Abstract
The purpose of this study was to determine whether budesonide– and indomethacin–hydroxypropyl-β-cyclodextrin (HPBCD) complexes could be formed using a supercritical fluid (SCF) process. The process involved the exposure of drug-HPBCD mixtures to supercritical carbon dioxide (SC CO2). The ability of the SCF process to form complexes was assessed by determining drug dissolution, drug crystallinity, and drug-excipient interactions. Drug dissolution was assessed using a HPLC assay. Crystallinity was assessed using powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC). Drug-excipient interactions were characterized using Fourier transform infrared spectroscopy (FTIR). Scanning electron microscopy (SEM) was used to determine any morphological changes. SC CO2 process did not alter the dissolution rate of pure drugs but resulted in two- and three-fold higher dissolution rates for budesonide– and indomethacin–HPBCD mixtures, respectively. SCF-processed mixtures exhibited a disappearance of the crystalline peaks of the drugs (PXRD), a partial or complete absence of the melting endotherm of the drugs (DSC), and a shift in the C=O stretching of the carboxyl groups of the drugs (FTIR), consistent with the loss of drug crystallinity and formation of intermolecular bonds with HPBCD. SEM indicated no discernible drug crystals upon physical mixing with or without SCF processing. Thus, budesonide– and indomethacin–HPBCD complexes with enhanced dissolution rate can be formed using a single-step, organic solvent-free SC CO2 process.
Keywords: Budesonide, Carbon dioxide, Complexation, Cyclodextrin, Indomethacin, Supercritical fluid
1. Introduction
The absorption of poorly soluble drugs across biological barriers is limited by their dissolution rate in the biological fluids (Horter and Dressman, 2001; Martinez and Amidon, 2002). Complexation of poorly soluble drugs with cyclodextrins is a useful approach to improve drug dissolution (Albers and Muller, 1995; Thompson, 1997; Pitha and Pitha, 1985; Loftsson and Brewster, 1996) and to improve drug stability (Koushik et al., 2001). The formulation methods currently employed for the preparation of drug-cyclodextrin complexes including granulation, kneading, solvent evaporation, grinding, milling, and compaction are often time-consuming and involve multi-stage processing (Albers and Muller, 1995; Thompson, 1997). Formulations derived from granulation and kneading result in residual water, which could act as a plasticizer that increases the molecular mobility and hence solid-state reactivity (Guo et al., 2000). The solvent evaporation method requires excessive organic solvent usage and results in high residual solvent content, often requiring additional processing steps for the removal of residual solvents. Methods, such as grinding, milling, and compaction, often used in pharmaceutical manufacturing for size reduction, are energy intensive and induce surface static charge on drug particles, thereby altering powder flow properties (Parrot, 1976; Garcia et al., 1998). To overcome some of these limitations, an environmentally benign processing method employing supercritical fluids (SCF) has been recently proposed for the preparation of drug-cyclodextrin complexes. The SCF process eliminates multistage processing and produces complexes without using organic solvents (Van Hees et al., 1999; Charoenchaitrakool et al., 2002).
By definition, a fluid whose temperature and pressure are simultaneously higher than its critical temperature and critical pressure is defined as a supercritical fluid. Supercritical fluids, by virtue of their liquid-like densities and gas-like viscosities and diffusivities, offer good solubilizing and mixing properties (York, 1999; Kompella and Koushik, 2001; Sunkara and Kompella, 2002). Moreover, in the supercritical region, these properties exhibit high rates of change in response to small changes in temperature and/or pressure. With slight changes in temperature and/or pressure, the physicochemical properties of a substance in the supercritical region can be adjusted from gas-like to liquid-like. As a result, the solvent power and diffusivity of the supercritical fluids can be varied enormously. Supercritical CO2 is the most commonly used SCF for pharmaceutical processing because it offers several advantages. These include its (a) low critical temperature of 31 °C, which allows processing of heat labile drugs, (b) low cost ($0.05–0.07 per lb), (c) nontoxic nature, (d) nonflammable nature, (e) recyclable nature, and (f) GRAS (generally recognized as safe) status (Kompella and Koushik, 2001; Sunkara and Kompella, 2002).
Budesonide (molecular weight: 430.5), a potent anti-inflammatory corticosteroid is currently marketed as a dry powder inhaler (DPI; Pulmicort®), an aqueous nasal spray (Rhinocort®), and an ileal release capsule (Entocort®) for the treatment of asthma, allergic rhinitis, and Crohn’s disease, respectively (Mutlu et al., 2002; Barnes, 2002). In addition, budesonide is of potential value as a chemopreventive agent for lung cancer (Wattenberg et al., 1997). For the first time, we observed that budesonide inhibits the expression of vascular endothelial growth factor (VEGF) and multidrug resistance-associated protein (MRP1), two key proteins that are overexpressed in various tumors including lung tumors (Bandi and Kompella, 2001; Bandi and Kompella, 2002). Budesonide, with a log P of 3.2, is practically insoluble in water (20 μg ml−1) at physiological pH, which may be limiting the therapeutic potential of budesonide. Development of budesonide formulations that can enhance drug solubility and dissolution rate in biological fluids will likely achieve higher tissue concentrations and effectiveness in some conditions. In addition, in the solution form, the drug is less likely to be cleared by the mucocilliary apparatus in the respiratory tract. Indomethacin (molecular weight: 357.8) is another anti-inflammatory agent, which is practically insoluble (2.5 μg ml−1) in water and has the ability to inhibit VEGF (Szabo et al., 2001). Thus, the objective of the present study was to determine whether HPBCD complexes of budesonide and indomethacin could be formed using a single-step, organic-solvent-free supercritical fluid process employing CO2 as the supercritical fluid.
2. Materials and methods
2.1. Materials
Budesonide, indomethacin, potassium dihydrogen phosphate, phenolphthalein, sodium carbonate, sodium phosphate (monobasic and dibasic), and sodium chloride were obtained from Sigma Chemical Co. (St. Louis, MO). Hydroxypropyl β-cyclodextrin (HPBCD) was a gift from American Maize Products (Indianapolis Boulevard, IN). HPLC grade methylene chloride and acetonitrile were obtained from Fisher Scientific (Pittsburgh, PA). Carbon dioxide (99.95%), obtained from Linweld (Lincoln, NE) was used as the supercritical fluid.
2.2. SCF process for the formation of drug-cyclodextrin complexes
Budesonide and indomethacin–HPBCD complexes were formed using the SCF set-up shown in Fig. 1. The SCF setup consisted of a high-pressure vessel (High Pressure Equipment Company, Erie, PA), a manual pump (High Pressure Equipment Company, Erie, PA), a CO2 cylinder, and valves (High Pressure Equipment Company, Erie, PA) at appropriate locations for regulating the CO2 flow. The pressure inside the high-pressure vessel was monitored using a pressure gauge (Psi-tronix, P&R Intra Supply, Inc., Atlanta, GA) and the vessel was maintained in a temperature-controlled bath. Accurately weighed amounts of drug-HPBCD mixtures were placed in the vessel and the vessel was tightly sealed. The vessel was placed in a water bath and the contents were allowed to equilibrate for 10 min. By opening the valves V1 and V2, CO2 from the cylinder was allowed to enter into the pre-cooled manually driven pump. During this stage, valves V3 and V4 remained closed. Once the pump was filled, as indicated by the pressure gauge, V1 was closed and V3 was opened. Upon opening of V3, CO2 from the pump entered into the high-pressure vessel. By manually operating the pump, the pressure inside the vessel could be increased or decreased. Once the pressure inside the high-pressure vessel reached a desired value, V3 was closed and the high-pressure vessel was allowed to remain in this condition for discrete time periods. Following the SCF-treatment period, V4 was opened to depressurize the system over 30 s. The conditions used for preparing budesonide–HPBCD (1:10%, w/w; 0.31:1 molar ratio) and indomethacin–HPBCD (1:5 and 1:10%, w/w; 0.85:1 and 0.35:1 molar ratios) complexes were–temperature: 40 °C; pressure: 211 bar; exposure time: 20 h. The same conditions were utilized to determine the effect of SCF processing on budesonide, indomethacin, and HPBCD alone. Under these optimized conditions, three batches (n = 3) of each formulation were prepared for further characterization.
Fig. 1.
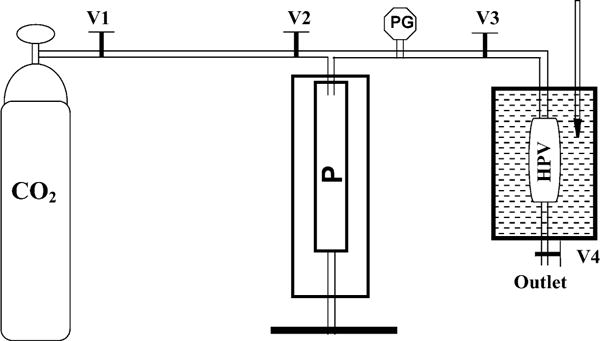
Supercritical fluid (SCF) setup for processing cyclodextrin complexes. CO2: carbon dioxide source; P: pump; PG: pressure gauge; HPV: high-pressure vessel; and V1, V2, V3, V4: valves 1, 2, 3, and 4, respectively.
2.3. Scanning electron microscopy (SEM)
The shape and surface characteristics of drugs, physical mixtures, and SCF-processed physical mixtures were visualized using a scanning electron microscope (JSM-5510, JEOL USA, Peabody, MA). Briefly, the samples were sputter coated with gold–palladium under an argon atmosphere using a gold sputter module in a high vacuum evaporator and the samples were examined using SEM set at 15 kV.
2.4. Powder X-ray diffraction analysis (PXRD)
The powder X-ray diffraction patterns of various powders were obtained using a Rigaku D/Max-B diffractometer (Rigaku, Slidell, LA) with Bragg-Brentano parafocusing geometry, a diffracted beam monochromator, and a conventional copper target X-ray tube set to 40 kV and 30 mA with Cu Kα radiation. The 2θ scan range was 5–35°, with a step size of 0.03° and the scan speed was 2.5° min−1.
2.5. Differential scanning calorimetry (DSC)
The DSC analysis for various powders was carried out using a differential scanning calorimeter (Shimadzu DSC-50 System, Shimadzu Scientific Instruments, Inc., Columbia, MD). During each scan, 1–2 mg of the sample was heated in a hermetically sealed aluminum pan at a heating rate of 10°C min−1 under a nitrogen atmosphere (flow rate 20 ml min−1), using an empty aluminum pan as the reference. All the samples were heated in the range of 25–350 °C.
2.6. Fourier transform infrared spectroscopic analysis (FTIR)
FTIR spectra for the various powders were obtained using a Nicolet FTIR spectrometer with a DTGS detector. Crystalline KBr (FTIR grade, 99%), obtained from Aldrich Chemical (San Francisco, LA) dried overnight at 120 °C before use. The samples for analysis were mixed with dry KBr at about 1:100 (sample: KBr) ratio and pressed to form KBr pellets. For each sample, 256 scans were collected at a resolution of 2 cm−1 over the wavenumber region 4000–400 cm−1.
2.7. Solubility studies
The solubility studies for various powders were performed according to the method of Higuchi and Connors (Higuchi and Connors, 1965). Briefly, the substances were added to screw capped vials containing 2 ml of phosphate buffered saline (pH 7.4; 5–15 mM) and the vials were allowed to shake at room temperature for 48 h in a thermostatically controlled shaking water bath. Following this, the vials were centrifuged at 15,000 × g for 15 min and the supernatants were estimated for budesonide or indomethacin using HPLC or UV-spectroscopy. The solubility of HPBCD was determined by using a previously described colorimetric assay (Makela et al., 1987).
2.8. Dissolution study
Dissolution experiments were carried out with a Hanson Dissolution Test Station (Model #64-700-121; Hanson Research, Ocean, NJ). The dissolution study was conducted at 37 °C in PBS (pH 7.4) using the basket method at a rotation speed of 50rpm. The dissolution study was performed for budesonide or indomethacin alone, physical mixtures, and the SCF-processed mixtures. Briefly 6 mg of budesonide or 3 mg of indomethacin or an equivalent amount of the SCF-processed complex or the physical mixture was filled in a #2 gelatin capsule, sealed, and rotated in a USP basket. At appropriate time intervals, 2 ml of the dissolution medium was withdrawn and immediately replaced with fresh medium. The dissolved budesonide or indomethacin was analyzed using a HPLC assay as described below.
2.9. HPLC assay
A previously described reverse phase HPLC method was used for quantifying budesonide (Martin et al., 2002). Indomethacin was also quantified using the same HPLC conditions. Budesonide and indomethacin were monitored at 250 and 318 nm, respectively. The run time for the assay was 12 min and the retention times for budesonide and indomethacin were 5.2 and 6.5 min, respectively.
2.10. Statistical analysis
Statistical analysis was performed by comparing mean values between the different treatments using two way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis using SPSS (version 8.0) software. Differences were considered statistically significant at P < 0.05.
3. Results
The investigations indicated that the SCF process was capable of formulating drug:HPBCD complexes with minimal loss of the drug and HPBCD. The powder yield at the end of SCF processing was 98.5 ± 0.1%, 95.7 ± 4%, and 98.7 ± 0.3% (mean ± S.D. for n = 3) for budesonide:HPBCD (1:10%, w/w), indomethacin:HPBCD (1:5%, w/w), and indomethacin:HPBCD (1:10%, w/w) mixtures, respectively. A HPLC assay indicated that the drug content in these SCF processed mixtures was 94, 89, and 96% of the initial values, respectively.
3.1. Morphology
SEM pictures of budesonide with and without SCF processing indicated small needle shaped crystals (Fig. 2B and C). SEM pictures of indomethacin with and without SCF processing indicated large plate-like crystals with irregular borders (Fig. 2F and G), which became inconspicuous following physical mixing with HPBCD with or without SCF-processing. Following SCF processing of the drug-HPBCD physical mixtures, occasional fusion or aggregation of particles was apparent (Fig. 2I).
Fig. 2.
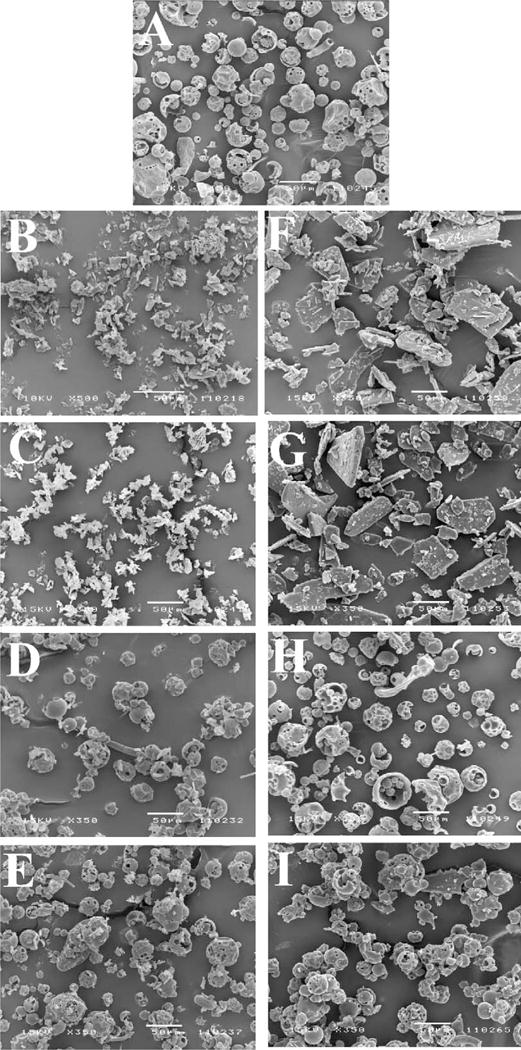
Scanning electron microscope (SEM) pictures of SCF-processed drug-HPBCD complexes: (A) HPBCD; (B) budesonide; (C) SCF-processed budesonide; (D) physical mixture of budesonide–HPBCD (1:10); (E) SCF-processed budesonide–HPBCD mixture (1:10); (F) indomethacin; (G) SCF-processed indomethacin; (H) physical mixture of indomethacin–HPBCD (1:10); and (I) SCF-processed indomethacin–HPBCD mixture (1:10). Conditions for SCF processing: temperature: 40°C; pressure: 211 bar; exposure time: 20 h.
3.2. Powder X-ray diffraction studies
The PXRD patterns for budesonide (2θ: 5.96, 11.96, 14.42, 15.35, 15.92, 18.35, and 22.79), indomethacin (2θ: 11.57, 19.58, 20.81, 21.77, 26.51, and 29.3), and SCF-processed budesonide/indomethacin indicated characteristic peaks (Fig. 3A and B), suggesting that budesonide and indomethacin exist in a crystalline form and SCF processing does not alter the crystallinity of these drugs. Physical mixtures of drug and HPBCD indicated crystalline drug peaks, although of a lower intensity. However, the PXRD pattern of the SCF-processed drug-HPBCD mixture was devoid of crystalline drug peaks (Fig. 3A and B).
Fig. 3.
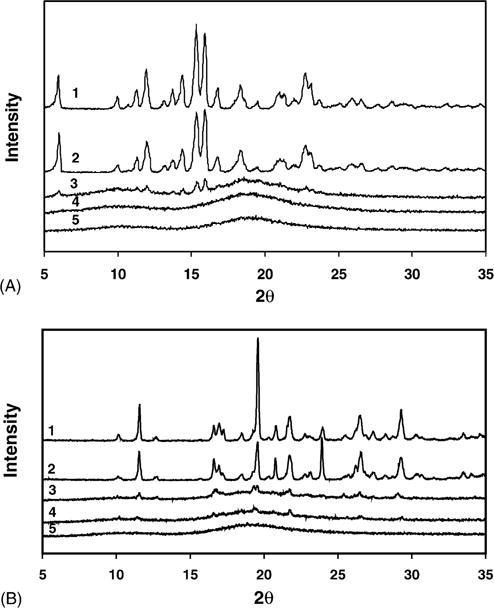
Powder X-ray diffraction (PXRD) analysis of (A) SCF-processed budesonide–HPBCD mixture and (B) SCF-processed indomethacin–HPBCD mixture. (A) PXRD patterns of (1) budesonide; (2) SCF-processed budesonide; (3) budesonide–HPBCD (1:10) physical mixture; (4) SCF-processed budesonide–HPBCD (1:10) mixture; and (5) HPBCD. (B) PXRD patterns of (1) indomethacin; (2) SCF-processed indomethacin; (3) indomethacin–HPBCD (1:10) physical mixture; (4) SCF-processed indomethacin–HPBCD (1:10) mixture; and (5) HPBCD. Conditions for SCF processing: temperature: 40 °C; pressure: 211 bar; exposure time: 20 h.
3.3. Differential scanning calorimetry (DSC) studies
The DSC curve for budesonide (255–260 °C) (Martin et al., 2002), indomethacin (159–161 °C), and SCF-processed budesonide/indomethacin indicated characteristic melting endotherms (Fig. 4A and B), indicating that budesonide and indomethacin exist in a crystalline form and SCF processing does not alter the crystallinity of these drugs. Although the physical mixture indicated endothermic peaks for budesonide (245–255 °C) and indomethacin (147–152 °C), the SCF-processed physical mixture indicated the absence of the drug peaks (Fig. 4A and B), suggesting the amorphous nature of drugs in the SC CO2-processed mixture. HPBCD did not exhibit an endothermic peak, consistent with its amorphous nature.
Fig. 4.
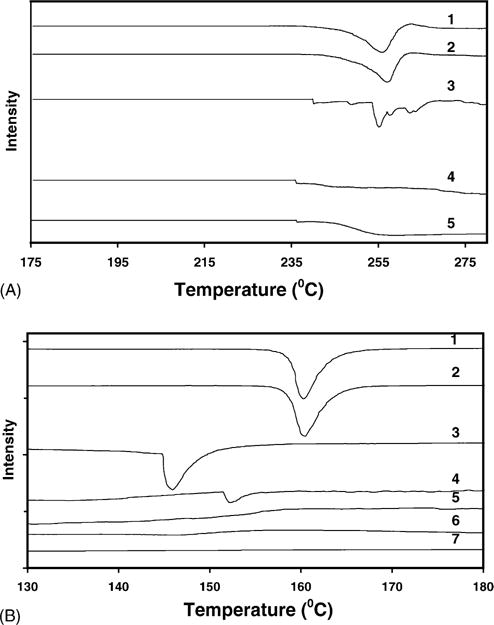
Differential scanning calorimetric (DSC) analysis of (A) SCF-processed budesonide–HPBCD mixture and (B) SCF-processed indomethacin–HPBCD mixture. (A) DSC analysis of (1) pure budesonide; (2) SCF-processed budesonide; (3) budesonide–HPBCD (1:10) physical mixture; and (4) SCF-processed budesonide–HPBCD mixture (1:10); and (5) pure HPBCD. (B) DSC analysis of (1) pure indomethacin; (2) SCF-processed indomethacin; (3) indomethacin–HPBCD (1:5) physical mixture; (4) indomethacin–HPBCD (1:10) physical mixture; (5) SCF-processed indomethacin–HPBCD (1:5) mixture; (6) SCF-processed indomethacin–HPBCD (1:10) mixture; and (7) HPBCD. Conditions for SCF processing: temperature: 40 °C; pressure: 211 bar; exposure time: 20 h.
3.4. Fourier transform infrared spectroscopy (FTIR) studies
3.4.1. Budesonide
FTIR spectrum of budesonide indicated carbonyl stretching bands at 1722 and 1673 cm−1 (Fig. 5a). The vibrations in the 1600–1900 cm−1 region of the FTIR spectrum indicate the C=O stretch. Budesonide contains two C=O groups; a dihydrobenzoquinone C=O group and an acetyl C=O group. Acetyl C=O and dihydrobenzoquinone C=O groups exhibit a stretch in the region of 1700–1900 and 1600–1750 cm−1, respectively. Thus, the bands at 1722 and 1673 cm−1 in the budesonide spectrum (Fig. 5A) correspond to the non-conjugated acetyl C=O stretch and conjugated dihydrobenzoquinone C=O groups, respectively. Budesonide–HPBCD physical mixture resulted in a similar spectrum. However, the SCF-processed budesonide–HPBCD mixture exhibited a shift in the conjugated C=O stretching band from 1673 to 1662cm−1, suggesting a possible change in the environment of the conjugated C=O group of budesonide.
Fig. 5.
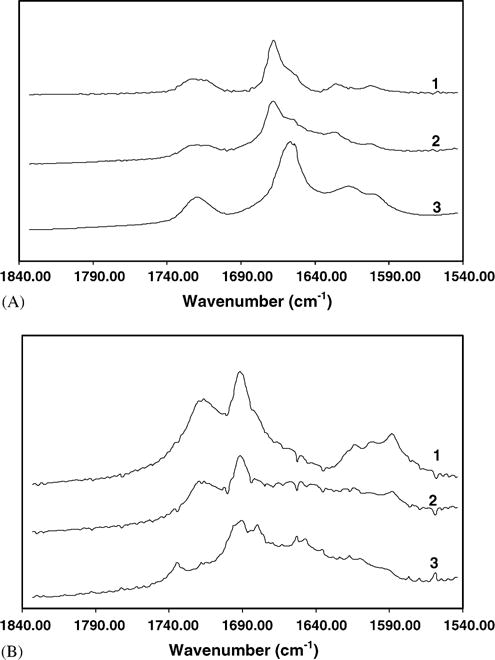
FTIR analysis of (A) SCF-processed budesonide–HPBCD mixture and (B) SCF-processed indomethacin–HPBCD mixture. (A) FTIR analysis of (1) pure budesonide; (2) budesonide–HPBCD (1:10) physical mixture; and (3) SCF-processed budesonide–HPBCD mixture. (B) FTIR analysis of (1) pure indomethacin; (2) indomethacin–HPBCD (1:10) physical mixture; and (3) SCF-processed indomethacin–HPBCD (1:10) mixture. Conditions for SCF processing: temperature: 40 °C; pressure: 211 bar; exposure time: 20 h.
3.4.2. Indomethacin
The FTIR spectrum of indomethacin indicated carbonyl-stretching bands at 1696, 1721, and 1724 cm−1 (Fig. 5B). Indomethacin contains a benzoyl carbonyl and an acid carbonyl group. Peaks at 1696 cm−1 correspond to the benzoyl carbonyl stretch and those at 1721 and 1724 cm−1 correspond to the acid carbonyl stretch (Taylor and Zografi, 1997). Although physical mixture exhibited a similar spectrum, the SCF-processed mixture indicated a shift in the benzoyl and acid carbonyl group peaks to 1690 and 1732 cm−1, respectively, indicating possible intermolecular interactions of indomethacin with HPBCD.
3.5. Aqueous solubility
Solubility studies at the end of 48 h indicated an aqueous solubility of 23.5 ± 1.3μg ml−1 and 2.53 ± 0.5 μg ml−1 for budesonide and indomethacin, respectively (Table 1A and Table 1B). For both the drugs, no further increase in solubility was observed at the end of 4 and 7 days. The observed budesonide solubility was in close agreement with that reported by Wiedmann et al. (2000). Budesonide–HPBCD physical mixture significantly increased budesonide solubility to 258.5 ± 53 μg ml−1 and SCF processed physical mixture further enhanced budesonide solubility to 529 ±51 μg ml−1 (Table 1A). On the other hand, indomethacin–HPBCD physical mixtures significantly increased indomethacin solubility with the solubilities being 25 ± 4.2 μg ml−1 and 70 ± 18μg ml−1 for the 1:5 and 1:10 ratios, respectively. SCF-processed physical mixtures (1:5 and 1:10) further enhanced indomethacin solubility to 50 ± 2.5 μg ml−1 and 117 ± 2.4μg ml−1, in the 1:5 and 1:10 mixtures, respectively (Table 1B). The solubility of HPBCD before (64 ± 7%, w/v) and after SCF processing (65 ± 4%, w/v) was not statistically different, suggesting that SC CO2 does not alter the solubility of HPBCD.
Table 1A.
Aqueous solubility of Budesonide
| Aqueous solubility (μg ml−1)a |
|
|---|---|
| Pure budesonide | 23.5 ± 2.3 |
| SCF-processed budesonide | 21.5 ± 1.6 |
| Budesonide–HPBCD mixture (1:10) | 258.5 ± 53b |
| SCF-processed budesonide–HPBCD mixture (1:10) | 529 ± 51c |
Solubility data at the end of 48 h expressed as mean ± S.D. for n = 3.
Indicates P < 0.05 between pure budesonide and untreated physical mixture.
Indicates P < 0.05 between untreated physical mixture and SCF-treated physical mixture.
Table 1B.
Aqueous Solubility of Indomethacin
| Aqueous solubility (μg ml−1)a |
|
|---|---|
| Pure indomethacin | 2.5 ± 0.5 |
| SCF-processed indomethacin | 4.1 ± 0.6b |
| Indomethacin–HPBCD mixture (1:5) | 24.5 ± 4.2c |
| SCF-processed indomethacin–HPBCD mixture (1:5) | 49.5 ± 2.5d |
| Indomethacin–HPBCD mixture (1:10) | 70 ± 18 |
| SCF-processed indomethacin–HPBCD mixture (1:10) | 116.6 ± 2.4ef |
Solubility data at the end of 48 h expressed as mean ± S.D. for n = 3.
Indicates P < 0.05 between pure indomethacin and SCF-processed indomethacin.
Indicates P < 0.05 between untreated physical mixture (1:5) and SCF-processed indomethacin.
Indicates P < 0.05 between untreated physical mixture (1:5) and SCF-processed physical mixture (1:5).
Indicates P < 0.05 between SCF-processed physical mixture (1:5) and SCF-processed physical mixture (1:10).
Indicates P < 0.05 between untreated physical mixture (1:10) and SCF-processed physical mixture (1:10).
3.6. Dissolution studies
3.6.1. Budesonide
The aqueous dissolution rate of budesonide was very low with 14.4 ± 1.4% dissolving at the end of 45 min. Upon SCF processing, the percentage of budesonide dissolved (21.3 ± 4.3) was not statistically different from pure budesonide, suggesting that SCF process did not influence the dissolution rate of budesonide. Compared to budesonide or SCF-processed budesonide, the physical mixture of budesonide with HPBCD significantly enhanced budesonide dissolution rate (53 ± 1%). SCF-processing further increased the dissolution rate to 87 ± 4% (Fig. 6A). The dissolution rate constants were in the order: budesonide ≤ SCF-processed budesonide < budesonide–HPBCD physical mixture < SCF-processed budesonide–HPBCD physical mixture (Table 2A).
Fig. 6.
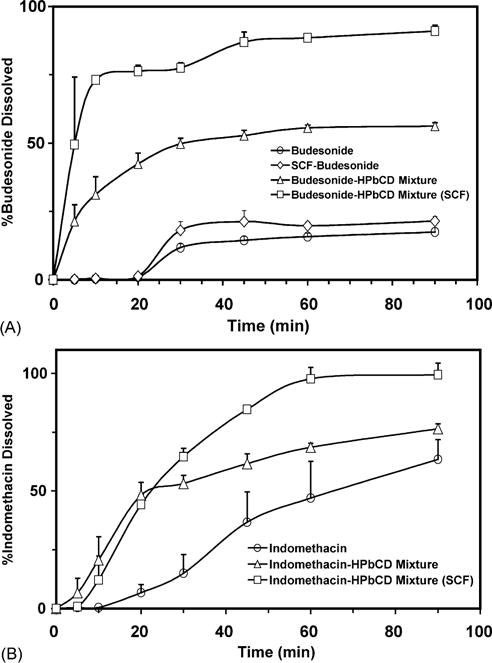
Drug dissolution profiles of (A) SCF-processed budesonide–HPBCD mixture and (B) SCF-processed indomethacin–HPBCD mixture. All the experiments were performed at 37 °C. Data is expressed as mean ± S.D. for n = 3. Conditions for SCF processing: temperature: 40 °C; pressure: 211 bar; exposure time: 20 h.
Table 2A.
Dissolution Rate Constants for Budesonide
| Dissolution rate constanta (k) (min−1) | |
|---|---|
| Budesonide | 0.003 ± 0.0005 |
| SCF-processed budesonide | 0.004 ± 0.001 |
| budesonide–HPBCD mixture (1:10) | 0.008 ± 0.001b |
| SCF-processed budesonide–HPBCD mixture (1:10) | 0.0158 ± 0.005c |
Data is expressed as mean ± S.D. for n = 3.
Indicates P < 0.05 between SCF-processed budesonide and untreated physical mixture.
Indicates P < 0.05 between untreated physical mixture and SCF-treated physical mixture.
3.6.2. Indomethacin
The percentage of indomethacin dissolved at the end of 45 min was 37 ± 13 (Fig. 6B). Compared to pure indomethacin, indomethacin–HPBCD physical mixture indicated an increase in the dissolution rate of indomethacin (62 ± 4.1%). The percentage of indomethacin dissolved with SCF-processed indomethacin–HPBCD physical mixture (85 ± 1) was significantly higher than indomethacin–HPBCD physical mixture (Fig. 6B). The dissolution rate constants were in the order: indomethacin ≤ indomethacin–HPBCD physical mixture < SCF-processed indomethacin–HPBCD physical mixture (Table 2B).
Table 2B.
Dissolution Rate Constants for Indomethacin
| Dissolution rate constanta (k) (min−1) | |
|---|---|
| Indomethacin | 0.014 ± 0.005 |
| Indomethacin–HPBCD mixture (1:10) | 0.018 ± 0.003 |
| SCF-processed indomethacin–HPBCD mixture (1:10) | 0.05 ± 0.003b |
Data is expressed as mean ± SEM for n = 3.
Indicates P < 0.05 between untreated physical mixture and SCF-processed physical mixture.
4. Discussion
The exposure of supercritical CO2 to budesonide– and indomethacin–HPBCD mixtures enhanced the drug dissolution rate compared to untreated physical mixtures. One possible reason for this improved dissolution rate is the formation of solid-state inclusion complexes upon SC CO2 treatment. There are two lines of evidence indicating that SC CO2 exposure facilitates the interaction between the drugs and HPBCD. First, a loss of drug crystallinity was observed with SCF-processing of drug-HPBCD mixtures. Second, a molecular interaction between the drugs and HPBCD in the solid state was observed.
Upon SC CO2 exposure to budesonide, the endothermic peak of budesonide in the DSC analysis was not altered suggesting that the SCF-processing had no effect on the crystalline behavior of budesonide (Fig. 4A). Untreated drug-HPBCD physical mixtures exhibited reduced endotherm intensities for budesonide and indomethacin, an observation consistent with the reduction in drug crystallinity as a result of blending amorphous cyclodextrins with crystalline drugs (Nozawa and Yamamoto, 1989). SCF-processing further reduced or eliminated the crystallinity of budesonide and indomethacin in drug-HPBCD mixture, an observation consistent with the formation of drug-HPBCD inclusion complexes (Van Hees et al., 1999; Charoenchaitrakool et al., 2002; Jain and Adeyeye, 2001; Becket et al., 1999; Tarpani et al., 2000) (Figs. 3 and 4). It appears that indomethacin assumed a new crystalline form in the physical mixtures. Indomethacin has been shown to exist in two monotropic forms, the stable γ form and the metastable α form, with melting points in the range of 159–161 °C and 150–153 °C, respectively (Imaizumi et al., 1983; Khan etal., 2000). Thus, in our study, pure indomethacin likely existed in the γ form and in untreated physical mixtures it transformed to the α form. HPBCD contains up to 8% moisture, which is known to transform indomethacin to the α form (Imaizumi et al., 1983; Khan et al., 2000). This is likely due to the incorporation of the moisture into the crystal lattice of the γ form followed by the plasticizing effect of the sorbed moisture, imparting the necessary molecular mobility for transformation into the α form.
FTIR studies indicated an altered environment of the interatomic (C=O) bonds in budesonide and indomethacin upon SCF processing of the drug-HPBCD mixtures (Fig. 5). The formation of intermolecular hydrogen bonds between the C=O group of the drug and the hydroxyl groups of HPBCD is a possible explanation for this. Our observations are consistent with Tarpani et al. (2000), who reported shifts in the carbonyl groups of zolpidem upon complexation with HPBCD.
The possible explanation for the drug-HPBCD inclusion complex formation is as follows. Upon SC CO2 treatment, HPBCD or drug undergoes a transformation to a molten state enabling the interaction between the two components. Previous studies indicated that an increase in the pressure of supercritical CO2 reduced the melting point and glass transition temperature of ibuprofen and methyl-β-cyclodextrin, respectively, and transformed the methyl-β-cyclodextrin to a molten phase (Charoenchaitrakool et al., 2002; Charoenchaitrakool et al., 2000). Such an effect, which happens due to the sorption of the CO2 into the solute lattice, is likely to facilitate the interaction between the drug and cyclodextrins in the supercritical phase. Solubilization of the drug in the supercritical CO2 also facilitates complex formation. Upon SCF processing of drug-HPBCD mixtures, a certain amount of drug can be dissolved in the supercritical CO2. The extent of drug solubility in the supercritical CO2 is dictated by the supercritical conditions (i.e., temperature and pressure). For instance, the solubility of ibuprofen (log P 3.0) in supercritical CO2 at 35 °C was enhanced 1.8-times when the supercritical pressure was increased from 130 to 220 bar (Charoenchaitrakool et al., 2000). This solubility enhancement in conjunction with the transformation of the cyclodextrins to a molten phase likely enhances drug-HPBCD interactions and complexation. Indeed, previous studies of Martin et al. (2002) suggested significant solubilities of budesonide at 40 °C and pressures greater than 139 bar in CO2. In addition, previous reports indicated the ability of SC CO2 to extract indomethacin (Bodmeier et al., 1995; Bleich and Muller, 1996). Following solubilization of the drug in supercritical CO2, inclusion and/or molecular dispersion of the drug in cyclodextrin are possible due to partitioning of the dissolved drug between the supercritical fluid phase and the hydrophobic cyclodextrin cavity followed by specific molecular interactions including hydrogen bonding and hydrophobic interactions between the drug and the cyclodextrin.
The ability of SC CO2 exposure to enhance drug dissolution in physical mixtures can also be explained by a change in the physical form of the drug and/or HPBCD to a more soluble form. SC CO2 treatment of pure drugs and excipients did not enhance the dissolution of either of these components. However, a possible transformation of the drug or excipient to a more soluble form when present as a physical mixture cannot be ruled out. Another possible explanation for the observed dissolution enhancement is the better mixing of drug and HPBCD powders in the presence of SC CO2. While these mechanisms may contribute to the enhanced drug dissolution rates in part, the data obtained in this study, especially the FTIR studies, clearly indicated the solid-state drug-HPBCD complex formation upon SC CO2 exposure.
The SCF process used in this study might yield free cyclodextrin carrier in the final product in addition to complexes. However, this process offers the advantage of a solvent-free single-step approach that provides high final product yields (≥96%).
5. Conclusions
This study indicated reduced drug crystallinity, altered environment of the C=O bonds in budesonide and indomethacin, and improved drug dissolution rate for SC CO2 exposed budesonide–HPBCD and indomethacin–HPBCD physical mixtures, indicating drug-HPBCD inclusion complex formation. This was achieved in a single step without the use of any organic solvents. Thus, SCF process is an efficient approach for the preparation of budesonide– and indomethacin–HPBCD complexes.
Acknowledgments
This work was supported in part by the NIH grants EY013842 and DK064172. The authors acknowledge the University of Nebraska Medical Center for awarding a graduate fellowship to Nagesh Bandi. The authors are thankful to Dr. Davar Khossravi for his critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers E, Muller BW. Cyclodextrin derivatives in pharmaceutics. Crit Rev Ther Drug Carrier Syst. 1995;12:311–337. doi: 10.1615/critrevtherdrugcarriersyst.v12.i4.20. [DOI] [PubMed] [Google Scholar]
- Bandi N, Kompella UB. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur J Pharmacol. 2001;425:109–116. doi: 10.1016/s0014-2999(01)01192-x. [DOI] [PubMed] [Google Scholar]
- Bandi N, Kompella UB. Budesonide reduces multidrug resistance-associated protein 1 expression in an airway epithelial cell line (Calu-1) Eur J Pharmacol. 2002;437:9–17. doi: 10.1016/s0014-2999(02)01267-0. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. The role of inflammation and anti-inflammatory medication in asthma. Respir Med. 2002;96(Suppl. A):S9–S15. [PubMed] [Google Scholar]
- Becket G, Schep LJ, Tan MY. Improvement of the in vitro dissolution of praziquantel by complexation with α-, β-, and γ-cyclodextrins. Int J Pharm. 1999;179:65–71. doi: 10.1016/s0378-5173(98)00382-2. [DOI] [PubMed] [Google Scholar]
- Bleich J, Muller BW. Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process. J Microencapsul. 1996;13:131–139. doi: 10.3109/02652049609052902. [DOI] [PubMed] [Google Scholar]
- Bodmeier R, Wang H, Dixon DJ, Mawson S, Johnston KP. Polymeric microspheres prepared by spraying into compressed carbon dioxide. Pharm Res. 1995;12:1211–1217. doi: 10.1023/a:1016276329672. [DOI] [PubMed] [Google Scholar]
- Charoenchaitrakool M, Dehghani F, Foster NR, Chan HK. Micronization by RESS to enhance the dissolution rates of poorly water-soluble pharmaceuticals. Ind Eng Chem Res. 2000;39:4794–4802. [Google Scholar]
- Charoenchaitrakool M, Dehghani F, Foster NR. Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-beta-cyclodextrin. Int J Pharm. 2002;239:103–112. doi: 10.1016/s0378-5173(02)00078-9. [DOI] [PubMed] [Google Scholar]
- Garcia TP, Taylor MK, Pande GS. Comparison of the performance of two sample thieves for the determination of the content uniformity of a powder blend. Pharm Dev Technol. 1998;3:7–12. doi: 10.3109/10837459809028474. [DOI] [PubMed] [Google Scholar]
- Guo Y, Byrn SR, Zografi G. Effects of lyophilization on the physical characteristics and chemical stability of amorphous quinapril hydrochloride. Pharm Res. 2000;17:930–935. doi: 10.1023/a:1007519003070. [DOI] [PubMed] [Google Scholar]
- Imaizumi H, Nambu N, Nagai T. Stability and several physical properties of amorphous and crystalline form of indomethacin. Chem Pharm Bull. 1983;28:2565–2569. doi: 10.1248/cpb.28.2565. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- Horter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Del Rev. 2001;46:75–87. doi: 10.1016/s0169-409x(00)00130-7. [DOI] [PubMed] [Google Scholar]
- Jain AC, Adeyeye MC. Hygroscopicity, phase solubility, and dissolution of various substituted sulfobutylether β-cyclodextrins (SBE) and Danazol-SBE inclusion complexes. Int J Pharm. 2001;212:177–186. doi: 10.1016/s0378-5173(00)00607-4. [DOI] [PubMed] [Google Scholar]
- Khan MA, Karnachi AA, Agarwal V, Vaithiyalingam SR, Nazzal S, Reddy IK. Stability characterization of controlled release coprecipitates and solid dispersions. J Controlled Release. 2000;63:1–6. doi: 10.1016/s0168-3659(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Koushik K. Preparation of drug delivery systems using supercritical fluid technology. Crit Rev Ther Drug Carrier Syst. 2001;18:173–199. [PubMed] [Google Scholar]
- Koushik KN, Bandi N, Kompella UB. Interaction of [D-Trp6, Des-Gly10] LHRH ethylamide and hydroxy propyl beta-cyclodextrin (HPBCD): thermodynamics of interaction and protection from degradation by alpha-chymotrypsin. Pharm Dev Technol. 2001;6:595–606. doi: 10.1081/pdt-120000297. [DOI] [PubMed] [Google Scholar]
- Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1 Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- Makela M, Korpela T, Laakso S. Colorimetric determination of beta-cyclodextrin: two assay modifications based on molecular complexation of phenolphthalein. J Biochem Biophys Methods. 1987;14:85–92. doi: 10.1016/0165-022x(87)90043-1. [DOI] [PubMed] [Google Scholar]
- Martin TA, Bandi N, Schulz R, Roberts CB, Kompella UB. Preparation of budesonide and budesonide-PLA microparticles using supercritical fluid precipitation technology. AAPS Pharm Sci Tech. 2002;3:3. doi: 10.1007/BF02830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Farhadi A, Keshavarzian A. New developments in the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 2002;11:365–385. doi: 10.1517/13543784.11.3.365. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Yamamoto A. Effects of roll mixing with beta-cyclodextrin on enhancing solubilities of water insoluble drugs. Pharm Acta Helv. 1989;64:24–29. [PubMed] [Google Scholar]
- Parrot EL. Milling. In: Lachman L, Mieberman HA, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy. Philadelphia: 1976. pp. 466–486. Chapter 15. [Google Scholar]
- Pitha J, Pitha J. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharm Sci. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- Sunkara G, Kompella UB. Drug delivery applications of supercritical fluid technology. Drug Del Technol. 2002;2:44–50. [PubMed] [Google Scholar]
- Szabo IL, Pai R, Soreghan B, Jones MK, Baatar D, Kawanaka H, Tarnawski AS. NSAIDs inhibit the activation of egr-1 gene in microvascular endothelial cells. A key to inhibition of angiogenesis? J Physiol Paris. 2001;95:379–383. doi: 10.1016/s0928-4257(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Tarpani G, Latrofa A, Franco M, Pantaleo MR, Sanna E, Massa F, Tuveri F, Liso G. Complexation of zolpidem with 2-hydroxypropyl-β-, methyl-β-, and 2-hydroxypropyl-γ-cyclodextrin: effect on aqueous solubility, dissolution rate, and ataxic activity in rat. J Pharm Sci. 2000;89:1443–1451. doi: 10.1002/1520-6017(200011)89:11<1443::aid-jps7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14:1691–1698. doi: 10.1023/a:1012167410376. [DOI] [PubMed] [Google Scholar]
- Thompson DO. Cyclodextrins—enabling excipients: their present and future use in pharmaceuticals. Crit Rev Ther Drug Carrier Syst. 1997;14:1–104. [PubMed] [Google Scholar]
- Van Hees T, Piel G, Evrard B, Otte X, Thunus L, Delattre L. Application of supercritical carbon dioxide for the preparation of a piroxicam-beta-cyclodextrin inclusion compound. Pharm Res. 1999;16:1864–1870. doi: 10.1023/a:1018955410414. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW, Wiedmann TS, Estensen R, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–5492. [PubMed] [Google Scholar]
- Wiedmann TS, Bhatia R, Wattenberg LW. Drug solubilization in lung surfactant. J Controlled Release. 2000;65:43–47. doi: 10.1016/s0168-3659(99)00230-8. [DOI] [PubMed] [Google Scholar]
- York P. Strategies for particle design using supercritical fluid technologies. Pharm Sci Technol Today. 1999;2:430–440. doi: 10.1016/s1461-5347(99)00209-6. [DOI] [PubMed] [Google Scholar]


