Abstract
RePORT International is a global network of research sites in India, Brazil, Indonesia, South Africa, China, and the Philippines dedicated to collaborative tuberculosis research in the context of HIV. A standardized research protocol (the Common Protocol) guides the enrollment of participants with active pulmonary tuberculosis and contacts into observational cohorts. The establishment of harmonized clinical data and bio-repositories will allow cutting-edge, large-scale advances in the understanding of tuberculosis, including identification of novel biomarkers for progression to active tuberculosis and relapse after treatment. The RePORT International infrastructure aims to support research capacity development through enabling globally-diverse collaborations. To that end, representatives from the RePORT International network sites, funding agencies, and other stakeholders gathered together in Brazil in September 2017 to present updates on relevant research findings and discuss ideas for collaboration. Presenters emphasized research involving biomarker identification for incipient tuberculosis, host immunity and pharmacogenomics, co-morbidities such as HIV and type 2 diabetes mellitus, and tuberculosis transmission in vulnerable and high-risk populations. Currently, 962 active TB participants and 670 household contacts have contributed blood, sputum, urine and microbes to in-country biorepositories. Cross-consortium collaborations have begun sharing data and specimens to analyze molecular and cytokine predictive patterns.
Keywords: transmission, pharmacogenomics, incipient tuberculosis, HIV, diabetes, biomarkers
1. Introduction to RePORT
The National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) has a history of supporting global high impact tuberculosis (TB) research, while expanding local capacity for conducting such research in high-burden settings. Toward that end, in 2012 the US Division of AIDS (DAIDS) launched a series of collaborative, bilaterally-funded research networks in TB high-burden countries starting with the government of India. Since then, government entities in Brazil, Indonesia, South Africa, China, and the Philippines have followed and provided co-funding along with DAIDS to form the Regional Prospective Observational Research for Tuberculosis (RePORT) International consortium of networks (Figure 1). RePORT consortia are comprised of study sites within each participating country. After a few years of site training and start-up, RePORT International has started to make substantive contributions to the global TB research agenda and increase capacity of scientists and institutions in high-burden settings to do high impact clinical research.
Figure 1.
Timeline of funding initiation for the RePORT International consortia Common Protocol. Parent research protocol initiation occurred on a different timeline.
The vision for RePORT International is to enable a standardized platform for data and specimen collection in prospective TB cohorts, forming the basis for collaborative biomarker and other scientific research of the future, including potential treatment and vaccine trials [1]. The standardized platform is formalized in the Common Protocol and associated documents, and includes a set of data elements with prescribed formatting and definitions (see RePORT Toolkit (https://www.reportinternational.org/). Some country projects began de novo with the Common Protocol, while others adopted it along with a data element strategy in the course of ongoing site-specific parent protocol cohort enrollments. The Common Protocol specifies enrollment of persons into two prospective cohorts: Cohort A is comprised of individuals with active pulmonary tuberculosis disease and Cohort B is comprised of household contacts of active pulmonary tuberculosis patients. These cohorts provide the opportunity to study patients at risk for treatment failure and relapse (Cohort A) and at risk for progression to TB disease (Cohort B). Several consortium sites have particular emphases, such as pediatric patients and persons with diabetes.
The third meeting of RePORT International took place in Rio de Janeiro, Brazil, September 12–13, 2017. Speakers included a combination of RePORT investigators and invited experts. In addition, as part of the group’s capacity-building goal, DAIDS funded six junior investigators who were competitively selected to attend the meeting and present ongoing research relating to RePORT projects. The meeting provided the opportunity to assess progress from the inception of RePORT International and prior meetings [2], with an aim to identify critical gaps in current TB research efforts and opportunities for leveraging the RePORT International infrastructure to address these gaps. The meeting also highlighted Brazilian scientists and their contributions to TB research.
2. Background and Progress of RePORT Consortia
Globally, TB is the leading cause of death from a single infectious agent [3]. The WHO estimates that 1.3 million persons died from TB in 2016, with an additional 0.4 million deaths due to TB in persons living with HIV [4]. There were 10.4 million new cases of TB of which more than 4 million were undiagnosed or untreated. Despite declines in worldwide TB mortality and incidence for over a decade, tremendous disparities exist in the global distribution of TB. Additional factors such as drug resistance and HIV co-infection further complicate progress towards global TB elimination targets. Each country involved in RePORT International is on at least one of three lists of WHO high burden countries (TB, MDR-TB, TB/HIV) that form the basis of focus for global action [5]. Factors such as inadequate sample size, lack of access to biological specimens, and poor data quality limit the ability of TB research in some high-burden settings to answer important translational research questions. Furthermore, a lack of compatibility between data elements from different national and international studies may limit attempts to combine existing study data. RePORT International was designed to mitigate these limitations by providing a platform to collect standardized clinical and laboratory data across many sites.
RePORT International consortia and research sites within each country are at various stages of obtaining regulatory approvals, enrolling subjects into their own parent protocols, co-enrolling eligible subjects into the RePORT International Common Protocol cohorts, and storing well characterized samples from TB patients and their contacts at different time points for future biomarker research (Table 1). In many cases, the DAIDS and country-specific government co-funding for RePORT sites occurs alongside substantial funding from other funding sources for site-specific projects, thereby furthering opportunities for advances in TB research. The financial contribution made by countries that are part of RePORT International ranges from self-funded to matching funds or up to about one-third of the study costs, and investigators from each site have a wide range of additional funding for parent site-specific projects.
Table 1.
RePORT Consortium Site Status and Common Protocol Enrollments as of August 2017
| Cohort A (Active TB) | Cohort B (Household Contacts) | |||||||
|---|---|---|---|---|---|---|---|---|
| # sites enrolling | # enrolled | # expected | % enrolled | # sites enrolling | # enrolled | # expected | % enrolled | |
| Brazil | 5 | 522 | 900 | 58 | 5 | 587 | 2700 | 22 |
| South Africa | 5 | 309 | 1115 | 28 | 3 | 42 | 1755 | 2 |
| India | 2 | 57 | 1700 | 3 | 2 | 49 | 5100 | 1 |
| Indonesia | 4 | 78 | 1357 | 6 | NA | NA | NA | NA |
| China | 7 | 2 | 160 | 1 | NA | NA | NA | NA |
| Total | 24 | 962 | 5232 | 18 | 9 | 670 | 9555 | 7 |
Brazil was the first country to start enrolling patients into the Common Protocol. RePORT Brazil sites are involved in numerous areas of research, including transcriptional profiling in TB and diabetes mellitus with a focus on eicosanoids and inflammatory vs. anti-inflammatory balance. Multiple studies are assessing TB-HIV co-infection, including transcriptional signatures of TB in advanced HIV and predictors of treatment toxicity, failure, and relapse in TB-HIV co-infection.
In India, RePORT sites represent a mix of urban and rural, geographically disparate populations, and bring unique interests and expertise to their parent projects. The specific areas of interest include the impact of diabetes on TB severity among adults, host and microbial factors associated with active and latent TB infection in adults and children, the role of cellular immunity in preventing progression to active TB, risk factors for treatment relapse and progression to active TB, and the role of the host determinants in the eicosanoid pathway as modulators of the inflammatory response, disease outcome, and treatment responsiveness in TB. In addition, a vaccine study on candidate VPM 1002, with a view to reduce relapse in subjects who have successfully completed TB treatment, has begun recruitment at multiple RePORT India sites (NCT03152903).
Research priorities for RePORT sites in South Africa include the search for biomarkers for the prediction of progression of infected individuals to active TB and TB treatment response, the evaluation of phenotypic drug tolerance in sputum, the characterization of neutrophil phenotypic markers and bioenergetics in culture conversion, community based active case finding with Xpert Omni, and quantifying infectiousness of undiagnosed TB cases. Areas of interest involving household contacts include the characterization of asymptomatic TB and its short-term progression and the identification of paucibacillary household contacts. Projects focused on pediatric TB include the evaluation of the performance of Xpert MTB/RIF Ultra in both sputum and stool specimens, the use of oral swabs for TB diagnosis, and biomarkers for TB diagnosis in children.
Under the Indonesia Research Partnership on Infectious Disease (INA-RESPOND) network, RePORT Indonesia has begun enrollment in Cohort A of the Common Protocol. This is linked to parent studies focused on assessment of new and previously treated TB among patients with drug-resistant TB in Indonesia.
The most recent consortium additions to RePORT International are China and the Philippines. In China, research sites and suitable parent studies have been identified for enrollment in Cohort A of the Common Protocol. The Philippine government has approved two projects and has sanctioned funding for the same under the RePORT umbrella. The sites are in the process of setting up and obtaining necessary regulatory and other approvals.
RePORT International consortia are involved in multiple areas of cutting-edge TB research, and the RePORT International meeting highlighted progress in the areas of TB-HIV co-infection, TB and type 2 diabetes mellitus (DM), host immune response to Mycobacterium tuberculosis infection and disease, and factors associated with M. tuberculosis transmission. The following sections feature some of the advances and opportunities for further investigation at and among RePORT International consortium sites highlighted during the conference, as well as challenges and shortcomings of RePORT International.
3. HIV-related Tuberculosis
3.1. TB-HIV in Brazil
Several RePORT International consortia have sites in regions with a substantial burden of TB-HIV co-infection. Dr. Valeria Rolla, a leader in the RePORT Brazil consortium, reported on the clinical importance of improved understanding and monitoring of TB and HIV drug-drug interactions in Brazil, particularly given the rapid expansion of access to antiretroviral therapy (ART). Rifampicin-based TB regimens and ART are associated with morbidity and mortality due to drug-induced hepatitis and other adverse reactions. These events may incur substantial additional costs because of added outpatient visits, tests, and in more serious instances, hospitalizations.
Efavirenz as a backbone of fixed-dose combination (FDC) HIV treatment is giving way to raltegravir in Brazilian patients with CD4 counts <100 cells/mm3 or who meet severe HIV disease criteria due to the increasing incidence of primary resistance to efavirenz in Brazil [6]. And while ART is generally better tolerated when the backbone includes non-nucleotide reverse trancriptase inhibitors (NNRTI) instead of protease inhibitors (PI), options that include PIs are critical when resistance has developed. Drug regimens for TB-HIV co-infected patients that are well tolerated, effective, and locally accessible are urgently needed. Dolutegravir is not currently recommended for TB patients in Brazil due to concern for drug-drug interactions and possible reduction of rifampicin concentrations [7]. The RePORT network is well suited to investigate the pharmacokinetic and pharmacogenomic basis for TB and HIV therapy for drugs like the PIs and dolutegravir, as well as assess implementation of such regimens.
3.2. TB-HIV Pharmacogenomics
Dr. David Haas discussed the pharmacogenomics of drugs used to treat TB and HIV, leading to discussions about the potential role for RePORT International in this area of research. Polymorphisms in drug metabolism and transport genes, immune response genes, and mitochondrial DNA confer inter-individual variability in efficacy, toxicity and/or pharmacokinetics of various antimicrobials. Regarding anti-TB drug pharmacogenetics, isoniazid is the most extensively studied. Loss-of-function NAT2 alleles are frequent in all populations, and individuals carrying one or two loss-of-function alleles have intermediate or slow acetylator phenotypes, respectively, and progressively greater plasma isoniazid exposure [8–11]. An association between such NAT2 polymorphisms and increased risk for isoniazid hepatotoxicity was shown in meta-analyses involving multiple TB treatment cohorts, with odds ratios ranging from ~2 to 5 [12–14]. A small randomized clinical trial of TB patients in Japan, which compared standard isoniazid dosing (5 mg/kg) to NAT2 genotype-guided dosing (2.5 mg/kg for slow, 5 mg/kg for intermediate, and 7.5 mg/kg for fast acetylators) [15], found that genotype-guided dosing yielded fewer treatment failures in rapid acetylators and less hepatotoxicity in slow acetylators, although treatment failure and hepatotoxicity rates were excessive. Beyond NAT2 there is not yet compelling evidence for genetic associations with isoniazid hepatotoxicity.
Data are limited regarding the pharmacogenetics of other widely prescribed anti-TB agents including rifamycins, pyrazinamide, ethambutol, and fluoroquinolones. Rifampicin is a substrate for the organic anion-transporting polypeptide 1B1, which is encoded by SLCO1B1 [16]. In a report from South Africa involving 57 patients, a frequent SLCO1B1 polymorphism was associated with reduced rifampicin bioavailability [17], although this was not confirmed in other populations [18,19]. It is not known whether immune response genes influence rifamycin hypersensitivity reactions. Pharmacogenomic associations with newer drugs such as bedaquiline have not yet been reported.
Pharmacogenetics is also relevant to many antiretroviral drugs and a genotype-driven drug-drug interaction occurs between anti-TB and antiretroviral drugs. Rifampicin induces hepatic cytochrome P450 (CYP) expression, and therefore should decrease plasma efavirenz exposure by inducing CYP2B6 [20]. However, plasma efavirenz exposure paradoxically increases in some patients receiving anti-TB therapy that includes rifampicin with isoniazid, particularly when both CYP2B6 and NAT2 loss-of-function genotypes are present [21–23]. This may reflect isoniazid inhibition of CYP2A6, a minor efavirenz elimination pathway that may assume a greater role in CYP2B6 slow metabolizers [22–24].
Pharmacogenomics may impact TB and HIV care considerably, given the immense global burden of TB and HIV. Genetic analyses involving well-phenotyped clinical datasets such as those established in RePORT International, which allow relationships between human genetic variants and clinical outcomes to be characterized, may ultimately lead to safer and more effective treatment regimens.
4. Tuberculosis and Diabetes Mellitus
Adequate TB control at the population level requires better understanding of interactions with potential co-morbid risk factors. The most significant acquired TB risks factors are undernutrition, smoking, HIV, and DM [25–28]. These co-morbidities increase the risk of developing active TB and adverse treatment outcomes. Globally, 15% of TB cases are estimated to be attributable to DM [29]. The number of people worldwide with DM is expected to substantially rise during the next 20 years, with the largest increase in countries where TB is already endemic, such as Brazil, India, China, and South Africa [30,31]. Rising DM prevalence presents challenges in clinical TB management since it is associated with higher risk of adverse outcomes and relapse after the initiation of anti-TB treatment [32–34].
4.1. TB-DM drug-drug interactions
Dr. Vidya Mave, a RePORT India investigator, focused on the effect of DM on the pharmacokinetics of rifampicin, isoniazid, and pyrazinamide and drug-drug interactions in the treatment of both diseases. Previous studies have shown conflicting reports on whether DM decreases the concentrations of anti-TB drugs. While earlier studies demonstrated that the presence of DM decreased rifampicin concentrations [35,36], a subsequent study showed that rifampicin, isoniazid, and pyrazinamide levels remain unchanged in DM when adjusted for body weight [37]. A study from Mexico showed that time to reach rifampicin Cmax was longer in TB patients with DM (3 hours vs. 2) and that rifampicin clearance was slower in patients with TB and DM [38]. A recent study from South India showed that post 2-hour rifampicin levels were unaffected by DM but the isoniazid and pyrazinamide levels were significantly lower [39].
These varied study findings demonstrate the importance of further characterizing the optimal treatment of patients with both TB and DM. Research priorities include: clarifying the effect of DM and glycated hemoglobin on delayed absorption, area under the curve, and Cmax of TB drugs; investigating the toxicity profile of TB drugs in patients with DM who may be older or have additional co-morbidities; whether high-dose or alternative rifamycins and diabetes drugs/control will be needed to optimize TB treatment outcomes; assessing metformin interactions and whether rifampicin increases metformin’s DM activity by increasing intrahepatic concentrations while concurrently decreasing its host-directed therapy activity by reducing systemic concentrations. The latter is particularly relevant given recent findings of decreased mortality during TB treatment associated with metformin use [40].
4.2. Inflammation and eicosanoids in TB-DM
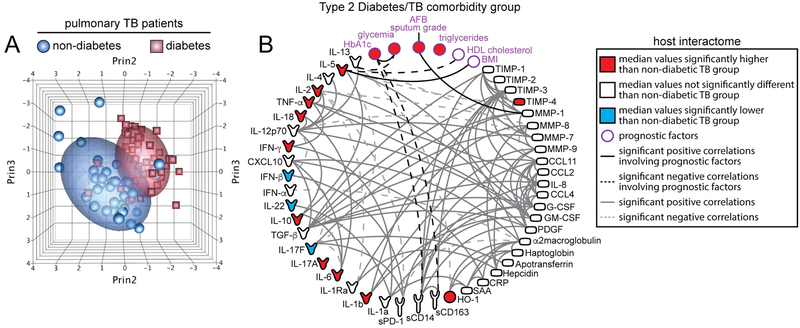
Dr. Bruno Andrade, a leader in the RePORT Brazil consortium, discussed how the etiology of increased risk of active TB in DM is likely multifactorial. Studies have identified specific cytokine abnormalities in persons with DM and pre-diabetes that likely contribute to both active and latent TB disease [41,42]. Patients with TB-DM display heightened levels of plasma biomarkers of inflammation, tissue remodeling, and oxidative stress, all of which could be driving increased susceptibility to adverse TB-related clinical outcomes (Figure 2) [43].
Figure 2. Biosignature of TB-diabetes comorbidity.
Plasma levels of several markers of inflammation, immune activation, growth factors, and tissue remodeling were compared between pulmonary TB patients with or without type-2 diabetes (n=44 patients per group). (A) Principal component analysis of candidate biomarkers for TB-diabetes comorbidity. All the biomarkers used in the principal component analysis models are displayed in (B). (B) Spearman correlation matrix displayed as circus plot, describing associations between the circulating concentration of the candidate biomarkers and previously described prognostic factors. Variables are colored in red or blue if the levels in patients with diabetes and TB were significantly higher or lower (P<.05) than in patients with TB without diabetes, respectively, using Mann-Whitney tests after adjusting for multiple measurements (Holm method). The data presented were generated in collaboration with Dr. Subash Babu (NIAID, NIH) and Pavan Kumar (National Institute for Research in Tuberculosis, Chennai, India) and obtained from samples from patients previously studied: Andrade BB, Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, et al. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest 2014;145: 1244–54. doi: 10.1378/chest.13–1799.
Understanding the TB-DM interaction and its impact on the host immune system could help enhance clinical management of patients with both diseases and have a positive impact on public health. Emerging evidence shows that lipid mediators such as eicosanoids (a diverse group of bioactive lipids derived from the enzymatic or non-enzymatic oxidation of arachidonic acid) play important roles in both TB and DM pathogenesis in experimental and clinical settings [44–47]. Lipid mediators of the eicosanoid family have been shown to influence the outcome of experimental M. tuberculosis infection [48]. Additional investigations using macrophages demonstrated that the balance between prostaglandins and leukotrienes/lipoxins influences the fate of macrophages infected with M. tuberculosis. In these in vitro studies, PGE2 drives macrophage apoptosis, which is associated with better control of M. tuberculosis infection, whereas lipoxin A4 triggers necrotic death of the infected cells, resulting in unrestrained mycobacterial growth.
Associations between the eicosanoid balance and TB clinical outcomes were demonstrated in patients with pulmonary TB in an exploratory study performed in India and China [49]. Interestingly, these findings seemed to be independent of the status of poor glycemic control as DM was an exclusion criterion for recruitment. In a proof-of-concept experiment using an in vivo model of progressive TB in mice, supplementation with synthetic PGE2 and simultaneous pharmacological blockage of 5-lypoxigenase substantially reduced lung pathology, mortality, and mycobacterial loads in lungs, indicating that manipulation of the eicosanoid balance can serve as a strategy to reduce TB burden [49]. These results demonstrate the importance of the eicosanoid balance in driving pathological inflammation in both TB and DM; however, no study to date has evaluated the longitudinal profile of expression of the eicosanoid pathway in patients with TB-DM.
The role of eicosanoid balance in HIV pathogenesis has not been described in detail, but preliminary studies suggest that differential expression of eicosanoid pathways may be critical in driving the inflammation associated with HIV, TB and DM. The RePORT International TB-DM working group is performing a longitudinal multi-site study simultaneously quantifying several eicosanoids to evaluate whether eicosanoid balance reflects TB disease activity and how this balance is influenced by HIV and/or DM. Characterizing the longitudinal eicosanoid profile could reveal signatures or even simple clinical characteristics to identify patients likely to benefit from an extended course of anti-TB therapy or host-directed therapies to accelerate microbiological cure. A better understanding of the contribution of DM and HIV to TB pathogenesis could allow for interventions to enhance TB treatment and prevention, targeted to persons at highest risk of poor outcomes.
4.3. Screening for TB-DM
Dr. Cesar Ugarte-Gil discussed how TB and DM bi-directional screening programs may provide opportunities to engage previously undiagnosed diabetic individuals or those not retained in care for DM, particularly in low- and middle-income countries (LMICs) where TB often garners more attention and funding than DM [50]. The yield of screening, however, is different among TB and DM patients: the detection of DM is higher in TB patients compared with the detection of TB among DM patients [51,52].
RePORT International provides opportunities to improve the understanding of TB-DM epidemiology. Internal RePORT funding has been mobilized to support collaborations between India, Brazil, and South Africa to better understand the molecular basis of TB-DM interactions. These and other projects are well-positioned to study the utility of bi-directional screening in LMIC settings and the evaluation of optimal screening tests for both diseases, including whether the implementation of point-of-care glycosylated hemoglobin in TB clinics and Xpert MTB/RIF in diabetes clinics can improve treatment outcomes in various populations [53]. Studies are also needed to assess whether improved blood glucose control in TB patients with DM can help improve TB treatment outcomes, and whether integrated TB and DM care programs are feasible and effective at improving patient care and outcomes. Furthermore, although some data suggest a higher mortality rate among MDR-TB patients with DM compared to those without DM [54], studies are needed to clarify how treatment responses, pharmacokinetics, and immune responses are affected in MDR-TB patients with DM.
5. Host Immune Response to M. tuberculosis Infection and TB Disease
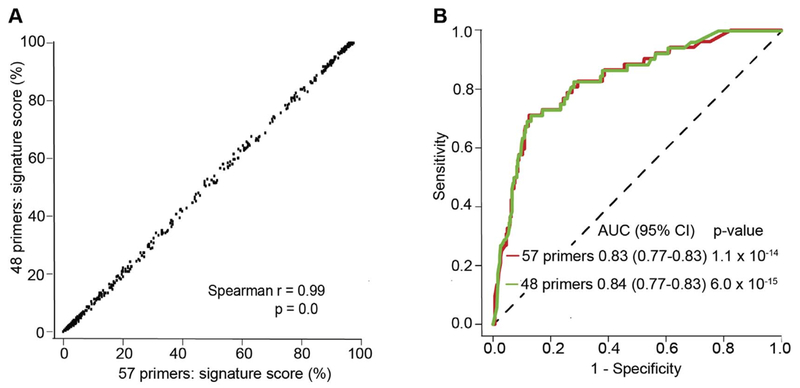
Progress in the understanding of human immune response to M. tuberculosis is critical for advancing diagnostics, host-directed therapies, and vaccine development. Dr. Mark Hatherill reported advances in the identification of transcriptomic signatures of M. tuberculosis. Identification of M. tuberculosis-infected individuals with incipient TB, before progression to infectious subclinical or clinical disease, is of crucial importance for effective prevention in TB endemic countries. Incipient TB may be clinically indistinguishable from quiescent infection, but may be differentiated on the basis of an immune signature characteristic of progression to disease [55]. A 16-gene host blood RNA signature that has been validated on a qRT-PCR platform (Biomark Fluidigm) discriminates progression to TB in cases from controls up to 18 months before diagnosis among South African adolescents [56]. An 11-gene signature shows similar performance characteristics (Figure 3) [57]. Since prognostic performance of these transcriptomic signatures is maximal immediately before the time of disease diagnosis, it is likely that the assays have diagnostic potential as triage tests. Indeed, when re-parameterized to published micro-array data, the 16-gene signature differentiates active TB disease from latently infected and uninfected individuals (area under the receiver operating curve 0.86 – 0.99) [56].
Figure 3. Performance of the reduced ACS 11-gene signature of TB risk as a classifier of TB disease from M.tb-infection in PBMC.
(A) Correlation of the signature scores generated from the original 57 primer-probe (16 genes) and the reduced, 48 primer-probe (11-genes) qRT-PCR transcriptomic signatures of risk of TB disease in progressors and controls from the Adolescent Cohort Study (ACS). The Spearman correlation coefficient is shown. (B) Performance of the original and reduced CoR signatures in classifying progressor and control samples from the Adolescent Cohort Study. In Darboe F, Mbandi SK, Thompson EG, Fisher M, Rodo M, van Rooyen M, Filander E, Bilek N, Mabwe S, Hatherill M, Zak DE, Penn-Nicholson A, Scriba TJ, SATVI Clinical Immunology Team. Diagnostic performance of an optimized transcriptomic signature of risk of tuberculosis in cryopreserved peripheral blood mononuclear cells. Tuberculosis 2018; 108:124–126. doi: 10.1016/j.tube.2017.11.001.
The utility of the 11-gene signature is being explored in a TB test & treat strategy, whereby different positivity thresholds might be used to triage individuals to curative, preventive, or no intervention, without the need for symptoms or a positive interferon-gamma release assay to allow entry into the TB screening algorithm [58]. Specifically, a high test threshold would be used as an indication for definitive investigation for active TB disease, an intermediate test threshold would be used as an indication for preventive therapy, and a negative test would eliminate the need for investigation or intervention. Such a test would allow mass community TB screening if the diagnostic and prognostic performance of these RNA signatures meets expectations. Other pre-requisites for success of a TB screen & treat strategy would include acceptable signature performance in both HIV-positive and HIV-negative persons; a highly effective short-course preventive therapy regimen that could be rolled out widely and rapidly; and devolution of testing to near point-of-care.
Preliminary data suggest RNA signature performance may be sub-optimal in HIV-positive patients who are not yet established on antiretroviral therapy [59]. Other evidence suggests that host blood RNA signatures respond rapidly to curative treatment and might be useful to predict TB treatment outcome [60]. Efforts are ongoing to reduce the RNA signature still further, to a 6-gene model or smaller, in order to transfer the assay to a point-of-care platform that would allow implementation of a TB test & treat strategy in the field [61].
6. Factors Associated with M. tuberculosis Transmission
6.1. Epidemiology of M. tuberculosis transmission
Effective transmission of M. tuberculosis depends on qualities of the infected host, the organism, and the exposed person. Dr. Robert Horsburgh noted that the proximity and duration of contact between an infectious host and a susceptible recipient are major predictors of disease transmission [62]. Households have been traditionally identified as important venues for TB transmission, but recent research has demonstrated that other locations, such as schools, workplaces and public transportation can also be effective transmission sites [63–66].
Infected hosts vary considerably in their ability to transmit the organism, but predictors of efficient host transmission are not well understood [67]. Factors related to the virulence of the organism that might facilitate efficient transmission are also poorly understood [68]. Some drug-resistant M. tuberculosis isolates appear less likely to be transmitted than drug-susceptible isolates, but others are quite fit and may persist in circulation, resulting in a high level of transmissibility of MDR-TB strains [69–71]. BCG vaccination and previous M. tuberculosis infection are both protective against infection (or re-infection), while exposed persons with HIV infection have increased risk of acquisition [72–75]. Prevention of transmission relies upon prompt detection and treatment of infected persons, as this can substantially shorten the duration of transmission [62]. Ongoing trials of latent MDR-TB infection treatment will hopefully provide important new regimens for prevention of MDR-TB.
Many important questions regarding the transmission of M. tuberculosis remain, including 1) What qualities make an infected host an efficient transmitter, 2) What pathogen virulence factors make for efficient transmission, 3) How can we more quickly identify persons with infectious TB disease, 4) What regimens can be used to treat latent MDR-TB infection, and 5) Can we identify the most important groups for targeted treatment of latent TB infection? RePORT International provides important opportunities for collaboration to answer several of these questions, with particular attention to household contact studies to identify risk factors for transmission and to collect isolates for in vitro studies of virulence of M. tuberculosis strains.
6.2. TB transmission in prison populations
As TB epidemics shift from more generalized settings to more concentrated environments or populations in many LMICs, numerous commentators suggest that future success in global TB elimination hinges upon the identification of high-risk groups that serve as reservoirs for broader population epidemics [76–78]. For example, prisons are likely to be a major reservoir for TB in many LMICs [79,80]. Dr. Julio Croda presented data showing that TB incidence in twelve prisons in Brazil (incident case rate 1,760 per 100,000 people) may drive broader population incidence (42 per 100,000 people) [79]. The research team used a combination of conventional case-control methods together with traditional molecular approaches to examine potential TB linkages between prisons and community settings. Among 240 cases of TB occurring over a period of 46 months, 180 (75%) occurred in the community and 60 (25%) occurred among prisoners [81]. Among those occurring in the community, 23% occurred among ex-prisoners, and previous incarceration was the strongest risk factor for TB in the community (Adjusted OR, 28.5).
Subsequently, the team matched TB notifications in the state to prison entrances and releases from 2007–2014; they verified 615 TB cases among 42,925 inmates. TB notifications rose steadily from the time of incarceration, peaking at five years, and upon release remained elevated (~5 time the general population rate) and slowly declined over the following seven years. Prisoners account for 8% of all TB cases in Brazil, a number that has grown over the past five years [82]. Even this may underestimate the importance of prisons, however, due to the high TB rates observed among ex-prisoners, who also contribute to onward transmission. Among 319 isolates for which genotyping by IS6110 restriction fragment length polymorphism was performed, 76% could be classified in clusters of 2 to 40 cases. The majority of clusters included prisoners or ex-prisoners and community members. Whole genome sequencing of 31 cases from the largest cluster demonstrated support for close phylogenetic relatedness for strains in and outside of prisons, suggesting the TB epidemic in prisons may be spilling over into the general population.
The work assessing TB in and around Brazilian prison populations has led to innovative methods to maximize efficiency and cost-effectiveness in screening large reservoirs of persons at high risk for TB. One such method that is being studied is the use of a mobile diagnostic unit with molecular testing of pooled sputum specimens, followed by individual testing of positive pools (as is done for detecting pathogens in donated blood). Collaborative studies among RePORT International sites could test the accuracy and efficiency of methods of pooled screening with the potential for global implementation. Although the Common Protocol does not currently allow for enrollment of prisoners, select sites or consortia may consider future modifications to allow such populations to be studied.
7. Priorities for further scientific inquiry
The RePORT International meeting not only highlighted numerous advances in multiple aspects of TB research in recent years, but also exposed critical knowledge gaps and opportunities for further study using the RePORT International platform (Table 2). The RePORT International infrastructure provides opportunities to identify and implement innovative approaches from well-established and junior investigators alike.
Table 2.
Meeting themes and priority questions for further research
| Theme | Questions |
|---|---|
| TB in vulnerable populations (children, pregnant women, prisoners, drug users) |
|
| Identification of incipient TB |
|
| Pharmacogenomics and pharmacovigilance are vital to treat TB and co-morbidities |
|
| Innovative approaches to understand TB transmission |
|
| Research findings should be operationalized |
|
| Regional variations in TB epidemics |
|
Detailed understanding of TB transmission remains a critical research priority. Innovative approaches are required to understand host and pathogen factors that determine why some people transmit TB (both drug-susceptible and drug-resistant) more efficiently than others. Advances could lead to targeted identification of persons who transmit tuberculosis, programmatic rapid hotspot detection, and recommendations for treatment of MDR-TB contacts. Dr. Helder Nakaya discussed how adapting GPS technology for mapping hotspots for mosquito-human malaria transmission could help TB transmission detection and prevention.
In order to optimize care for TB patients and direct public health programs and policy, a greater understanding of context-specific social determinants of TB is needed [83]. Heather Ewing presented results from a cutting edge survey platform to study knowledge, attitudes and perceptions toward TB infection and disease in Brazil. Data analysis demonstrated that increased knowledge of TB could be associated with increased stigmatization of TB. The findings suggested that more study is needed to understand complex factors that contribute to stigma and perceptions of TB infection and disease among those diagnosed and their contacts, the contribution of healthcare worker communication about TB, and behavioral drivers of seeking care and completing treatment for TB infection and disease.
8. Challenges and shortcomings
The RePORT International meeting also provided the opportunity to identify important challenges faced by RePORT International as a whole and member sites. Because RePORT International is structured around a Common Protocol, but often embedded within parent studies, investigators and government funders must determine whether the efforts to maintain Common Protocol enrollments and collection of biological specimens are justifiable and sustainable, rather than a distraction from their own respective research and funding priorities. Due to the implementation of the Common Protocol, site-specific parent studies may experience delays, and Common Protocol enrollments may similarly be delayed due to activities related to site-specific studies. RePORT International was intended to provide opportunities and resources for parent studies and newly added cross-consortium studies to succeed, though some sites have found that the requirements provide competing interests. While the Common Protocol is intended to dovetail into site-specific studies, the integration with data and specimen collection of previously established site-specific studies has been challenging, and ongoing work to consolidate data has allowed site-specific studies and Common Protocol data fields to become more closely aligned over time.
The actual governance of shared data and biological specimens is complex, particularly navigating current and evolving local and national intellectual property rights across all countries involved. Some countries have noted confusion about management responsibilities of the international and local funders, including legal obligations and mechanisms of accountability. Furthermore, the prioritization of access to biological specimens remains an important consideration, along with balancing long-term goals of assuring sufficient quantities of samples for cross-consortium analyses with complete datasets (and therefore unused samples for long periods of time) versus immediate access to samples from Consortium members or outside investigators.
Individual sites and member countries have also experienced challenges. Funding delays and challenges coordinating logistics have led to delays in recruitment for the Common Protocol. In some cases, unanticipated delays in implementing the Common Protocol meant that parent studies had completed recruitment, leading to lower enrollment into the Common Protocol than planned. Some Principal Investigators had difficulty coordinating activities with the cycle of the funding bodies, necessitating funding from other sources to retain staff during such watershed periods. Among laboratory concerns, several sites found that specimen containers were not compatible with the freezers for storage in the RePORT biorepository for that country. Questions about common methods for sample quality assessment have been raised, and assurance of quality control for sample handling in local laboratories and country biorepositories has also been a concern. One country identified a location for the biorepository, but after the project began found out that they will need to find another location for the biorepository. The RePORT International meetings and organizational calls have provided opportunities to discuss and work to address these challenges.
Conclusions
The RePORT International network of sites provides a robust opportunity for cutting-edge, collaborative TB research in TB high burden countries. The collaborative design of RePORT International allows participating countries to study important TB research questions at a scale that would not otherwise be possible through enrollment of patients in local studies and harmonized observational cohorts. Partnerships that include co-funding between US and participating country governments promote country-specific interest in TB research and development of research capacity in multiple TB high-burden settings across the globe. Regular consortium-specific communication and meetings, along with calls for supplemental research applications allow investigator and country-specific research priorities to be highlighted and pursued and challenges to be addressed. Furthermore, RePORT International has made it a priority to encourage junior investigators from consortium sites to engage in the robust collaborative environment provided by the RePORT infrastructure, thus further creating opportunities for building sustainability and research capacity in TB high-burden settings. As enrollment in the Common Protocol cohorts increases in the near future, investigators will be able to leverage valuable RePORT International clinical and specimen data to make critical progress in the global fight against TB.
Acknowledgements
The authors would like to thank Kimberly Booher for her contributions to the preparation of the manuscript.
RePORT International is funded by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH), and serves all the consortia. The NIH and local governments jointly fund the individual RePORT consortia in a bilateral manner. Country-specific co-funders include:
RePORT India: The Indian Department of Biotechnology (DBT), the Ministry of Science and Technology, and the Indian Council of Medical Research (ICMR)
RePORT Brazil: The Ministry of Health and Department of Science and Technology (Departamento de Ciência e Tecnologia do Ministério da Saúde [DECIT]), Secretaria de Ciência, Tecnologia e Insumos Estratégicos (SCTIE), Ministério da Saúde
RePORT Indonesia: National Institute of Health Research and Development (NIHRD), Ministry of Health (Indonesia)
RePORT South Africa: South African Medical Research Council (SAMRC), with support from the South African Department of Science and Technology (DST) and Department of Health (DOH)
RePORT China: China Innovation Alliance on Tuberculosis Diagnosis and Treatment (Beijing), and China Ministry of Science and Technology, with co-funding from six China Clinical Trials Consortuim (CTCTC) member centers
RePORT Philippines: University of the Philippines, Manila-Philippines National Institutes of Health
This work was supported as follows: Dr. van der Heijden was supported by the National Institutes of Health (NIH) [K08 AI106420] and the U.S. Civilian Research and Development Foundation (CRDF) [OISE-16–62061-1]. Dr. Andrade was supported by the NIH [U01AI14018, R01AI20790], CRDF Global [DAA3–17-63145–1], Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil [028/2010]. Drs. Andrews and Croda were supported by the NIH [R01AI130058], and Dr. Croda was also supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil [401824/2016–0 and 420672/2017–6]. Dr. Haas was supported by the NIH [R01AI077505, P30 AI110527]. Dr. Hatherill was supported by CRDF Global [OISE-16–62054-1] and the South African Medical Research Council (SAMRC Collaborating Centre for TB and HIV). Dr. Horsburgh was supported by the Providence/Boston Center for AIDS Research [P30AI042853], the Boston University/Rutgers Tuberculosis Research Unit [U19AI111276], and the U.S.-India Vaccine Action Program (VAP) Initiative on Tuberculosis (CRDF Global/NIAID). Dr. Mave was supported by the Indo (DBT)-US (NIH) [USB1–31147-XX-13 CRDF/NIH], and the Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks [U01AI069497]. Dr. Sugiyono was supported by the NIAID at NIH [contract numbers HHSN261200800001E and HHSN261201500003I]. Dr. Ugarte-Gil was supported by the TANDEM project, which is funded by the European Union’s Seventh Framework Programme (FP7/2007–2013) [305279].
NIH/NIAID/DAIDS (sponsor) representatives contributed to an agenda-setting process that was led by the RePORT Brazil site and the RePORT International Coordinating Center at FHI 360.
Abbreviations:
- DAIDS
Division of AIDS
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- TB
tuberculosis
- RePORT
Regional Prospective Observational Research for Tuberculosis
- WHO
World Health Organization
- HIV
human immunodeficiency virus
- MDR-TB
multidrug-resistant tuberculosis
- INA-RESPOND
Indonesia Research Partnership on Infectious Disease
- DM
type 2 diabetes mellitus
- ART
antiretroviral therapy
- FDC
fixed-dose combination
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- DNA
deoxyribonucleic acid
- Cmax
maximum concentration
- PGE2
prostaglandin E2
- LMIC
low and middle-income country
- RNA
ribonucleic acid
- qRT-PCR
quantitative real-time polymerase chain reaction
- GPS
global positioning system
Footnotes
Declarations of interest:
All authors: none
References
- [1].Hamilton CD, Swaminathan S, Christopher DJ, Ellner J, Gupta A, Sterling TR, et al. RePORT International: Advancing Tuberculosis Biomarker Research Through Global Collaboration. Clin Infect Dis 2015;61Suppl 3: S155–9. doi: 10.1093/cid/civ611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Geadas C, Stoszek SK, Sherman D, Andrade BB, Srinivasan S, Hamilton CD, et al. Advances in basic and translational tuberculosis research: Proceedings of the first meeting of RePORT international. Tuberculosis (Edinb) 2017;102: 55–67. doi: 10.1016/j.tube.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. The Top 10 Causes of Death. WHO Fact Sheet, updated January 2017 Retrieved May 2, 2018, 2018, from http://origin.who.int/mediacentre/factsheets/fs310/en/
- [4].World Health Organization (Producer). (2017, 12 November 2017). Global Tuberculosis Report 2017. WHO/HTM/TB/2016.23. Retrieved from http://www.who.int/tb/publications/global_report/en/
- [5].World Health Organization (Producer). (2015, 18 August 2016). Use of high burden country lists for TB by WHO in the post-2015 era. WHO/HTM/TB/2015.29. Retrieved from http://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf?ua=1
- [6].Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos, updated 27 September 2017. (2017, 24 March 2018). Retrieved from http://www.aids.gov.br/pt-br/pub/2013/protocolo-clinico-e-diretrizes-terapeuticas-para-manejo-da-infeccao-pelo-hiv-em-adultos
- [7].Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr 2013;62: 21–7. doi: 10.1097/QAI.0b013e318276cda9 [DOI] [PubMed] [Google Scholar]
- [8].Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, et al. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. [Research Support, Non-U.S. Gov’t]. Hepatology 2002;35: 883–9. doi: 10.1053/jhep.2002.32102 [DOI] [PubMed] [Google Scholar]
- [9].Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003;37: 924–30. doi: 10.1053/jhep.2003.50144 [DOI] [PubMed] [Google Scholar]
- [10].Roy PD, Majumder M, & Roy B. Pharmacogenomics of anti-TB drugs-related hepatotoxicity. Pharmacogenomics 2008;9: 311–21. [DOI] [PubMed] [Google Scholar]
- [11].Ramachandran G, & Swaminathan S. Role of pharmacogenomics in the treatment of tuberculosis: a review. Pharmacogenomics and Personalized Medicine 2012;5: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun F, Chen Y, Xiang Y, & Zhan S. Drug-metabolising enzyme polymorphisms and predisposition to anti-tuberculosis drug-induced liver injury: a meta-analysis. The International Journal of Tuberculosis and Lung Disease 2008;12: 994–1002. [PubMed] [Google Scholar]
- [13].Wang P, Xie S, Hao Q, Zhang C, & Jiang B. NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis. The International Journal of Tuberculosis and Lung Disease 2012;16: 589–95. [DOI] [PubMed] [Google Scholar]
- [14].Cai Y, Yi J, Zhou C, & Shen X. Pharmacogenetic study of drug-metabolising enzyme polymorphisms on the risk of anti-tuberculosis drug-induced liver injury: a meta-analysis. PLoS One 2012;7: e47769. doi: 10.1371/journal.pone.0047769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Azuma J, Ohno M, Kubota R, Yokota S, Nagai T, Tsuyuguchi K, et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol 2013;69: 1091–101. doi: 10.1007/s00228-012-1429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest 2003;33 Suppl 2: 1–5. [DOI] [PubMed] [Google Scholar]
- [17].Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother 2011;55: 4122–7. doi: 10.1128/AAC.01833-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeremiah K, Denti P, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrob Agents Chemother 2014;58: 3468–74. doi: 10.1128/AAC.02307-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramesh K, Hemanth Kumar AK, Kannan T, Vijayalakshmi R, Sudha V, Manohar Nesakumar S, et al. SLCO1B1 gene polymorphisms do not influence plasma rifampicin concentrations in a South Indian population. Int J Tuberc Lung Dis 2016;20: 1231–5. doi: 10.5588/ijtld.15.1007 [DOI] [PubMed] [Google Scholar]
- [20].Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, & Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 2003;306: 287–300. [DOI] [PubMed] [Google Scholar]
- [21].Kwara A, Lartey M, Sagoe KW, & Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS 2011;25: 388–90. doi: 10.1097/QAD.0b013e3283427e05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis 2015;211: 197–205. doi: 10.1093/infdis/jiu429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luetkemeyer AF, Rosenkranz SL, Lu D, Grinsztejn B, Sanchez J, Ssemmanda M, et al. Combined effect of CYP2B6 and NAT2 genotype on plasma efavirenz exposure during rifampin-based antituberculosis therapy in the STRIDE study. Clin Infect Dis 2015;60: 1860–3. doi: 10.1093/cid/civ155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009;19: 300–9. doi: 10.1097/FPC.0b013e328328d577 [DOI] [PubMed] [Google Scholar]
- [25].Odone A, Houben RM, White RG, & Lonnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol 2014;2: 754–64. doi: 10.1016/S2213-8587(14)70164-0 [DOI] [PubMed] [Google Scholar]
- [26].Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007;11: 1049–61. [PubMed] [Google Scholar]
- [27].Oni T, Youngblood E, Boulle A, McGrath N, Wilkinson RJ, & Levitt NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015;15: 20. doi: 10.1186/s12879-015-0750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lonnroth K, Roglic G, & Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol 2014;2: 730–9. doi: 10.1016/S2213-8587(14)70109-3 [DOI] [PubMed] [Google Scholar]
- [29].International Diabetes Federation. (2013). IDF Diabetes Atltas; 6th edition, 2013, from http://www.idf.org/diabetesatlas [Google Scholar]
- [30].Sen T, Joshi SR, & Udwadia ZF. Tuberculosis and diabetes mellitus: merging epidemics. J Assoc Physicians India 2009;57: 399–404. [PubMed] [Google Scholar]
- [31].van Crevel R, van de Vijver S, & Moore DAJ. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol 2017;5: 457–68. doi: 10.1016/S2213-8587(16)30081-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 2007;45: 428–35. doi: 10.1086/519841 [DOI] [PubMed] [Google Scholar]
- [33].Reis-Santos B, Gomes T, Locatelli R, de Oliveira ER, Sanchez MN, Horta BL, et al. Treatment outcomes in tuberculosis patients with diabetes: a polytomous analysis using Brazilian surveillance system. PLoS One 2014;9: e100082. doi: 10.1371/journal.pone.0100082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang JY, Lee MC, Shu CC, Lee CH, Lee LN, Chao KM, et al. Optimal duration of anti-TB treatment in patients with diabetes: nine or six months? Chest 2015;147: 520–8. doi: 10.1378/chest.14-0918 [DOI] [PubMed] [Google Scholar]
- [35].Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis 2006;43: 848–54. doi: 10.1086/507543 [DOI] [PubMed] [Google Scholar]
- [36].Heysell SK, Moore JL, Keller SJ, & Houpt ER. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 2010;16: 1546–53. doi: 10.3201/eid1610.100374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ruslami R, Nijland HM, Adhiarta IG, Kariadi SH, Alisjahbana B, Aarnoutse RE, et al. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother 2010;54: 1068–74. doi: 10.1128/AAC.00447-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Medellin-Garibay SE, Cortez-Espinosa N, Milan-Segovia RC, Magana-Aquino M, Vargas-Morales JM, Gonzalez-Amaro R, et al. Clinical Pharmacokinetics of Rifampin in Patients with Tuberculosis and Type 2 Diabetes Mellitus: Association with Biochemical and Immunological Parameters. Antimicrob Agents Chemother 2015;59: 7707–14. doi: 10.1128/aac.01067-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kumar AK, Chandrasekaran V, Kannan T, Murali AL, Lavanya J, Sudha V, et al. Anti-tuberculosis drug concentrations in tuberculosis patients with and without diabetes mellitus. Eur J Clin Pharmacol 2017;73: 65–70. doi: 10.1007/s00228-016-2132-z [DOI] [PubMed] [Google Scholar]
- [40].Degner NR, Wang JY, Golub JE, & Karakousis PC. Metformin Use Reverses the Increased Mortality Associated With Diabetes Mellitus During Tuberculosis Treatment. Clin Infect Dis 2018;66: 198–205. doi: 10.1093/cid/cix819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kumar NP, Banurekha VV, Nair D, Sridhar R, Kornfeld H, Nutman TB, et al. Coincident pre-diabetes is associated with dysregulated cytokine responses in pulmonary tuberculosis. PLoS One 2014;9: e112108. doi: 10.1371/journal.pone.0112108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumar NP, George PJ, Kumaran P, Dolla CK, Nutman TB, & Babu S. Diminished systemic and antigen-specific type 1, type 17, and other proinflammatory cytokines in diabetic and prediabetic individuals with latent Mycobacterium tuberculosis infection. J Infect Dis 2014;210: 1670–8. doi: 10.1093/infdis/jiu329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Andrade BB, Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, et al. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest 2014;145: 1244–54. doi: 10.1378/chest.13-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab 2012;15: 518–33. doi: 10.1016/j.cmet.2012.01.023 [DOI] [PubMed] [Google Scholar]
- [45].Luo P, & Wang MH. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat 2011;95: 1–10. doi: 10.1016/j.prostaglandins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hardwick JP, Eckman K, Lee YK, Abdelmegeed MA, Esterle A, Chilian WM, et al. Eicosanoids in metabolic syndrome. Adv Pharmacol 2013;66: 157–266. doi: 10.1016/B978-0-12-404717-4.00005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Filgueiras LR, Brandt SL, Wang S, Wang Z, Morris DL, Evans-Molina C, et al. Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci Signal 2015;8: ra10. doi: 10.1126/scisignal.2005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest 2005;115: 1601–6. doi: 10.1172/JCI23949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014;511: 99–103. doi: 10.1038/nature13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lonnroth K, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health 2010;15: 1300–14. doi: 10.1111/j.1365-3156.2010.02632.x [DOI] [PubMed] [Google Scholar]
- [51].Laurence Y, Critchley J, Livia R, Grint D, Hill P, Panduru N, et al. Costs per accurate diagnosis of bi-directional screening in Indonesia and Romania: integrating tuberculosis and diabetes services. 2017. Presented at the 48th Union World Conference on Lung Health. [Google Scholar]
- [52].Alisjahbana B, Ronacher K, Ugarte-Gil C, Riza A, Grint D, Critchley J, et al. Can bi-directional screening for diabetes and tuberculosis efficiently identify cases of co-morbidity? Abstract PD-687–27. 2016. Presented at the 47th Union World Conference on Lung Health. [Google Scholar]
- [53].Pan SC, Ku CC, Kao D, Ezzati M, Fang CT, & Lin HH. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. Lancet Diabetes Endocrinol 2015;3: 323–30. doi: 10.1016/s2213-8587(15)00042-x [DOI] [PubMed] [Google Scholar]
- [54].Ugarte-Gil C, Alarcon V, Figueroa C, Moore D, & Golub J. Epidemiological characteristics and treatment outcomes among Peruvian MDR-TB patients with and without diabetes, Abstract PD-863–28. 2016. Presented at the 47th Union World Conference on Lung Health. [Google Scholar]
- [55].Petruccioli E, Scriba TJ, Petrone L, Hatherill M, Cirillo DM, Joosten SA, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J 2016;48: 1751–63. doi: 10.1183/13993003.01012-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zak DE, Penn-Nicholson A, Scriba TJ, Thompson E, Suliman S, Amon LM, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. The Lancet 2016. doi: 10.1016/s0140-6736(15)01316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Darboe F, Mbandi SK, Thompson EG, Fisher M, Rodo M, van Rooyen M, et al. Diagnostic performance of an optimized transcriptomic signature of risk of tuberculosis in cryopreserved peripheral blood mononuclear cells. Tuberculosis (Edinb) 2018;108: 124–6. doi: 10.1016/j.tube.2017.11.001 [DOI] [PubMed] [Google Scholar]
- [58].Penn-Nicholson A, Scriba TJ, Hatherill M, White RG, & Sumner T. A novel blood test for tuberculosis prevention and treatment. S Afr Med J 2016;107: 4–5. doi: 10.7196/SAMJ.2016.v107.i1.12230 [DOI] [PubMed] [Google Scholar]
- [59].Darboe F, Mbandi SK, Yende-Zuma N, Thompson E, Duffy F, Fisher M, et al. A transcriptomic risk signature predicts subclinical TB in HIV-infected persons on highly active antiretroviral therapy 2018. Presented at the Keystone Symposium Tuberculosis: Translating Scientific Findings for Clinical and Public Health Impact, Whistler, Canada. [Google Scholar]
- [60].Thompson EG, Du Y, Malherbe ST, Shankar S, Braun J, Valvo J, et al. Host blood RNA signatures predict the outcome of tuberculosis treatment. Tuberculosis (Edinb) 2017;107: 48–58. doi: 10.1016/j.tube.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Suliman S, Thompson E, Sutherland J, Weiner Rd J, Ota MOC, Shankar S, et al. Four-gene Pan-African Blood Signature Predicts Progression to Tuberculosis. Am J Respir Crit Care Med 2018. doi: 10.1164/rccm.201711-2340OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yates TA, Khan PY, Knight GM, Taylor JG, McHugh TD, Lipman M, et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016;16: 227–38. doi: 10.1016/S1473-3099(15)00499-5 [DOI] [PubMed] [Google Scholar]
- [63].Middelkoop K, Mathema B, Myer L, Shashkina E, Whitelaw A, Kaplan G, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis 2015;211: 53–61. doi: 10.1093/infdis/jiu403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Buu TN, van Soolingen D, Huyen MN, Lan NN, Quy HT, Tiemersma EW, et al. Tuberculosis acquired outside of households, rural Vietnam. Emerg Infect Dis 2010;16: 1466–8. doi: 10.3201/eid1609.100281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Horna-Campos OJ, Consiglio E, Sanchez-Perez HJ, Navarro A, Cayla JA, & Martin-Mateo M. Pulmonary tuberculosis infection among workers in the informal public transport sector in Lima, Peru. Occup Environ Med 2011;68: 163–5. doi: 10.1136/oem.2009.051128 [DOI] [PubMed] [Google Scholar]
- [66].Glynn JR, Guerra-Assuncao JA, Houben RM, Sichali L, Mzembe T, Mwaungulu LK, et al. Whole Genome Sequencing Shows a Low Proportion of Tuberculosis Disease Is Attributable to Known Close Contacts in Rural Malawi . PLoS One 2015;10: e0132840. doi: 10.1371/journal.pone.0132840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ypma RJ, Altes HK, van Soolingen D, Wallinga J, & van Ballegooijen WM. A sign of superspreading in tuberculosis: highly skewed distribution of genotypic cluster sizes. Epidemiology 2013;24: 395–400. doi: 10.1097/EDE.0b013e3182878e19 [DOI] [PubMed] [Google Scholar]
- [68].Middelkoop K, Bekker LG, Mathema B, Myer L, Shashkina E, Whitelaw A, et al. Factors affecting tuberculosis strain success over 10 years in a high TB- and HIV-burdened community. Int J Epidemiol 2014;43: 1114–22. doi: 10.1093/ije/dyu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Grandjean L, Gilman RH, Martin L, Soto E, Castro B, Lopez S, et al. Transmission of Multidrug-Resistant and Drug-Susceptible Tuberculosis within Households: A Prospective Cohort Study. PLoS Med 2015;12: e1001843; discussion e. doi: 10.1371/journal.pmed.1001843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Knight GM, Colijn C, Shrestha S, Fofana M, Cobelens F, White RG, et al. The Distribution of Fitness Costs of Resistance-Conferring Mutations Is a Key Determinant for the Future Burden of Drug-Resistant Tuberculosis: A Model-Based Analysis. Clin Infect Dis 2015;61Suppl 3: S147–54. doi: 10.1093/cid/civ579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shah NS, Auld SC, Brust JC, Mathema B, Ismail N, Moodley P, et al. Transmission of Extensively Drug-Resistant Tuberculosis in South Africa. N Engl J Med 2017;376: 243–53. doi: 10.1056/NEJMoa1604544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58: 470–80. doi: 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- [73].Getahun H, Matteelli A, Chaisson RE, & Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015;372: 2127–35. doi: 10.1056/NEJMra1405427 [DOI] [PubMed] [Google Scholar]
- [74].Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, & Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001;358: 1687–93. doi: 10.1016/S0140-6736(01)06712-5 [DOI] [PubMed] [Google Scholar]
- [75].Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, & Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis 2012;54: 784–91. doi: 10.1093/cid/cir951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Dowdy DW, Golub JE, Chaisson RE, & Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A 2012;109: 9557–62. doi: 10.1073/pnas.1203517109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Basu S, Stuckler D, & McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg 2011;84: 30–7. doi: 10.4269/ajtmh.2011.10-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dowdy DW, Azman AS, Kendall EA, & Mathema B. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin Infect Dis 2014;59: 1123–9. doi: 10.1093/cid/ciu506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, & Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med 2010;7: e1000381. doi: 10.1371/journal.pmed.1000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stuckler D, Basu S, McKee M, & King L. Mass incarceration can explain population increases in TB and multidrug-resistant TB in European and central Asian countries. Proc Natl Acad Sci U S A 2008;105: 13280–5. doi: 10.1073/pnas.0801200105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sacchi FP, Praca RM, Tatara MB, Simonsen V, Ferrazoli L, Croda MG, et al. Prisons as reservoir for community transmission of tuberculosis, Brazil. Emerg Infect Dis 2015;21: 452–5. doi: 10.3201/eid2103.140896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bourdillon PM, Goncalves CC, Pelissari DM, Arakaki-Sanchez D, Ko AI, Croda J, et al. Increase in Tuberculosis Cases among Prisoners, Brazil, 2009–20141. Emerg Infect Dis 2017;23: 496–9. doi: 10.3201/eid2303.161006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pedrazzoli D, Boccia D, Dodd PJ, Lonnroth K, Dowdy DW, Siroka A, et al. Modelling the social and structural determinants of tuberculosis: opportunities and challenges. Int J Tuberc Lung Dis 2017;21: 957–64. doi: 10.5588/ijtld.16.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]