Abstract
Exposure to heavy metals may lead to hearing impairment. However, experimental studies have not explored this issue with and without noise exposure in mature animals with environmentally relevant doses. The aim of this study was to investigate ototoxicity produced by lead (Pb) and cadmium (Cd) and noise, singly and in combination, in the adult CBA/CaJ mouse. Metals were delivered via drinking water (0.03 mM, 1 mM, and 3 mM Pb; or 30, 100, and 300 μM Cd) for 12 weeks, resulting in environmentally- and occupationally-relevant mean (± standard deviations) blood levels of Pb (2.89 ± 0.44, 38.5 ± 4.9, and 60.1 ± 6.6 μg/dl, respectively) and Cd (1.3 ± 0.23, 6.37 ± 0.87, 27.2 ± 4.1 μg/L, respectively). Metal treatment was also combined with a noise exposure consisting of a 105 dB broadband (2–20 kHz) stimulus for 2 hr or a sham exposure. Auditory performance was determined by comparing auditory brainstem responses (ABR) and otoacoustic emissions (DPOAE) at baseline and after 11 weeks of metal treatment. Metal-exposed animals did not develop significant auditory deficits and did not exhibit morphological damage to cochlear hair cells. In contrast, noise-exposed animals, including those exposed to combinations of metals and noise, demonstrated significant hair cell loss, reduced DPOAE amplitudes, and ABR threshold shifts of 42.2 ± 13 dB at 32 kHz (105 dB noise alone). No significant potentiation or synergistic effects were found in groups exposed to multiple agents. This study establishes a highly reproducible adult mouse model that may be used to evaluate a variety of environmental exposure mixtures.
Keywords: ototoxicity, lead, cadmium, hearing loss, exposure mixtures
Introduction
Worldwide, over 360 million people are estimated to suffer from hearing loss (HL) (WHO 2013), and non-age related HL ranks as the fifth cause of years lived with disability globally (Stevens et al. 2013). Hearing loss in almost any form exerts detrimental impact at all stages of life. Even mild losses in children are associated with poorer speech perception and significantly lowered performance on basic skills tests (Tharpe et al. 2009, Rodrigues et al. 2015). In adults, HL may impair a wide variety of adverse social, psychological, educational, clinical and occupational outcomes (Seidman and Standring 2010).
Exposures to high levels of noise produce HL (EPA 1974), and other environmental agents may also cause or contribute to HL (Fechter 2004, Guthrie et al. 2015). Auditory toxicity (ototoxicity) resulting from exposures to certain clinically relevant drugs (Schacht et al. 2012) is well established. Research from the past and present identified another potential source of HL: exposure to non-essential heavy metals, including lead (Pb) and cadmium (Cd) (Schwartz and Otto 1987, Vyskocil et al. 2012), which are common environmental and occupational contaminants in industrialized communities (Guan et al. 2010, Yorita Christensen 2012). Pb is present in paint in US homes built before 1977 and exposure may occur through the water supply in older homes, soil and household dust (Pichery et al. 2011), children’s toys (Greenway and Gerstenberger 2010), and even through some food products (Berger et al. 2013). Cd exposure may occur through contact with contaminated soils and dusts, tobacco smoke (Cosselman et al. 2015), and through dietary intake (Tellez-Plaza et al. 2012).
Contradictory information exists on the consequences of prolonged low-level heavy metal exposures in adults. Several reports suggested that Pb may be ototoxic in human adults (Hwang et al. 2009, Park et al. 2010) but not all studies agree (Counter and Buchanan 2002). The potential ototoxicity of Cd in human adults has been addressed in one study (Choi et al. 2012). Epidemiological evidence also suggests that HL associated with Pb and Cd exposures in humans might be synergistically or additively affected by concurrent noise exposure (Farahat et al. 1997; Wu et al. 2000).
Lead has also been implicated as a potential ototoxicant in developing animals, including mice (Jones et al. 2008; Fortune and Lurie 2009; Prins et al. 2010) and monkeys (Rice 1997) and acute Pb exposures may generate auditory dysfunction in guinea pigs (Tuncel et al. 2002). Similarly, Cd in drinking water at 5, 15, or 150 ppm produced ototoxic effects in 30-day exposures in two rat and one mouse study (Ozcaglar et al. 2001; Agirdir et al. 2002; Kim et al. 2008). However, as with Pb, not all studies of Cd demonstrated ototoxicity (Whitworth et al. 1999). These discrepancies largely arise from the lack of an established animal model to guide a consistent approach to potential ototoxic exposures by heavy metals.
While detrimental neurological effects of metals have been established during development in both children (Sharma and Mogra 2014) and animals (Jones et al. 2008), though this is controversial as some studies show no consistent effects (Buchanan et al. 2011; Taylor et al. 2018). Adult animal models using higher occupational exposures to Pb have been exploited to a lesser extent. Therefore, adult animal models are necessary to address ambiguities in the epidemiological findings and explore potential causal connections between exposure to Pb and/or Cd and HL. In order to close these knowledge gaps, the objective of this study was to evaluate and quantify, in a well-controlled animal model, the relationship between Pb and Cd exposure and ototoxicity as measured by auditory threshold shifts and cochlear hair cell loss. Based upon environmental and occupational exposure parameters, the effects of the heavy metals were evaluated singly, in tandem, and also in combination with noise.
Methods
Animals:
The University of Michigan’s University Committee on the Use and Care of Animals approved all animal protocols for this work. Routine care for animals was provided by the University of Michigan’s Unit for Laboratory Animal Medicine (ULAM). Mice were housed in a containment facility with unlimited access to both food and treatment water. Treated water bottles were changed twice a week. The facility maintained a 12-hr light-dark cycle. In-cage 72-hr noise exposure measurements were taken monthly using a personal noise dosimeter (Spark 706RC, Larson Davis, Depew, NY). Average ambient sound pressure levels (SPL) were consistently at or below 60 dBA, a level sufficient to eliminate the risk of noise-induced hearing loss in mice (Reynolds et al. 2010, Ohlemiller et al. 2011).
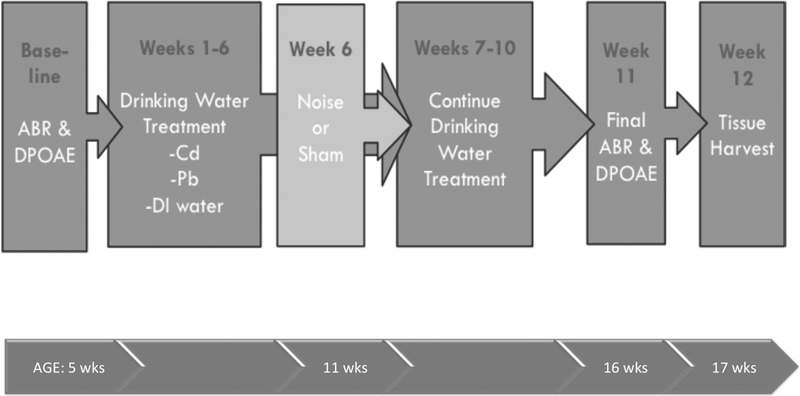
CBA/CaJ mice arrived from the Jackson Laboratory (Bar Harbor, ME) at 4 weeks of age weighing 23 g and were allowed one week for acclimation. All mice were housed 5 to a cage if possible. Baseline auditory brainstem response (ABR) measurements at age 5 weeks determined hearing thresholds before chemical treatments began at 6 weeks of age (Figure 1). A treatment schedule beginning no earlier than age 5 weeks was selected to coincide with mouse reproductive maturity and comparable to the age of young workers in the US. Treatments continued for 11 weeks. Sacrifice by anesthesia and decapitation followed by blood, bone, liver, kidney, and cochlear tissue harvest occurred at 17 weeks of age. Noise exposures were administered during the sixth week of treatment (11 weeks of age). Final hearing thresholds were measured during week 11 of treatment (16 weeks of age).
Figure 1.
Timeline of exposures and treatments along with corresponding age of mice.
All animals were visually inspected daily and weighed weekly to verify consistent growth. No cases of overt toxicity from Pb or Cd were observed. Animals were sacrificed after final hearing assessments for pathological analysis, cochlear assessment, and tissue Pb and Cd measurements. One control mouse died during our experiment due to acute obstructive uropathy unrelated to the experimental protocols. All other mice completed the full experimental treatment.
Treatments:
Mouse treatment groups and total group size throughout this study are shown in Table 1. This study was run in three stages. The first stage used various levels of Pb, Cd, and noise treatments to determine the level of treatment that caused damage. During the second stage, doses of 3 mM Pb, 300 uM Cd, and 105 dB noise were selected to investigate effects of these treatments under concurrent dosing regimens. The third stage exclusively investigated the DPOAE outcome. Controls were included in each period thus larger numbers of control animals are reflected in Table 1. Each treatment group included at least 6 mice to allow for health outcomes to show through individual variability. Only male mice were used due to documented estrus-related hearing fluctuations in female mice (Willott et al. 2008).
Table 1.
Description of treatment groups.
| Group | N |
|---|---|
| Total | 150 |
| Control (no Pb, no Cd, sham noise) | 16 |
| Pb (no Cd, sham noise) | |
| 3 mM | 16 |
| lmM | 8 |
| 0.03 mM | 8 |
| Cd (no Pb, sham noise) | |
| 300 μM | 16 |
| 100 μM | 7 |
| 30 μM | 6 |
| Noise (no Pb, no Cd) | |
| 108 dB | 7 |
| 105 dB | 15 |
| 102 dB | 7 |
| 300 μM Cd + 3 mMPb (sham noise) | 9 |
| 3 mM Pb + 105 dB Noise (no Cd) | 14 |
| 300 μM Cd + 105 dB Noise (no Pb) | 14 |
| 300 μM Cd + 3 mM Pb + 105 dB Noise | 7 |
Pb exposure
An aqueous 2% w/v lead acetate solution (Fisher Scientific, Waltham, MA; #429132) was diluted into highly purified and filtered water (Merck Millipore, Billerica, MA Milli-Q System). Final concentrations of Pb in drinking water were 0.03 mM, 1 mM, and 3 mM; concentrations were selected to obtain serum levels representing those seen in US workers from average environmental, high environmental and legal limits of occupational exposures, respectively.
Cd exposure
Cd in the aqueous form of CdCl2 (VWR, Radnor, PA; # 101443–260) was diluted into Milli-Q water to final concentrations of 30 μM, 100 μM, or 300 μM, again selected to achieve serum levels to represent US exposures ranging from environmental to occupational.
Combined exposure to Pb and Cd consisted of treated water containing the highest doses of Pb (3 mM) and Cd (300 μM). Random samples from each concentration were taken from all water bottles at monthly intervals to verify consistency in concentrations over the study.
Noise exposure
A single two-hr noise exposure was administered in week 5 of the chemical treatment period. Using a method previously described (Vicente-Torres and Schacht 2006), mice were housed in individual wire cages in a ventilated chamber with a loudspeaker mounted at the top of the chamber. Two or three awake animals were simultaneously exposed to 2 hours of broadband white noise (2 to 20 kHz) at intensities of 102, 105, or 108 dB SPL. Noise levels were confirmed within the wire cage with sound level meters before and during exposure (B&K sound level meter model 2231, with type 4155 1/2” microphone).
Pathophysiology:
Auditory Brainstem Response (ABR)
For ABR testing, animals were anesthetized with intraperitoneal injections of ketamine (65 mg/kg), xylazine (7 mg/kg), and acepromazine (2 mg/kg) to insure relaxation and immobilization as described in previous literature (Sha et al. 2008). Additional injections of ketamine and xylazine were administered as necessary to maintain anesthesia for the duration of the examination; an injection of glycopyrrolate (0.2 mg/kg) was administered to aid recovery. The ear canals and tympanic membranes of all animals were evaluated for signs of obstruction or infection prior to hearing assessments. No obstructions or infections were observed in any of the mice.
Needle electrodes were inserted subdermally at the vertex of each mouse’s skull equidistant to each external auditory meatus, a reference electrode was inserted below the pinna of the left ear, and a ground electrode was inserted contralaterally (Wu et al. 2001; Sha et al. 2008). Sound stimuli were carried in a closed acoustic system to the left external auditory meatus and then transmitted through an ear bar connected to a Beyer DT-48 transducer (Beyer Dynamic, Farmingdale, NY, USA). The test output was transmitted to an amplifier, viewed via oscilloscope, and recorded using SigGen software (Tucker-Davis Technologies, Gainsville, FL USA). Thresholds were determined at low, mid-range, and high frequencies (8, 16, and 32 kHz, respectively) by progressive reductions in sound intensity by 10 dB SPL steps initially, and 5 dB SPL steps near threshold. Thresholds were defined as the lowest stimulus at which a positive waveform was seen (Hurd et al. 2011) and threshold shifts were calculated for individual animals as the difference between measurement at baseline and the conclusion of the experiment (chemical treatment week 11). Pilot work included preliminary analyses for groups suspected to show the largest changes of final ABR peak I through V amplitude and latency data as well as inter-peak latencies (I-II, II-III, III-IV, IV-V, and I-V) were calculated using MATLAB (Mathworks, Natick, MA) at 80 dB for frequencies of 8, 16, and 32 kHz for control mice, and those in the highest Pb and Cd treatment groups. Due to inconsistencies in interpreting ABR waveforms of noise-exposed animals, we excluded any animal with noise treatment from these analyses.
Distortion Product Otoacoustic Emissions (DPOAEs)
DPAOEs were collected at the same time points as ABR (i.e., baseline and eleventh week of treatment). DPOAE tests were run as described previously (Karolyi et al. 2007, Hurd et al. 2011) at 32 kHz following administration of anesthetics as described above for ABR. The ratio of the intensity of the primary tones f1 and f2, remained constant at f2/f1 = 1.2. F1 intensity was adjusted in 5–10 dB SPL increments, while f2 intensity was held 10 dB SPL below f1. Tucker-Davis Technologies System II (Gainsville, FL) hardware and SigGen/BioSig software captured responses and presented stimuli tones. Amplitude shifts were calculated by subtracting the final amplitude from the initial.
General Pathology:
Immediately following euthanasia via deep anesthesia with ketamine (100 mg/kg) all animals were exsanguinated via cardiac puncture to collect up to 1 ml blood. Upon exsanguination, 0.5 ml blood was placed in a trace metals analysis tube (Becton Dickinson, Franklin Lakes, NJ #368381). Any remaining blood was placed in to a serum separator tube, spun for two min and frozen in preparation for blood chemistry analysis. Blood serum was analyzed for markers of kidney and liver function: creatinine, aspartate amino transferase (AST), albumin, alanine amino transferase (ALT), total bilirubin, blood urea nitrogen (BUN), and alkaline phosphatase (ALP). Mice were necropsied and gross visual inspection was made for systemic damage. Liver and kidneys were removed during necropsy on all mice and placed into formalin fixative for at least 24 hr before they were trimmed and stained with eosin and hemotoxylin. Right femur and right tibia bones were collected, scraped of extraneous tissues with a ceramic blade, weighed and stored under refrigeration. Cochleae were collected and placed on ice in phosphate buffered saline (PBS) for dissection.
Tissue analysis
A University of Michigan Unit for Laboratory Animal Medicine (ULAM) veterinary pathologist blinded to treatment status evaluated liver and kidney slides for organ-specific lesions. ULAM also completed blood chemistry panel analyses. Blood samples were analyzed for Cd and Pb levels using inductively coupled plasma mass spectrometry (ICP-MS) at the Michigan Department of Health (Lansing, MI). Cochlear bone Pb and Cd levels were determined from the entire cochlear-vestibular apparatus along with a portion of the temporal bone. Right and left cochlea were weighed together and digested in OPTIMA grade nitric acid. Tibia and Femur bones were similarly digested Pb and Cd content was determined using ICP-MS by the Michigan Department of Health. Detection limits were 0.05 mg/kg Pb and 0.05 mg/kg Cd for the bone and cochlear metal analyses.
Hair cell counts
Surface preparations of cochleae were examined to determine numbers of all cochlear inner and outer hair cells. Both left and right auditory systems were examined using a light microscope for abnormalities and signs of infection. The round window, oval window, and apex were opened to perfuse the entire cochlea with 4% paraformaldehyde in 10 mM phosphate-buffered saline (PBS) at pH 7.4. Cochleae were then decalcified with 4% EDTA in 10 mM PBS, stained with rhodamine phalloidin to label actin and hair cells were counted along the entire length of the cochlea (Matt et al. 2012; Wu et al. 2001). Average outer hair cell losses were tallied in the apex, middle section, and base.
Statistics:
Data were compiled, and descriptive statistics computed, in Excel (Microsoft, Redmond WA). Further analyses were run using R 3.3.1 (Vienna, Austria) and figures were generated in GraphPad Prism 6 (San Diego, CA). Counts of mice with ABR threshold shifts >20 dB, a level that has been used as a threshold for human hearing impairment (Vos et al. 2015), were also computed. Means across exposure groups were compared using Student’s t-test and one way ANOVA using a Bonferroni correction for multiple comparisons; distributional comparisons were conducted via χ2. Results were considered significant at α = 0.05. Blood serum above or below established normal values (Charles River Laboratories International 2011) were counted were pooled from similar treatment groups using Pearson’s Chi-squared test with Yates’ continuity.
Results
Animals:
Pilot work in CBA/J male mice revealed a number of ear infections not identifiable during visual inspection before performing ABR and observed after cochlear dissections. The CBA/CaJ strain of mouse was therefore selected for this investigation due to their low incidence of ear infections compared to other mouse strains (McGinn et al. 1992) and stable hearing thresholds into advanced age (12–18 months) (Sha et al. 2008, Ohlemiller et al. 2011). Our study noted only one mouse with an ear infection on the right (non-ABR) side out of a total 150 mice undergoing ABR and cochlear dissection. Body weight measures were maintained throughout the study and no overt changes of health were noted. As reported in pathology results, hepatotoxicity and systemic toxicity were not found at this dose.
Treatments:
All control water samples tested below the limit of detection (0.2 mg/L) for both Pb and Cd. All Pb treatment water tested below limits of detection (LOD) for Cd and all Cd water tested below LOD for Pb. Water samples showed that mean treatment concentrations of Pb and Cd were within 25% error below the intended concentrations, and no individual samples tested above the intended concentrations of Pb or Cd. Mean water sample concentrations (all results reported to two significant figures) of Pb were 0.023, 0.91, and 2.7 mM (4.8, 190, and 550 mg/L) for the 0.03, 1, and 3 mM treatment groups, respectively. Mean Cd water sample concentrations were 27, 93, and 240 μM (3.0, 11, and 27 mg/L) for the 30, 100, and 300 μM treatment groups, respectively. Mean water sample concentrations for the combined Pb and Cd treatment groups were 2.4 mM (510 mg/L) and 280 μM (31 mg/L) for Pb and Cd, respectively.
Noise
Pilot study results indicated that noise exposures of 102 dB SPL produced no hearing damage, while 105 dB or 108 dB SPL exposures averaged similar threshold shifts (approximately 35–40 dB). Therefore, the 105 dB SPL exposure, which produced a robust threshold shift without a full elimination of auditory responses, was selected for evaluation of exposures in combination with metals in order to optimize our ability to assess possible potentiation effects of Pb and Cd on hearing thresholds.
Pathophysiology
ABR Baseline Levels
Baseline thresholds decreased with increasing test frequency, as is typical for CBA/CaJ mice. Variability in baseline thresholds was low for the 147 mice that underwent ABR. The highest threshold mean (± SD) of 26 ± 3 dB was observed at 8 kHz, compared to 17 ± 3 dB at 16 kHz and 17 ± 3 dB at 32 kHz.
ABR Threshold Shifts
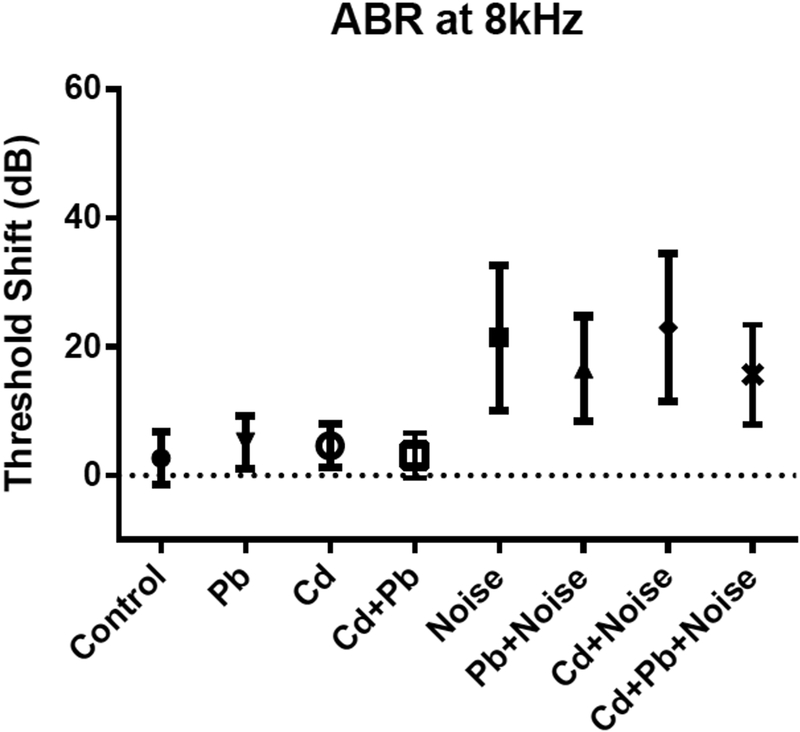
No significant changes from baseline thresholds were observed in the control animals at 8 and 32 kHz (Table 2 also shown in Figures 2–4). Threshold shift variability in the metal treatment groups was similar at all frequencies to that of the control group. No Pb or Cd treatment groups (including single and combination metal exposures) displayed threshold shifts at any frequency that differed significantly from control, though changes from baseline thresholds among the Pb and Cd single treatment groups were higher on average at 8 and 32 kHz than those of controls. Only 1 of 47 mice (2.1%) in a combination of the control, all Cd single treatment, and all Pb single treatment groups experienced a 20-dB or greater threshold shift at 32 kHz, and only 2 of 42 (4.7%) experienced such a shift at 8 kHz.
Table 2.
Threshold shift averages (dB) and counts of shifts equal to or over 20 dB by treatment groups.
| 32 kHz | 8 kHz | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | N | Avg Shift | SD | N Shifts ≥20dB | N | Avg Shift | SD | N Shifts ≥20dB |
| Control | 15 | 3.9 | 4.7 | 0 | 15 | 2.6 | 4.1 | 0 |
| Pb | ||||||||
| 3 mM | 16 | 3.9 | 4.3 | 0 | 11 | 5.2 | 4.1 | 0 |
| l mM | 8 | 6.6 | 5 | 0 | 8 | 12.75 | 5.6 | 2 |
| 0.03 mM | 8 | 6.3 | 8.9 | 1 | 8 | 8.0 | 4.9 | 0 |
| Cd | ||||||||
| 300 μM | 16 | 4.5 | 5.3 | 0 | 11 | 4.6 | 3.5 | 0 |
| 100 μM | 6 | 5.5 | 3.3 | 0 | 5 | 9.4 | 5.6 | 0 |
| 30 μM | 6 | 7.8 | 4.4 | 0 | 6 | 10.7 | 6.7 | 0 |
| Noise | ||||||||
| 108 dB | 6 | 41.0 | 18.8 | 5 | 6 | 25.2 | 16.5 | 3 |
| 105 dB | 15 | 42.2 | 13.5 | 14 | 10 | 21.4 | 11.3 | 5 |
| 102 dB | 7 | 15.3 | 6.5 | 1 | 7 | 13 | 3.3 | 0 |
| 300 μM Cd + 3 mM Pb | 9 | 0.8 | 3.5 | 0 | 9 | 3.1 | 3.4 | 0 |
| 3 mM Pb + 105 dB Noise | 14 | 38.9 | 12.3 | 13 | 9 | 16.6 | 8.2 | 2 |
| 300 μM Cd + 105 dB Noise | 14 | 39.3 | 8 | 14 | 9 | 23 | 11.5 | 6 |
| 300 μM Cd + 3 mM Pb + 105 dB Noise | 7 | 29.6 | 18 | 5 | 7 | 15.7 | 7.8 | 2 |
Figure 2.
Mean and standard deviations for ABR threshold shifts at 8 kHz for single treatment and mixture groups with 3 mM Pb, 300 uM Cd, and 105 dB.
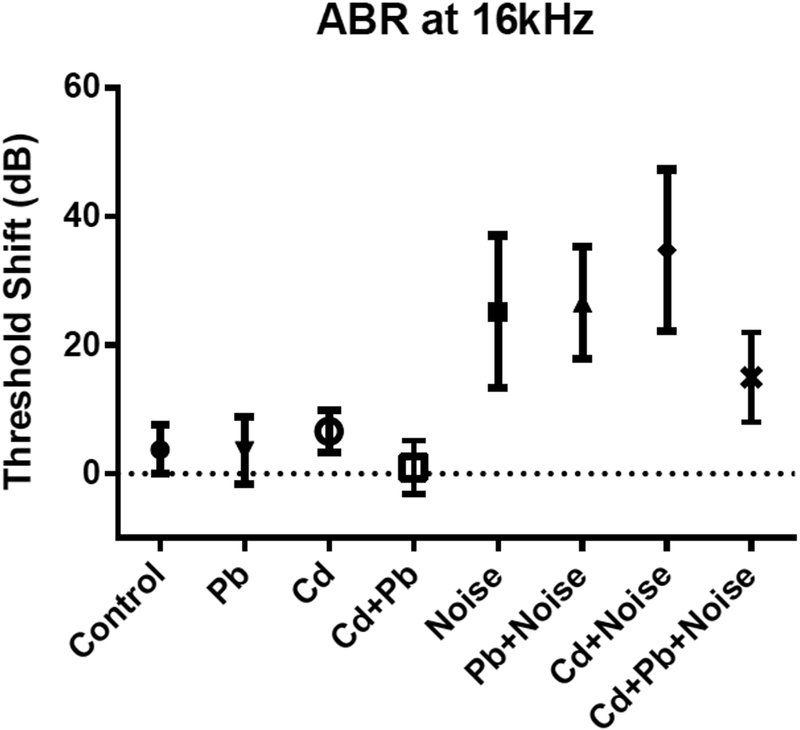
Figure 3.
Mean and standard deviations for ABR Threshold shifts at 16 kHz for single treatment and mixture groups with 3 mM Pb, 300 μM Cd, and 105 dB.
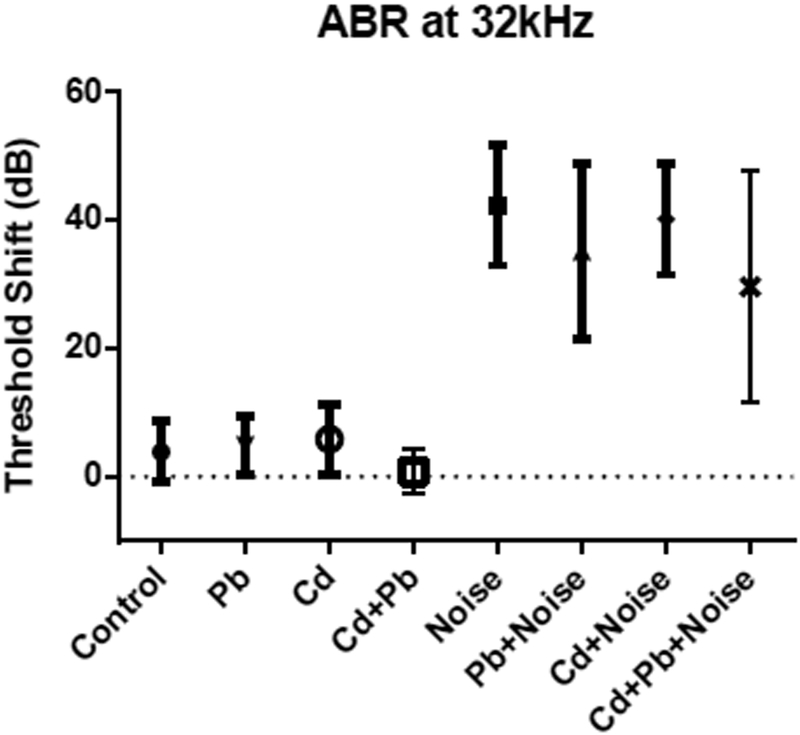
Figure 4.
Mean and standard deviations for ABR Threshold shifts at 32 kHz for single treatment and mixture groups with 3 mM Pb, 300 μM Cd, and 105 dB.
Noise-exposed mice showed threshold shifts much larger and more consistent than those from Cd, Pb, or Cd+Pb treatments, and which differed significantly from control (Table 2; results for controls, 105 dB noise exposures, and highest Pb and Cd treatments singly or in combination are shown in Figures 2–4). Mice exposed to 102 dB only exhibited alterations from baseline thresholds 10–11 dB greater than controls at 8 and 16 kHz; however, these differences were not significant. Threshold shifts in the noise-only treatment groups of 105 dB and 108 dB were not significantly different at any frequency. Average threshold shifts were significantly different from controls at 8, 16, and 32 kHz in mice treated with 105 dB noise alone or 105 dB noise in combination with Pb, Cd, or Pb and Cd (data not shown). Threshold shifts in the 105 dB noise plus Pb and/or Cd exposure groups did not differ significantly from the 105 dB noise-only treatment group.
The Cd+Pb+Noise group unexpectedly showed significantly lower changes from baseline thresholds than the Cd+Noise treatment at 16 kHz,. Changes remained lower, though not significant at 8 kHZ and 32 kHz; differences ranged from 7.3 dB at 8 kHz to 19.8 dB at 16 kHz. The fractions of animals experiencing substantial threshold shifts (greater or equal to 20 dB) were similar among mice treated with mixtures of noise, Pb, and Cd, compared to mice exposed singly to 105 dB noise.
ABR Peak and Latency:
ABR waves I, II, III, IV, and V amplitudes and latencies were analyzed as well as inter-peak I-II, IIII, I-IV, and I-V. Preliminary findings did not show significance of any wave peaks or latencies in the highest treatment groups compared with controls, so we did not pursue further deeper analysis. No significant differences from the control group were found among mice treated with 3 mM Pb or 300 μM Cd.
DPOAE Thresholds:
DPOAE amplitude shifts at 32 kHz among the Pb, Cd, 105 dB, Pb+Noise, and Cd+Noise groups were similar to those seen in ABR at 32 kHz (Table 3). DPOAE shifts among Pb and Cd treatment groups were not significantly different from zero, and mean differences of amplitude shifts among noise-exposed mice were not significantly different. Mice exposed to noise displayed significantly higher threshold shifts than mice treated with Cd and Pb. One mouse in the noise-only exposure group did not experience a DPOAE threshold shift; this created a larger SD in this group compared to other treatment groups. DPOAE amplitude shift averages for Noise+Pb [28 ± 9.5 dB SPL] were similar to Noise+Cd [26 ± 11.7 dB SPL] and not significantly different.
Table 3.
DPOAE Average Amplitude Shifts (dB) and Matched ABR Threshold Shifts (dB) at 32 kHz.
| Group | Threshold Shifts | Amplitude Shifts | |||
|---|---|---|---|---|---|
| N | ABR | SD | DPOAE | SD | |
| Pb | 5 | 2 | 3.1 | −1.1 | 2.6 |
| Cd | 4 | 0.5 | 2.5 | 0.6 | 0.9 |
| Noise | 5 | 42.2 | 21.0 | −21.9 | 15.7 |
| Pb+Noise | 5 | 45.8 | 4.3 | −27.7 | 9.5 |
| Cd+Noise | 5 | 37.8 | 7.3 | −26.1 | 11.7 |
Tissue Assessment:
Blood levels of metals
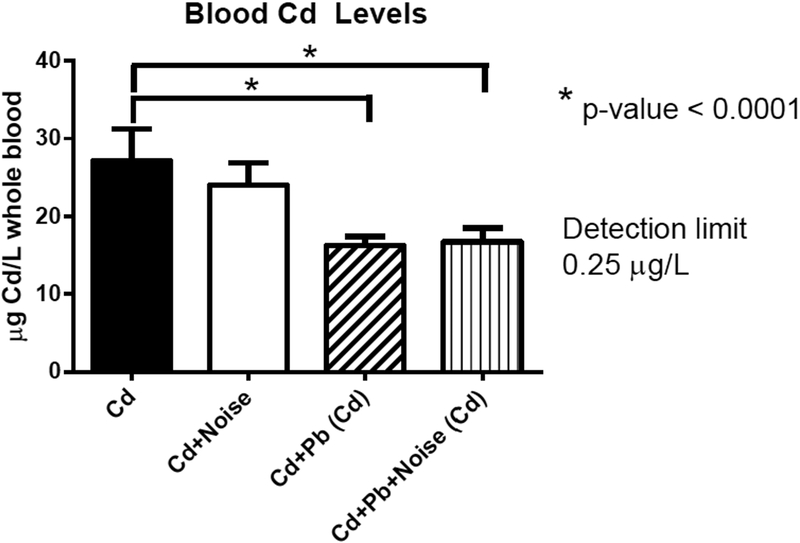
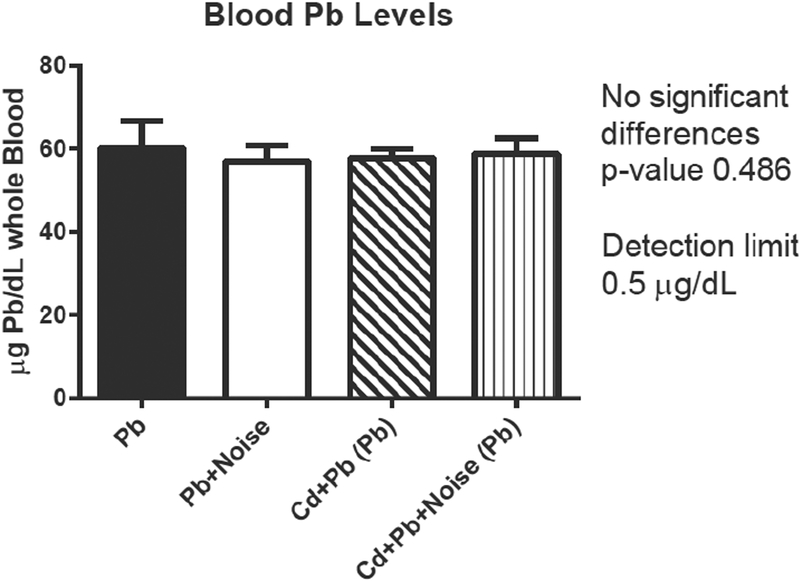
Pb blood levels at sacrifice were not altered by addition of noise or Cd treatment. No mice from control or Cd treatment groups had detectable levels of Pb while all mice treated with Pb had detectable levels of Pb in their blood. Blood Pb levels were significantly different among the three Pb-only treatment groups (Table 4). The mean blood Pb levels across the combination exposure groups (using 3 mM Pb) were not significantly different from each other or the single treatment of 3 mM Pb. All mice treated with Cd had detectable levels of blood Cd. Three mice not in a Cd treatment group (one control, two exposed to Pb only) had a detectable level of blood Cd. Blood Cd levels differed significantly among the exposure groups (Table 4 and Figure 5).
Table 4.
Concentrations of metals in blood by treatment group.
| Treatment group | N | Whole Blood Metals | |||
|---|---|---|---|---|---|
| Pb (μg/dL)^ | Cd (μg/L)^^ | ||||
| Avg | SD | Avg | SD | ||
| Control | 16 | ND | - | ND* | - |
| Pb | |||||
| 3mM | 11 | 60.1 | 6.6 | ND | - |
| lmM | 8 | 38.5 | 4.9 | ND** | - |
| 0.03 mM | 8 | 2.89 | 0.44 | ND*** | - |
| Cd | |||||
| 300 μM | 12 | ND | - | 27.2 | 4.1 |
| 100 μM | 7 | ND | - | 6.37 | 0.87 |
| 30 μM | 6 | ND | - | 1.3 | 0.23 |
| Noise | |||||
| 108 dB | 7 | ND | - | ND | - |
| 105 dB | 10 | ND | - | ND | - |
| 102 dB | 6 | ND | - | ND | - |
| 300 μM Cd + 3 mM Pb | 9 | 57.8 | 2.2 | 16.2 | 1.2 |
| 3 mM Pb + 105 dB Noise | 9 | 57.0 | 3.9 | ND | - |
| 300 μM Cd + 105 dB Noise | 9 | ND | - | 24 | 2.9 |
| 300 μM Cd + 3 mM Pb + 105 dB Noise | 7 | 58.9 | 3.9 | 16.7 | 1.8 |
Detection limit is 0.5
Detection limit is 0.25
One sample above DL: 0.26
One samples above DL: 0.32
One sample above DL: 0.27
Figure 5.
Blood Cd levels (μg/L) by treatment groups for single treatment and mixture groups with 3 mM Pb, 300 μM Cd, and 105 dB.
In combination treatment, Pb levels were unchanged in mice concurrently exposed to Cd (Figure 6). In contrast, mean levels of blood Cd in mice were altered by addition of Pb treatment, though not among noise-exposed animals (Figure 5). Blood Cd levels for the 300 μM treatment were significantly decreased when combined with Pb exposures. Both the Cd+Pb groups and the Cd+Pb+Noise group exhibited significantly lower blood Cd levels than Cd treatments alone.
Figure 6.
Blood Pb levels (μg/dl) by treatment groups for single treatment and mixture groups with 3 mM Pb, 300 μM Cd, and 105 dB.
Bones and cochleae:
All mice had detectable levels of Pb in their tibia and femur, though levels were 1,000-fold higher among the Pb treatment groups than non-Pb-groups. Only mice treated with Cd displayed detectable levels of Cd (Table 5). Among Pb treatment groups, Pb bone levels in the femur were higher than those in the tibia. Mean femur Pb was significantly higher in the Pb-only group than the Cd+Pb group; however this was not the case for tibia Pb. Bone Cd levels were not significantly different between Cd treatment groups.
Table 5.
Concentrations of metals (mg/kg) in bone.
| Femur | Tibia | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Pb | Cd | ||||||
| Treatment Group | N | Avg | SD | Avg | SD | Avg | SD | Avg | SD |
| Control | 5 | 0.16* | 0.14 | ND** | - | 0.16 | 0.06 | ND | - |
| 3 mM Pb | 12 | 287 | 45 | ND^ | - | 216 | 51 | ND | - |
| 300 μM Cd | 11 | 0.13^^ | 0.01 | 0.27 | 0.03 | 0.18 | 0.04 | 0.23 | 0.03 |
| 300 μM Cd + 3 mM Pb | 9 | 236 | 24 | 0.26 | 0.02 | 207 | 23 | 0.21 | 0.02 |
All five samples run in control groups showed levels of Pb were detectable and above the limit of detection of 0.05 mg/kg
All five samples in control groups showed levels of Cd were below the limit of detection of 0.05 mg/kg.
Two femur and tibia samples run in Pb treatment groups showed levels of Cd were below the limit of detection of 0.05 mg/kg. Results were similar to control levels.
Two femur and tibia samples run in Cd treatment groups showed levels of Pb were detectable and above the limit of detection of 0.05 mg/kg. Results were similar to control levels.
As with bones, detectable levels of Pb were found in all cochlear tissue and bone samples, while detectable levels of Cd were observed only in the cochlear tissue and bone of Cd treatment groups (Table 6). Levels of cochlear Pb were not significantly different among non-Pb-treatment groups, and levels of cochlear Cd were not significantly different among non-Cd-treatment groups.
Table 6.
Concentrations of metals (mg/kg) in cochlea and adjoining tissue
| Cochlea | |||||
|---|---|---|---|---|---|
| Pb | Cd | ||||
| Treatment Group | N | Avg | SD | Avg | SD |
| 105 dB Noise | 5 | 0.250 | 0.153 | ND* | - |
| 3 mM Pb | 5 | 185.0 | 34.9 | ND* | - |
| 300 μM Cd | 4 | 0.228 | 0.04 | 0.287 | 0.066 |
All Pb treatments and Noise treatment groups had levels of Cd that were not detectable at the limit of detection of 0.05 ppm or mg/kg.
Cochlear Assessments:
Significant cochlear hair cell losses were only seen in noise-exposed groups (Table 7). Numerous outer hair cells were missing in the basal area of the cochlea among all noise exposure groups, while no outer hair cells were missing in the apex and mid ranges of all treatment groups. There was a dose-dependent rise in mean loss of basal hair cells with increasing noise exposure; mean levels were 3 ± 2, 6 ± 4, and 14 ± 17 hair cells missing for the 102, 105, and 108 dB exposure groups, respectively. Among combination noise and metal treatment groups, mean basal hair cell loss was highest in the Cd+Noise group [12 ± 10] and lowest in the Pb+Noise group [3 ± 2]; the Cd+Pb+Noise group fell between these [7 ± 5]. Inner hair cells were noted to be intact under all treatment conditions.
Table 7.
Cochlea Cytogram Missing Outer Hair Cell Counts
| Apex | Middle | Base | |||||
|---|---|---|---|---|---|---|---|
| Treatment Group | N | Avg | SD | Avg | SD | Avg | SD |
| Control | 15 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.4 |
| 3 mM Pb* | 11 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 |
| 300 μM Cd* | 12 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 |
| 102 dB Noise | 7 | 0.2 | 0.2 | 0.3 | 0.3 | 3.1 | 2.3 |
| 105 dB Noise* | 10 | 0.2 | 0.2 | 0.4 | 0.2 | 5.8 | 4.4 |
| 108 dB Noise | 8 | 0.3 | 0.1 | 1.6 | 2.6 | 13.8 | 16.7 |
| Cd+Pb | 9 | 0.1 | 0.3 | 0.2 | 0.2 | 0.7 | 1.2 |
| Pb+Noise | 9 | 0.3 | 0.3 | 0.3 | 0.3 | 2.9 | 2.3 |
| Cd+Noise | 9 | 0.2 | 0.1 | 0.5 | 0.4 | 11.6 | 9.6 |
| Cd+Pb+Noise | 6 | 0.1 | 0.1 | 0.3 | 0.3 | 7.3 | 5.0 |
Group treatment levels used for mixtures (3 mM Pb, 300 μM Cd, & 105 dB Noise)
General Pathology
Histology:
Only mice treated with Pb showed signs of kidney distress, but even the levels noted histologically were not indicative of major systemic health problems. Karyomegaly in the S3 tubular epithelium, which is known to be a characteristic lesion of Pb exposure in mice, was present in 91% of mice treated with 3 mM Pb alone, 86% of mice in the Cd+Pb+Noise group, and in 100% of mice in the Pb+Noise and Cd+Pb groups. Intranuclear inclusions were also present in the highest Pb treatment groups with similar proportions to karyomegaly. One mouse in the 108 dB Noise treatment group displayed both these lesions; that was the only mouse without Pb treatment found to exhibit these lesions. Karyomegaly was absent in the 0.03 mM Pb group and rare (13% of mice) in the 1 mM Pb group. No other lesions were characteristic of any treatment group. Mild (affecting under 10% of tubules in the tissue) tubular hyperplasia occurred in over 50% of animals in all but one treatment group and lesions observed displayed no regeneration or fibrosis. Mild hepatic lesions also presented in a wide range of treatment groups at low levels. Two control animals presented with both centrilobular or random degeneration and necrosis multifocal neutrophilic with mononuclear inflammation. All other groups had 0 to 2 mice with similar lesions; no dose-response patterns were noted. Histology data is shown in Table 8.
Table 8.
Histology data in counts of mice with observed lesions
| Renal Lesions | Hepatic Lesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | N | THP | KM | TD | MII | FM | INI | ANY RENAL LESION | DGN | MFI | ANY HEPATIC LESION |
| Control | 16 | 9 | 0 | 4 | 0 | 0 | 0 | 9 | 2 | 2 | 2 |
| 102 dB Noise | 7 | 5 | 0 | 2 | 1 | 0 | 0 | 6 | 1 | 2 | 2 |
| 105 dB Noise* | 10 | 9 | 0 | 5 | 1 | 0 | 0 | 9 | 1 | 1 | 1 |
| 108 dB Noise | 7 | 3 | 1 | 4 | 1 | 0 | 1 | 4 | 0 | 0 | 0 |
| 30 μM Cd | 6 | 4 | 0 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| 100 μM Cd | 7 | 6 | 0 | 4 | 1 | 0 | 0 | 6 | 1 | 1 | 2 |
| 300 μM Cd* | 12 | 9 | 0 | 6 | 1 | 0 | 0 | 9 | 1 | 1 | 1 |
| Cd+Noise | 9 | 5 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| 0.03 mM Pb | 8 | 8 | 0 | 3 | 0 | 0 | 0 | 8 | 1 | 1 | 1 |
| l mM Pb | 8 | 7 | 1 | 4 | 0 | 0 | 1 | 7 | 2 | 2 | 2 |
| 3 mM Pb* | 11 | 9 | 10 | 6 | 0 | 0 | 10 | 11 | 0 | 0 | 0 |
| Pb+Noise | 9 | 8 | 9 | 1 | 0 | 4 | 8 | 9 | 0 | 1 | 1 |
| Cd+Pb | 9 | 7 | 9 | 1 | 0 | 0 | 9 | 9 | 0 | 1 | 1 |
| Cd+Pb+Noise | 7 | 6 | 6 | 0 | 0 | 2 | 6 | 7 | 0 | 0 | 0 |
Group treatment levels used for mixtures (3 mM Pb, 300 μM Cd, & 105 dB Noise)
DGN - degeneration and necrosis, centrilobular and random
FM - focal mineralization
INI - intranuclear inclusions
KM - karyomegaly, tubular epithelium, S3
MFI - multifocal neutrophilic and mononuclear inflammation
MII - mononuclear interstitial inflammation
TD - tubular degeneration
THP - simple tubular hyperplasia
Blood serum results
Serum ALP levels at sacrifice tested at biologically relevant low levels in Cd treated mice at higher treatments (4 of 9, or 44 %, in the 300 μM Cd group, 3 of 9, or 33 %, in the Cd+Noise group, and 1 of 12, or 8.3 %, among controls) though these levels were not significantly different from controls.
Most mice showed total bilirubin counts below normal values of 0.12 to 0.58 mg/dl. At sacrifice, blood serum levels of creatinine, AST, ALT, and BUN were not always within the normal reference ranges but were not significantly different between treatment groups and did not show dose-response changes with increasing treatment levels.
Discussion
Previous studies exploring the ototoxic interaction of chemicals with noise have used rats (Mäkitie et al. 2003). An alternative animal model such as the mouse is increasingly important in modern research making up about 90% of species used today in animal studies and was thus selected. Molecular tools as well as transgenic strains render this model more versatile than any other species for research. In addition, exploration of interactions between genetic and environmental factors in mice offers a much more flexible and yet highly controlled experimental approach than does epidemiological modelling (Mauderly 1993), and the relevance of this research can be increased even further through the use of transgenic mice mimicking human disorders (Vandamme 2014).
In the CBA/CaJ adult mouse model presented here blood levels of Pb and Cd relevant to occupational situations in the US were achieved and simulated environmental exposures that occur in certain areas internationally. Although levels of Pb in the general environment decline in the US, higher levels may still be experienced in certain occupations and remain important to public health. Further, recent research suggests that exposure to noise (both occupational and recreational) may have more severe consequences than previously assumed (Rodrigues et al. 2015). Even low-level exposure that only initiates temporary threshold shifts in young but mature hearing systems may produce earlier and greater age-related hearing losses (Kujawa and Liberman 2006). Globally, adults in low- and middle-income nations have high levels of noise exposures as well as high Pb and Cd exposures in both occupational and community settings (Nelson et al. 2005, Laborde et al. 2015).
With regards to occupational exposures to Pb, the current OSHA Permissible Exposure Limit for Pb exposures among workers is 50 μg/dl; in our study, the exposures achieved in the highest Pb-exposed group was equivalent to 60 μg/dl. The levels of Cd in our highest-exposed mice (27 μg/L) were double the OSHA occupational limit (10 μg/L), and Cd exposures in combined treatment of Pb and Cd still resulted in levels above the same Cd OSHA limit. When environmental exposures are considered, levels in treated mice were above the updated CDC Community Action Limit for Pb (5 μg/dl) (CDC 2012). Even exposures below this CDC limit are coming under increasing scrutiny as research continues to demonstrate the importance of cumulative effects (CDC 2009; Spivey 2007).
Our data appears to be the first study to test for Pb and Cd levels in the mouse cochlea. It was found that levels were detectable and similar to bone levels in the tibia and femur, though these were more similar for Cd than Pb. Mean Pb levels in the cochlea were 36% less than in the femur and 17% less than Pb in tibia. Detectable levels of Pb in non-Pb-treated mice were identified. This Pb exposure may originate from their feed, which contained detectable levels of Pb in our tests. Cd concentrations in bone and cochlea were not detectable in mice that were not treated with Cd. Most animals not exposed to Pb or Cd did not show detectable levels in their blood. However, three mice exposed to the control, or Pb alone showed detectable levels of Cd in their blood. All bone samples, regardless of Pb treatment status or not, showed detectable levels of Pb. Contamination in animal food has been previously shown as is a likely contributor to the few mice which showed detectable levels of Cd and an ongoing exposure to low levels of Pb (Mesnage et al. 2015, Adamse et al. 2017). For mice treated with a combination of Pb and Cd lowered concentrations of both Pb and Cd were observed in the femur and tibia; this is likely due to competing metal uptake. Cochleae from these mice were not tested for metals.
Mixture-based research such as this is increasingly recognized as a critical area for epidemiological research. Therefore, our study included a thorough analysis of the ototoxicity of Pb and Cd in combination with noise. In agreement with previous investigations (Vicente-Torres and Schacht 2006, Minami et al. 2007), noise exposures produced significant detriments to thresholds in nearly all mice, validating the model. In contrast, our study did not identify any significant differences in hearing loss risk resulting from metal exposure, thus exposing a potential difference between developing and mature animals. It is also possible that Pb or Cd, are more ototoxic in humans, or that longer-term exposures are required in order to produce hearing deficits in older animals.
It is noteworthy that our model explored cochlear histology but did not investigate peripheral or central neurological damage that may disrupt auditory processing. Fortune and Lurie (2009) noted alterations to the superior olivary complex and normal monoaminergic expression albeit in developing mice. These are exciting areas for future studies to examine as Pb treatment may well distort auditory temporal processes. Further, subthreshold damage at a young age might manifest as increased hearing loss as the animals age (Kujawa and Liberman 2009). A long-term follow-up would be an important contribution to our understanding of metal toxicity.
The results of our study appear to disagree with two previously published studies that showed ototoxicity due to Pb exposure. The first used 6 monkeys, treated from birth to age 13, and pure tone thresholds to establish ototoxicity (Rice 1997). Results of this study demonstrated normal thresholds in three monkeys and lowered thresholds at high frequencies in three monkeys (Rice 1997). The second study investigated hearing loss in Wistar rats exposed to 4 mg/kg Pb acetate by gavage for 30 days (Liu et al. 2011). HL was demonstrated as an increased latency in the ABR peaks from I to V (Liu et al. 2011). This was not found in analysis of ABR peaks within our study. Assuming the daily amount of water consumed by one 30 g mouse is about 4 ml (Bachmanov et al. 2002), the Pb dose mice in our study received was about 82.67 mg/kg, a higher level than given to the rats above. However, differences in absorption due to developmental exposure times, species differences, digestion times, and stress levels due to mode of administration may also alter this comparison.
In contrast to Pb, Cd is not as well-studied. Kim et al. (2008; 2009; 2011) demonstrated ototoxic properties attributed to Cd. In vitro, over half of hair cells were apoptotic, detected through the use of TUNEL staining, following 24 hr direct exposure of organ of Corti explants from rats to Cd (10 μM) (Kim et al. 2008). This study further explored the ABR threshold shifts in mice following 150 mg/L (1334 μM) Cd in drinking water for 30 days. Thresholds of control mice at 32 kHz were near 30 dB and averaged near 55 dB following 30 days of Cd treatment. A likely reason for the discrepancy to our study is the dosing by Kim et al. (2008) which is over 4-fold higher than the levels administered in our study and should have resulted in comparatively higher serum levels. Increases of ALP in mouse blood due to Cd treatment noted in our highest Cd treatment group were also observed previously by Adefegha et al. (2015).
Conclusions
This comprehensive study design with both positive (noise) and negative (water) controls confirms the findings of many previous studies regarding the severity and consistency of noise-related damage to auditory function (Johnson 1993, Barden et al. 2012). For combination treatment, a similar strategy to studies on the interactions of styrene and noise was used (Mäkitie et al. 2003; Venet et al. 2015). To the best of our knowledge, our study is the first of its kind to use mice and had the longest treatment period of experiments of this type identified in a literature search. Therefore, this investigation offers a novel model for examining interactions within the auditory and vestibular (Klimpel et al. 2017) systems.
It is significant that a CBA/CaJ adult mouse model was developed with blood levels of Pb and Cd relevant to human health in real-world mixture exposure settings. Interestingly, these mice did not demonstrate significant ototoxic effects of Pb or Cd singly or in combination. It is also important that throughout the chronic exposure all animals maintained good general health and only showed subtle pathological changes in renal parameters parallel to those seen in rodents (Payne and Saunders 1978) and comparable to observed renal outcomes in humans (Fels et al. 1994). The use of this robust adult model might advance toxicology knowledge and research methods on mixed exposures.
Future studies investigating ototoxicity of metals and possible interactions of chemical agents with noise need to further explore the complex pathways of signals and cell bodies in auditory neurons and ascending pathways for a more complete understanding of health outcomes due to ototoxicant interactions. Metals are known neurotoxicants (Karri et al. 2016; Andrade et al. 2017); it is not unreasonable that changes in auditory perception may be due to auditory synaptopathy, which may be aggravated by noise exposures (Fernandez et al. 2015), or central effects. One example of a chemical producing adverse effects in the brain stem following noise exposure is 1,3-dinitrobenzene (Ray et al. 1992). Finally, it is possible that the CBA/CaJ mouse may not be sufficiently vulnerable to demonstrate effects potentially seen in more sensitive human populations. Species and strain differences are important and well documented variables in research and deserve further exploration.
Acknowledgements
The authors would like to acknowledge Ann Kendall, Sue DeRemer, Karin Halsey, and Diane Prieskorn for valuable guidance and mentorship. Additionally, this project could not have taken place without Chrystina James, Katarina Klimpel, Krittika Mittal, and Kan Sun and their assistance on this project.
Funding
This work was supported by grants from the National Institute for Deafness and Communication Disorders at the National Institutes of Health [R03DC013378, R01-DC010412, R01-DC011294, P30-DC05188], a Rackham Merit Fellowship, and a University of Michigan – Environmental Toxicology and Epidemiology Program grant funded through the National Institute of Environmental Health Sciences [T32 ES007062].
Footnotes
Disclosure
The authors declare no conflicts of interest related to this research.
References
- Adamse P, (Ine) Van der Fels-Klerx HJ, and de Jong J. 2017. Cadmium, lead, mercury and arsenic in animal feed and feed materials - trend analysis of monitoring results. Food Addit. Contam. Part A DOI 10.1080/19440049.2017.1300686. [DOI] [PubMed] [Google Scholar]
- Adefegha SA, Omojokun OS, and Oboh G. 2015. Modulatory effect of protocatechuic acid on cadmium induced nephrotoxicity and hepatoxicity in rats in vivo. Springerplus 4:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirdir BV, Bilgen I, Dinc O, Ozçaglar HÜ, Fienk F, Turhan M, and Oner G. 2002. Effect of zinc ion on cadmium-induced auditory changes. Biol. Trace Elem. Res 88:153–163. [DOI] [PubMed] [Google Scholar]
- Andrade VM, Aschner M, and Marreilha dos Santos AP. 2017. Neurotoxicity of Metal Mixtures Pages 227–265 in Aschner M and Costa LG (eds), Advances in Neurobiology. Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, and Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet 32:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden EK, Rellinger EA, Ortmann AJ, and Ohlemiller KK. 2012. Inheritance Patterns of Noise Vulnerability and “Protectability” in (C57BL/6J × CBA/J) F1 Hybrid Mice. J. Am. Acad. Audiol 23:332–340. [DOI] [PubMed] [Google Scholar]
- Berger Ritchie JA and Gerstenberger SL. 2013. An evaluation of lead concentrations in imported hot sauces. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 48:530–538. [DOI] [PubMed] [Google Scholar]
- Buchanan LH, Counter SA, and Ortega F. 2011. Environmental lead exposure and otoacoustic emissions in Andean children. J. Toxicol. Environ. Heal. - Part A Curr. Issues 74:1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2012. Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.” Page Morbidity Mortality Weekly. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2009. Adult Blood Lead Epidemiology and Surveillance -- United States, 2005–2007. MMWR. Morb. Mortal. Wkly. Rep 58:365–369. [PubMed] [Google Scholar]
- Charles River Laboratories International. 2011. Research Models: C57BL/6 Mice. [Google Scholar]
- Choi Y-H, Hu H, Mukherjee B, Miller J, and Park SK. 2012. Environmental Cadmium and Lead Exposures and Hearing Loss in US Adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ. Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, and Kaufman JD. 2015. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol 12:627–642. [DOI] [PubMed] [Google Scholar]
- Counter SA and Buchanan LH. 2002. Neuro-ototoxicity in andean adults with chronic lead and noise exposure. J. Occup. Environ. Med 44:30–8. [DOI] [PubMed] [Google Scholar]
- EPA. 1974. Information on Levels of Environmental Noise Requisite to Protect Public Health and Welfare with an Adequate Margin of Safety. [Google Scholar]
- Farahat TM, Abdel-Rasoul GM, El-Assy AR, Kandil SH, and Kabil MK. 1997. Hearing thresholds of workers in a printing facility. Environ. Res 73:189–92. [DOI] [PubMed] [Google Scholar]
- Fechter LD 2004. Promotion of noise-induced hearing loss by chemical contaminants. J. Toxicol. Environ. Heal. - Part A 67:727–740. [DOI] [PubMed] [Google Scholar]
- Fels LM, Herbort C, Pergande M, Jung K, Hotter G, Roselló J, Gelpi E, Mutti A, De Broe M, Stolte H, Rosello J, Gelpi E, Mutti A, De Broe M, and Stolte H. 1994. Nephron target sites in chronic exposure to lead. Nephrol Dial Transpl. 9:1740–1746. [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PWC, Lall K, Liberman MC, and Kujawa SG. 2015. Aging after Noise Exposure: Acceleration of Cochlear Synaptopathy in “Recovered” Ears. J. Neurosci 35:7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune T and Lurie DI. 2009. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. J. Comp. Neurol 513:542–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway JA and Gerstenberger S. 2010. An evaluation of lead contamination in plastic toys collected from day care centers in the Las Vegas Valley, Nevada, USA. Bull. Environ. Contam. Toxicol 85:363–6. [DOI] [PubMed] [Google Scholar]
- Guan H, Piao F-Y, Li X-W, Li Q-J, Xu L, and Yokoyama K. 2010. Maternal and fetal exposure to four carcinogenic environmental metals. Biomed. Environ. Sci 23:458–65. [DOI] [PubMed] [Google Scholar]
- Guthrie OW, Wong BA, McInturf SM, Reboulet JE, Ortiz PA, and Mattie DR. 2015. Inhalation of Hydrocarbon Jet Fuel Suppress Central Auditory Nervous System Function. J. Toxicol. Environ. Heal. Part A 78:1154–1169. [DOI] [PubMed] [Google Scholar]
- Hurd EA, Adams ME, Layman WS, Swiderski DL, Beyer LA, Halsey KE, Benson JM, Gong TW, Dolan DF, Raphael Y, and Martin DM. 2011. Mature middle and inner ears express Chd7 and exhibit distinctive pathologies in a mouse model of CHARGE syndrome. Hear. Res 282:184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y-H, Chiang H-Y, Yen-Jean M-C, and Wang J-D. 2009. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci. Total Environ 408:43–9. [DOI] [PubMed] [Google Scholar]
- Johnson A-C 1993. The ototoxic effect of toluene and the influence of noise, acetyl salicylic acid, or genotype. A study in rats and mice. Scand. Audiol Suppl 39:1–40. [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Luebke AE, and Lurie DI. 2008a. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J. Comp. Neurol 506:1003–17. [DOI] [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Luebke AE, and Lurie DI. 2008b. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J. Comp. Neurol 506:1003–1017. [DOI] [PubMed] [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Parlow AF, Raphael Y, Dolan DF, and Camper SA. 2007. Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm. Genome 18:596–608. [DOI] [PubMed] [Google Scholar]
- Karri V, Schuhmacher M, and Kumar V. 2016. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol 48:203–213. [DOI] [PubMed] [Google Scholar]
- Kim S-J, Jeong H-J, Myung N-Y, Kim M, Lee J-H, So H, Park R-K, Kim H-M, Um J-Y, and Hong S-H. 2008a. The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ. Health Perspect 116:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Myung N-Y, Shin B-G, Lee J-H, So H-S, Park R-K, Um J-Y, and Hong S-H. 2011. Protective effect of a Chrysanthemum indicum containing formulation in cadmium-induced ototoxicity. Am. J. Chin. Med 39:587–600. [DOI] [PubMed] [Google Scholar]
- Kim S-J, Shin B-G, Choi I-Y, Kim D-H, Kim M-C, Myung N-Y, Moon P-D, Lee J-H, An H-J, Kim N-H, Lee J-Y, So H-S, Park R-K, Jeong H-J, Um J-Y, Kim H-M, and Hong S-H. 2009. Hwanggunchungyitang prevents cadmium-induced ototoxicity through suppression of the activation of caspase-9 and extracellular signal-related kinase in auditory HEI-OC1 cells. Biol. Pharm. Bull 32:213–9. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, Park RK, Kim HM, Um JY, and Hong SH. 2008b. The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ. Health Perspect 116:854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel KE, Lee MY, King WM, Raphael Y, Schacht J, and Neitzel RL. 2017. Vestibular dysfunction in the adult CBA/CaJ Mouse after Lead and Cadmium Treatment. Environ. Toxicol 32:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG 2006. Acceleration of Age-Related Hearing Loss by Early Noise Exposure: Evidence of a Misspent Youth. J. Neurosci 26:2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG and Liberman MC. 2009. Adding Insult to Injury : Cochlear Nerve Degeneration after “ Temporary ” Noise-Induced Hearing Loss 29:14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde A, Tomasina F, Bianchi F, Bruné MN, Buka I, Comba P, Corra L, Cori L, Duffert CM, Harari R, Iavarone I, McDiarmid MA, Gray KA, Sly PD, Soares A, Suk WA, and Landrigan PJ. 2015. Children’s health in Latin America: The infuence of environmental exposures. Environ. Health Perspect 123:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang K, Wu S, Ji X, Li N, Liu R, and Gao X. 2011. Lead-induced hearing loss in rats and the protective effect of copper. Biol. Trace Elem. Res 144:1112–1119. [DOI] [PubMed] [Google Scholar]
- Mäkitie AA, Pirvola U, Pyykkö I, Sakakibara H, Riihimäki V, and Ylikoski J. 2003. The ototoxic interaction of styrene and noise. Hear. Res 179:9–20. [DOI] [PubMed] [Google Scholar]
- Matt T, Ng CL, Lang K, Sha S-H, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, and Bottger EC. 2012. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc. Natl. Acad. Sci 109:10984–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauderly JL 1993. Toxicological approaches to complex mixtures. Environ. Health Perspect. 101:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MD, Bean-Knudsen D, and Ermel RW. 1992. Incidence of otitis media in CBA/J and CBA/CaJ mice. Hear. Res 59:1–6. [DOI] [PubMed] [Google Scholar]
- Mesnage R, Defarge N, Rocque L-M, Spiroux de Vendômois J, and Séralini G-E. 2015. Laboratory Rodent Diets Contain Toxic Levels of Environmental Contaminants: Implications for Regulatory Tests. PLoS One 10:e0128429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami SB, Yamashita D, Ogawa K, Schacht J, and Miller JM. 2007. Creatine and tempol attenuate noise-induced hearing loss. Brain Res. 1148:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DI, Nelson RY, Concha-Barrientos M, and Fingerhut M. 2005. The global burden of occupational noise-induced hearing loss. Am. J. Ind. Med 48:446–458. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Rybak Rice ME, Rellinger EA, and Ortmann AJ. 2011. Divergence of noise vulnerability in cochleae of young CBA/J and CBA/CaJ mice. Hear. Res 272:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilinçarslan S, and Oner G. 2001. Effects of cadmium on the hearing system. Acta Otolaryngol. 121:393–7. [DOI] [PubMed] [Google Scholar]
- Park SK, Elmarsafawy S, Mukherjee B, Spiro A, Vokonas PS, Nie H, Weisskopf MG, Schwartz J, and Hu H. 2010. Cumulative lead exposure and age-related hearing loss: the VA Normative Aging Study. Hear. Res 269:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BJ and Saunders LZ. 1978. Heavy metal nephropathy of rodents. Vet. Pathol. Suppl 15 Suppl 5:51–87. [PubMed] [Google Scholar]
- Pichery C, Bellanger M, Zmirou-Navier D, Glorennec P, Hartemann P, and Grandjean P. 2011. Childhood lead exposure in France: benefit estimation and partial cost-benefit analysis of lead hazard control. Environ. Health 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JM, Brooks DM, Thompson CM, and Lurie DI. 2010. Chronic low-level Pb exposure during development decreases the expression of the voltage-dependent anion channel in auditory neurons of the brainstem. Neurotoxicology 31:662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DE, Brown AW, Cavanagh JB, Nolan CC, Richards HK, and Wylie SP. 1992. Functional/metabolic modulation of the brain stem lesions caused by 1,3-dinitrobenzene in the rat. Neurotoxicology 13:379–88. [PubMed] [Google Scholar]
- Reynolds RP, Kinard WL, Degraff JJ, Leverage N, and Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J. Am. Assoc. Lab. Anim. Sci 49:592–597. [PMC free article] [PubMed] [Google Scholar]
- Rice DC 1997. Effects of lifetime lead exposure in monkeys on detection of pure tones. Fundam. Appl. Toxicol 36:112–8. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Amorim M, Silva MV, Neves P, Sousa A, and Inácio O. 2015. Sound Levels and Risk Perceptions of Music Students During Classes. J. Toxicol. Environ. Heal. Part A 78:825–839. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, and Rybak LP. 2012. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat. Rec. (Hoboken) 295:1837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J and Otto D. 1987. Blood lead, hearing thresholds, and neurobehavioral development in children and youth. Arch. Environ. Health 42:153–60. [DOI] [PubMed] [Google Scholar]
- Seidman MD and Standring RT. 2010. Noise and quality of life. Int. J. Environ. Res. Public Health 7:3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, and Schacht J. 2008. Age-related auditory pathology in the CBA/J mouse. Hear. Res 243:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R and Mogra S. 2014. Lead as a developmental toxicant: a review. Int. J. Pharm. Sci. Res 5:636–642. [Google Scholar]
- Spivey A 2007. The weight of lead. Effects add up in adults. Environ. Health Perspect 115:A30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, and Finucane M. 2013. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur. J. Public Health 23:146–152. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Emond AM, Lingam R, and Golding J. 2018. Prenatal lead, cadmium and mercury exposure and associations with motor skills at age 7 years in a UK observational birth cohort. Environ. Int 117:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, and Guallar E. 2012. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ. Health Perspect 120:1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharpe AM, Sladen DP, Dodd-Murphy J, and Boney SJ. 2009. Minimal hearing loss in children: mininal but not inconsequential. Pages 80–93 Seminars in hearing. [Google Scholar]
- Tuncel U, Clerici WJ, and Jones RO. 2002. Differential ototoxicities induced by lead acetate and tetraethyl lead. Hear. Res 166:113–23. [DOI] [PubMed] [Google Scholar]
- Vandamme TF 2014. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci 6:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet T, Campo P, Thomas A, Cour C, Rieger B, and Cosnier F. 2015. The tonotopicity of styrene-induced hearing loss depends on the associated noise spectrum. Neurotoxicol. Teratol 48:56–63. [DOI] [PubMed] [Google Scholar]
- Vicente-Torres MA and Schacht J. 2006a. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J. Neurosci. Res 83:1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Torres MA and Schacht J. 2006b. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J. Neurosci. Res 83:1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Hansen GM, Hanson SW, Heuton KR, Higashi H, Kassebaum N, Kyu H, Laurie E, Liang X, Lofgren K, Lozano R, MacIntyre MF, Moradi-Lakeh M, Naghavi M, Nguyen G, Odell S, Ortblad K, Roberts DA, Roth GA, Sandar L, Serina PT, Stanaway JD, Steiner C, Thomas B, Vollset SE, Whiteford H, Wolock TM, Ye P, Zhou M, Ãvila MA, Aasvang GM, Abbafati C, Ozgoren AA, Abd-Allah F, Aziz MIA, Abera SF, Aboyans V, Abraham JP, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NME, Aburto TC, Achoki T, Ackerman IN, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Arnlov J, Agardh EE, Al Khabouri MJ, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Ameli O, Amini H, Ammar W, Anderson BO, Anderson HR, Antonio CAT, Anwari P, Apfel H, Arsenijevic VSA, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu S, Basu A, Baxter A, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta Z, Bienhoff K, Bikbov B, Bin Abdulhak A, Blore JD, Blyth FM, Bohensky MA, Basara BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brauer M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Briggs ADM, Brooks PM, Brown J, Brugha TS, Buchbinder R, Buckle GC, Bukhman G, Bulloch AG, Burch M, Burnett R, Cardenas R, Cabral NL, Campos-Nonato IR, Campuzano JC, Carapetis JR, Carpenter DO, Caso V, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang JC, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Chugh SS, Cirillo M, Coggeshall M, Cohen A, Colistro V, Colquhoun SM, Contreras AG, Cooper LT, Cooper C, Cooperrider K, Coresh J, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Dandona R, Dandona L, Dansereau E, Dantes HG, Dargan PI, Davey G, Davitoiu DV, Dayama A, De La Cruz-Gongora V, De La Vega SF, De Leo D, Del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, DeVeber GA, Dharmaratne SD, Diaz-Torne C, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duber H, Durrani AM, Edmond KM, Ellenbogen RG, Endres M, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farzadfar F, Fay DFJ, Felson DT, Fereshtehnejad SM, Fernandes JG, Ferri CP, Flaxman A, Foigt N, Foreman KJ, Fowkes FGR, Franklin RC, Furst T, Futran ND, Gabbe BJ, Gankpe FG, Garcia-Guerra FA, Geleijnse JM, Gessner BD, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gona P, De Cosio TG, Gosselin RA, Gotay CC, Goto A, Gouda HN, Guerrant RL, Gugnani HC, Gunnell D, Gupta R, Gupta R, Gutierrez RA, Hafezi-Nejad N, Hagan H, Halasa Y, Hamadeh RR, Hamavid H, Hammami M, Hankey GJ, Hao Y, Harb HL, Haro JM, Havmoeller R, Hay RJ, Hay S, Hedayati MT, Pi IBH, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu H, Hu G, Huang JJ, Huang C, Huiart L, Husseini A, Iannarone M, Iburg KM, Innos K, Inoue M, Jacobsen KH, Jassal SK, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Joseph J, Juel K, Kan H, Karch A, Karimkhani C, Karthikeyan G, Katz R, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Khader YS, Khalifa SEAH, Khan EA, Khan G, Khang YH, Khonelidze I, Kieling C, Kim D, Kim S, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs L, Knudsen AK, Kokubo Y, Kosen S, Kramer A, Kravchenko M, Krishnamurthi RV, Krishnaswami S, Defo BK, Bicer BK, Kuipers EJ, Kulkarni VS, Kumar K, Kumar GA, Kwan GF, Lai T, Lalloo R, Lam H, Lan Q, Lansingh VC, Larson H, Larsson A, Lawrynowicz AEB, Leasher JL, Lee JT, Leigh J, Leung R, Levi M, Li B, Li Y, Li Y, Liang J, Lim S, Lin HH, Lind M, Lindsay MP, Lipshultz SE, Liu S, Lloyd BK, Ohno SL, Logroscino G, Looker KJ, Lopez AD, Lopez-Olmedo N, Lortet-Tieulent J, Lotufo PA, Low N, Lucas RM, Lunevicius R, Lyons RA, Ma J, Ma S, MacKay MT, Majdan M, Malekzadeh R, Mapoma CC, Marcenes W, March LM, Margono C, Marks GB, Marzan MB, Masci JR, Mason-Jones AJ, Matzopoulos RG, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, McMahon BJ, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah G, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Moffitt TE, Ibrahim NM, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Montine TJ, Moore AR, Moran AE, Morawska L, Mori R, Moschandreas J, Moturi WN, Moyer M, Mozaffarian D, Mueller UO, Mukaigawara M, Murdoch ME, Murray J, Murthy KS, Naghavi P, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KMV, Nash D, Nejjari C, Neupane SP, Newman LM, Newton CR, Ng M, Ngalesoni FN, Nhung NT, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Omer SB, Opio JN, Ortiz A, Pandian JD, Panelo CIA, Papachristou C, Park EK, Parry CD, Caicedo AJP, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pellegrini CA, Pereira DM, Perez-Ruiz FP, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips D, Phillips B, Piel FB, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman SU, Raju M, Rakovac I, Rana SM, Razavi H, Refaat A, Rehm J, Remuzzi G, Resnikoff S, Ribeiro AL, Riccio PM, Richardson L, Richardus JH, Riederer AM, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Ronfani L, Rothenbacher D, Roy N, Ruhago GM, Sabin N, Sacco RL, Ksoreide K, Saha S, Sahathevan R, Sahraian MA, Sampson U, Sanabria JR, Sanchez-Riera L, Santos IS, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schoettker B, Schneider IJC, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Servan-Mori EE, Shackelford K, Shaheen A, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shepard DS, Shi P, Shibuya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh JA, Singh L, Skirbekk V, Sliwa K, Soljak M, Soneji S, Soshnikov SS, Speyer P, Sposato LA, Sreeramareddy CT, Stoeckl H, Stathopoulou VK, Steckling N, Stein MB, Stein DJ, Steiner TJ, Stewart A, Stork E, Stovner LJ, Stroumpoulis K, Sturua L, Sunguya BF, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tan F, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, Te Ao BJ, Temesgen AM, Ten Have M, Tenkorang EY, Terkawi AS, Theadom AM, Thomas E, Thorne-Lyman AL, Thrift AG, Tleyjeh IM, Tonelli M, Topouzis F, Towbin JA, Toyoshima H, Traebert J, Tran BX, Trasande L, Trillini M, Truelsen T, Trujillo U, Tsilimbaris M, Tuzcu EM, Ukwaja KN, Undurraga EA, Uzun SB, Van Brakel WH, Van De Vijver S, Van Dingenen R, Van Gool CH, Varakin YY, Vasankari TJ, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubramanian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Waller S, Wallin MT, Wan X, Wang L, Wang J, Wang Y, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KR, Westerman R, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CDA, Wong JQ, Wong H, Woolf AD, Wright JL, Wurtz B, Xu G, Yang G, Yano Y, Yenesew MA, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Kim KY, Zaki MES, Zhang Y, Zhao Z, Zhao Y, Zhu J, Zonies D, Zunt JR, Salomon JA, and Murray CJL. 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil A, Truchon G, Leroux T, Lemay F, Gendron M, Gagnon F, El Majidi N, Boudjerida A, Lim S, Emond C, and Viau C. 2012. A weight of evidence approach for the assessment of the ototoxic potential of industrial chemicals. Toxicol. Ind. Health 28:796–819. [DOI] [PubMed] [Google Scholar]
- Whitworth CA, Hudson TE, and Rybak LP. 1999. The effect of combined administration of cadmium and furosemide on auditory function in the rat. Hear. Res 129:61–70. [DOI] [PubMed] [Google Scholar]
- WHO. 2013. Multi-country assessment of national capacity to provide hearing care. [Google Scholar]
- Willott JF, VandenBosche J, Shimizu T, Ding D-L, and Salvi R. 2008. Effects of exposing C57BL/6J mice to high- and low-frequency augmented acoustic environments: auditory brainstem response thresholds, cytocochleograms, anterior cochlear nucleus morphology and the role of gonadal hormones. Hear. Res 235:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Shen C, Lai J, Goo C, Ko K, Chi H-Y, Chang P-Y, and Liou S-H. 2000. Effects of Lead and Noise Exposures on Hearing Ability. Arch. Environ. Heal. An Int. J 55:109–114. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, and Schacht J. 2001. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res 158:165–178. [DOI] [PubMed] [Google Scholar]
- Yorita Christensen KL 2012. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int. J. Hyg. Environ. Health. [DOI] [PubMed] [Google Scholar]