 0.10 |
| DPN, n (%) | 281 (94.3) | 54 (90.0) | 45 (100.0) | 58 (93.5) | 124 (94.6) | 0.33 |
| PAD, n (%) | 293 (98.3) | 59 (98.3) | 44 (97.8) | 62 (100.0) | 128 (97.7) | 0.88 |
| TC, mmol/L | 3.89 ± 1.23 | 3.73 ± 0.95 | 4.08 ± 1.25 | 3.97 ± 1.12 | 3.86 ± 1.38 | 0.91 |
| LDL-C, mmol/L | 2.31 ± 1.07 | 2.20 ± 0.79 | 2.32 ± 0.86 | 2.30 ± 1.10 | 2.37 ± 1.02 | 0.62 |
| Serum albumin, g/L | 32.79 ± 5.20 | 35.48 ± 4.01 | 35.13 ± 4.43 | 34.12 ± 5.28 | 30.13 ± 4.67 | 0.01 |
| Insulin use, n (%) | 287 (96.3) | 56 (93.3) | 44 (97.8) | 59 (95.2) | 128 (97.7) | 0.31 |
| Wagner grade, n (%) | ||||||
| 2 | 36 (12.1) | 7 (11.6) | 5 (11.1) | 9 (14.5) | 15 (11.5) | 0.17 |
| 3 | 96 (32.2) | 26 (43.3) | 15 (33.3) | 23 (51.1) | 32 (24.4) | |
| 4 | 166 (55.7) | 27 (45.0) | 25 (55.5) | 30 (48.4) | 84 (64.1) | |
| Degree of infection, n (%) | ||||||
| Mild | 35 (11.7) | 10 (16.7) | 8 (17.8) | 11 (17.7) | 6 (4.6) | 0.01 |
| Moderate | 123 (41.3) | 27 (45.0) | 25 (55.5) | 29 (46.8) | 42 (32.1) | |
| Severe | 140 (47.5) | 23 (38.3) | 12 (26.7) | 22 (35.5) | 83 (63.3) | |
| eGFR categories, n (%) | ||||||
| ≥ 90 | 166 (55.7) | 30 (50.0) | 18 (40.0) | 32 (51.6) | 86 (65.6) | 0.01 |
| 60–89 | 76 (25.5) | 11 (18.3) | 12 (26.7) | 25 (40.3) | 28 (21.4) | |
| 30–59 | 33 (11.1) | 10 (16.7) | 8 (17.8) | 3 (4.8) | 12 (9.2) | |
| 15–29 | 23 (7.7) | 9 (15.0) | 7 (15.5) | 2 (3.2) | 5 (3.8) | |
SBP systolic blood pressure, DBP diastolic blood pressure, CHD coronary heart disease, DPN diabetic peripheral neuropathy, PAD peripheral artery disease, TC total cholesterol, LDL-C low density lipoprotein cholesterol
Patients with higher baseline HbA1c level tended to be younger, had shorter duration of foot ulcer, lower serum albumin level, higher eGFR, and more severe infection (all P < 0.05). However, there was no significant difference in BMI, duration of diabetes, proportion of insulin use, prevalence of comorbidities, and distribution of Wagner grade among the four groups.
During the 1-year follow-up period, 40 individuals (13.4%) died, among them 24 from cardiac and cerebral vascular events, 6 as a result of uncontrolled sepsis, 5 after multiple organ dysfunction, 5 from cancer. In the remaining 258 living patients, 168 (65.1%) achieved ulcer healing.
Baseline HbA1c and Ulcer Healing
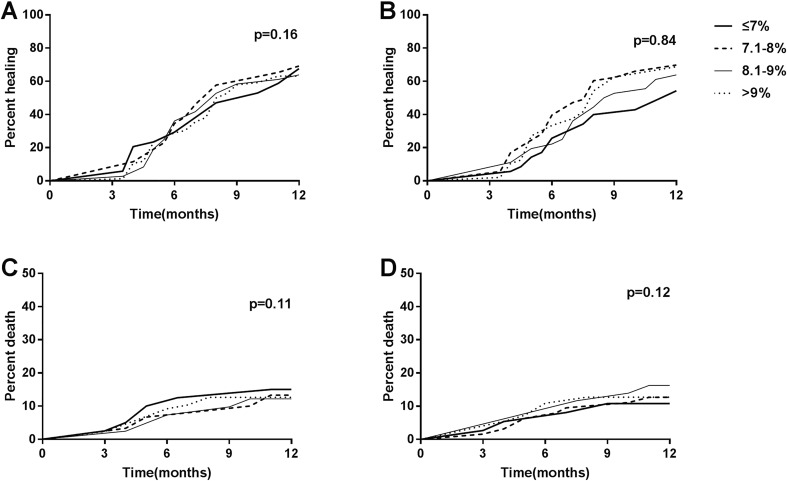
Baseline HbA1c measurements among the 258 living patients were categorized as quartiles (≤ 7.0%, 7.1–8.0%, 8.1–9.0%, > 9.0%). The prevalence of ulcer healing for each quartile was similar as shown in the Kaplan–Meier curve (P > 0.05) (Fig. 1a). Univariate logistic regression analysis also showed no significant increase in incidence of ulcer healing in the three higher quartiles compared with the reference group (baseline HbA1c ≤ 7%). Furthermore, no relationship between baseline HbA1c and ulcer healing was detected (P > 0.05), after adjusting for a series of confounders.
Fig. 1.
Kaplan–Meier curves of ulcer healing among different a baseline HbA1c groups or b groups stratified by mean HbA1c during treatment. Kaplan–Meier curves of death among different c baseline HbA1c groups or d groups stratified by mean HbA1c during treatment. HbA1c measurements were categorized as quartiles (≤ 7.0%, 7.1–8.0%, 8.1–9.0%, > 9.0%). Thick solid line, HbA1c ≤ 7.0%. Dashed line, HbA1c 7.1–8.0%. Thin solid line, HbA1c 8.1–9.0%. Dotted line, HbA1c > 9.0%. Log-rank test was used to make comparisons of ulcer healing and death among different groups stratified by baseline HbA1c or mean HbA1c during treatment
Mean HbA1c During Treatment and Ulcer Healing
As displayed in Table 2, univariate analyses of data from the 258 surviving participants did not show any association between mean HbA1c during treatment and ulcer healing (P > 0.05), similarly to the result of the log-rank test (P > 0.05) (Fig. 1b). There was no significant difference in ulcer healing rate among the four groups stratified by mean HbA1c during treatment, after adjusting for age, sex, and BMI (model 1) or further adjusting for Wagner grade, degree of infection, previous history of amputation, and peripheral neuropathy (model 2). However, when adjusting for more confounders in model 3, the second quartile (7.0% < mean HbA1c ≤ 8.0%) exhibited a greater chance of ulcer healing compared with the reference group (mean HbA1c ≤ 7.0%), with odds ratio of 2.01 (95% CI 1.02–3.96, P < 0.05). But a similar association did not appear in the higher quartiles.
Table 2.
Association between mean HbA1c during treatment and ulcer healing among alive patients
| Mean HbA1c after admission (%) | N | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| ≤ 7.0 | 51 | Ref | Ref | Ref | Ref | ||||
| 7.1–8.0 | 81 | 1.57 (0.80, 3.09) | 0.19 | 1.53 (0.77, 3.02) | 0.22 | 1.60 (0.80, 3.20) | 0.08 | 2.01 (1.02, 3.96) | < 0.05 |
| 8.1–9.0 | 54 | 1.30 (0.66, 2.56) | 0.54 | 1.21 (0.60, 2.44) | 0.42 | 1.12 (0.57, 2.20) | 0.46 | 1.28 (0.61, 2.69) | 0.68 |
| > 9.0 | 72 | 1.14 (0.55, 2.36) | 0.52 | 0.95 (0.46, 1.96) | 0.67 | 1.04 (0.50, 2.08) | 0.60 | 1.18 (0.62, 2.24) | 0.43 |
Model 1 adjusted for age, sex, BMI
Model 2 adjusted for age, sex, BMI, Wagner grade, degree of infection, DPN, previous history of amputation
Model 3 adjusted for age, sex, BMI, Wagner grade, previous history of amputation, previous history of stroke, eGFR categories, smoking status, plasma albumin concentration
Then, subgroup analysis was performed further stratified by baseline HbA1c level of ≤ 8.0% and > 8.0%. Table 3 shows the association of mean HbA1c during treatment and ulcer healing in patients with baseline HbA1c ≤ 8.0%. As all of the mean HbA1c values during treatment were lower than 9.0% in this subgroup, the HbA1c measurements were categorized as tertiles (≤ 7.0%, 7.1–8.0%, > 8.0%). Those with an average HbA1c between 7% and 8% during treatment manifested a 2.48 times higher probability of ulcer healing (P = 0.01), compared with reference group (mean HbA1c ≤ 7.0%). Whereas, there was no significant difference in chance of ulcer healing between the highest tertile and the reference group (P > 0.05). After adjustment for a series of confounders, the chance for ulcer healing in the middle tertile was augmented, whereas the chance for ulcer healing did not change significantly in the highest tertile.
Table 3.
Association between mean HbA1c during treatment and ulcer healing among alive patients with baseline HbA1c ≤8% (n = 90)
| Mean HbA1c after admission (%) | N | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| ≤ 7.0 | 30 | Ref | Ref | Ref | Ref | ||||
| 7.1–8.0 | 37 | 2.48 (1.24, 4.96) | 0.01 | 2.31 (1.15, 4.62) | 0.02 | 2.14 (1.12, 4.09) | 0.02 | 3.01 (1.32, 6.86) | 0.01 |
| > 8.0 | 23 | 1.23 (0.47, 3.20) | 0.68 | 1.41 (0.51, 3.90) | 0.50 | 1.01 (0.32, 3.16) | 0.99 | 1.26 (0.40, 3.97) | 0.70 |
Model 1 adjusted for age, sex, BMI
Model 2 adjusted for age, sex, BMI, Wagner grade, degree of infection, DPN, previous history of amputation
Model 3 adjusted for age, sex, BMI, Wagner grade, previous history of amputation, previous history of stroke, eGFR categories, smoking status, plasma albumin concentration
However, in the subgroup with baseline HbA1c > 8.0%, no significant relationship between mean HbA1c during treatment and ulcer healing was observed in any of the models (all P > 0.05). According to unadjusted or adjusted analysis, the healing rate of ulcers was not lower in higher HbA1c groups compared to the HbA1c ≤ 7.0% group during the follow-up period.
Baseline HbA1c or Mean HbA1c and Death
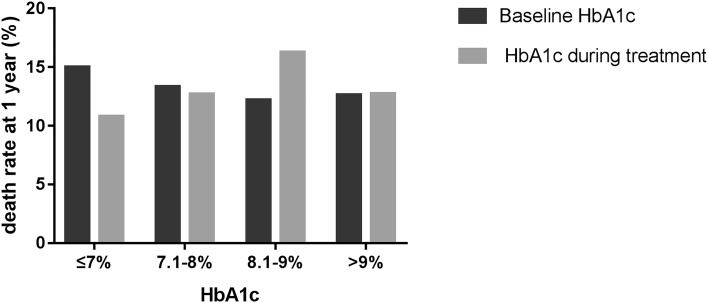
There was no significant difference in accumulated death rates for 1 year among different HbA1c categories stratified by baseline HbA1c or mean HbA1c during treatment (P > 0.05) (Fig. 2). In addition, results from the log-rank test also did not show a significant difference in healing rate trend over time among the four groups stratified by baseline HbA1c or mean HbA1c during treatment (all p > 0.05) (Figs. 1c, d).
Fig. 2.
1-year accumulated death rate of patients with DFU at different glycemic level. Dark gray, groups stratified by baseline HbA1c. Light gray, groups stratified by mean HbA1c during treatment
In other words, no correlation was revealed between baseline HbA1c or average HbA1c during treatment and the mortality within 1 year.
Discussion
According to our analysis, a level of HbA1c that ranged from 7.0% to 8.0% during treatment would be more helpful for wound healing in DFU patients than other levels. It seemed that there was no association between baseline HbA1c levels and ulcer healing, but better baseline HbA1c level would benefit wound healing if the HbA1c is maintained between 7.0% and 8.0% during treatment. No significant increase or decrease of the mortality was indicated among different HbA1c control levels. Consistent with previous literature, for patients with baseline HbA1c < 7.0%, elevated HbA1c during treatment was associated with accelerated wound healing [8], indicating that a slightly higher glycemic level was better for wound healing in DFU patients.
Although previous studies have suggested that lower baseline HbA1c level correlates with advanced wound healing [19], most studies noted that baseline HbA1c level was not associated with lower extremity wound healing in patients with diabetes. When baseline HbA1c was divided into < 6.5%, 6.5–8.0%, > 8.0%, as described by Fesseha et al. [8], initial blood glucose level displayed no relationship with wound healing, based on the analysis using different adjusted models. A similar outcome was obtained in our investigation. By baseline HbA1c data analysis, not grouped, another study showed that HbA1c at study inclusion did not predict the risk of delayed healing among a pooled analysis of 586 subjects with neuropathic DFU [20].
DFU was characterized by poor healing outcome and hyperglycemia was identified as the culprit of impaired wound healing. Pre-existing diabetic angiopathy and neuropathy resulting from previous exposure to hyperglycemia have been recognized as the major causes of delayed wound healing. Besides, current exposure to hyperglycemia is also linked to impaired wound healing processes. Plenty of evidence showed that hyperglycemia-related advanced glycation end-products play a crucial role in disturbing the normal wound healing process, with underlying mechanisms like raised oxidative stress, altered cellular proliferation and apoptosis, and altered interaction between cell and extracellular matrix [5, 21].
The existence of foot ulcers attracts more attention to hyperglycemia. However, ideal blood glucose targets could not be easily achieved, considering many uncontrolled factors like infection, other concomitant diseases, etc. These may be found in the baseline characteristics of study patients in our study or the studies referred to above. After work by multidisciplinary team, modifiable factors like infection and organ dysfunctions were managed. Blood glucose should be controlled at a reasonable level, not only for the wound healing but also for the whole body.
Only a few studies focused on the benefit of blood glucose control to wound healing during the DFU treatment. Research by Fesseha et al. [8] showed that increase of HbA1c during the treatment compared to admission was associated with a hazard ratio (HR) of nearly 2 for wound healing in DFU patients with baseline HbA1c < 7.5%, while no associated benefit was observed for DFU patients with baseline HbA1c over 7.5%. For the purpose of wound healing, this demands that blood glucose be controlled at higher levels if the initial HbA1c value is less than 7.5%. In our study, HbA1c controlled within 7.0–8.0% during DFU treatment is beneficial for wound healing; this advantage was even more evident in DFU patients with baseline HbA1c less than 8.0%. Additionally, in contrast to those with HbA1c controlled at less than 7%, the wound healing rate was almost the same or slightly better in DFU patients with HbA1c higher than 8.0% during DFU treatment.
Tight blood glucose control characterized by lower HbA1c level often leads to more frequent episodes of hypoglycemia as some large clinical trials demonstrated [22, 23]. For elderly diabetic patients with long diabetes duration, such as patients with DFU, fluctuation of blood glucose is relatively more prevalent [24]. In general, mortality rate increases as the glycemic level elevates for patients with diabetes. However, the mortality pattern was different in various disease statuses of diabetes. For older patients with diabetes, mortality risk was significantly higher in those with an HbA1c > 8.0% compared with those with an HbA1c < 6.5% [25]. For patients with diabetes and chronic kidney disease, a U-shaped relationship between HbA1c and mortality was observed; HbA1c < 6.0% and ≥ 9.0% were associated with higher risk of death [26]. Patients suffering from DFU, which is believed to be the advanced stage of diabetes, are clearly different from ordinary diabetic patients. The association between HbA1c and mortality may be unique in patients with DFU. There was still no relevant study focused on the association of blood glucose level and mortality in the population with DFU. According to our results of 1-year follow-up, mortality was not associate significantly with glycemic control level.
Several limitations of our study should not be neglected. First, this is a single-center observational study; all participants were enrolled from one hospital, leading to inevitable selection bias. Second, subjects enrolled in this study were hospitalized patients with relatively worse conditions. Clinical patients with minor wounds and better general conditions were not included; thus, the results cannot be extended to the general population with diabetic foot ulcers. Third, the follow-up period was not long enough, so that it is impossible to observe more long-term outcomes. In spite of these shortcomings, our study still reflects the relationship of glycemic control and outcomes in such a specific population with severe DFU and poor general condition. Therefore, the importance of this study should not be negated.
Conclusions
In summary, there was no difference in wound healing rate among groups of different baseline HbA1c levels, but good control of blood glucose at baseline would be beneficial if future HbA1c could be controlled reasonably. Slightly higher levels of HbA1c within 7.0–8.0% during treatment were associated with higher ulcer healing rate in patients with DFU, particularly for those with better glycemic control at baseline. Besides, in contrast to HbA1c less than 7.0%, the wound healing rate under the condition of HbA1c higher than 8.0% during DFU treatment was almost the same or a little better. These results suggested that HbA1c should be maintained higher than 7.0% for the glycemic control of DFU patients, and the best range for HbA1c should be within 7.0–8.0%. Meanwhile, we did not observe elevated mortality among lower or higher glycemic level groups. These results about the influence of reasonable blood glucose control should be further verified with large-sample, multicenter clinic trials.
Acknowledgements
The authors are grateful to the participants of this study for their invaluable contributions to this work.
Funding
No funding or sponsorship was received for this study or the publication of this article. The article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Zhengyi Tang designed the study and reviewed the manuscript. Shumin Wang, Yang He, Lei Xu, and Shanshan Zhang collected data. Jiali Xiang analyzed the data and drafted manuscript.
Disclosures
Jiali Xiang, Shumin Wang, Yang He, Lei Xu, Shanshan Zhang, and Zhengyi Tang declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the institutional review board of Ruijin Hospital affiliated to the Shanghai Jiao Tong University School of Medicine and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individuals included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7314986.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Game FL, Attinger C, Hartemann A, et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes/Metabol Res Rev. 2016;32(Suppl 1):75–83. doi: 10.1002/dmrr.2700. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A. Evaluation and management of lower-extremity ulcers. N Engl J Med. 2018;378(3):302. doi: 10.1056/NEJMc1715237. [DOI] [PubMed] [Google Scholar]
- 4.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012;41(3):384–397. doi: 10.1007/s12020-012-9619-x. [DOI] [PubMed] [Google Scholar]
- 5.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17(4):461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 7.Moffat AD, Worth ER, Weaver LK. Glycosylated hemoglobin and hyperbaric oxygen coverage denials. Undersea Hyperb Med. 2015;42(3):197–204. [PubMed] [Google Scholar]
- 8.Fesseha BK, Abularrage CJ, Hines KF, et al. Association of hemoglobin A1c and wound healing in diabetic foot ulcers. Diabetes Care. 2018;41(7):1478–1485. doi: 10.2337/dc17-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paprott R, Schaffrath Rosario A, Busch MA, et al. Association between hemoglobin A1c and all-cause mortality: results of the mortality follow-up of the German National Health Interview and Examination Survey 1998. Diabetes Care. 2015;38(2):249–256. doi: 10.2337/dc14-1787. [DOI] [PubMed] [Google Scholar]
- 10.Skriver MV, Stovring H, Kristensen JK, Charles M, Sandbaek A. Short-term impact of HbA1c on morbidity and all-cause mortality in people with type 2 diabetes: a Danish population-based observational study. Diabetologia. 2012;55(9):2361–2370. doi: 10.1007/s00125-012-2614-1. [DOI] [PubMed] [Google Scholar]
- 11.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 12.Ghanassia E, Villon L, Thuan Dit Dieudonne JF, Boegner C, Avignon A, Sultan A. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5-year follow-up study. Diabetes Care. 2008;31(7):1288–1292. doi: 10.2337/dc07-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiner T, Kessels AG, Nelemans PJ, et al. Peripheral arterial disease: comparison of color duplex US and contrast-enhanced MR angiography for diagnosis. Radiology. 2005;235(2):699–708. doi: 10.1148/radiol.2352040089. [DOI] [PubMed] [Google Scholar]
- 14.Morbach S, Furchert H, Groblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021–2027. doi: 10.2337/dc12-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115–124. doi: 10.1016/S2213-8587(15)00508-2. [DOI] [PubMed] [Google Scholar]
- 17.Wagner FW., Jr The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 18.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013;103(1):2–7. doi: 10.7547/1030002. [DOI] [PubMed] [Google Scholar]
- 19.Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011;131(10):2121–2127. doi: 10.1038/jid.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol. 2000;136(12):1531–1535. doi: 10.1001/archderm.136.12.1531. [DOI] [PubMed] [Google Scholar]
- 21.Peppa M, Raptis SA. Glycoxidation and wound healing in diabetes: an interesting relationship. Curr Diabetes Rev. 2011;7(6):416–425. doi: 10.2174/157339911797579188. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 23.Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012;14(1):79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- 24.Murata GH, Duckworth WC, Shah JH, Wendel CS, Hoffman RM. Sources of glucose variability in insulin-treated type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES) Clin Endocrinol. 2004;60(4):451–456. doi: 10.1111/j.1365-2265.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 25.Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC. Hemoglobin A1c and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988–2011) Diabetes Care. 2017;40(4):453–460. doi: 10.2337/dci16-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Winkelmayer WC, Nally JV., Jr Diabetes control and the risks of ESRD and mortality in patients with CKD. Am J Kidney Dis. 2017;70(2):191–198. doi: 10.1053/j.ajkd.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.