Abstract
Purpose of review:
The obesity epidemic is a global health crisis of staggering proportion. Excess body weight is a major risk factor for the development of cardiovascular disease (CVD). We review temporal trends in obesity rates, pertinent pathophysiology to understand mechanisms of disease, and treatment strategies in the context of reducing cardiovascular risk.
Recent findings:
The prevalence of obesity is increasing in recent decades and is driven by a complex interplay of economic, environmental, and biological factors. In developed countries, changes in foodintake, such as increased consumption of energy-dense and added sugar have contributed significantly to weight gain. Single nucleotide variations in genes and alterations in the gut microbiome have been associated with the obese phenotype. The description of an obesity paradox in patients with CVD may have several explanations, including limitations of body mass index (BMI) to assess adiposity, selection bias, and lead-time bias with earlier onset of disease. Evidence-based treatments for weight loss include lifestyle intervention, pharmacotherapy, and bariatric surgery. Data on the long-term effects of these therapies on cardiovascular risk are limited.
Summary:
Overweight and obesity are associated with increased cardiovascular morbidity and mortality over the lifespan. Despite our increasing understanding of biological and environmental drivers of obesity, more work is needed in developing effective prevention strategies and implementation of evidence-based treatments to promote cardiovascular health and reduce cardiovascular risk. Ultimately, efforts to prevent and postpone cardiovascular morbidity should include focus on maintenance of normal BMI (primordial prevention) for a longer and healthier life, free of CVD.
Keywords: Overweight, obesity, cardiovascular disease risk
Introduction
The obesity epidemic is one of the greatest global health crises of our time. In the United States (US) alone, more than a third of the population is obese [1], and it is expected that by year 2030, that figure may rise to as much as 50% [2]. Moreover, data from the pediatric population demonstrate a disturbing increase in the rates of overweight and obesity over the past several decades [3] leading to a greater proportion of life lived with obesity and obesity-associated morbidity. Multiple factors drive this trend: sedentary lifestyle, abundance of calorie-rich nutrient-poor food products in the marketplace, and continued barriers to access to care [4].
Obesity has been well-described as a risk factor for the development of a number of cardiovascular (CV) risk factors, including hypertension, type II diabetes, and dyslipidemia [5]. In addition, obesity is increasingly recognized as an independent risk factor for the development of cardiovascular disease (CVD) morbidity and mortality [6]. In fact, between 1980 and 2000, higher body mass index (BMI) resulted in approximately 25,905 additional deaths due to coronary heart disease, which were offset by improvements in other risk factors (e.g. smoking cessation and systolic blood pressure reduction) [7]. While national CVD mortality rates declined overall in the past several decades, the rate of decline has decelerated, raising concern about the contribution of the obesity epidemic potentially reversing progress made in CVD [8]. In this article, we will review the temporal trends in obesity rates, explore the pathophysiology underlying the development of overweight and obese, and discuss the clinical approach to identifying, preventing and treating obesity to reduce CVD risk.
Temporal Trends in Obesity in the US and Globally
Overweight and obesity in adults is commonly defined using BMI categories as follows: overweight (BMI 25.0 – 29.9 kg/m2) and obese class I (BMI 30.0 – 34.9 kg/m2), class II (BMI 35.0 – 39.9 kg/m2), and class III (BMI ≥ 40 kg/m2). Sex-specific BMI-for-age Centers for Disease Control and Prevention (CDC) growth charts are used for US youth, with overweight defined as 85th to < 95th percentile and obese defined as ≥ 95th percentile. Importantly, the American Heart Association has identified BMI of less than 85th percentile in youths (ages 2–19 years) and < 25 kg/m2 in adults (ages ≥ 20 years) as 1 of the 7 components of ideal cardiovascular health [9]. In 2013 to 2014, 63.1% of youth and 29.6% of adults met these criteria [10].
Data from the National Health and Nutrition Examination Surveys (NHANES) indicated the prevalence of obesity was 39.6% among adults and 18.5% among children in the US in 2015–2016 [11]. These figures demonstrate an approximately 30% higher prevalence compared to when the annual survey began in 1999 with greatest increases in the prevalence of severe obesity (Figure 1). Important race-sex disparities in obesity are present with greater rates among minority populations; strikingly, more than half of both black and Hispanic women are obese [11].
Figure 1.
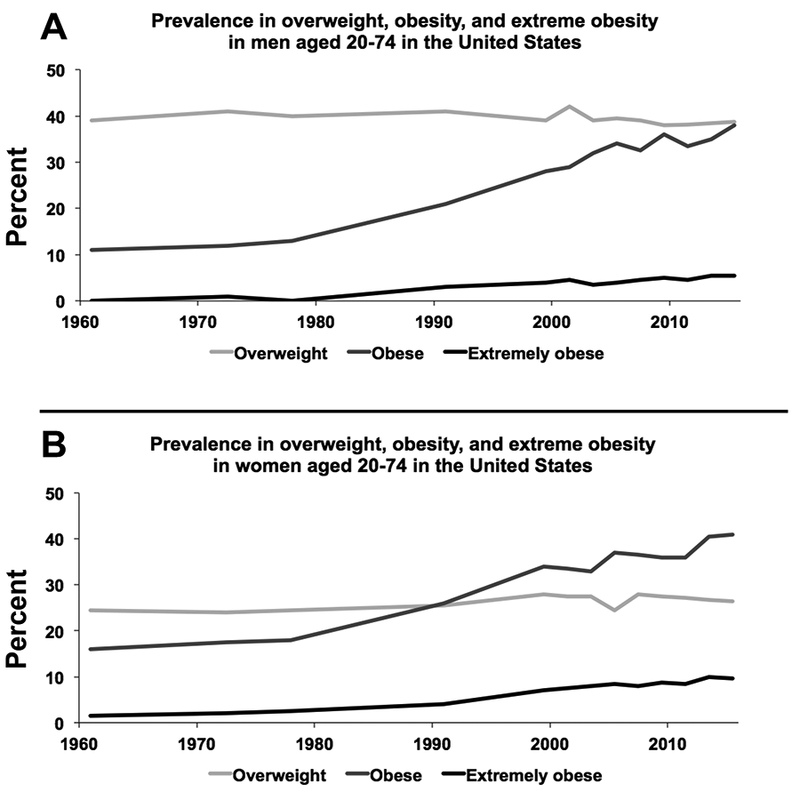
Obesity trends in adult men (A) and women (B) in the United States. Overweight: BMI ≥ 25 kg/m2 (light grey); Obese: BMI ≥ 30 kg/m2 (dark grey); Extremely obese: BMI ≥ 40 kg/m2 (black). Data source: National Health and Nutrition Examination Survey (NHANES).
Changes in food supply
Changing food supply has been implicated as a major contributing factor to the obesity epidemic. Economic development results in increased buying power and reduced cost of food, which influences dietary habits [12]. In the US, high caloric density of the food supply has significantly contributed to the excess weight gain of the population [13]. According to data from the US Department of Agriculture (USDA), in 2010 there were roughly 4,000 kilocalories per person per day available in the US food supply, a figure which has been rising steadily since the 1960s [14]. Which foods in particular may be to blame for our progressive weight gain is less clear: increasing consumption of calorie-rich fast food items and sugar-sweetened beverages [15] has occurred despite also increased intake of fruits and vegetables [16]. During the years 1985–2000, a marked increase in carbohydrates per person in the US food supply coincided with the emergence of low- and reduced-fat food options [14]; whilethe rise in obesity clearly coincides with the substitution of simple and refined sugars into the food supply, obesity trends were increasing before 1985 and have persisted despite the introduction of low- and reduced-fat food options.
The Global Obesity Burden
From 1980 to 2015, the global prevalence of obesity increased among adults of both genders and across all socio-economic strata [17]. The overall prevalence of obesity in 2015 was 12.0% among adults, generally higher in women than men across all age groups. Worldwide, the US has the highest prevalence of childhood obesity (12.7%) among the 20 most populous countries. Globally, elevated BMI contribute to more than 4 million deaths and 120 million disability-adjusted life years annually, the vast majority of which are directly attributed to subsequent development of CVD [17].
Pathophysiology
Overweight and obesity may also be described as the obvious manifestations of an imbalance between energy consumption and energy expenditure. However, this simple observation is complicated by the multitude of biologic and environmental factors that drive each side of this energy-balance equation. It is widely agreed that there are multiple mechanisms which drive the pathophysiology of obesity: physiological, behavioral, genetic and epigenetic, environmental and socio-economic. A better understanding of the complex interplay between the intrinsic and extrinsic factors that cause obesity is needed to understand the contemporary temporal trends in obesity rates in the US and globally.
Pathogenesis and molecular mechanisms
Major biological systems that have been implicated as dysfunctional and contributing to the pathogenesis of obesity include central regulation of food intake in the hypothalamus, dysregulated hormonal signaling, and changes in gut microbiome.
The hypothalamus maintains whole body energy homeostasis by integrating hunger and satiety signals from peripheral tissues to influence food intake or expenditure. Neuroimaging studies indicate that the hypothalamus communicates with areas of the brain involved with processing external sensory information, emotion, and reward-based decision making, and that these pathways are influenced by conditions of fasting, feeding, leanness and obesity [18].
Hormones released from the organs of the gastrointestinal tract, adipose tissue, and pancreas play a key role in regulation of the gut-brain axis, mediating appetite, food consumption, and satiety [19]. These hormones act on central pathways in the hypothalamus, midbrain and brainstem to influence behavior and response to feeding; dysregulation of hormonal action in obesity demonstrates that these pathways hold potential as therapeutic targets.
Leptin, a hormone synthesized and released from adipocytes, regulates fatty acid metabolism by stimulating hydrolysis of stored and circulating triglyceride and inhibiting lipogenesis. It acts centrally to curb appetite, diminishing food reward and promoting satiety during eating [20]. Rodent models deficient in either leptin or leptin-receptor are prone to develop overweight and type II diabetes [21]. In humans, genetic mutation resulting in loss of this hormone or its receptor results in early onset of severe obesity, and leptin resistance has been proposed as a contributing factor for hyperphagia observed in Prader-Willi syndrome [22]. In obesity, elevated levels of circulating leptin are thought to promote leptin resistance, reducing the hormone’s ability to curb appetite and weight gain [23]. Resistance to leptin in obesity may explain why treatment with leptin supplementation failed to reduce eating and weight gain in a clinical trial [24].
Insulin is produced in beta cells of the pancreas and is a key regulator of glucose homeostasis; insulin rises in response to feeding to drive glucose uptake into muscle, liver, and adipose tissue where it is either utilized for adenosine triphosphate (ATP) production or stored as glycogen or triglyceride. Similar to leptin, obese individuals have been observed to have increased serum levels of fasting and postprandial insulin compared to lean counterparts [25,26]. Emerging evidence suggests that the central action of insulin is an important modulator of hunger and satiety, and that individuals with peripheral insulin resistance also have an attenuated central response to insulin signaling which may contribute to overeating [27].
Glucagon-like peptide 1 (GLP-1) is released from intestinal cells after a meal, stimulating glucose-dependent insulin secretion, inhibiting glucagon release, and suppressing food intake. Obesity is associated with lower fasting GLP-1 and an attenuated postprandial release [28]. As will be discussed, liraglutide, a GLP-1 receptor agonist, is FDA-approved for the treatment of obesity, acting by slowing gastric emptying and promoting gastric distension.
Other peripheral hormonal signals whose actions are altered in the setting of obesity include ghrelin, peptide YY (PYY), and cholecystokinin (CCK). Taken together, these peripheral hormone signals influence appetite and feeding behaviors via communication with the central nervous system and hold potential to be exploited as possible pharmacologic targets for anti-obesity therapies [19].
Role of the gut microbiome
Over the past decade, studies have suggested a link between weight gain and gut microbiota composition. Germ-free mice with sterile gastrointestinal tracts receiving gut microbiota from genetically obese mice demonstrated increased weight gain compared to littermates receiving gut microbiota from lean mice [29]. Similar results were demonstrated when germ-free mice were transplanted with gut microbiota from identical human twins discordant for obesity [30]. Changing the composition of the gut microbiome with antibiotic treatment affected diet-induced weight gain and insulin sensitivity [31]. These results suggest that gut microbes influence weight gain, either by affecting energy absorption from ingested food or through endocrine signaling to the host [32]. Consistent with animal models, alterations in the human gut microbiome have been observed in obesity [29].
Genetic predisposition to obesity
Heritability of obesity has been estimated between 40 and 70%, suggesting a significant genetic susceptibility [33]. As opposed to single-gene disorders such as sickle cell anemia or cystic fibrosis, obesity is associated with many different genes located across the genome [34]. Genome-wide association studies (GWASs) have identified more than 500 genetic loci associated with obesity and visceral fat deposition. Loci containing variants associated with high BMI include genes highly expressed in the central nervous system (CNS, hypothalamus, pituitary gland, and hippocampus), skeletal muscles, and immune cells [35], implicating these organ systems in the etiology of obesity. Loci with gene variants associated with visceral fat distribution (as measured by waist-to-hip ratio) implicate processes such as adipogenesis and insulin signaling [36]. Identifying specific genes within these loci, and translating them into new biology offering insight into the pathophysiology of obesity remains an important challenge within the fields of genetics and physiology [34].
Social determinants of obesity
The relationship between socioeconomic status and obesity is complex. At the global level as a country develops and accumulates wealth, the prevalence of overweight and obesity among its populace increases [37]; notable examples include China [38] and India [39]. However, an examination of the distribution of disease burden within the US demonstrates a strong association between low-income areas and prevalence of obesity. Data from US counties reveals that obesity rates are positively associated with poverty rates; indeed, counties with poverty rates greater than 35% have obesity rates that are more than double those of wealthy counties [40]. One possible explanation is that “food deserts” (areas where the population experiences a lack of access to healthy foods) and “food-swamps” (areas of relatively abundant high-calorie fast food and junk food) are disproportionately concentrated in low-income areas [41]. Importantly, food swamps are suggested to have a greater impact on the development of obesity than food deserts [42].
Cardiovascular morbidity and mortality among the overweight and obese
Overweight and obesity are associated with significantly higher risk of cardiovascular morbidity, including coronary artery disease (CAD), heart failure (HF), atrial fibrillation, and sudden cardiac death [5]. Elevated BMI in adolescence is associated with higher mortality from CVD later in life [43], underscoring the importance of prevention and early intervention in the pediatric population. Obesity is also associated with significantly higher all-cause mortality compared to normal weight [44], with increased death from cancer and CVD as the primary causes [45].
However, controversy exists about overweight and obesity and mortality outcomes. In the study by Flegal et al, greater longevity was observed in the overweight group (BMI 25–30 kg/m2) compared to the normal BMI group [44]. Possible explanations for the observed protective effect of overweight include the inclusion of participants with comorbidities at baseline, specifically prevalent CVD, which may contribute to selection and survival bias due to protopathic bias (reverse causation) related to unintentional weight loss. Furthermore, the observation that after diagnosis of CVD, individuals with excess weight tend to live longer than individuals with normal BMI (the so-called “obesity paradox”), has led some to conclude that excess weight may have a protective effect on CVD. However, a recent study examining both morbidity and mortality from CVD over the lifespan demonstrated no difference in longevity between overweight and normal BMI groups and overweight was associated with earlier onset of CVD, resulting in increased number of years lived with CVD [6]. Thus, the “obesity paradox” may be a result of earlier CVD onset. A study of a large European cohort came to a similar conclusion, that excess weight beyond normal BMI is associated with worse CVD outcomes [46].
Assessment of adiposity
The most commonly employed screening measure for overweight and obesity is BMI, defined as body weight in kilograms divided by height in meters squared. This metric derives from the long-ago established observation that weight empirically scales as height squared, and has the advantage of being quickly and easily determined in clinical settings. Obtaining a detailed weight history from a patient can also yield important prognostic information, as the presence of overweight and obesity during childhood and adolescence has been suggested to confer increased CVD risk into adulthood [47]. Recent evidence suggests that historical maximum BMI is more strongly associated with mortality risk than single baseline BMI assessment [48].
However, the use of BMI as a surrogate marker for overall adiposity has significant drawbacks. BMI does not differentiate between adipose tissue and lean body mass. BMI also does not determine body fat distribution, an important consideration given that central adiposity and visceral fat pose a higher risk for the development of CVD, metabolic syndrome, and diabetes than peripherally-distributed fat [49]. Alternative anthropometric indices have been proposed to better assess overall adiposity and distribution: waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) as well as imaging modalities to better estimate composition and body fat percentage.
BMI varies as a predictor of adiposity between race, gender, and age
An examination of NHANES data revealed significant quantitative differences in adiposity as predicted by BMI across racial groups. For instance, for individuals of a given BMI, non-Hispanic blacks were found to have significantly lower percent body fat than either non-Hispanic white or Mexican-American individuals [50]. Investigators also observed differences between genders and over the course of the lifespan: BMI tends to predict higher percentage body fat in women compared to men and in older compared to younger individuals. The varied relationship between BMI and adiposity has led to disagreements on the appropriate body weight guidelines for these populations. Furthermore, it highlights the need for population-specific methods of correcting BMI to accurately screen for those at-risk. In a study of 1078 subjects from 4 ethnic groups (South Asians, Chinese, Aboriginals, and Europeans), ethnic-specific BMI cut points were derived and were approximately 6 kg/m2 lower among non-European groups than those found among Europeans [51].
Imaging modalities to assess body composition
Given our increasingly nuanced understanding of the endocrine function of adipose tissue which is dependent on its location within the body, a more precise approach for assessing body fat distribution in the clinical setting may be warranted to help identify patients who are at higher risk. Computed tomography (CT), magnetic resonance imaging MRI), and dual-energy x-ray absorptiometry (DEXA) offer a significant advantage over conventional anthropometry in their ability to more accurately account for total adiposity, differentiate subcutaneous versus visceral adipose tissue, and detect foci of ectopic fat deposition in organs such as the liver and heart [52]. These imaging modalities have already demonstrated superiority to BMI in trials for the purposes of assessing risk [53] as well as evaluating response to weight loss therapy [54]. However, the cost of these diagnostic tools remains a significant drawback, preventing more widespread adoption of imaging into current clinical practice.
Prevention and Treatment of Obesity
The prevention of obesity in an individual starts prior to birth. Early life maternal exposures including pre-conception weight and gestational weight gain are two of the most important determinants of childhood obesity [54]. Maternal obesity has been linked not only to adverse outcomes for the mother, but for the offspring as well. During pregnancy, weight gain in excess of Institute of Medicine guidelines [56] is associated with resultant fetal macrosomia and an increased risk for the offspring to develop obesity during childhood and adulthood [57]. Pregnant women should be counseled during pregnancy on appropriate gestational weight gain to minimize subsequent risk to the child [58]. In the post-partum period, breastfeeding (compared to formula feeding) is associated with reduced risk of obesity in later life [59].
At the population level, it is critical to recognize that the overabundance of calorie-dense, nutrient-poor, and inexpensive foods leads to overconsumption. A number of recent public policy efforts address obesity prevalence: taxes on sugar-sweetened beverages and limitations of restaurant portion sizes have recently been proposed in several major cities and implemented with varying degrees of success [60]. Section 4205 of the Affordable Care Act mandated clear labeling of calorie count and nutrition information at chain restaurants and vending machines to enable informed decision-making among consumers [61]. Partnerships between governments and private sector are needed to reduce the number of so-called “food deserts” and “food swamps” which are disproportionately concentrated in at-risk areas. While many studies have tested strategies in schools, work environments, and communities that might prevent the rise of BMI, so far these measures have had little success in curbing rising rates of overweight and obesity [62], and evidence for use of economic policies to prevent obesity remains limited [16].
Treatment Strategies
The obvious and fundamental goal in the treatment of overweight and obesity is weight loss, which is recommended in individuals with BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of weight-related comorbidity [63]. The goal of initial weight loss should be 5–10% over the first 6 months. When weight-loss therapy is initiated, the importance of frequent follow up with the patient to monitor weight loss progression cannot be overemphasized. Indeed, in several studies examining response to weight-loss therapies in obesity, the rate of initial weight loss was the most important predictor of continued and sustained weight loss [64]. While lifestyle modification is widely considered to be the foundation of weight loss therapy, supplementation with pharmacotherapy or even surgery is often warranted.
Lifestyle modification
Fundamental to the treatment of overweight and obesity, lifestyle modification is comprised of behavioral training, dietary change, and increased physical activity [65]. The Look AHEAD trial provides evidence supporting the efficacy of lifestyle intervention, delivered as a series of group or individual face-to-face sessions, resulting in 5% weight loss in a majority of participants in the treatment arm at 8-year follow up [66]. Behaviors that varied directly with amount of weight lost included number of sessions attended, number of meal replacements used, and amount of physical activity. The US Preventive Services Task Force recommends referral to lifestyle treatment for patients with obesity and other CVD risk factors [67].
The primary endpoint of Look AHEAD, which was conducted in obese and overweight individuals with type II diabetes, was the rate of macrovascular events: nonfatal myocardial infarction, nonfatal stroke, death, or hospitalization for angina. While the trial did demonstrate that meaningful weight loss with lifestyle intervention improved several cardiovascular risk factors (glycemic control, lipids and systolic blood pressure) the trial was stopped early due to futility for failing to reduce the risk of cardiovascular events [68]. Currently, there is a paucity of data whether weight loss achieved through lifestyle modification translates into long-term prevention of CVD and other obesity-related comorbidities [69].
Pharmacotherapy
Currently, there are five FDA-approved pharmacologic agents for the treatment of obesity, indicated in individuals with BMI greater than 30 kg/m2 or greater than 27 kg/m2 with presence of co-morbid conditions [70]: 1) Phentermine-topiramate: a combination of atypical amphetamine analogue and anti-epileptic which acts centrally to suppress appetite and inhibit taste; 2) Orlistat: a pancreatic lipase inhibitor that blocks hydrolysis and absorption of ingested fat; 3) Lorcaserin: an agonist of central serotonin 2C receptors which suppresses appetite; 4) Naltrexone-bupropion: a combination of mu opioid receptor antagonist and dopamine-norepinephrine uptake inhibitor which also acts to suppress appetite and dopaminergic reward pathway; and 5) Liraglutide: a glucagon-like peptide 1 analog, initially improved in diabetes, which slows gastric emptying and increases satiety
While there have been several cardiovascular outcomes trials (CVOTs) examining the safety of these agents, there has been little investigation to date measuring cardiovascular outcomes among obese patients who have achieved weight loss with any of these agents. A recent meta-analysis by Khera and Pandey et al. found that these pharmacologic agents had only a modest effect on various cardiometabolic risk factors: systolic blood pressure, fasting glucose, hemoglobin A1c and lipid profile [71]. However, the use of liraglutide as therapy for weight loss poses an especially intriguing option in light of the recent LEADER trial, which demonstrated a mortality benefit when liraglutide was added to standard care in patients with type II diabetes and high cardiovascular risk [72]. Whether such benefit might extend to non-diabetic obese patients with no pre-existing CVD remains to be explored.
Bariatric Surgery
For patients with a BMI greater than 40 kg/m2, or greater than 35 kg/m2 with comorbidity, bariatric surgery is indicated [73]. While the number of bariatric surgeries performed in the US grew rapidly in the first decade of the 21st century, that number has since plateaued and has not kept pace with the increase in prevalence of morbid obesity. Indeed, it is estimated that only 1% of the patient population who meets the indication for surgery receives the operation [74]. Weight loss after bariatric surgery is often profound, with average reduction in BMI of 17 kg/m2 and 22 kg/m2 (at 3 years) for sleeve gastrectomy and Roux-en-Y, respectively [75]. A study of obese adults in Sweden receiving bariatric surgery demonstrated a nearly 50% reduction in fatal and non-fatal cardiovascular events over a mean follow up of 15 years [75] as well as improvement in comorbidity budren, including remission of type II diabetes and obstructive sleep apnea; not insignificantly, patients also report an improved quality of life [76]. Of particular interest, the improvement seen in blood glucose control and reduction of micro- and macro-vascular complications in diabetics receiving bariatric surgery has led to the emerging concept of “metabolic surgery”, where surgery may be indicated in patients with BMI 30–35 kg/m2 due to benefits independent of weight loss [77,78].
Conclusion
If current trends continue, the majority of the US population will be obese by 2030. Obesity is associated with a significantly higher burden cardiovascular morbidity and mortality through the life course with earlier onset of disease and greater proportion of life lived with CVD. Despite our increasing understanding of the biological and environmental drivers of weight gain, we have yet to develop effective, feasible and scalable prevention strategies to combat this growing epidemic to reduce cardiovascular risk and promote cardiovascular health. Further, evidence-based treatments for obesity are underutilized and additional data are needed on associated cardiovascular outcomes.
Footnotes
Compliance with Ethical Standards Conflict of Interest
Ryan Lahey declares no conflicts of interest; Sadiya S. Khan reports grants from NIH/NHBLI, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. [DOI] [PubMed] [Google Scholar]
- 3.*Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016; 315(21):2292–2299.Report on increasing obesity prevalence in recent decades among the US pediatric population.
- 4.*Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(15): 1492.A review with emphasis on management which may be of special interest to clinicians.
- 5.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;52(21): 1925–1932. [DOI] [PubMed] [Google Scholar]
- 6.*Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–287.A large-scale population-based study demonstrating increased CVD morbidity and mortality associated with overweight and obesity.
- 7.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–98. [DOI] [PubMed] [Google Scholar]
- 8.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, et al. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. JAMA. 2017;317(19):1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1–8. [PubMed] [Google Scholar]
- 12.Zobel EH, Hansen TW, Rossing P, von Scholten BJ. Global changes in food supply and the obesity epidemic. Curr Obes Rep. 2016;5(4):449–455. [DOI] [PubMed] [Google Scholar]
- 13.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–56. [DOI] [PubMed] [Google Scholar]
- 14.USDA Economic Research Service. Food calories and macronutrients per capita per day. United States Department of Agriculture: Center for Nutrition Policy and Promotion. 2014. https://www.cnpp.usda.gov/USFoodSupply-1909-2010. Accessed 25 May 2018.
- 15.Bowman SA, Vinyard BT. Fast food consumption of U.S. adults: impact on energy and nutrient intakes and overweight status. J Am Col Nutr. 2004;23(2):163–8. [DOI] [PubMed] [Google Scholar]
- 16.Sturm R, An R. Obesity and economic environments. CA Cancer J Clin. 2014;64(5):337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, et al. Obesity: pathophysiology and intervention. Nutrients. 2014;6(11):5153–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10(2):131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqi IS, O’Rahilly S. Recent advances in the genetics of severe childhood obesity. Arch Dis Child. 2000;83(1):31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101(6):545–559. [DOI] [PubMed] [Google Scholar]
- 24.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26(4):504–509. [DOI] [PubMed] [Google Scholar]
- 25.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, Bonadonna RC. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93(6):2122–2128. [DOI] [PubMed] [Google Scholar]
- 26.Björntorp P Obesity, atherosclerosis and diabetes mellitus. Verh Dtsch Ges Inn Med. 1987;93:443–448. [DOI] [PubMed] [Google Scholar]
- 27.Tiedemann LJ, Schmid SM, Hettel J, Giesen K, Francke P, Büchel C, Brassen S. Central insulin modulates food valuation via mesolimbic pathways. Nat Commun. 2017;8:16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018. 20(Suppl 1):5–21. [DOI] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;44(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 30.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajpal DK, Klein JL, Mayhew D, Boucheron J, Spivak AT, Kumar V, et al. Selective spectrum antibiotic modulation of the gut microbiome in obesity and diabetes rodent models. PLoS One. 2015;10(12):e0145499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rastelli M, Knauf C, Cani PD. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity (Silver Spring). 2018;26(5):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10(6):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.*Loos RJ. The genetics of adiposity. Curr Opin Genet Dev. 2018;50:86–95.A review of the current knowledge regarding the genetic determinants of overweight and obesity.
- 35.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196.Through GWASs, identified numerous novel loci associated with visceral adiposity, shedding new light on underlying pathophysiological mechanisms.
- 37.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–352. [DOI] [PubMed] [Google Scholar]
- 38.Chen C Overview of obesity in Mainland China. Obes Rev. 2008;9(Suppl 1):14–21. [DOI] [PubMed] [Google Scholar]
- 39.Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, et al. Prevalence of generalized & abdominal obesity in urban & rural India--the ICMR-INDIAB Study (Phase-I) [ICMR- NDIAB-3]. Indian J Med Res. 2015;142(2):139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine JA. Poverty and obesity in the U.S. Diabetes. 2011;60(11):2667–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Story M, Kaphingst KM, Robinson-O’Brien R, Glanz K. Creating healthy food and eating environments: policy and environmental approaches. Annu Rev Public Health. 2008;29:253–272. [DOI] [PubMed] [Google Scholar]
- 42.Cooksey-Stowers K, Schwartz MB, Brownell KD. Food swamps predict obesity rates better than food deserts in the United States. Int J Environ Res Public Health. 2017;14(11):E1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.*Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374(25):2430–40.A population-based study which found an association between elevated BMI in adolescence and cardiovascular mortality later in adulthood.
- 44.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–7. [DOI] [PubMed] [Google Scholar]
- 45.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iliodromiti S, Celis-Morales CA, Lyall DM, Anderson J, Gray SR, Mackay DF, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296,535 adults of white European descent. Eur Heart J. 2018;39(17):1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu E, Ley SH, Manson JE, Willett W, Satija A, Hu FB, Stokes A. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med. 2017;166(9):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sam S Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. Horm Mol Biol Clin Investig. 2018. doi: 10.1515/hmbci-2018-0014. [DOI] [PubMed] [Google Scholar]
- 50.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, et al. Defining obesity cut points in a multiethnic population. Circulation. 2007;115(16):2111–8. [DOI] [PubMed] [Google Scholar]
- 52.Andreoli A, Garaci F, Cafarelli FP, Guglielmi G. Body composition and clinical practice. Eur J Radiol. 2016;85(8):1461–8. [DOI] [PubMed] [Google Scholar]
- 53.Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ,4, Di Gravio C, et al. Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obesity (Lond). 2018;42(4):850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baum T, Cordes C, Dieckmeyer M, Ruschke S, Franz D, Hauner H, et al. MR-based assessment of body fat distribution and characteristics. Eur J Radiol. 2016;85(5):1512–8. [DOI] [PubMed] [Google Scholar]
- 55.Trandafir LM, Temneanu OR. Pre and post-natal risk and determination of factors for childhood obesity. J Med Life. 2016;9(4):386–391. [PMC free article] [PubMed] [Google Scholar]
- 56.Olson C Achieving a healthy weight gain during pregnancy. Annu Rev Nutr. 2008;28:411–423. [DOI] [PubMed] [Google Scholar]
- 57.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112(5):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams CB, Mackenzie KC, Gahagan S. The effect of maternal obesity on the offspring. Clin Obstet Gynecol. 2014;57(3):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. [DOI] [PubMed] [Google Scholar]
- 60.*Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2):7–12.Provides insight into obesity trends in various countries.
- 61.111th Congress (2009–2010). H.R.3590: Patient Protection and Affordable Care Act. https://www.congress.gov/bill/111th-congress/house-bill/3590. Accessed 25 May 2018.
- 62.Wang Y, Wu Y, Wilson RF, Bleich S, Cheskin L, Weston C, et al. Childhood obesity prevention programs: comparative effectiveness review and meta-analysis. Rockville (MD): AHRQ Comparative Effectiveness Reviews; 2013. [PubMed] [Google Scholar]
- 63.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 64.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. [DOI] [PubMed] [Google Scholar]
- 65.*Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947–56.A review of current clinical management of overweight and obesity, including lifestyle modification, pharmacotherapy, and surgery.
- 66.Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014;22(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeFevre ML US Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(8):587–93. [DOI] [PubMed] [Google Scholar]
- 68.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunkley AJ, Charles K, Gray LJ, Camosso-Stefinovic J, Davies MJ, Khunti K. Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab. 2012;14(7):616–625. [DOI] [PubMed] [Google Scholar]
- 70.*Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84(12):951–958.An overview of the current FDA-approved pharmacotherapies for weight loss.
- 71.Khera R, Pandey A, Chandar AK, Murad MH, Prokop LJ, Neeland IJ, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9(2)159–191. [DOI] [PubMed] [Google Scholar]
- 74.American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 25 May 2018.
- 75.*Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.The first study to demonstrate cardiovascular benefit of bariatric surgery, conducted in a small Swedish population.
- 76.Sjöström L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 77.Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304. [DOI] [PubMed] [Google Scholar]
- 78.Frühbeck G Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11(8):465–77. [DOI] [PubMed] [Google Scholar]


