Abstract
Introduction
Once-weekly semaglutide is a novel glucagon-like peptide-1 (GLP-1) analogue for the treatment of type 2 diabetes that was associated with greater reductions in glycated hemoglobin (HbA1c) and body mass index (BMI) versus once-daily GLP-1 analogue liraglutide in a recent network meta-analysis (NMA). The aim of the present study was to assess the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus liraglutide 1.2 mg in Estonia.
Methods
Outcomes were projected over patient lifetimes using the IQVIA CORE Diabetes Model (version 9.0), with baseline cohort characteristics sourced from SUSTAIN 3 and changes in HbA1c, systolic blood pressure (SBP), and BMI associated with once-weekly semaglutide and liraglutide derived from the NMA. Patients were assumed to receive once-weekly semaglutide or liraglutide for 5 years before intensifying to basal insulin. Treatment effects were applied for the first 5 years, after which HbA1c increased to 7.0%, SBP followed a natural progression, and BMI reverted to baseline for the remainder of the analysis. Costs were expressed in euros (EUR) and estimated from a healthcare payer perspective. Utilities associated with diabetes and diabetes-related complications were taken from published sources.
Results
Once-weekly semaglutide 1 mg was associated with improvements in quality-adjusted life expectancy of 0.13 quality-adjusted life years (QALYs) versus liraglutide 1.2 mg. Direct costs were EUR 67 higher with once-weekly semaglutide, due to the increased acquisition cost, but this was mostly offset by cost savings due to avoidance of diabetes-related complications. Once-weekly semaglutide 1 mg was therefore associated with an incremental cost-effectiveness ratio of EUR 523 per QALY gained versus liraglutide 1.2 mg, which falls well below a willingness-to-pay threshold of EUR 52,390 per QALY gained (three times the Estonian GDP per capita).
Conclusion
Once-weekly semaglutide was considered highly cost-effective versus liraglutide 1.2 mg for the treatment of patients with type 2 diabetes in Estonia.
Funding
Novo Nordisk A/S.
Plain Language Summary
Plain language summary available for this article.
Keywords: Cost, Cost-effectiveness, Diabetes mellitus, Estonia, GLP-1 analogue, GLP-1 receptor agonist, Liraglutide, Semaglutide
Plain Language Summary
Multifactorial treatments that target both reductions in blood sugar levels [measured via glycated hemoglobin (HbA1c)] and body weight are becoming increasingly important for the treatment of type 2 diabetes, with studies demonstrating that short-term improvements in these outcomes are associated with a reduction in the risk of long-term diabetes-related complications.
In a recent network meta-analysis (NMA), once-weekly semaglutide was associated with greater efficacy versus once-daily liraglutide, with greater improvements in HbA1c and body weight in adult type 2 diabetes patients with inadequate glycemic control on multiple oral antidiabetic medications (OADs).
With the prevalence and costs associated with diabetes spiraling, and healthcare payer budgets coming under increasing pressure, choosing cost-effective treatments is becoming increasingly important.
The present analysis assessed the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus liraglutide 1.2 mg for the treatment of adult type 2 diabetes patients with inadequate glycemic control on OADs from a healthcare payer perspective in Estonia.
Once-weekly semaglutide 1 mg was associated with improved life expectancy and quality-adjusted life expectancy versus liraglutide 1.2 mg over patient lifetimes. Total costs were marginally higher with once-weekly semaglutide 1 mg, with the increased acquisition cost mostly offset by cost savings due to avoidance of diabetes-related complications.
Once-weekly semaglutide 1 mg therefore offers a highly cost-effective alternative to liraglutide 1.2 mg for the treatment of adult type 2 diabetes patients with inadequate glycemic control on OADs in Estonia.
Introduction
Diabetes is associated with a significant clinical and economic burden in Estonia, with between 7% and 9% of the adult population affected, more than 2000 people per year hospitalized with the condition, and diabetes-related healthcare expenditure totaling USD 87 million in 2017 [1–5]. Improved glycemic control, measured via glycated hemoglobin (HbA1c), remains the key treatment target for patients with type 2 diabetes, with lowered HbA1c associated with a reduced incidence of long-term diabetes-related complications in landmark studies [6–10]. However, short-term improvements in systolic blood pressure (SBP) and body weight have also been shown to substantially reduce the risk of long-term complications [11–14]. Additionally, patients express a preference for treatments that do not increase body weight and require fewer injections [15, 16]. Therefore, treatments that target a variety of factors are becoming increasingly popular.
In Estonia, a high proportion of patients with type 2 diabetes fail to achieve glycemic control targets, with 50% found to have an HbA1c level greater than 7.0% and 61% not achieving an HbA1c value below 6.5% in 2009 [17, 18]. Similarly, patients with type 2 diabetes often struggle to maintain a normal weight, with only 6% of patients below a body mass index (BMI) of 25 kg/m2 and more than 90% classified with BMIs greater than 27 kg/m2 [17]. Additionally, only 37% of patients have an SBP of less than 140 mmHg [17]. Glycemic control and reductions in weight and SBP are particularly important for reducing the risk of cardiovascular complications, which is substantially higher in patients with type 2 diabetes compared with the general population. The risk of death from cardiovascular complications is approximately two to three times higher in patients with type 2 diabetes versus people with no history of the disease, while cardiovascular disease is responsible for 52% of deaths in patients with type 2 diabetes [19, 20]. A 1% reduction in mean HbA1c has been associated with a 16% risk reduction for heart failure, a 4% risk reduction for myocardial infarction, and a 12% risk reduction for stroke, while modest weight losses of between 5% and 10% have been linked with significant improvements in cardiovascular disease risk factors [21, 22]. This exemplifies the need for treatments that target improvements in multiple clinical outcomes, not solely glycemic control.
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of diabetes treatments that have been associated with improved glycemic control and weight loss versus a variety of comparators [23–26]. In Estonia, the costs of GLP-1 receptor agonists are reimbursed for patients with type 2 diabetes with a BMI ≥ 35 kg/m2, with once-daily injectable liraglutide 1.2 mg currently the most frequently used GLP-1 analogue [4].
Once-weekly semaglutide is a novel GLP-1 analogue that is approved for use in the European Union. Its safety and efficacy have been assessed versus a variety of comparators, and at different stages of the type 2 diabetes treatment algorithm, throughout the SUSTAIN clinical trial program [27, 29]. However, no head-to-head comparison data of once-weekly semaglutide versus liraglutide are available, with the recently completed SUSTAIN 10 trial yet to be published [30]. To fill this data gap, a network meta-analysis (NMA) conducted in adult patients with inadequate glycemic control on one or two oral antidiabetic medications (OADs) has been published [31]. The NMA was based on a systematic literature review and assessed the changes from baseline in HbA1c, SBP, and body weight in patients with inadequate glycemic control on one or two OADs, based on a Bayesian framework [31]. A total of 26, 15, and 25 studies were included in the HbA1c, SBP, and body weight networks, respectively. These showed that once-weekly semaglutide 1 mg was associated with statistically significant reductions in HbA1c and body weight and statistically nonsignificant reductions in SBP versus liraglutide 1.2 mg [31].
Healthcare in Estonia is almost wholly provided by a national health insurance service, known as the Estonian Health Insurance Fund, which is funded through taxation of the population and businesses. Approximately 95% of patients are covered through this mandatory insurance, which intends to cover at least 75% of the total healthcare expenditure for patients [32]. The aim of the present study was to assess the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus liraglutide 1.2 mg for the treatment of adult patients with type 2 diabetes with inadequate glycemic control on OADs, based on data from the NMA, from an Estonian Health Insurance Fund perspective.
Methods
Model Overview
The evaluation of cost-effectiveness was performed using the IQVIA CORE Diabetes Model (version 9.0), an internet-based, interactive computer model developed to project long-term health outcomes and economic consequences of implementing interventions for the treatment of type 1 and type 2 diabetes [33, 34]. Long-term outcomes projected by the model have been validated against real-life data, both at the time of initial publication in 2004 and in a more recent 2014 study [34, 35]. Outputs from the model include life expectancy (measured in life years), quality-adjusted life expectancy [measured in quality-adjusted life years (QALYs)], cumulative incidence and time to onset of diabetes-related complications, direct medical costs, and cost-effectiveness scatterplots and acceptability curves. Diabetes-related complications include cardiovascular events (angina, stroke, myocardial infarction, congestive heart failure, and peripheral vascular disease), renal complications (microalbuminuria, gross proteinuria, and end-stage renal disease), retinopathy diseases (macular edema, cataract, severe vision loss, and background and proliferative retinopathy), and hypoglycemic events (severe and nonsevere), as well as ulcers, amputations, and neuropathy. Where an intervention is associated with clinical benefits and a cost increase, cost-effectiveness is assessed in the form of an incremental cost-effectiveness ratio (ICER), calculated as the incremental cost per unit of effect gained by using the novel intervention instead of the comparator.
Analyses were performed over a 50-year time horizon to capture all relevant long-term complications and associated costs and to assess their impact on life expectancy and quality of life, as recommended in guidelines for the assessment of cost-effectiveness of diabetes interventions [36]. In all base-case and sensitivity analyses, mortality was considered as a result of diabetes-related complications, with background mortality based on Estonia-specific life tables [37]. The UKPDS 68 risk equations were applied to predict the risk of cardiovascular complications [38]. A first-order Monte Carlo approach, capturing 1000 identical patients who are run through the model 1000 times, was used for base-case and sensitivity analyses, while a second-order Monte Carlo approach, with sampling applied to patient cohort characteristics, treatment effects, costs, utilities, and probabilities of events, was used for probabilistic sensitivity analysis (PSA). Clinical and cost outcomes were discounted at 5.0% per annum, in line with the guidelines for the assessment of medicinal products in the Baltic states [39].
Clinical Data
Baseline cohort characteristics were based on the subgroup of patients with a BMI ≥ 35 kg/m2 in the SUSTAIN 3 clinical trial, with data extracted in a post hoc analysis (Table 1). This trial was chosen as it was used to inform the once-weekly semaglutide 1 mg arm of the NMA [31]. The proportion of patients using tobacco products (18.1%) was based on the trial data, but the number of cigarettes smoked per day was assumed to be the same as the general population in Estonia [4]. Similarly, mean weekly alcohol consumption was taken from Estonia-specific data for the general population [4].
Table 1.
Baseline cohort characteristics of patients with a BMI ≥ 35 kg/m2 in SUSTAIN 3
| Characteristic | Mean (standard deviation) |
|---|---|
| Age at onset (years) | 53.94 (10.52) |
| Duration of diabetes (years) | 7.83 (5.19)a |
| Percentage male (%) | 43.21 |
| HbA1c (%) | 8.37 (0.98) |
| Systolic blood pressure (mmHg) | 134.63 (14.37) |
| Diastolic blood pressure (mmHg) | 81.06 (8.42) |
| Total cholesterol (mg/dL) | 188.63 (42.18) |
| HDL cholesterol (mg/dL) | 47.10 (12.13) |
| LDL cholesterol (mg/dL) | 105.08 (36.66) |
| Triglycerides (mg/dL) | 196.23 (135.50) |
| BMI (kg/m2) | 41.04 (5.35) |
| Percentage smokers (%) | 18.12 |
| Cigarettes per day | 13.00b |
| Alcohol consumption (oz/week) | 4.66b |
All data were taken from SUSTAIN 3, unless otherwise indicated
BMI body mass index, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
aRounded to 8.00 in the analysis, as the model only accepts integer values for the duration of diabetes
bBased on a 2017 health technology assessment of GLP-1 receptor agonists [4]
Physiological parameter treatment effects applied in the first year of the analysis with once-weekly semaglutide 1 mg and liraglutide 1.2 mg were based on data from the NMA (Table 2). A random-effects model was used to assess changes in HbA1c, and fixed-effects models were used to assess changes in systolic blood pressure and body weight. These showed that once-weekly semaglutide 1 mg was associated with statistically significant reductions in HbA1c [− 1.5%, (95% confidence interval − 1.7 to − 1.2) versus − 0.9% (− 1.1 to − 0.6)] and body weight [− 3.8 kg (− 4.4 to − 3.3) versus − 1.8 kg (− 2.4 to − 1.2)] and statistically nonsignificant reductions in SBP [− 6.3 mmHg (− 9.3 to − 3.3) versus − 4.5 mmHg (− 7.2 to − 1.7)] versus liraglutide 1.2 mg [31]. Due to limitations in the published data, the NMA was based on all patients with diabetes receiving the study medications, so the present analysis assumes that the treatment effects are equivalent in patients with a BMI ≥ 35 kg/m2. The outcomes included in the NMA that were applicable to an analysis using the IQVIA CORE Diabetes Model, encompassing changes from baseline in HbA1c, SBP, and body weight (converted to BMI) versus placebo, were applied in both treatment arms, with both statistically significant and nonstatistically significant differences included in line with modeling guidelines [40]. Where parameters were not included in the NMA, inputs were assumed to be 0 in both arms to ensure that these did not drive cost-effectiveness outcomes.
Table 2.
Treatment effects included in the analysis
| Parameter | Mean (standard error) | |
|---|---|---|
| Once-weekly semaglutide 1 mg | Liraglutide 1.2 mg | |
| HbA1c (%) | − 1.47 (0.12)* | − 0.87 (0.12) |
| Systolic blood pressure (mmHg) | − 6.28 (1.52) | − 4.45 (1.39) |
| BMI (kg/m2) | − 1.35 (0.10)* | − 0.64 (0.10) |
BMI body mass index, HbA1c glycated hemoglobin
*Statistically significant difference at 95% confidence level versus liraglutide 1.2 mg
Treatment Duration, Switching, and Long-Term Parameter Progression
Patients were assumed to receive once-weekly semaglutide or liraglutide for the first 5 years of the analysis, in line with a 2017 health technology assessment of GLP-1 receptor agonists in Estonia [4]. After 5 years, treatment with once-weekly semaglutide or liraglutide was discontinued and patients were assumed to intensify to basal insulin therapy with insulin glargine U100 (Lantus®). This assumption recognizes that intensification from GLP-1 receptor agonists to basal insulin therapy will be required for patients to maintain glycemic control over the long term, due to the progressive nature of type 2 diabetes. Benefits in HbA1c and BMI associated with once-weekly semaglutide or liraglutide treatment were assumed to persist for the 5 years that patients received these treatments. On intensification to basal insulin therapy, HbA1c was brought to 7.0% in both treatment arms (based on guidelines released by the European Association for the Study of Diabetes) and BMI reverted to baseline for the remainder of the analysis [33, 41]. SBP was assumed to follow the UKPDS progression equation for the duration of the analysis. This resulted in a balanced cost-effectiveness analysis, with differences in HbA1c and BMI only maintained while there were differences in costs. Alternative treatment switching and parameter progression assumptions were explored in sensitivity analyses.
Costs, Resource Use, and Utilities
Costs were estimated from an Estonian healthcare payer perspective, specifically the Estonian National Health Insurance Fund, and expressed in euros (EUR). Unit costs of diabetes medications and consumables were based on retail prices, with calculations reflecting the acquisition cost reimbursed by the Estonian Health Insurance Fund [for needles, self-monitoring of blood glucose (SMBG) test strips, and SMBG lancets], and the maximum reimbursement quantities depending on the type of therapy patients received (for SMBG test strips and SMBG lancets).
Diabetes medication resource use was based on the trials from which the data were taken for the NMA in each arm of the analysis. Concomitant medication use (including metformin, sulfonylurea, and thiazolidinedione) was based on the semaglutide 1 mg arm of the SUSTAIN 3 trial, and was assumed to be equal in both treatment arms. It was assumed that each patient received the defined daily dose (DDD) of each concomitant medication, with sulfonylurea treatment assumed to be glimepiride and thiazolidinedione treatment assumed to be pioglitazone. Patients receiving sulfonylurea were assumed to use three SMBG tests per week, but no SMBG use was directly associated with once-weekly semaglutide or liraglutide. Liraglutide required one needle per day for administration, but no needles were required in the once-weekly semaglutide arm, as these are included in the pack. Following intensification after 5 years, patients were assumed to receive the DDD (40 IU) of insulin glargine U100 (Lantus), with concomitant medication use equal in both treatment arms. Patients were assumed to use one needle and one SMBG test per day. Resource use was used to calculate annual treatment costs (Table 3).
Table 3.
Annual pharmacy costs in the base-case analysis
| Item | Once-weekly semaglutide 1 mg | Liraglutide 1.2 mg | Basal insulin (intensification) |
|---|---|---|---|
| Annual medication costs | 1367.86 | 1156.50 | 493.14 |
| Annual metformin costs | 50.40 | 50.40 | 50.40 |
| Annual glimepiride costs | 18.55 | 18.55 | 18.55 |
| Annual pioglitazone costs | 13.29 | 13.29 | 13.29 |
| Annual needle costs | 0.00 | 45.86 | 45.86 |
| Annual SMBG testing costs | 25.04 | 25.04 | 137.24 |
| Total annual costs | 1475.15 | 1309.65 | 758.48 |
All costs are expressed in euros (EUR)
SMBG self-monitoring of blood glucose
The costs of diabetes-related complications in the year of the event and the annual follow-up costs were taken from a 2017 health technology assessment of GLP-1 receptor agonists, with the exception of the cost of severe hypoglycemia, which was taken from the insulin degludec assessment by the Estonian National Health Insurance Fund [4, 42].
Quality-of-life utilities associated with diabetes and diabetes-related complications were sourced from a 2014 review by Beaudet et al., while disutilities relating to hypoglycemia were taken from a 2013 publication by Evans et al. (published after the literature searches by Beaudet et al. had been conducted) [43, 44].
Sensitivity Analyses
As the long-term extrapolation of clinical and cost outcomes from short-term data is associated with uncertainty, sensitivity analyses were performed on key parameters to assess the robustness of the base-case findings. Analyses were performed with only statistically significant differences in treatment effects applied. The influence of the time horizon on projected outcomes was investigated via simulations with substantially shorter time horizons of 10 and 20 years applied, for which it should be noted that not all complications and costs were captured, as a 50-year time horizon was required for all modeled patients to have died. The effect of discounting on cost-effectiveness outcomes was assessed by applying discount rates of 0% and 10% in separate analyses. Simulations were prepared with only the HbA1c treatment difference between the treatment arms applied, to evaluate the impact of only this treatment effect on clinical and cost outcomes (i.e., changes in systolic blood pressure and BMI were the same in both arms).
Alternative parameter progressions were explored, with BMI differences between the treatments maintained for patient lifetimes, and HbA1c in both treatment arms following the UKPDS progression equation from the start of the analysis. To assess variations in the treatment effects, the upper and lower 95% confidence interval limits of the estimated treatment differences of HbA1c and BMI were applied in four separate analyses. Alternative treatment switching patterns were explored by bringing treatment switching forward to the end of year 3 in both arms, and having it occur when HbA1c reached 7.5% following the application of the UKPDS progression equation from the first year of the analysis. The effect of overestimating or underestimating the costs of diabetes-related complications was assessed by increasing and decreasing these costs by 10%.
In 2014, an update to the IQVIA CORE Diabetes Model was released, incorporating data from the UKPDS 82 for several risk equations, and an analysis using this version of the model was performed. Although this version of the model has been validated, the model proprietors suggest that the update is used in a sensitivity analysis, with the previous version used for base-case analyses [35]. Further analyses tested the effect of using a larger BMI disutility, giving a greater impact to weight changes in the analysis, and alternative hypoglycemia disutilities, giving greater impact to nonsevere hypoglycemic events but smaller impact to severe events [45, 46]. Additionally, an analysis was performed with a diminishing hypoglycemia disutility model applied [47].
PSA was performed using the predefined function in the IQVIA CORE Diabetes Model to capture statistical uncertainty, with sampling applied to parameter inputs such as baseline characteristics, treatment effects, event risks, costs and utilities. These parameters were sampled from distributions, with the simulation run using a second-order Monte Carlo approach; 1000 unique cohorts, each containing 1000 patients, were run through the model to produce 1000 data points. The proportion of these points that fell under the willingness-to-pay threshold of EUR 52,390 per QALY gained was calculated, in addition to the mean outcomes.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Base-Case Analysis
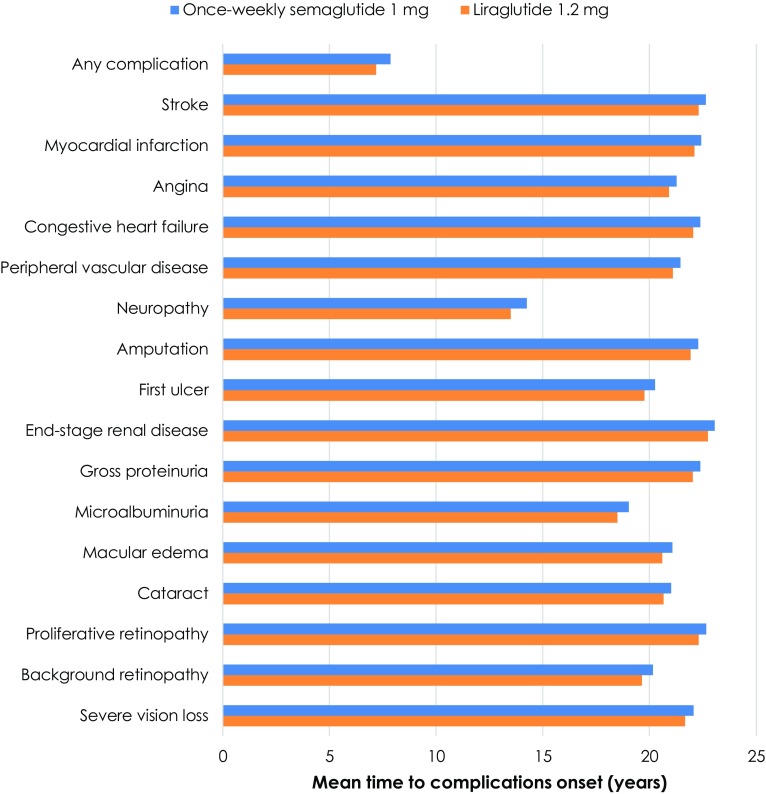
Long-term projections in patients with inadequate glycemic control on OADs indicated that once-weekly semaglutide 1 mg was associated with improvements in discounted life expectancy and discounted quality-adjusted life expectancy of 0.12 years and 0.13 QALYs, respectively, versus liraglutide 1.2 mg (Table 4). Improved clinical outcomes were a result of reduced cumulative incidence and delayed time to onset of diabetes-related complications with once-weekly semaglutide. Mean time to onset of any diabetes-related complication in the analysis was approximately 0.7 years longer with once-weekly semaglutide 1 mg compared with liraglutide 1.2 mg, with benefits observed across all micro- and macrovascular complications included in the analysis (Fig. 1).
Table 4.
Long-term cost-effectiveness outcomes in the base-case analysis
| Health outcomes | Once-weekly semaglutide 1 mg | Liraglutide 1.2 mg | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 12.41 (0.13) | 12.29 (0.13) | + 0.12 |
| Discounted quality-adjusted life expectancy (QALYs) | 7.77 (0.08) | 7.64 (0.08) | + 0.13 |
| Discounted direct costs (EUR) | 25,183 (795) | 25,116 (881) | + 67 |
| ICER based on life expectancy and direct costs | EUR 561 per life year gained | ||
| ICER based on quality-adjusted life expectancy and direct costs | EUR 523 per QALY gained | ||
Values are means (standard deviations)
EUR euros, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life years
Fig. 1.
Mean time to onset of diabetes-related complications
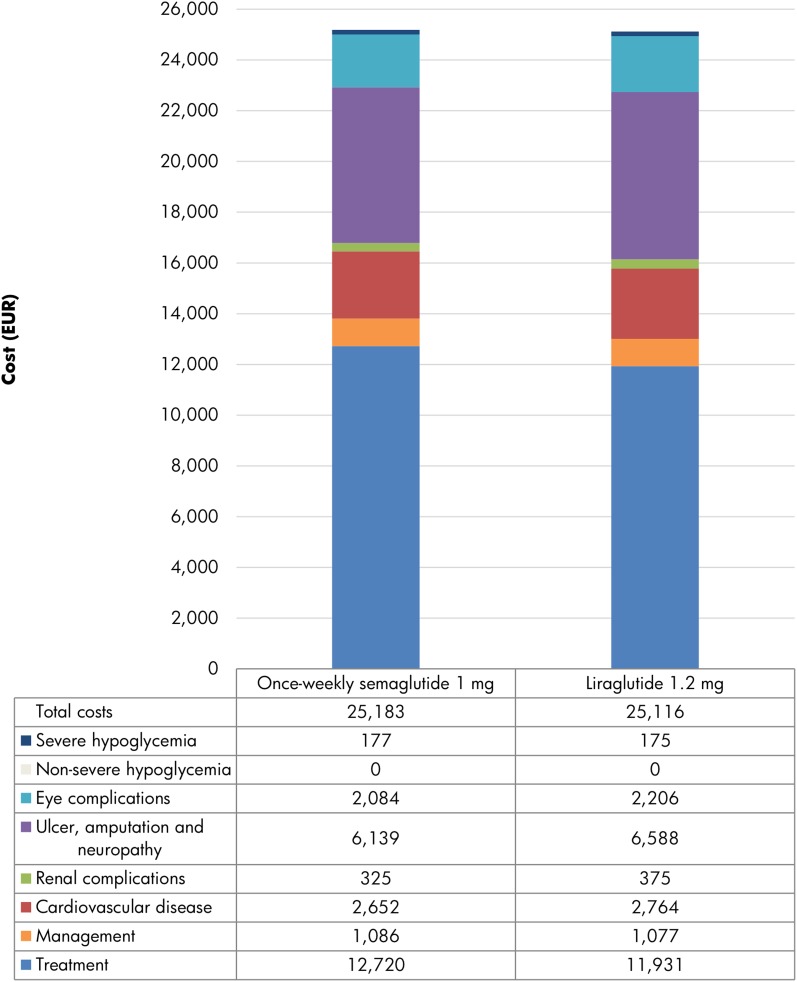
Total direct costs were projected to be EUR 67 higher with once-weekly semaglutide 1 mg versus liraglutide 1.2 mg over patient lifetimes, driven by the higher acquisition costs over the first 5 years of the analysis and the increased survival and further treatment of patients in the long term (Fig. 2). Higher acquisition costs were mostly offset by cost savings due to the avoidance of diabetes-related complications with once-weekly semaglutide, most notably those relating to ulcers, amputation, and neuropathy (mean cost savings of EUR 449 per patient).
Fig. 2.
Direct costs over patient lifetimes. EUR euros
With improved clinical outcomes at an increased cost from a healthcare payer perspective, once-weekly semaglutide 1 mg was associated with an ICER of EUR 523 per QALY gained versus liraglutide 1.2 mg. This falls well below the suggested willingness-to-pay threshold of EUR 52,390 per QALY gained in Estonia (based on three times the Estonian GDP per capita [EUR 17,463], as recommended by the World Health Organization), and once-weekly semaglutide 1 mg was therefore considered highly cost-effective versus liraglutide 1.2 mg [48, 49].
One-Way and Multi-Way Sensitivity Analyses
Sensitivity analyses showed that the base-case findings were robust to changes in the input parameters and assumptions used, with once-weekly semaglutide 1 mg remaining well below the suggested willingness-to-pay threshold of EUR 52,390 per QALY gained across all scenarios (Table 5). Including only the statistically significant differences between the treatment arms, specifically HbA1c and BMI, resulted in slightly decreased clinical benefits but also reduced incremental costs with once-weekly semaglutide 1 mg, leading to an ICER of EUR 195 per QALY gained versus liraglutide 1.2 mg. Shortening the time horizon to 10 and 20 years (compared with the 50 years used in the base-case analysis) resulted in reduced clinical benefits and increased incremental costs, yielding ICERs of EUR 7354 and EUR 1561 per QALY gained, respectively, for once-weekly semaglutide 1 mg versus liraglutide 1.2 mg. These outcomes exemplify the fact that once-weekly semaglutide improves long-term outcomes, and that these benefits are not fully captured over shorter time horizons. Altering the discount rate also reflected these long-term benefits, with once-weekly semaglutide associated with greatly increased clinical benefits and cost savings when discount rates of 0% were applied, meaning it was considered dominant versus liraglutide 1.2 mg. Conversely, clinical benefits decreased and incremental costs increased when discount rates of 10% were applied, leading to an ICER of EUR 3380 per QALY gained for once-weekly semaglutide.
Table 5.
Sensitivity analysis results
| Analysis | Discounted quality-adjusted life expectancy (QALYs) | Discounted direct costs (EUR) | ICER (EUR per QALY gained) | ||||
|---|---|---|---|---|---|---|---|
| Once-weekly semaglutide 1 mg | Liraglutide 1.2 mg | Difference | Once-weekly semaglutide 1 mg | Liraglutide 1.2 mg | Difference | ||
| Base-case | 7.77 | 7.64 | + 0.13 | 25,183 | 25,116 | + 67 | 523 |
| Statistically significant differences only | 7.76 | 7.64 | + 0.12 | 25,139 | 25,116 | + 23 | 195 |
| 20-year time horizon | 6.79 | 6.69 | + 0.09 | 19,329 | 19,186 | + 143 | 1561 |
| 10-year time horizon | 4.70 | 4.64 | + 0.06 | 12,356 | 11,934 | + 423 | 7354 |
| 0% discount rates | 14.08 | 13.80 | + 0.28 | 54,245 | 54,661 | − 416 | Once-weekly semaglutide dominant |
| 10% discount rates | 5.11 | 5.04 | + 0.07 | 15,060 | 14,812 | + 248 | 3380 |
| HbA1c difference only | 7.73 | 7.64 | + 0.09 | 25,148 | 25,116 | + 32 | 356 |
| BMI difference maintained for patient lifetimes | 7.81 | 7.64 | + 0.17 | 25,205 | 25,116 | + 89 | 535 |
| UKPDS HbA1c creep for duration of the analysis (no change upon treatment intensification) | 7.31 | 7.21 | + 0.11 | 29,765 | 29,548 | + 217 | 2077 |
| Upper 95% CI of HbA1c estimated treatment difference | 7.80 | 7.64 | + 0.16 | 24,936 | 25,116 | − 180 | Once-weekly semaglutide dominant |
| Lower 95% CI of HbA1c estimated treatment difference | 7.74 | 7.64 | + 0.09 | 25,553 | 25,116 | + 437 | 4769 |
| Upper 95% CI of BMI estimated treatment difference | 7.78 | 7.64 | + 0.13 | 25,176 | 25,116 | + 60 | 453 |
| Lower 95% CI of BMI estimated treatment difference | 7.76 | 7.64 | + 0.12 | 25,233 | 25,116 | + 117 | 1019 |
| Treatment switching at 3 years | 7.72 | 7.62 | + 0.10 | 24,260 | 24,119 | + 141 | 1398 |
| Treatment switching at 7.5% HbA1c threshold (using UKPDS progression) | 7.26 | 7.14 | + 0.12 | 28,725 | 28,295 | + 430 | 3542 |
| Cost of complications + 10% | 7.77 | 7.64 | + 0.13 | 26,322 | 26,328 | − 6 | Once-weekly semaglutide dominant |
| Cost of complications −10% | 7.77 | 7.64 | + 0.13 | 24,140 | 24,004 | + 136 | 1064 |
| UKPDS 82 risk equations applied | 7.93 | 7.87 | + 0.06 | 21,204 | 20,813 | + 391 | 6568 |
| Lee et al.’s BMI disutility applied | 7.00 | 6.86 | + 0.13 | 25,183 | 25,116 | + 67 | 504 |
| Diminishing hypoglycemia disutility applied | 7.73 | 7.60 | + 0.13 | 25,183 | 25,116 | + 67 | 525 |
| Currie et al.’s hypoglycemia disutility applied | 7.83 | 7.70 | + 0.13 | 25,183 | 25,116 | + 67 | 520 |
BMI body mass index, CI confidence interval, EUR euros, HbA1c glycated hemoglobin, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year
Applying only the difference in HbA1c versus liraglutide 1.2 mg in the once-weekly semaglutide 1 mg arm showed that greater reductions with once-weekly semaglutide were a substantial contributor to improved clinical outcomes versus liraglutide 1.2 mg, with only slightly reduced clinical benefits and incremental costs, resulting in an ICER of EUR 356 per QALY gained. Maintaining the BMI difference between the treatment arms after intensification increased the clinical benefit and incremental costs associated with once-weekly semaglutide, yielding an ICER of EUR 535 per QALY gained. Application of the UKPDS HbA1c progression equation resulted in reduced quality-adjusted life expectancy in both treatment arms, with increased incremental costs with once-weekly semaglutide compared with liraglutide 1.2 mg, leading to an ICER of EUR 2077 per QALY gained.
Use of the upper limit of the 95% confidence interval of the estimated treatment differences in HbA1c resulted in increased clinical benefits and cost savings with once-weekly semaglutide, meaning it was considered dominant versus liraglutide 1.2 mg. Application of the lower limit of the 95% confidence interval had the converse effect, with clinical benefits reduced and incremental costs increased. Use of the upper limit of the 95% confidence interval of the estimated treatment differences in BMI resulted in maintained clinical benefits from the base-case analysis and comparable incremental costs, while application of the lower limit of the 95% confidence interval led to slightly reduced clinical benefits and increased incremental costs.
Treatment switching at 3 years, rather than the 5 years as in the base-case, resulted in smaller clinical benefits and increased incremental costs with once-weekly semaglutide, yielding an ICER of EUR 1398 per QALY gained versus liraglutide 1.2 mg. Application of the UKPDS HbA1c progression with treatment switching when HbA1c exceeded 7.5% led to slightly reduced clinical benefits and increased incremental costs for once-weekly semaglutide 1 mg versus liraglutide 1.2 mg.
Increasing the costs of treating diabetes-related complications resulted in small cost savings with once-weekly semaglutide, meaning it was considered dominant versus liraglutide 1.2 mg. Reducing the costs of complications had the converse effect, with incremental costs increased.
Using the UKPDS 82 risk equations to predict cardiovascular events resulted in smaller clinical benefits with once-weekly semaglutide 1 mg compared with the base-case analysis, with incremental costs increased. Application of alternative utilities relating to hypoglycemia and BMI resulted in only minor changes to clinical outcomes, and ICERs remained similar to the base-case analysis.
Probabilistic Sensitivity Analysis
PSA, performed to capture statistical uncertainty, showed similar mean results to the base-case but increased measures of variance around the mean outcomes. Once-weekly semaglutide 1 mg was associated with a mean incremental improvement in quality-adjusted life expectancy of 0.08 QALYs and higher mean costs of EUR 168 per patient versus liraglutide 1.2 mg. Therefore, once-weekly semaglutide 1 mg was associated with an ICER of EUR 2103 per QALY gained versus liraglutide 1.2 mg in the PSA. Based on the suggested willingness-to-pay threshold of EUR 52,390 per QALY gained, the modeling analysis indicated that the probability of once-weekly semaglutide 1 mg being cost-effective versus liraglutide 1.2 mg was 73.4% (Fig. 3).
Fig. 3.
Cost-effectiveness acceptability curve from the probabilistic sensitivity analysis. EUR euros, QALY quality-adjusted life year
Discussion
The present analysis found once-weekly semaglutide 1 mg to be a highly cost-effective treatment option versus liraglutide 1.2 mg for the treatment of patients with type 2 diabetes with a BMI ≥ 35 kg/m2 in Estonia. Life expectancy and quality-adjusted life expectancy were both improved with once-weekly semaglutide at a small cost increase over patient lifetimes from a healthcare payer perspective. Greater reductions in short-term clinical outcomes of HbA1c, body weight, and SBP resulted in a reduced cumulative incidence and delayed time to onset of long-term diabetes-related complications, leading to cost savings that mostly offset the higher acquisition costs associated with once-weekly semaglutide.
The positive impact of improvements in HbA1c and body weight on the risk of cardiovascular disease has been well documented [21, 22, 50]. Moreover, once-weekly semaglutide has been associated with additional cardiovascular benefits in the SUSTAIN 6 clinical trial, reducing the risk of a major cardiovascular event (a composite endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) compared with placebo plus standard of care [51]. Liraglutide has also been associated with a reduced risk of cardiovascular disease versus placebo in the LEADER trial [52]. The present analysis did not capture the impacts on cardiovascular disease events identified in SUSTAIN 6 and LEADER, as risk equations based on these studies have not been incorporated into health economic models of diabetes.
Treatment guidelines for type 2 diabetes in Estonia recommend the introduction of a GLP-1 receptor agonist as either a second-line therapy in patients receiving oral monotherapy with an HbA1c ≥ 8.5%, or a fourth-line therapy in patients receiving oral triple therapy of metformin plus either sulfonylurea or thiazolidinedione and a dipeptidyl peptidase-4 (DPP-4) inhibitor or a sodium-glucose co-transporter 2 (SGLT-2) inhibitor with an HbA1c > 7.0% [53]. The present analysis assessed the cost-effectiveness of once-weekly semaglutide in patients with inadequate glycemic control on one or two OADs, and found that once-weekly semaglutide 1 mg was a highly cost-effective option versus liraglutide 1.2 mg for those patients. Once-weekly semaglutide has also been shown to improve short-term outcomes versus both the DPP-4 inhibitor sitagliptin and the SGLT-2 inhibitor empagliflozin in SUSTAIN 2 and an NMA, respectively, and it could therefore be argued that once-weekly semaglutide is eligible to be used earlier in the treatment algorithm [54, 55]. Indeed, recent 2018 guidelines from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend GLP-1 receptor agonist therapy as the first-line injectable medication for treating type 2 diabetes [56]. Moreover, since once-weekly semaglutide requires fewer injections than once-daily liraglutide, and since patient preference is for simpler treatment regimens with fewer injections, treatment with once-weekly semaglutide could potentially improve patient adherence and alter preferences towards injectable GLP-1 receptor agonist therapy, given the benefits in HbA1c and body weight these treatments offer [15, 16, 23–26, 54, 55].
A limitation of the study was the reliance on relatively short-term clinical trial data to make long-term projections. However, this is common to a number of health economic analyses and, in the absence of long-term clinical trial data, extrapolation of short-term data remains one of the best available options to model chronic diseases. Indeed, projecting outcomes over patient lifetimes is recommended in the guidance for cost-effectiveness studies for patients with type 2 diabetes [36]. Additionally, the present analysis was conducted using a published and extensively validated model, with numerous sensitivity analyses displaying the robustness of the base-case results [34, 35].
The use of data from an NMA, rather than a head-to-head clinical trial, could also be considered a potential shortcoming of the analysis. However, selection of the most appropriate comparator (in this case the most widely used GLP-1 receptor agonist in Estonia) was the first priority, and the use of evidence synthesis, using recommended methodologies, is becoming increasingly important and accepted for health technology assessment globally [57, 58].
A further limitation is that the NMA relied on published data that only reported outcomes for all patients, as the study publications identified by the reviewers did not report data for patients with BMI ≥ 35 kg/m2 [31]. The present analysis applied the treatment effects for all patients in patients with BMI ≥ 35 kg/m2, and this assumption of equivalent efficacy across these two populations represents a potential weakness. However, subgroup analyses have shown that once-weekly semaglutide is consistently efficacious across patient subgroups, with reductions in HbA1c and body weight observed in patients with higher BMIs similar to those seen in the full populations throughout the SUSTAIN clinical trials [59–61]. Therefore, while quantifying the impact of applying treatment effects from all patients in patients with BMI ≥ 35 kg/m2 is difficult, it is unlikely to change the conclusion that once-weekly semaglutide 1 mg is cost-effective versus liraglutide 1.2 mg.
Conclusions
Once-weekly semaglutide 1 mg represents a highly cost-effective treatment option versus liraglutide 1.2 mg for the treatment of type 2 diabetes patients with inadequate glycemic control on one or two OADs in Estonia.
Acknowledgements
Funding
The present cost-effectiveness analysis and article processing charges were supported by funding from Novo Nordisk A/S. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Monika Russel-Szymczyk is an employee of Novo Nordisk Pharma Sp. z o.o. Girtel Liidemann is an employee of Novo Nordisk A/S Eesti Filiaal. Vallo Volke has served at speakers’ bureaus and received travel grants from AstraZeneca, Eli Lilly, Aventis, and Novo Nordisk. Samuel Malkin is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support the preparation of the analysis. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support the preparation of the analysis.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7370441.
References
- 1.Rajasalu T, Vilimaa T. Suhkurtõve levimus eesti täiskasvanud rahvastikus [The prevalence of diabetes in the Estonian adult population] Eesti Arst. 2008;87:337–341. [Google Scholar]
- 2.Eglit T, Rajasalu T, Lember M. Prevalence of diabetes and impaired glucose regulation in Estonia. Diabet Med. 2011;28(4):504–505. doi: 10.1111/j.1464-5491.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health Development. Tervisestatistika ja terviseuuringute andmebaas [Health Statistics and Health Research Database]. Esmashaigusjuhud soo ja vanuserühma järgi [Primary cases by gender and age group]. 2017. http://pxweb.tai.ee/PXWeb2015/pxweb/et/02Haigestumus. Accessed 14 Sep 2018.
- 4.Juus E, Volke V, Roosimaa M, Lutsar K, Lukka M, Kiivet R. Tervisetehnoloogia hindamise raport TTH33: GLP-1 retseptori agonistide kliiniline tõenduspõhisus ja kulutõhusus 2. tüüpi diabeedi ravis [Health Technology Assessment Report TTH33: The clinical evidence-based and cost-effectiveness of GLP-1 receptor agonists in the treatment of type 2 diabetes]. 2017. https://tervis.ut.ee/en/health-technology-assessment/hta-reports. Accessed 2 Apr 2018.
- 5.International Diabetes Federation. Diabetes atlas, 8th edn. 2017. http://www.diabetesatlas.org/across-the-globe.html. Accessed 20 Aug 2018.
- 6.Ismail-Beigi F, Craven T, Banerji MA, ACCORD trial group et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, MacMahon S, Chalmers J, ADVANCE Collaborative Group et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMicm066227. [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed]
- 9.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. [DOI] [PubMed]
- 10.Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. [DOI] [PubMed]
- 11.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 12.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney PM, Blackwell L, Collins R, Cholesterol Treatment Trialists’ Collaborators et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res. 2007;16(7):1251–1265. doi: 10.1007/s11136-007-9226-0. [DOI] [PubMed] [Google Scholar]
- 16.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 17.Rätsep A, Kalda R, Lember M. Meeting targets in type 2 diabetes care contributing to good glycaemic control. A cross-sectional study from a primary care setting in Estonia. Eur J Gen Pract. 2010;16(2):85–91. doi: 10.3109/13814788.2010.481017. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html. Accessed 22 Aug 2018.
- 19.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 21.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149–58. [DOI] [PMC free article] [PubMed]
- 24.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–17. [DOI] [PubMed]
- 25.Russell-Jones D, Vaag A, Schmitz O, Liraglutide Effect and Action in Diabetes 5 [LEAD-5] met + SU Study Group et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152–160. doi: 10.2337/diaspect.29.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Medicines Agency. EPAR summary for the public: Ozempic/semaglutide. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004174/WC500244166.pdf. Accessed 1 Jun 2018.
- 28.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66. [DOI] [PubMed]
- 29.Pratley RE, Aroda VR, Lingvay I, SUSTAIN 7 investigators et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 30.US National Library of Medicine. Research study comparing a new medicine semaglutide to liraglutide in people with type 2 diabetes (SUSTAIN 10). ClinicalTrials.gov Identifier: NCT03191396. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03191396. Accessed 8 Oct 2018.
- 31.Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving 1–2 oral anti-diabetic drugs. Diabetes Ther. 2018;9(3):1149–1167. doi: 10.1007/s13300-018-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai T, Habicht T, Kahur K, Reinap M, Kiivet R, van Ginneken E. Estonia: health system review. Health Syst Transit. 2013;15(6):1–196. [PubMed]
- 33.Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 34.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–S40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 35.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 37.Statistics Estonia. RV046: Probability of dying and number of survivors by sex and age 2017. http://andmebaas.stat.ee/Index.aspx?lang=en. Last Accessed 7 May 2018.
- 38.Clarke PM, Gray AM, Briggs A, UK Prospective Diabetes Study (UKDPS) Group, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59. [DOI] [PubMed]
- 39.Behmane D, Lambot K, Irs A, Steikunas N. Balti riikide juhis ravimite farmakoökonoomiliseks hindamiseks [Guide for the pharmacoecological assessment of medicinal products in the Baltic States]. https://www.sm.ee/sites/default/files/content-editors/eesmargid_ja_tegevused/Tervis/Ravimid/balti_juhis_ravimite_farmakooekonoomiliseks_hindamiseks.pdf. Accessed 2 Apr 2018.
- 40.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 42.Estonian National Health Insurance Fund. Assessment of Tresiba (October 2017; number 3-14/12235-5). Tallinn: Estonian National Health Insurance Fund; 2017.
- 43.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–470. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–6. [DOI] [PubMed]
- 46.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 47.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 48.Statistics Estonia. GDP at current prices per capita, year. http://www.stat.ee/68594/?highlight=capita. Accessed 10 May 2018.
- 49.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Org. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laiteerapong N, Ham SA, Gao Y et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care. 2018;dc171144. [DOI] [PMC free article] [PubMed]
- 51.Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 52.Marso SP, Daniels GH, Brown-Frandsen K, LEADER Trial Investigators et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ambos A, Raie E, Kiudma T, et al. 2. tüüpi diabeedi Eesti ravijuhend 2016 [Estonian Therapeutic Guidelines for Type 2 Diabetes 2016] Eesti Arst. 2016;97:465–473. [Google Scholar]
- 54.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 55.Sharma R, Wilkinson L, Vrazic H, et al. Comparative efficacy of once-weekly semaglutide and SGLT-2 inhibitors in type 2 diabetic patients inadequately controlled with metformin monotherapy: a systematic literature review and network meta-analysis. Curr Med Res Opin. 2018;34(9):1595–1603. doi: 10.1080/03007995.2018.1476332. [DOI] [PubMed] [Google Scholar]
- 56.Davies MJ, D’Alessio DA, Fradkin J et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;dci180033. [DOI] [PMC free article] [PubMed]
- 57.European Network for Health Technology. Guideline—comparators & comparisons: direct and indirect comparisons. February 2013. http://www.eunethta.eu/sites/5026.fedimbo.belgium.be/files/Direct%20and%20indirect%20comparisons.pdf. Accessed 13 Dec 2017.
- 58.Dias SW, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, 2011; last updated April 2012. http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/TSD1-Introduction.final_.08.05.12.pdf. Accessed 13 Dec 2017.
- 59.Bain S, Araki E, Desouza C, Garg S, Rose L, Tsoukas G, Bergan EQ, Derving Karsbøl J, Devries JH. Semaglutide reduces HbA1c across baseline HbA1c subgroups across SUSTAIN 1-5 clinical trials. Diabetes. 2017;66(Suppl 1):A298–A299. [Google Scholar]
- 60.Leiter L, Charpentier G, Chaykin L, et al. Semaglutide reduces body weight across baseline BMI subgroups across SUSTAIN 1–5. Can J Diabetes. 2017;41(5):S6. doi: 10.1016/j.jcjd.2017.08.020. [DOI] [Google Scholar]
- 61.Viljoen A, Bluher M, Chow FCC, et al. Semaglutide reduces body weight vs. dulaglutide across baseline BMI subgroups in SUSTAIN 7. Diabetes. 2018;67(Suppl:1).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.