Abstract
OBJECTIVES:
The primary objective of this study was to evaluate the effects of high tibial osteotomy (HTO) on subchondral bone structure assessed with magnetic resonance (MR)-based trabecular bone imaging and the correlations of these effects with functional outcome and clinical symptoms.
METHODS:
Patients with varus malalignment (6.2±2.2°) and without a history of knee surgery (n=22; 3 women; 48.7±10.3 years) were included into this prospective study. 1.5T MR imaging was performed before and on average 1.5 years after HTO (amount of correction 4.7±2.5°) and histomorphometric parameters of the trabecular bone were calculated for the medial/ lateral tibia and femur. Functional outcome was assessed with validated scores focusing on sports activity including the Lysholm Score, Tegner Activity Scale and the adapted Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Score.
RESULTS:
Apparent trabecular number significantly decreased in all compartments of the tibiofemoral joint when comparing values before and on average 1.5 years after HTO (P<0.05 for all). Decrease in apparent trabecular number was significantly higher within the medial tibia compared to the lateral compartment (mean difference −0.24 mm−1 (95% confidence interval (CI): −0.33, −0.14 mm−1); P<0.001). Apparent trabecular bone thickness significantly increased within 1.5 years after HTO in the lateral femur (P=0.002) and tibia (P <0.001). The Lysholm Score and Tegner Scale demonstrated an improvement of functional outcome, and the adapted WOMAC demonstrated an improvement of pain, stiffness and physical function within 1.5 years after HTO (P<0.01), with the improvement of WOMAC correlating significantly with changes in trabecular bone thickness within the medial tibia (r= −0.48; P=0.01).
CONCLUSION:
These findings indicate a reversal of the previous subchondral bone alterations in patients with varus malalignment after undergoing HTO, while pronounced subchondral changes were associated with a better functional outcome.
LEVEL OF EVIDENCE:
3
Keywords: Knee, MRI, Bone, Osteotomy
INTRODUCTION
Osteoarthritis is a degenerative joint disease affecting approximately three quarters of the US population over the age of 65 years.1 Previous studies have shown that osteoarthritis occurs more frequently in the medial than in the lateral compartment.2–4 Moreover, cartilage lesions and meniscal lesions are more prevalent in the medial side of the knee joint,2 which may be due to the fact that the weight bearing region is located within the medial compartment.5 Yet, one of the main reasons for medial tibial femoral osteoarthritis is varus malalignment of the lower limb6. One of the potential treatment options available for early medial tibiofemoral osteoarthritis is high tibial osteotomy (HTO).7, 8 HTO comprises a mild valgus correction and a shift of the mean bearing axis (line of Mikulicz) into the lateral compartment.9 This interventional procedure has shown excellent results in previous studies regarding the improvement of clinical symptoms and postoperative radiographic findings.9, 10 Moreover, a previous study showed no significant structural changes in the articular cartilage of the lateral compartment or the lateral meniscus six months after HTO had been performed.11 Meniscal and cartilage degeneration are associated with subchondral bone alterations.12 Young asymptomatic patients with knee malalignment and consequently altered loading conditions showed early subchondral trabecular bone changes assessed with magnetic resonance imaging- (MRI-) based histomorphometric and texture parameters of trabecular bone.13 A previous experimental model showed that the degree of osteoarthritis was associated with alterations of the subchondral bone plate microstructure and that HTO did not affect the lateral compartment in the short term.14 This underlined the fact that knee malalignment is a risk factor for the development of knee osteoarthritis. However, the actual effects of HTO on the subchondral bone structure beneath the tibiofemoral joint surfaces remain fairly unknown until today.
Therefore, purpose of this prospective study was to assess the longitudinal changes of the trabecular bone structure after HTO was performed in subjects with varus malalignment and to examine the correlations between these changes in the trabecular bone structure and functional outcome as well as clinical symptoms.
METHODS
Subjects
In this study, 22 subjects (3 women and 19 men) with a mean age of 49 ±10 years were included. Patients included in this study showed a median Kellgren Lawrence Score of 2 (range 1; 3) within the medial compartment and a median Kellgren Lawrence Score of 1 (range 0; 1) within the lateral compartment, when assessing the radiographic status of osteoarthritis. Moreover, all subjects showed a varus malalignment (6.2±2.2°) of their knees, assessed by using weight bearing longstanding radiographs of the recorded leg, as described previously.13 For preoperative planning of the procedure in order to define the amount of axis correction through high tibial osteotomy, throughout the entire width, the tibia plateau was defined as adjacent 5 %-areas, with the medial border of the tibia plateau being defined as 0 %, and the zone between 50 and 65% of the tibia plateau width postoperatively, as previously described.15 The corrected position if the WBL achieved with HTO in these patients varied between 50.1% and 62.5% (57.8%±4.5%), resulting in a corresponding mechanical femorotibial angle of 1.5° (0.5°–2.7°) of valgus. Study protocol and informed consent documentation was reviewed and approved by the local institutional review board. All methods were performed in accordance with the relevant guidelines and regulations and informed consent was provided by all subjects.
Surgical technique
Medial opening-wedge HTO was performed in every patient included in this study, in accordance with the standard techniques by experienced orthopaedic surgeons. The preoperative digital planning was performed using a landmark-based software (mediCAD, Hectec GmbH, Germany), and the degree of the correction was adjusted according to the criteria published previously. 15 Medial opening-wedge HTO was performed using a biplanar technique as described previously 16 and an internal fixation locking plate (Tomofix, DePuySynthes, Switzerland; or Peek-Power-Plate ® -II. generation-, Arthrex Inc., USA) was used for the fixation of the osteotomy. All procedures were performed under general anesthesia. Intravenous antibiotics (cefuroxim, 1.5 mg) and standard thromboembolic prophylaxis (low-dose heparin) were used.
The post-operative rehabilitation consisted of free range of motion immediately after surgery, with partial weight-bearing (20 kg) for 2 weeks. After two weeks postoperatively, weight-bearing was increased by 20 kg each week, until full weight-bearing was reached. Sport activities were allowed at 3 months after surgery.
MR imaging
Magnetic resonance (MR) imaging was performed in all patients the day before the HTO procedure (range; 1 day to 3 days) as well as on average 1.5 years after HTO and removal of osteosynthesis material (range, 10 months to 20 months). MR images were acquired using a 1.5T MR system (Avanto, Siemens Healthineers, Erlangen, Germany) before and on average 1.5 years after HTO was performed, using the same 8-changel knee coil (Medical Advances Milwaukee, WI). The following sequences were used for imaging analysis: (i) 2D proton density-weighted (PD) turbo spin-echo (TSE) sequence with fat suppression (FS) in the coronal plane (3130ms/40ms, repetition time (TR)/ echo time (TE); 16cm, FOV; bandwidth of 75 hertz per pixel; slice thickness 3mm); (ii) 2D PD-weighted TSE sequences with FS in the sagittal plane (3760ms/44ms, TR/TE; 16cm, FOV; bandwidth of 110 hertz per pixel; slice thickness 3mm); (iii) 3D dual-echo steady-state (DESS) gradient-echo sequence (16.3ms/4.7ms, TR/TE, 16cm, FOV; in-plane resolution 0.5 × 0.5mm2; slice thickness of 0.7mm; bandwidth of 64 hertz per pixel; acquisition time 6 minutes), and a (iv) PD-weighted TSE sequences with FS in the axial plane (4430ms/41ms, TR/TE; 16cm, FOV; bandwidth of 70 hertz per pixel; slice thickness 3mm). Trabecular bone imaging was performed using a three dimensional T1-weighted fast low-angle shot sequence (5.7ms/11.7ms, TE/TR; 16 cm, FOV; in-plane resolution 0.25×0.25 mm², slice thickness 1 mm, bandwidth of 64 hertz per pixel, acquisition time 6 minutes).
Image analysis
All studies were analyzed by two radiologists (A.S.G., B.J.S., each with 5 years of experience) in consensus on a standard PACS workstation in order to assess the presence of lesions within the subchondral bone.17 No subchondral defects were diagnosed in our study population.
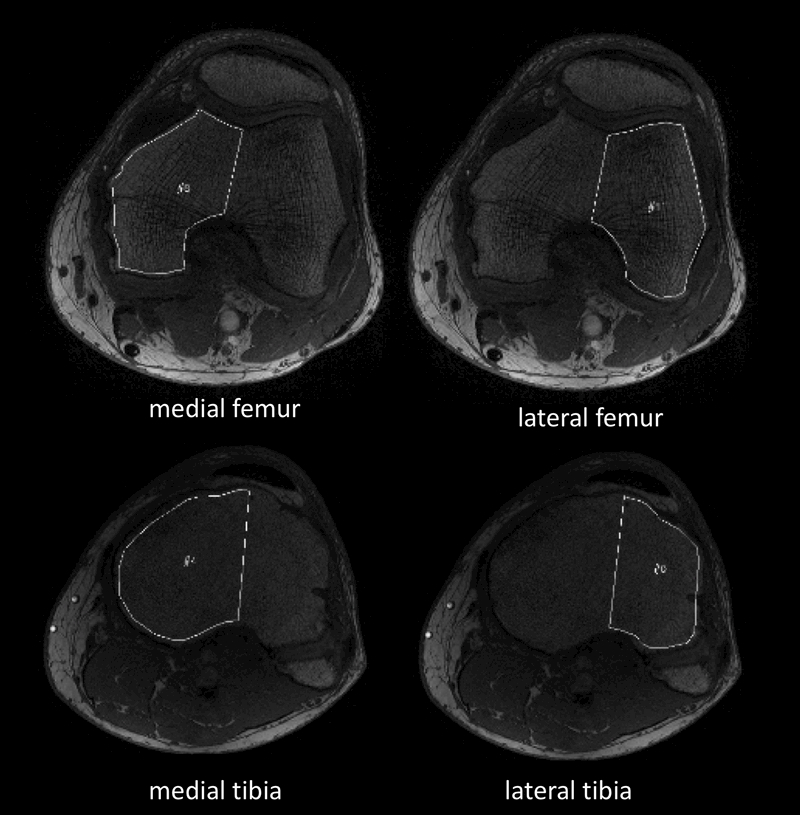
All trabecular bone analyses were performed by one reader and for reproducibility purposes partly again by a second reader (A.S.G. and J.Z.), using an in-house developed program as described previously.13 For this the axial images of the fast low angle shot sequences were transferred to a remote Linux work station. The in-house interface description language (IDL)-based program was used to manually segment the subchondral bone of the four compartments of the tibiofemoral joint (medial tibia (mt), medial femur (mf), lateral tibia (lt), lateral femur (lf)). Regions of interest (ROI) were all drawn in 30 consecutive sections each, as described previously.12, 13, 18, 19 The first ROI was drawn within the subchondral bone adjacent to the tibiofemoral joint line in each compartment, the remaining consecutive sections were defined either more proximally to the first section within the distal femur or more distally to the first section within the proximal tibia (Fig. 1). In order to calculate the histomorphometric of the trabecular bone, ROIs were assessed using a dual threshold algorithm, as described previously.12, 18, 19 Afterwards, histomorphometric parameters were calculated using the mean intercept length method,20 labeling the values as apparent (app.) due to the values calculated from sequences with a limited spatial resolution13: apparent bone fraction (app.BF), apparent trabecular number (app.TbN), apparent trabecular space (app.TbSp), and apparent trabecular thickness (app.TbTh).
Figure 1.
Representative MR images of the left knee of a subject with varus knee alignment before undergoing high tibial osteotomy, showing the corresponding segmented compartments: medial femur, lateral femur, medial tibia and lateral tibia.
Functional outcome
For the evaluation of the functional outcome, validated activity scores were used (Lysholm Score, Tegner Activity Score and adapted Western Ontario and MacMaster Universities (WOMAC) Score (maximum 100 points)).21–24 Of note, higher WOMAC values indicate higher levels of pain, stiffness and impaired physical function, therefore, a negative change over 1.5 years indicates an improvement in clinical performance. The adapted WOMAC score used was a version of the WOMAC score adapted to the German language to assess symptoms and physical functional disability associated with osteoarthritis.22 The Visual Analogue Scale (VAS) was used for the evaluation of pain levels while performing sports. Scores ranged from 0 and 10 (“0” indicating no pain and “10” indicating the severest imaginable pain).25
Statistical analysis
Statistical analysis was performed with SPSS software (version 23; IBM, Armonk, NY, USA) using a two-sided 0.05 level of significance. Differences between the trabecular bone parameters as well as functional outcome measured before and on average 1.5 years after HTO were assessed using a paired t-test. One-way analyses of variance were used in order to determine statistically significant change in histomorphometric trabecular parameters over 1.5 years after HTO (Δapp.BF, Δapp.TbN, Δapp.TbSp, Δapp.TbTh) between compartments using Bonferroni correction for multiple comparisons. Correlations between histomorphometric trabecular parameters and pre-/postoperative leg alignment, degree of correction of leg alignment as well as BMI were calculated using Pearson’s correlation coefficients. To compare histomorphometric trabecular parameters before and on average 1.5 years after HTO (Δapp.BF, Δapp.TbN, Δapp.TbSp, Δapp.TbTh) after distributing patients into the categories normal weight (≤25.0 kg/m2) and overweight (>25 kg/m2), independent samples t-tests were used for numerical data. Correlations between histomorphometric trabecular parameters (at baseline and on average 1.5 years after HTO) and change in functional outcome between the time point before surgery and on average 1.5 years after HTO (Lysholm Score, Tegner Activity Score, and adapted WOMAC Score), subjective VAS scores and BMI were assessed by using Spearman׳s rank correlation coefficient. The values of the trabecular bone parameters as well as the functional outcome are presented as mean ± standard deviation.
Reproducibility
Both for the intra- and interrater reproducibility, trabecular measurements were assessed for each parameter in each compartment in 4 randomly selected patients by two readers (A.S.G. and J.Z.).
RESULTS
Subchondral trabecular analysis
At baseline, the apparent trabecular thickness was significantly higher in the medial compartment (medial tibia (mt), medial femur (mf); mean, 0.51±0.12mm) compared to the lateral compartment (lateral tibia (lt), lateral femur (lf); 0.33±0.12mm; P=0.002), suggesting a higher trabecular thickness within the weight bearing medial compartments. Analogously, apparent bone fraction was significantly higher in the medial compartment (0.53±0.17; lateral compartment: 0.43±0.09; P<0.001), whereas the apparent trabecular space was significantly lower in the medial compartment (0.41±0.12mm) compared to the lateral compartment (0.52±0.16mm; P<0.001). Values of trabecular bone structure parameters for each compartment are displayed in Table 1.
Table 1.
Trabecular parameters before HTO and 1.5 years after HTO for each compartment (lateral femur (lf), lateral tibia (lt), medial femur (mf), medial tibia (mt); values given as mean ±SD).*
| compartment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lf | lt | mf | mt | |||||||||
| parameters | pre HTO | post HTO | P-value | pre HTO | post HTO | P-value | pre HTO | post HTO | P-value | pre HTO | post HTO | P-value |
| app. bone fraction | 0.46 ± 0.10 | 0.52 ± 0.15 | 0.41 | 0.41 ± 0.10 | 0.49 ± 0.08 | <0.001 | 0.51 ± 0.09 | 0.56 ± 0.12 | 0.03 | 0.55 ± 0.10 | 0.67 ± 0.19 | 0.14 |
| app. trabecular thickness | 0.49 ± 0.10 | 0.56 ± 0.12 | 0.002 | 0.40 ± 0.06 | 0.52 ± 0.15 | <0.001 | 0.52 ± 0.07 | 0.59± 0.12 | 0.002 | 0.51 ± 0.16 | 0.48 ± 0.17 | 0.03 |
| app. trabecular number | 0.91 ± 0.09 | 0.82 ± 0.13 | 0.26 | 0.99 ± 0.12 | 0.91 ± 0.12 | 0.01 | 0.92 ± 0.09 | 0.86 ± 0.10 | 0.001 | 1.05 ± 0.12 | 0.82 ± 0.16 | <0.001 |
| app. trabecular space | 0.51 ± 0.11 | 0.48 ± 0.11 | 0.20 | 0.53 ± 0.12 | 0.48 ± 0.09 | 0.05 | 0.46 ± 0.13 | 0.45 ± 0.12 | 0.60 | 0.35 ± 0.09 | 0.32 ± 0.11 | 0.20 |
P-values <0.05 are in bold
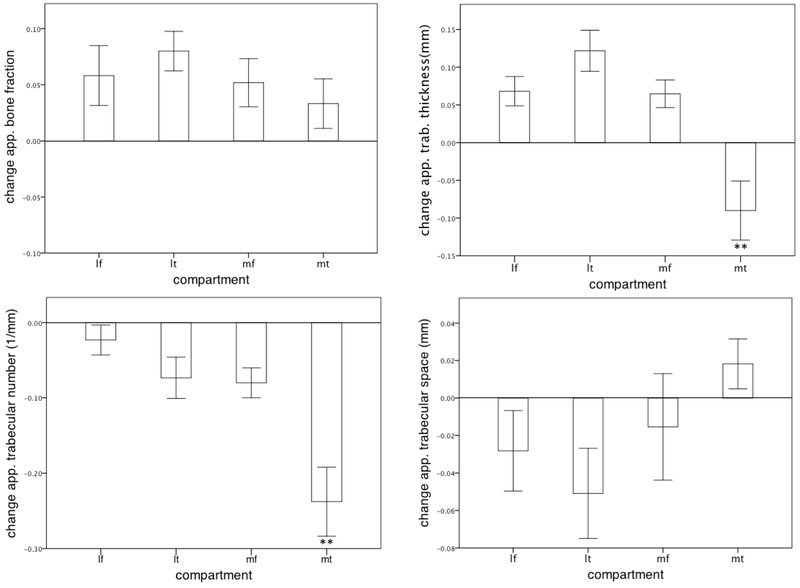
Within 1.5 years after HTO, apparent trabecular bone thickness significantly increased in the lateral compartments compared to baseline values measured immediately prior to HTO (lateral femur: mean difference 0.07mm ± 0.03mm, P=0.002; lateral tibia: mean difference 0.12mm ± 0.06mm, P <0.001). The same effect was found for the apparent bone fraction (lateral femur: mean difference 0.06mm ± 0.05mm, P=0.041; lateral tibia: mean difference 0.08 ± 0.04mm, P<0.001). The apparent trabecular number decreased within all compartments of the tibiofemoral joint (Table 1; Fig. 2).
Figure 2. Change of trabecular parameters over 1.5 years.
Columns indicating the mean values of change of trabecular parameters (whiskers indicating the standard deviation) between the preoperative measurements and the measurements performed within 1.5 years after HTO for each compartment (lateral femur (lf), lateral tibia (lt), medial femur (mf), medial tibia (mt); **P-values <0.05).
When comparing changes of apparent trabecular number in medial and lateral compartments, longitudinal decrease of apparent trabecular number was significantly higher in the medial tibia compared to the lateral tibia (mean difference −0.24 ± 0.1 mm−1; P<0.001), suggesting reversal of the subchondral alterations within the medial tibia over 1.5 years after HTO.
In contrast, the apparent trabecular space between baseline and 1.5 years after the HTO procedure increased within the medial tibia (0.02±0.081mm) and decreased within the other tibiofemoral compartments (lf, −0.03±0.08mm; lt, −0.05±0.09mm; mf, −0.02±0.06mm). Changes in apparent trabecular thickness over 1.5 years after HTO differed significantly between compartments, decreasing in the medial tibia (−0.10±0.08mm), and increasing in all other tibiofemoral compartments (lf, 0.07±0.09mm; lt, 0.12±0.12mm; mf, 0.07±0.07mm; P<0.001; Fig. 3).
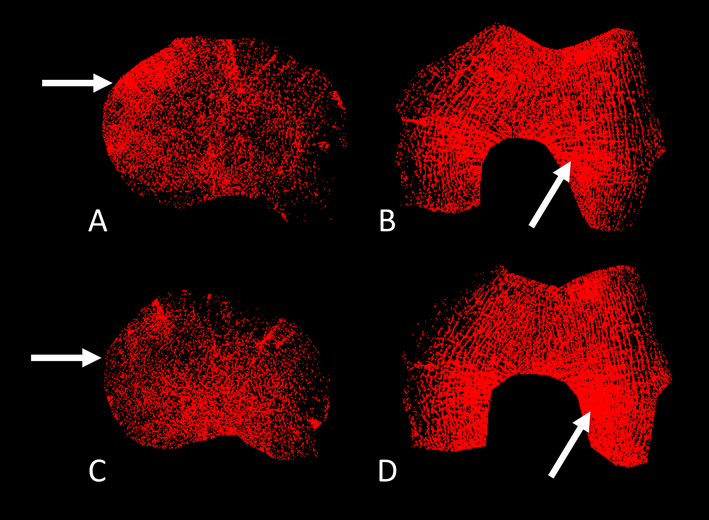
Figure 3.
MR-derived trabecular thickness maps showing change of trabecular thickness in the bone of the medial and lateral tibia (A, C) and the medial and lateral femur condyle (B, D). Maps generated immediately before HTO (A, B) and at 1.5-year follow-up (C, D). Horizontal white arrows indicating decrease in trabecular thickness over 1.5 years in the medial tibia. Oblique white arrows indicating increase of trabecular thickness over 1.5 years within the lateral femur.
The postoperative leg alignment correlated significantly with the change of apparent bone fraction (r=−0.20, P=0.01; Table 2), the change of apparent trabecular number (r=0.17, P=0.03) and the change of apparent trabecular space (r=0.22, P=0.007) over 1.5years after HTO. Moreover, the degree of correction of the leg alignment correlated significantly with the change in apparent bone fraction (r=0.29, P=0.001) as well as with the change of apparent trabecular space (r=−0.28, P<0.001), suggesting a correlation between the degree of correction and the trabecular bone parameters bone fraction and apparent trabecular space as well as suggesting the greater the degree of correction of the leg alignment achieved with HTO is, the greater the increase in bone fraction and the more the trabecular space decreases over all compartments.
Table 2.
Change of trabecular parameters over all compartments between the time point before HTO and 1.5 years after HTO correlated with the leg alignment preoperatively and postoperatively as well as with the degree of correction.
| Leg alignment preoperatively | Leg alignment postoperatively | Degree of correction | ||
|---|---|---|---|---|
| Δapp. bone fraction | Correlation Coefficient | −0.10 | −0.20 | 0.29 |
| P-value | 0.22 | 0.01 | 0.001 | |
| Δapp. trabecular thickness | Correlation Coefficient | 0.07 | 0.04 | 0.09 |
| P -value | 0.39 | 0.61 | 0,29 | |
| Δapp. trabecular number | Correlation Coefficient | 0.09 | 0.17 | −0.10 |
| P -value | 0.27 | 0.03 | 0.24 | |
| Δapp. trabecular space | Correlation Coefficient | 0.14 | 0.22 | 0.28 |
| P -value | 0.10 | 0.007 | <0.001 |
P-values <0.05 are in bold
In a sub-analysis, in which patients were categorized into the groups overweight (n=17; BMI > 25.0 kg/m2; mean BMI±SD, 28.4±3.8 kg/m2) as well as normal weight (n=5; BMI ≤ 25.0 kg/m2; mean BMI±SD, 22.8±0.9 kg/m2), the overweight group showed significantly higher apparent trabecular thickness (P=0.008, Table 3) as well as a lower apparent trabecular number (P=0.002) before HTO. Postoperatively the apparent trabecular thickness was still elevated in the overweight group compared the normal group, yet all other parameters as well as the change of all trabecular parameters over 1.5 years after HTO showed no significant difference between the overweight and normal weight group (P>0.05). None of the patients included in this study were underweight. Moreover, there were significant correlations between BMI and all preoperative trabecular bone parameters (bone fraction, r=0.47, P<0.001; trabecular thickness, r=0.49, P<0.001; trabecular number, r=0.34, P<0.001; trabecular space, r=−0.36, P<0.001) as well as all postoperative trabecular bone parameters (bone fraction, r=0.44, P<0.001; trabecular thickness, r=0.53, P<0.001; trabecular number, r=0.17, P<0.001; trabecular space, r=−0.28, P<0.001), suggesting a dependence of the trabecular bone on the BMI of the patient treated.
Table 3.
Overall trabecular parameters before HTO, 1.5 years after HTO and the change between the time points after patients were distributed into the categories overweight (BMI > 25.0 kg/m2) and normal weight (BMI ≤ 25.0 kg/m2).
| BMI | P-value | ||
|---|---|---|---|
| Overweight group (n=17) | Normal weigt group (n=5) | ||
| Preoperative parameters | |||
| app. bone fraction | 0.53 ± 0.12 | 0.50 ± 0.10 | 0.19 |
| app. trabecular thickness | 0.55 ± 0.06 | 0.46 ± 0.04 | 0.008 |
| app. trabecular number | 0.97 ± 0.07 | 1.06 ± 0.06 | 0.002 |
| app. trabecular space | 0.46 ± 0.21 | 0.46 ± 0.17 | 0.90 |
| Postoperative parameters | |||
| app. bone fraction | 0.60 ± 0.17 | 0.54 ± 0.12 | 0.54 |
| app. trabecular thickness | 0.61 ± 0.06 | 0.50 ± 0.07 | 0.002 |
| app. trabecular number | 0.91 ± 0.05 | 0.97 ± 0.04 | 0.13 |
| app. trabecular space | 0.44 ± 0.12 | 0.45 ± 0.11 | 0.75 |
| Change in parameters over 1.5 years | |||
| app. bone fraction | 0.07 ± 0.13 | 0.04 ± 0.11 | 0.31 |
| app. trabecular thickness | 0.07 ± 0.06 | 0.04 ± 0.06 | 0.41 |
| app. trabecular number | (−0.07) ± 0.07 | (−0.09) ± 0.06 | 0.57 |
| app. trabecular space | (−0.02) ± 0.20 | (−0.01) ± 0.18 | 0.62 |
P-values <0.05 are in bold
Functional scores
The evaluated functional scores showed excellent and good results at the time of follow-up after HTO in comparison to the functional scores assessed before HTO: In all patients, the Tegner Activity Score increased significantly from 3.64±1.18 before HTO to 4.45±0.80 1.5 years after HTO (P=0.01), suggesting an increase in physical activity. In addition, the adapted WOMAC score improved significantly, from overall 19.77±15.90 before HTO to 7.82±10.14 after HTO (P<0.001) and the Lysholm Score improved significantly from 64.05±16.54 to 82.81±18.09 after 1.5 years after HTO (P<0.001). Furthermore, the VAS for pain was 4.32±2.10 before HTO and 1.50±2.10 after 1.5 years after HTO (P<0.001), suggesting an improvement of pain after HTO. The improvement in WOMAC from before until 1.5 years after HTO correlated significantly with the amount of change in the apparent bone fraction (r=−0.48, P=0.01; Table 4) within the medial tibia, suggesting less worsening or even an improvement in functional outcome with an increased bone fraction in the medial tibia. Moreover there was a statistical trend found for the correlation between the improvement of WOMAC and the apparent trabecular space (r=0.44, P=0.06) as well as the apparent trabecular number (r=0.39, P=0.07). The apparent trabecular thickness (r=−0.16; P=0.49) showed no statistically significant correlation with WOMAC. On the other hand change in apparent bone fraction and trabecular thickness showed a substantial negative correlation with the activity score Lysholm in the lateral tibia (P=0.04 and P=0.01, respectively; Table 4), suggesting improved functional outcome with lower turnover within the lateral tibia.
Table 4.
Change of functional outcome scores between the time point before HTO and 1.5 years after HTO correlated with change in trabecular parameters within the same period of time in the medial tibia and in the lateral tibia.
| Change of parameters over 1.5 years medial tibia | Change in functional outcome over 1.5 years after HTO | ||||
|---|---|---|---|---|---|
| ΔVAS | ΔLysholm | ΔWOMAC | ΔTegner | ||
| Δapp. bone fraction | Correlation Coefficient | −0.32 | 0.19 | −0.48 | 0.14 |
| P-value | 0.14 | 0.39 | 0.01 | 0.54 | |
| Δapp. trabecular thickness | Correlation Coefficient | −0.32 | 0.02 | −0.16 | 0.24 |
| P -value | 0.18 | 0.94 | 0.49 | 0.29 | |
| Δapp. trabecular number | Correlation Coefficient | 0.22 | −0.08 | 0.39 | 0.08 |
| P -value | 0.33 | 0.72 | 0.07 | 0.72 | |
| Δapp. trabecular space | Correlation Coefficient | 0.28 | −0.21 | 0.44 | −0.22 |
| P -value | 0.20 | 0.37 | 0.06 | 0.33 | |
| Change of parameters over 1.5 years lateral tibia | |||||
|---|---|---|---|---|---|
| ΔVAS | ΔLysholm | ΔWOMAC | ΔTegner | ||
| Δapp. bone fraction | Correlation Coefficient | 0.18 | −0.43 | −0.02 | −0.21 |
| P -value | 0.42 | 0.04 | 0.93 | 0.37 | |
| Δapp. trabecular thickness | Correlation Coefficient | 0.38 | −0.53 | 0.02 | −0.18 |
| P -value | 0.08 | 0.01 | 0.92 | 0.42 | |
| Δapp. trabecular number | Correlation Coefficient | −0.32 | 0.25 | −0.01 | 0.13 |
| P -value | 0.15 | 0.27 | 0.95 | 0.55 | |
| Δapp. trabecular space | Correlation Coefficient | 0.03 | 0.24 | −0.01 | 0.08 |
| P -value | 0.91 | 0.22 | 0.96 | 0.72 |
P-values <0.05 are in bold
Reproducibility
The interrater reproducibility was assessed between the two readers (A.S.G. and J.Z.). ICCs for single compartments were as follows: 0.96 (0.93–0.99) for the lateral femur, 0.97 (0.92–0.99) for the lateral tibia, 0.96 (0.92–0.99) 1.51% for the medial femur and 0.95 (0.91–0.97) for the medial tibia.
For intrarater reproducibility analysis, the same reader performed repeated measurements in 4 randomly selected patients with readings separated by at least 14 days. Intrarater ICCs were calculated for each compartment: 0.97 (0.95–0.99) for the lateral femur, 0.98 (0.97–0.99) for the lateral tibia, 0.96 (0.94–0.99) for the medial femur, 0.97 (0.96–0.99) for the medial tibia and 0.96 (0.94–0.99) 1.10% over all compartments.
DISCUSSION
In our study, the effect of weight distribution changes induced by HTO on trabecular subchondral bone in individuals with varus malalignment were assessed, using MR-based trabecular bone imaging as well as by assessing correlations between these changes and functional outcome and clinical symptoms. Within 1.5 years after HTO, the subchondral bone of the medial tibia showed the highest decrease of apparent trabecular thickness and trabecular number as well as the lowest increase of apparent bone fraction in comparison to the other tibiofemoral compartments. Moreover, the change in apparent bone fraction correlated significantly with an improvement of pain, stiffness and physical function as measured by the WOMAC score, suggesting a direct link between the change in subchondral bone structure and clinical outcome.
To our best knowledge, this study is the first to examine the longitudinal changes of subchondral trabecular bone structure of the tibiofemoral joint in combination with the functional outcome and clinical symptoms in individuals with varus knee alignment before and on average 1.5 years after HTO. A previous study found highest values of apparent trabecular thickness in the medial tibia in individuals with mild and severe varus malalignment compared to individuals with neutral knee alignment or valgus knee alignment.13 Similar results had been observed previously after assessing the associations between the medial joint space narrowing scores and the apparent trabecular thickness as well as the apparent trabecular number in the proximal medial tibia.26 In neutrally aligned knees, the medial compartment absorbs 60–70% of the compressive force during weight bearing.5 Since even greater forces are found in the medial compartment in subjects with varus malalignment, consequently bone formation is induced in the medial compartment, and bone resorption is caused by decreased forces within the lateral compartment.13, 27–29 Also, our findings that trabecular thickness and number were significantly higher in overweight patients compared to normal weight patients are in line with these previous findings, since increased joint loading and alterations in gait patterns are the cause of even greater biomechanical forces in overweight patients in comparison to normal weight patients.30–33 Moreover, the fact that our study showed that correlations of pre- and postoperative trabecular findings with BMI were significant supports this concept.
Therefore, previous studies have considered overweight and knee malalignment to be the major risk factors for progression of osteoarthritis.29, 34 Also it has previously been reported in an ovine study that high degrees of osteoarthritis correlated with alterations of the subchondral bone plate microstructure.14 Moreover the same study found that increase of loading on the lateral tibiofemoral compartment after HTO with valgus overcorrection showed a 1.3 fold increase in specific bone surface of the lateral compartment compared to the group undergoing varus correction within a time period of six months, indicating the remodeling of subchondral bone after increase of load in the articular subchondral spongiosa.14 Similar findings have been reported previously in patients with varus malalignment undergoing HTO, showing a decrease of the pathological density patterns within the medial compartment in previous quantitative CT studies.35, 36 These findings are supported by our study, showing a decrease in trabecular thickness within the medial tibia, which was significantly lower compared to the increase in trabecular thickness, which was found in the other tibiofemoral compartments, after an average . Moreover it is known that in osteoarthritis, trabecular bone increases by 10 to 15%,37 due to the result of trabecular thickening, becoming apparent on radiographs as subchondral sclerosis.38 It has been suggested previously that bone remodeling and compression fractures are a main component in the process of subchondral sclerosis.26 Also, studies have shown that trabecular bone of subjects with osteoarthritis shows less content of highly mineralized bone compared to subjects from a matched control group.37, 39 The subchondral bone in osteoarthritis shows high rates of remodeling, resulting in not fully mineralized subchondral bone due to the high turnover. In our study, an association between the postoperative leg alignment as well as the degree of correction achieved by HTO and the changes in trabecular parameters over 1.5 years after HTO were found. In addition to these findings, our study suggests a correlation between the changes in the subchondral bone structure and the functional outcome as well as clinical symptoms. These findings are supported by previous studies suggesting that pathological changes in subchondral bone are associated with pain,40–43 since subchondral bone can perceive nociceptive stimuli, in contrast to aneural cartilage, and therefore is a relevant component in the development of clinical symptoms.
A decrease in mineralization may result in lower density of the bone in osteoarthritis as reported previously.18, 37 Therefore the effects of the change of the apparent trabecular thickness and the apparent trabecular number within the medial tibia of patients with varus knee malalignment undergoing HTO may be interpreted as the reversal of the previous pathological subchondral bone alterations as well as bone formation within the lateral compartment due to load redistribution through HTO. This assumption is underlined by the changes in apparent bone fraction being lowest within the medial tibia within 1.5 years after HTO was performed.
Our study has some limitations. For the assessment of clinical symptoms and physical functional disability, the adapted WOMAC score was used, which is considered to be a score for the assessment of clinical symptoms and disability cause by osteoarthritis. Moreover, we did not include a control cohort with trabecular bone measurements during the same period of time, therefore changes within the trabecular architecture parameters in patients with varus knee alignment undergoing HTO could not be compared to the parameters of a control group not undergoing treatment. Moreover, in future studies the quantitative assessment of cartilage quality is needed in order to assess the possible interactions between cartilage and subchondral bone in knees after HTO.
CONCLUSION
In summary, our study demonstrated early trabecular bone changes detected using MRI within 1.5 years after HTO in patients with varus malalignment. Our findings may indicate a reversal of the previous subchondral bone alterations in patients with varus knee malalignment after undergoing HTO. Moreover, our findings suggest that changes in subchondral bone are correlated with the improvement in functional outcome and clinical symptoms within 1.5 years after patients had undergone HTO procedure.
WHAT ARE THE NEW FINDINGS.
Early trabecular bone changes after high tibial osteotomy in patients with varus alignment can be detected using magnetic resonance imaging.
Subchondral bone alterations in patients with varus malalignment may reverse after undergoing high tibial osteotomy.
Changes in subchondral bone are associated with the improvement of functional outcome and clinical symptoms after patients with varus knee malalignment undergo the high tibial osteotomy procedure.
Acknowledgements
This study was funded by the National Institutes of Health (NIH); contract grant numbers: NIH P50-AR060752 and NIH R01-AR064771.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
REFERENCES
- 1.Lawrence RC, Hochberg MC, Kelsey JL, et al. Estimates of the prevalence of selected arthritic and musculoskeletal diseases in the United States. The Journal of rheumatology. 1989;16(4):427–41. [PubMed] [Google Scholar]
- 2.Eckstein F, Wirth W, Hudelmaier MI, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis research & therapy. 2009;11(3):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anandacoomarasamy A, Leibman S, Smith G, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Annals of the rheumatic diseases. 2012;71(1):26–32. [DOI] [PubMed] [Google Scholar]
- 4.Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Annals of the rheumatic diseases. 1993;52(7):520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP. Dynamics of knee malalignment. The Orthopedic clinics of North America. 1994;25(3):395–403. [PubMed] [Google Scholar]
- 6.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA : the journal of the American Medical Association. 2001;286(2):188–95. [DOI] [PubMed] [Google Scholar]
- 7.Lobenhoffer P, Agneskirchner JD. Improvements in surgical technique of valgus high tibial osteotomy. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2003;11(3):132–8. [DOI] [PubMed] [Google Scholar]
- 8.McNamara I, Birmingham TB, Fowler PJ, Giffin JR. High tibial osteotomy: evolution of research and clinical applications--a Canadian experience. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(1):23–31. [DOI] [PubMed] [Google Scholar]
- 9.Spahn G, Hofmann GO, von Engelhardt LV, et al. The impact of a high tibial valgus osteotomy and unicondylar medial arthroplasty on the treatment for knee osteoarthritis: a meta-analysis. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(1):96–112. [DOI] [PubMed] [Google Scholar]
- 10.Lustig S, Scholes CJ, Costa AJ, Coolican MJ, Parker DA. Different changes in slope between the medial and lateral tibial plateau after open-wedge high tibial osteotomy. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(1):32–8. [DOI] [PubMed] [Google Scholar]
- 11.Madry H, Ziegler R, Orth P, et al. Effect of open wedge high tibial osteotomy on the lateral compartment in sheep. Part I: Analysis of the lateral meniscus. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(1):39–48. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, Schooler J, Zuo J, et al. Trabecular bone structure and spatial differences in articular cartilage MR relaxation times in individuals with posterior horn medial meniscal tears. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum T, Sauerschnig M, Penzel J, et al. Early changes of trabecular bone structure in asymptomatic subjects with knee malalignment. Journal of computer assisted tomography. 2014;38(1):137–41. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler R, Goebel L, Seidel R, et al. Effect of open wedge high tibial osteotomy on the lateral tibiofemoral compartment in sheep. Part III: analysis of the microstructure of the subchondral bone and correlations with the articular cartilage and meniscus. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2015;23(9):2704–14. [DOI] [PubMed] [Google Scholar]
- 15.Feucht MJ, Minzlaff P, Saier T, et al. Degree of axis correction in valgus high tibial osteotomy: proposal of an individualised approach. International orthopaedics. 2014;38(11):2273–80. [DOI] [PubMed] [Google Scholar]
- 16.Hinterwimmer S, Beitzel K, Paul J, et al. Control of posterior tibial slope and patellar height in open-wedge valgus high tibial osteotomy. The American journal of sports medicine. 2011;39(4):851–6. [DOI] [PubMed] [Google Scholar]
- 17.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(12):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenkrantz G, Lindsey CT, Dunn TC, et al. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(12):997–1005. [DOI] [PubMed] [Google Scholar]
- 19.Bolbos RI, Zuo J, Banerjee S, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(10):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 21.van der Made AD, Reurink G, Gouttebarge V, Tol JL, Kerkhoffs GM. Outcome After Surgical Repair of Proximal Hamstring Avulsions: A Systematic Review. The American journal of sports medicine. 2015;43(11):2841–51. [DOI] [PubMed] [Google Scholar]
- 22.Stucki G, Meier D, Stucki S, et al. [Evaluation of a German version of WOMAC (Western Ontario and McMaster Universities) Arthrosis Index]. Zeitschrift fur Rheumatologie. 1996;55(1):40–9. [PubMed] [Google Scholar]
- 23.Amstutz HC, Thomas BJ, Jinnah R, et al. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. The Journal of bone and joint surgery American volume. 1984;66(2):228–41. [PubMed] [Google Scholar]
- 24.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. The American journal of sports medicine. 1982;10(3):150–4. [DOI] [PubMed] [Google Scholar]
- 25.Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res. 1989;38(5):286–8. [PubMed] [Google Scholar]
- 26.Lo GH, Tassinari AM, Driban JB, et al. Cross-sectional DXA and MR measures of tibial periarticular bone associate with radiographic knee osteoarthritis severity. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(7):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis--a biomechanical perspective. J Sci Med Sport. 2004;7(3):347–57. [DOI] [PubMed] [Google Scholar]
- 28.Englund M The role of biomechanics in the initiation and progression of OA of the knee. Best practice & research Clinical rheumatology. 2010;24(1):39–46. [DOI] [PubMed] [Google Scholar]
- 29.Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis and rheumatism. 2009;61(4):459–67. [DOI] [PubMed] [Google Scholar]
- 30.Koonce RC, Bravman JT. Obesity and osteoarthritis: more than just wear and tear. The Journal of the American Academy of Orthopaedic Surgeons. 2013;21(3):161–9. [DOI] [PubMed] [Google Scholar]
- 31.Jungmann PM, Kraus MS, Alizai H, et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis care & research. 2013;65(12):1942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andriacchi TP, Favre J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Current rheumatology reports. 2014;16(11):463. [DOI] [PubMed] [Google Scholar]
- 33.Pamukoff DN, Lewek MD, Blackburn JT. Greater vertical loading rate in obese compared to normal weight young adults. Clin Biomech (Bristol, Avon). 2016;33:61–5. [DOI] [PubMed] [Google Scholar]
- 34.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008;59(9):1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Gerbl M The subchondral bone plate. Adv Anat Embryol Cell Biol. 1998;141:III–XI, 1–134. [DOI] [PubMed] [Google Scholar]
- 36.Muller-Gerbl M, Putz R, Hodapp NH, Schulta E, Wimmer B. Computed tomography-osteoaboorptiometry: a method of assessing the mechanical condition of the major joints in a living subject. Clin Biomech (Bristol, Avon). 1990;5(4):193–8. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Aspden RM. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Annals of the rheumatic diseases. 1997;56(4):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burr DB. The importance of subchondral bone in the progression of osteoarthritis. J Rheumatol Suppl. 2004;70:77–80. [PubMed] [Google Scholar]
- 39.Grynpas MD, Alpert B, Katz I, Lieberman I, Pritzker KP. Subchondral bone in osteoarthritis. Calcified tissue international. 1991;49(1):20–6. [DOI] [PubMed] [Google Scholar]
- 40.Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):1033–40. [DOI] [PubMed] [Google Scholar]
- 41.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. The Journal of rheumatology. 2001;28(6):1330–7. [PubMed] [Google Scholar]
- 42.Lo GH, McAlindon TE, Niu J, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17(12):1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. [DOI] [PubMed] [Google Scholar]