Abstract
Current clinical options for the treatment of neovascular disorders of the posterior segment of the eye have several drawbacks. Photocoagulation lasers can impair peripheral and night vision. Photodynamic therapies as well as intravitreal macromolecule injections (Macugen® and Lucentis®) require frequent, invasive administrations. Above all, only modest improvement in vision is achieved with any of the existing treatments. In order to overcome these limitations in the long run, this study investigated the antiangiogenic potential of AQ4, a low molecular weight anthracenedione. The results indicate that AQ4 enters the cell nucleus and inhibits proliferation of choroid-retina endothelial (RF/6A) cells and human retinal pigment epithelial (ARPE-19) cells under hypoxic (1% O2) as well as normoxic (21% O2) conditions. The IC50 for these effects ranges from 5.5 to 6.3 µM. AQ4 does not affect the viability of nondividing RF/6A or ARPE-19 cells up to 0.1 mM. Further, AQ4 (20 µM) reduces vascular endothelial growth factor (VEGF) protein secretion by about 50% in ARPE-19 cells under normoxia as well as hypoxia, by reducing VEGF transcription. AQ4 arrests the growth of endothelial cells in S phase, consistent with interference of AQ4 with DNA replication. These results for the first time suggest that AQ4 can potentially alleviate the neovascularization of choroid/retina by a dual mechanism of inhibiting the proliferation of endothelial cells and by reducing mitogenic VEGF stimulus from retinal pigment epithelial cells.
Keywords: AQ4, Antiproliferative, Anti-VEGF, Retina, Choroid
1. Introduction
Inhibition of angiogenesis is of therapeutic value for vision threatening ocular disorders such as age related macular degeneration (ARMD) (Zhang and Ma, 2007). The treatment options for ocular neovascularization range from non-specific thermal laser photocoagulation of new blood vessels to prophylactic approach of intravitreal injection of vascular endothelial growth factor (VEGF) targeted macromolecules (Bylsma and Guymer, 2005). While, the former damages the overlying outer retina as well as retinal pigment epithelium (RPE), the latter is not likely to completely abolish neovascularization, as multiple growth factors are involved in the pathogenesis of neovascularization (Das and McGuire, 2003). Further, prevention of endothelial cell proliferation and migration by agents such as anecortave acetate has broader ability to inhibit angiogenesis by acting at a level subsequent to any inciting stimulus (Bylsma and Guymer, 2005). An ideal treatment should inhibit new blood vessel formation as well as provide prophylaxis to prevent recurrence of the neovascularization. In this study, we investigate the bi-pronged approach of preventing endothelial cell proliferation along-with inhibition of retinal pigment epithelial VEGF secretion by an investigational anticancer drug, AQ4.
Multiple anti-cancer agents have been reported to prevent proliferation of noncancerous cells of eye. The cytostatic activity of drugs such as 5-fluorouracil (Akarsu et al., 2003), mitomycin C (Chen et al., 1990; Kitazawa et al., 1991; Palmer, 1991), mitoxantrone (Tilleul et al., 1997), cytosine arabinoside (Al-Aswad et al., 1999), and idarubicin (Heilmann et al., 1999) is employed in ophthalmology to improve the clinical outcome of glaucoma filtration surgery. Anticancer agents increase the bleb survival subsequent to the surgery by inhibiting the proliferation of fibroblasts. An analogous approach could be applied to prevent the proliferation of retinal cells.
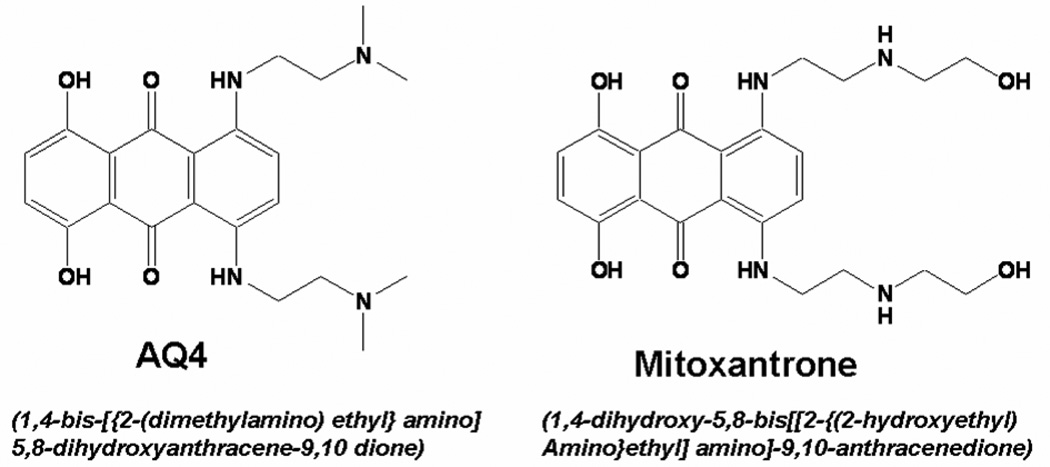
AQ4, (1,4-bis[{2-dimethylamino)ethyl}amino] 5, 8-dihydroxyanthracene-9,10-dione), is an anthracenedione anti-cancer agent (McKeown et al., 1995) similar to mitoxantrone (Fig. 1). Mitoxantrone is clinically used as an antineoplastic and immunosuppressive agent (Scott and Figgitt, 2004). In addition, mitoxantrone has antiangiogenic activity in rat corneas (Polverini and Novak, 1986). The antiangiogenic activity of mitoxantrone can be attributed to inhibition of prostaglandin E2 (PGE2) secretion (Frank and Novak, 1986), a potent stimulator of retinal VEGF secretion (Cheng et al., 1998). Owing to structure activity relationship, we hypothesized AQ4 inhibits choroid-retina endothelial (RF/6A) cell proliferation and reduces vascular endothelial growth factor (VEGF) secretion from retinal pigment epithelial (RPE) cells.
Figure 1. Chemical structure of AQ4 and mitoxantrone.
Multiple cell types in the eye including retinal pigment epithelial (RPE) cells produce VEGF (Murata et al., 1996). Our earlier work demonstrated induction of VEGF secretion from ARPE-19 cells by a lipid peroxidation product (Ayalasomayajula and Kompella, 2002), and inhibition of VEGF secretion by budesonide (Kompella et al., 2003), a corticosteroid, and celecoxib (Amrite et al., 2006), a cyclooxygenase-2 inhibitor, in ARPE-19 cells. Thus, ARPE-19 cells are useful as a model for similar analysis with AQ4. Further, rhesus Macaque choroid-retina derived endothelial cell line is a useful cell based model in angiogenesis research (Gendron et al., 2001; Kasai et al., 2004).
Thus, using well established models of choroid-retina endothelial (RF/6A) cells and retinal pigment epithelial (ARPE-19) cells, this study investigated antiproliferative and VEGF secretion inhibitory potential of AQ4, with an objective to add a low molecular weight drug molecule to the armamentarium for the management of neovascular ocular diseases.
2. Materials and Methods
2.1. Materials
AQ4 was provided by Novacea Inc., San Francisco, CA. Dulbecco's modified Eagle's medium (DMEM:F/12; 1:1), Ham’s F12 nutrient mixture, fetal bovine serum, penicillin–streptomycin and L-glutamine were obtained from Gibco-BRL (Grand Island, NY). Cell culture flasks and plates were purchased from Corning Inc. (Corning, NY). BrDU assay kit was purchased from Calbiochem (San Diego, CA). The antibodies for VEGF ELISA were purchased from Research Diagnostics Inc. (Flanders, NJ). 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), propidium iodide, ribonuclease A, EDTA, and Triton-X 100 were purchased from Sigma (St. Louis, MO).
2.2. Methods
2.2.1. Cell culture
Human (Homo sapiens) retinal pigment epithelial cells, ARPE-19, and monkey (Macacca mulatta) choroid-retina endothelial cells, RF/6A, were obtained from ATCC (Manassas, VA). The cell lines were maintained using the subculture protocol recommended by ATCC at 37°C and 5% CO2. Specifically, ARPE-19 cells were cultured in DMEM:F12 (1:1) supplemented with 10% v/v fetal bovine serum (FBS), 2% v/v L-glutamine (200 mM) and 1% v/v penicillin-streptomycin (a mixture containing 10,000 units/ml of penicillin G sodium and 10,000 µg/ml streptomycin sulphate). RF/6A cells were cultured in Ham’s F12 nutrient mixture supplemented with 10% v/v fetal bovine serum (FBS), 2% v/v L-glutamine (200 mM) and 1% v/v penicillin-streptomycin (a mixture containing 10,000 units/ml of penicillin G sodium and 10,000 µg/ml streptomycin sulphate).
Throughout the manuscript, normoxia and hypoxia refer to 21% and 1% O2, respectively. For all the studies under hypoxia, the treatment solutions were incubated under hypoxia (1% O2) for 12 h to allow the dissolved oxygen in the solutions to come in equilibrium with the culture conditions.
2.2.2. Cell uptake and clearance of AQ4
Confocal microscopy was used to study cellular entry and clearance of AQ4 by RF/6A and ARPE-19 cells. Confluent cells cultured in Delta T4 dishes (Bioptechs, Inc. Butler, PA) were incubated with AQ4 (20 µM) under normoxia for 3 h. The cells were washed thrice with PBS pH 7.4 and images taken in non-fixed cells with Zeiss LSM 410 confocal laser scanning microscope (Goettinger, Germany) using 647 nm argon/krypton laser (Smith et al., 1997b).
2.2.3. Antiproliferative activity of AQ4 under normoxia and hypoxia
The antiproliferative activity of AQ4 was studied by bromodeoxyuridine (BrDU) assay. BrDU, a thymidine analogue, is incorporated into newly synthesized DNA strands of proliferating cells. A reduction in percentage of BrDU incorporation is indicative of reduction in percentage of cells proliferating. A BrDU assay kit from Calbiochem (San Diego, CA) was used for the studies and the assay carried out according to manufacturer’s protocol. Specifically, the cells were seeded in a 96 well plate at a seeding density of 1 × 104 cells/well and allowed to attach overnight. Two types of negative controls were used: blank (wells without any cells) and background (wells with cells but no BrDU label). The cells were exposed to various concentrations of AQ4 for 12 h under normoxia and hypoxia. BrDU label (20 µl) was added to each well (except background wells), 5 h prior to the completion drug treatment duration. The cells were fixed, permeabilized and the DNA denatured, to enable binding of anti-BrDU antibody. The cells were incubated with anti-BrDU antibody for 1 h. The unbound antibody was washed away and horseradish peroxidase- conjugated goat anti-mouse antibody was added into each well and incubated for 30 min. Finally, chromogenic substrate tetra-methylbenzidine (TMB) was added and the absorbance of color developed measured by using a microplate reader at dual wavelength of 450 nm and 540 nm.
2.2.4. Cell cycle arrest byAQ4 under normoxia and hypoxia
The effect of AQ4 on cell cycle was assessed by DNA content analysis using flow cytometry. The cells were plated in T 25 flasks at a seeding density of 100,000 cells/well and were allowed to attain 60% confluency. The cells were then treated with IC50 concentration of AQ4 (Normoxia: 6.3 µM and hypoxia: 5.8 µM) for 12 hours. The cells treated with serum free media under normoxia and hypoxia served as respective controls. Treated cells and controls were fixed in 70% ethanol at 4°C for 1 h and subsequently stained overnight with propidium iodide (50 µg/ml in PBS, pH 7.4) at 4°C. The DNA content analysis was carried out using a BD FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) equipped with CellQuest (Becton-Dickinson, San Jose, CA) data acquisition software. The data was analyzed using Modfit LT software (Verity Software House, Topsham, ME).
2.2.5. Cytotoxicity of AQ4 under normoxia and hypoxia
The cytotoxicity of AQ4 was assessed using MTT assay. The cells were plated in a 96-well plate at a seeding density of 1000 cells/well and allowed to reach confluency (8 days). Confluent cells were incubated with solutions of AQ4 ranging in concentration from 1 nM to 1 mM for 12 h under normoxia and hypoxia, subsequent to 12 h quiescence. The media containing drug was aspirated out and 200 µl of fresh serum free medium was added to each well. MTT reagent (20 µl of 5 mg/ml MTT dissolved in PBS pH 7.4) was added to each well and incubated at 37°C for 4 h. The medium was aspirated out and the formazan crystals formed were dissolved in 200 µl of DMSO. The absorbance of the color developed was measured at 540 nm using a microplate reader.
2.2.6. VEGF secretion inhibitory activity of AQ4 under normoxia and hypoxia
VEGF secretion studies were performed as previously described (Amrite et al., 2006; Ayalasomayajula and Kompella, 2003) using an ELISA method capable of detecting VEGF165 and VEGF121 isoforms, under normoxia and hypoxia. Briefly, ARPE-19 cells were seeded into a 96-well plate at a seeding density of 1000 cells per well and cultured until confluency. The confluent cells were treated with 100 nM to 20 µM AQ4 solution in serum free media, subsequent to 12 h quiescence. The culture media was collected at 12 h and the secreted VEGF quantified using an ELISA Kit purchased from Research Diagnostics, Inc. (Flanders, NJ). The VEGF levels were normalized to total protein content in the cell lysates.
2.2.7. VEGF mRNA analysis using real time polymerase chain reaction (real time PCR) under normoxia and hypoxia
ARPE-19 cells cultured in T25 flasks were divided into 4 groups: control normoxia, AQ4 (20 µM) normoxia, control hypoxia and AQ4 (20 µM) hypoxia (n = 3). Controls groups were incubated with serum free media. The cells were incubated with the respective treatments for 12 h. Following treatment, total RNA was isolated from cells using RNeasy® protect mini kit (Qiagen Inc., Valencia, CA). The RNA (2 µg) isolated from various groups of cells were converted into cDNA by reverse transcription. Real time – PCR was carried out using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Bedford, MA). The reactions were performed with 2× SYBR Green PCR master mix (Applied Biosystems, Bedford, MA), in the presence of 80 ng cDNA and 500 nM of specific primer sets. Samples were analyzed in triplicates. Amounts of input RNAs in each sample were corrected for by dividing threshold cycle (Ct) of each specific gene by the Ct for 18s rRNA. Fold values were calculated as 2−ΔCt, where ΔCt = Ct for the specific gene – Ct for 18s rRNA in the same RNA. The samples with the lowest expression were set to 1.0 fold and other data were adjusted to that baseline. The primers used were sense 5’- TGGATCCATGAACTTTCTGCTGTC-3’ and antisense 5’- TCACCGCCTTGGCTTGTCACAT-3’ for human VEGF165 and VEGF121.
2.2.8. Statistical analysis
Comparison of means of various groups was done using non- parametric statistical analysis. Comparison of two groups was carried out using Mann Whitney test. However, for comparison of more than two groups, Kruskal Wallis non-parametric ANOVA was employed. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. AQ4 enters retinal cell nuclei within 3 h and persists in cells for at least 2 h
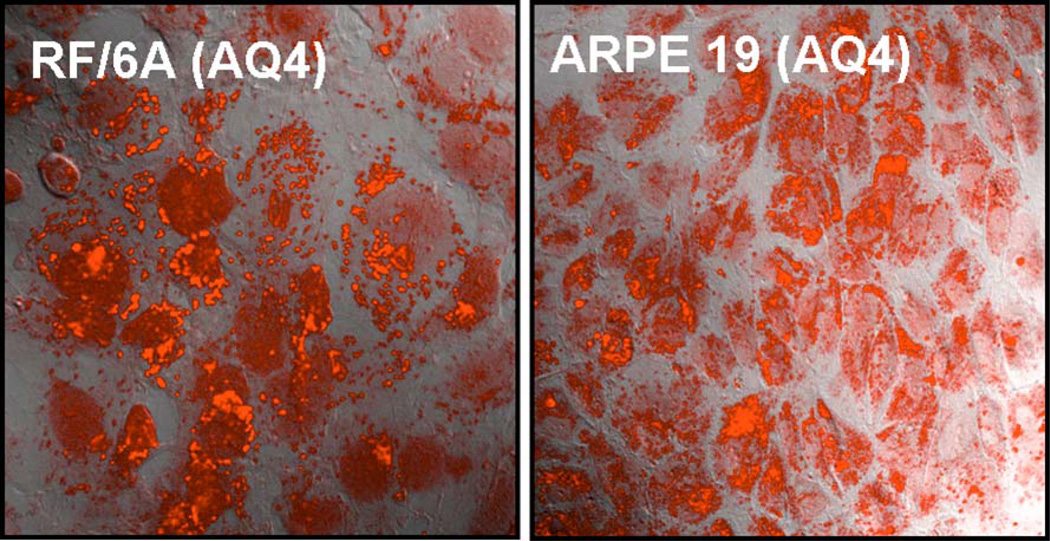
AQ4 fluoresces within choroid-retina endothelial and retinal pigment epithelial cells at an excitation wavelength of 647 nm. The Z-axis series for cells treated with AQ4 confirmed that fluorescence was intracellular. Upon overlay of confocal image with DIC image, AQ4 was observed in retinal cell cytoplasm as well as in the nucleus (Fig. 2) after 3 h of drug exposure.
Figure 2. Accumulation of AQ4 in RF/6A and ARPE-19 cells.
The cells were incubated with AQ4 (20 µM) for 3 h under normoxia. Following drug exposure, cells were washed and fresh serum free medium was added. Confocal images were obtained at an excitation wavelength of 647 nm. Magnification: 63×.
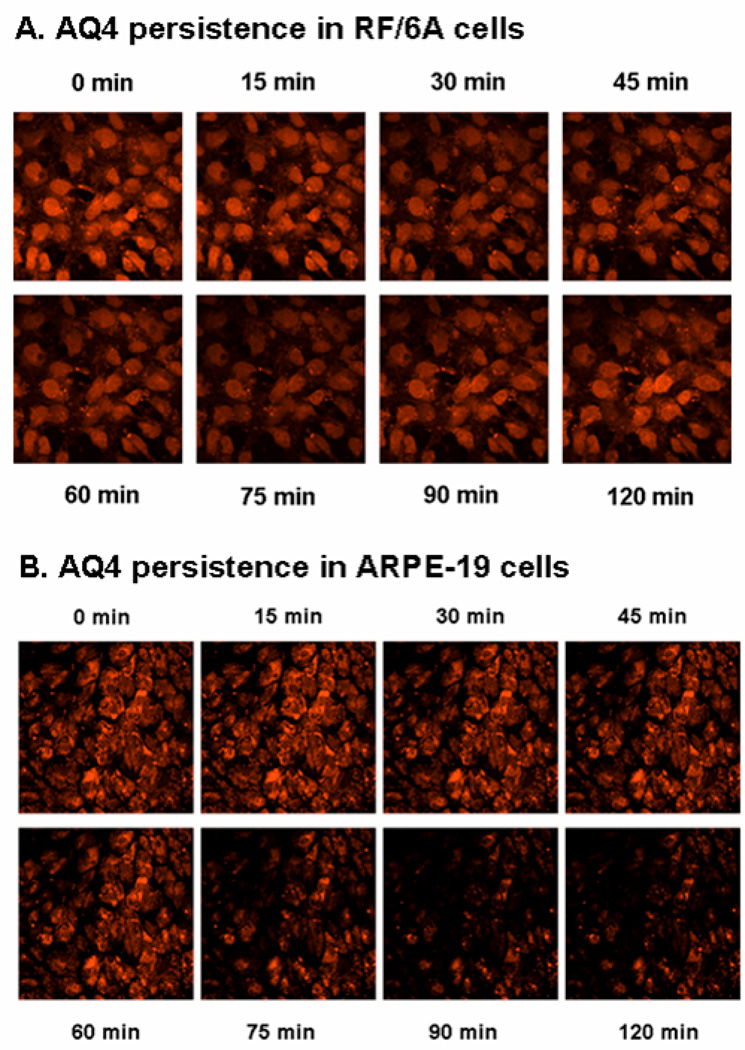
The fluorescence intensity of AQ4 in retinal cells does not bleach upon exposure to the confocal laser for 2 h (data not shown). Thus, confocal microscopy can be used for at least 2 h in live retinal cells for imaging AQ4. AQ4 was demonstrated to be retained within both types of retinal cells for at least 2 h following drug exposure. Significant disappearance of AQ4 from the cells was not seen until 75 min (Fig. 3).
Figure 3. Retention of AQ4 in (A) RF/6A and (B) ARPE-19 cells.
The cells were incubated with AQ4 (20 µM) for 3 h under normoxia. Following drug exposure, cells were washed and fresh serum free medium was added. Confocal images were obtained every 15 min up to 2 h post treatment at an excitation wavelength of 647 nm. Magnification: 63×.
3.2. AQ4 inhibits proliferation of endothelial and retinal pigment epithelial cells under normoxia and hypoxia
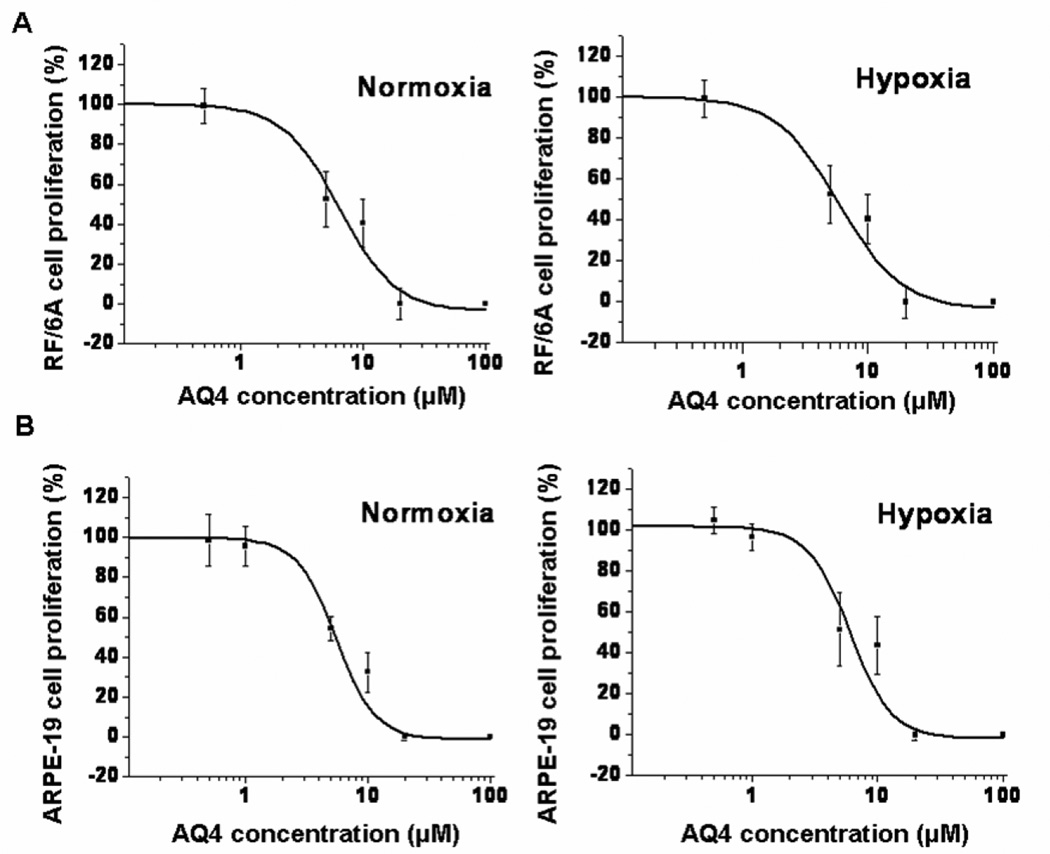
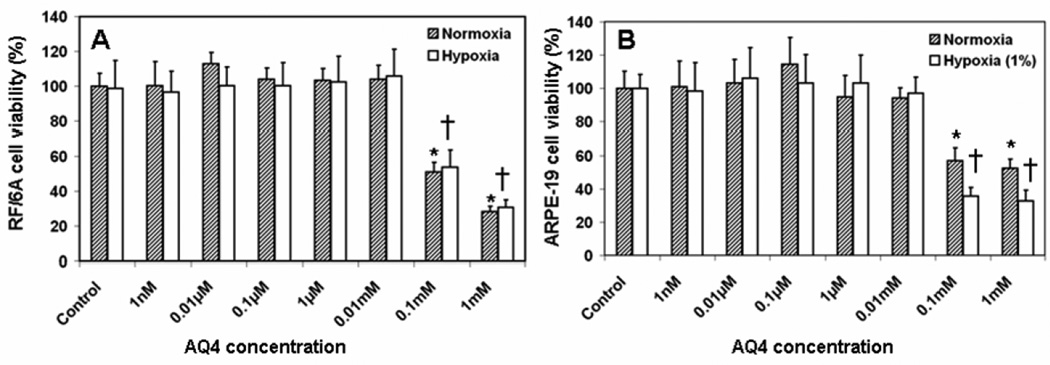
AQ4 reduced the proliferation (measured as % BrDU incorporation compared to serum free media treated cells) of sub-confluent RF/6A as well as ARPE-19 cells under both normoxic and hypoxic conditions, in a dose dependent manner (Fig. 4). In RF/6A cells, a significant reduction in percentage proliferating cells could be detected at 1 µM and above with AQ4 under normoxia as well as hypoxia. No difference was observed between normoxia and hypoxia groups, suggesting the susceptibility of even hypoxic retinal cells to antiproliferative effects of AQ4. The data exhibited a typical sigmoidal fit. AQ4 IC50 in the two cell lines was observed to be of a similar order of magnitude. The IC50 of AQ4 in RF/6A cells under normoxia and hypoxia was found to be 6.38 ± 1.51 µM and 5.84 ± 1.08 µM, respectively. The IC50 of AQ4 in ARPE-19 cells under normoxia and hypoxia was 5.51 ± 0.49 µM and 6.05 ± 1.35 µM, respectively. Further, no cytotoxicity in non-dividing (confluent) RF/6A and ARPE-19 cells was observed at antiproliferative concentrations of AQ4 (Fig. 6), suggesting specificity of cytotoxicity to only dividing cells.
Figure 4. Antiproliferative effect of AQ4 in (A) RF/6A and (B) ARPE-19 cells under normoxia and hypoxia.
BrDU incorporation was determined in both cells after 12 h incubation with various concentrations of AQ4 under normoxia and hypoxia. The percentages were calculated relative to BrDU incorporation in serum free medium treated controls. Data are expressed as mean ± s.d. for n = 8.
Figure 6. Effect of AQ4 on viability of non-dividing (A) RF/6A and (B) ARPE-19 cells.
Confluent monolayers of both cell types were treated with various concentrations of AQ4 or serum free medium (controls) for 12 h under normoxia and hypoxia. The percentage cell viability was determined using MTT assay. Data are expressed as mean ± s.d. for n = 8. * P < 0.05 as compared to normoxia control. † P < 0.05 as compared to hypoxia control.
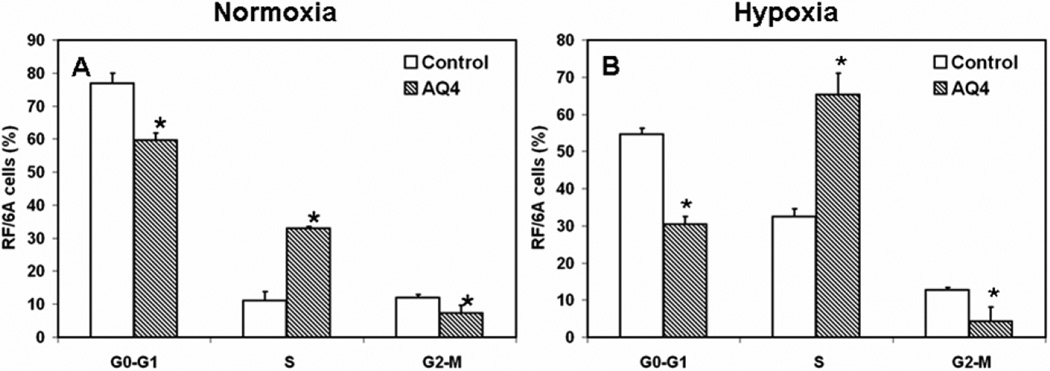
3.3. AQ4 causes S phase cell cycle arrest in endothelial cells under normoxia and hypoxia
AQ4 IC50 concentration (Normoxia: 6.3 µM and hypoxia: 5.8 µM) causes S-phase cell cycle arrest in choroid-retina endothelial cells (Fig. 5). The percentage of RF/6A cells in S phase of cell cycle, 12 h post treatment with AQ4 was significantly higher as compared to serum free media treated control groups. Specifically, the percentage of RF/6A cells in S phase in control and AQ4 treated groups was 11.1 ± 2.6 and 32.9 ± 0.6 under normoxia, while under hypoxia the percentage was 32.5 ± 2.0 and 65.3 ± 5.7, respectively.
Figure 5. Effect of AQ4 on cell cycle of RF/6A cells under normoxia and hypoxia.
RF/6A cells were treated with IC50 concentration of AQ4 or serum free medium (control) under normoxia (6.3 µM) and hypoxia (5.8 µM) for 12 h. The cells were fixed with 70% ethanol and stained with propidium iodide. DNA content was then analyzed by flow cytometry. Data are expressed as mean ± s.d. for n = 3. * P < 0.05 as compared to serum free medium treated controls.
3.4. AQ4 inhibits VEGF secretion from retinal pigment epithelial cells under normoxia and hypoxia
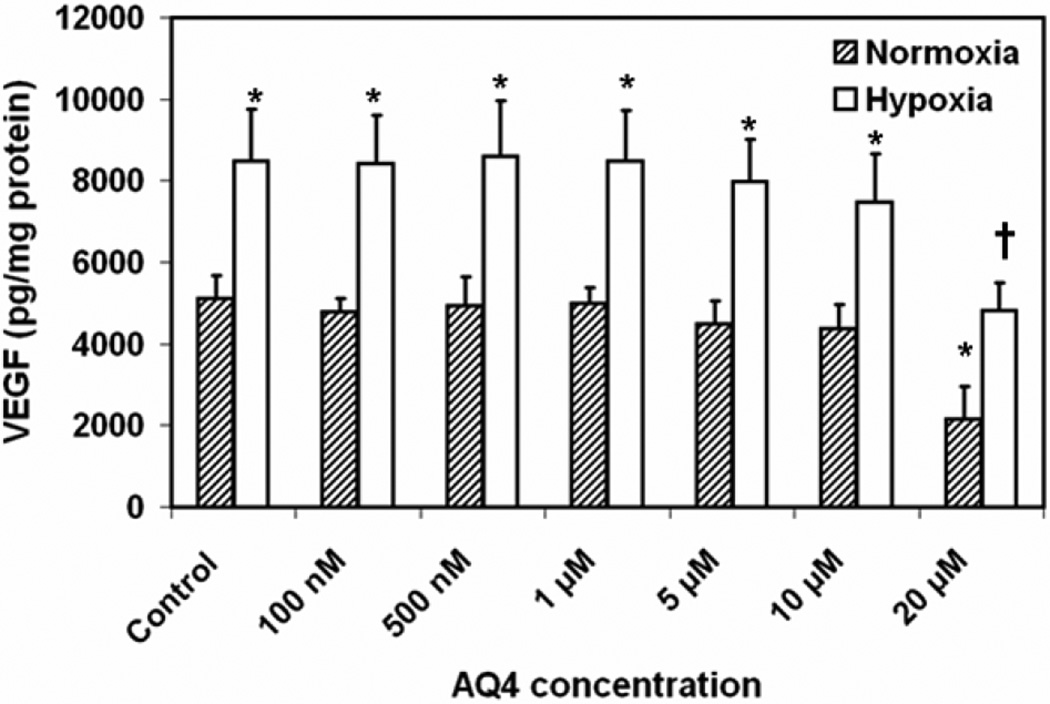
Treatment of ARPE-19 cells with AQ4 (20 µM) for 12 h decreases the VEGF secretion by 50% under normoxia as well as hypoxia as compared to the respective controls (Fig. 7). Specifically, the VEGF levels in the cell culture supernatant decreased from 5125 ± 535 in untreated cells to 2144 ± 810 pg/mg protein in cells treated with 20 µM AQ4 under normoxic culture conditions. In cells cultured under hypoxic conditions, VEGF levels reduced from 8495 ± 1252 in untreated cells to 4820 ± 687 pg/mg protein in AQ4 treated cells. As expected, hypoxia significantly elevated VEGF secretion from ARPE-19 cells.
Figure 7. Effect of AQ4 on VEGF secretion from ARPE-19 cells.
Secreted VEGF levels were quantified in cell culture supernatants following treatment of confluent ARPE-19 cells with various concentrations of AQ4 or serum free medium (controls) under normoxia and hypoxia. The VEGF levels were normalized to the total protein content. Data are expressed as mean ± s.d. for n = 6. * P < 0.05 as compared to normoxia control. † P < 0.05 as compared to hypoxia control.
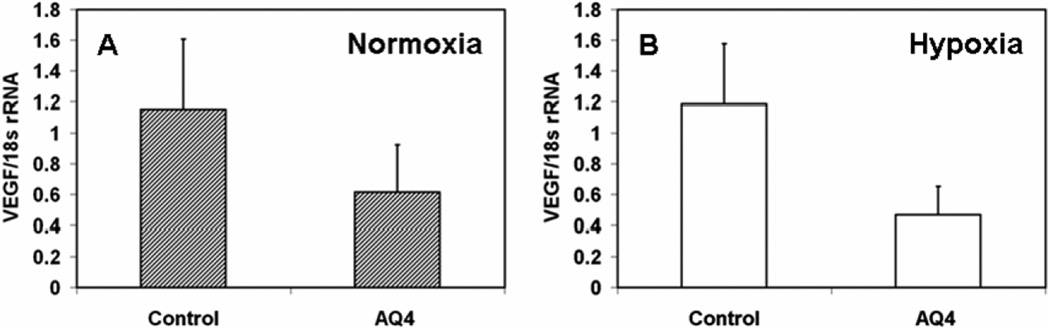
3.5. AQ4 reduces VEGF mRNA in retinal pigment epithelial cells under normoxia and hypoxia
Compared to serum free media treated controls, 12 h exposure of AQ4 (20 µM) reduced VEGF mRNA levels normalized to 18s rRNA by 40 and 60%, under normoxia and hypoxia, respectively, in ARPE-19 cells (Fig. 8). Thus, AQ4 reduces VEGF secretion in ARPE-19 cells by interfering with VEGF transcription.
Figure 8. Effect of AQ4 on VEGF mRNA levels in ARPE-19 cells under (A) normoxia and (b) hypoxia.
Confluent ARPE-19 cells were treated with AQ4 (20 µM) under normoxia and hypoxia. VEGF mRNA levels relative to 18s rRNA levels were quantified using real time PCR. Untreated cells under normoxia and hypoxia served as respective controls. Data are expressed as mean ± s.e.m. for n = 3.
4. Discussion
In this study, we have for the first time demonstrated that AQ4 enters the cytoplasm and nuclei of choroid-retina endothelial cells as well as RPE cells, inhibits endothelial cell proliferation, arrests the endothelial cell cycle in S phase, does not reduce the viability of non-dividing cells, and suppresses VEGF secretion by reducing VEGF transcription in RPE cells. Further, we demonstrated that AQ4 prevents proliferation of RPE cells. Several of these effects were observed at low micromolar concentrations under normoxia as well as hypoxia, indicating the potential value of AQ4 as a therapeutic agent for treating neovascular disorders of the posterior segment of the eye.
Pharmacodynamic characteristics of drug molecules are dictated by the ability of the drug to reach its intracellular target of action. We used 647 nm argon/krypton laser line based confocal microscopy method to study the drug uptake, subcellular distribution, and persistence in retinal cells (Smith et al., 1997b). Within 3 h, AQ4 enters the cell cytoplasm as well as nuclei of both cell types (RF/6A and ARPE-19) tested in this study (Fig. 2). Nuclear entry of AQ4 in cells is required for pharmacological effect, since the mechanism of action of anthracenediones involves intercalation with replicating DNA followed by inhibition of DNA and RNA polymerase and topoisomerase (Patterson, 1993). Further, AQ4 is retained well in the cells, as indicated by a slow decline of AQ4 fluorescence in both cell types, during a 2 h drug clearance study (Fig. 3). Similar results have been previously reported in SV40-transformed human fibroblast cell lines, MRC5-V1 and AT5BIVA, exposed to AQ4 (Smith et al., 1997b).
AQ4 reduces the proliferation of choroid-retina endothelial cells in a dose dependent manner, with an IC50 of 6.3 and 5.8 µM under normoxia and hypoxia, respectively (Fig. 4A). Hypoxia has been implicated in the pathogenesis of neovascular ocular disorders (Mousa et al., 1999; Nguyen et al., 2004; Nyengaard et al., 2004). Hypoxic subpopulations within a tumor cell mass are resistant to the cytotoxicity of chemotherapeutic agents (Shannon et al., 2003; Tomida and Tsuruo, 1999). Thus, it is noteworthy that AQ4 exerts similar antiproliferative effects under normoxic as well as hypoxic conditions.
Moreover, AQ4 reduces the proliferation of RPE cells with an IC50 of 5.5 and 6.0 µM under normoxia and hypoxia, respectively (Fig. 4B). Inhibition of RPE cell proliferation is of therapeutic value in the management of age related macular degeneration (Hoffmann et al., 2005; Hoffmann et al., 2004; Kaven et al., 2001; King et al., 2005). Since AQ4 does not show any cytotoxicity in non-dividing cells at the antiproliferative concentrations (Fig. 6), it is expected to prevent proliferation of endothelial and pigment epithelial cells in vivo, without affecting non-dividing cells.
Further, DNA content analysis using flow cytometry showed that AQ4 arrests endothelial cell cycle in S-phase (Fig. 5), consistent with previous reports that AQ4 inhibits DNA replication by DNA intercalation and topoisomerase II inhibition (Smith et al., 1997a).
This is the first study to demonstrate that an anthracenedione (AQ4) antitumor agent can inhibit VEGF secretion. We observed that AQ4 reduces VEGF secretion from ARPE-19 cells under normoxia as well as hypoxia, by about 50% at 20 µM (Fig. 7). This reduction in VEGF secretion is probably an outcome of reduced VEGF transcription, as evidenced by real-time PCR analysis in this study (Fig. 8). Although an exact mechanism cannot be attributed to the VEGF secretion inhibitory activity, it is likely that it involves interference with prostaglandin secretion.
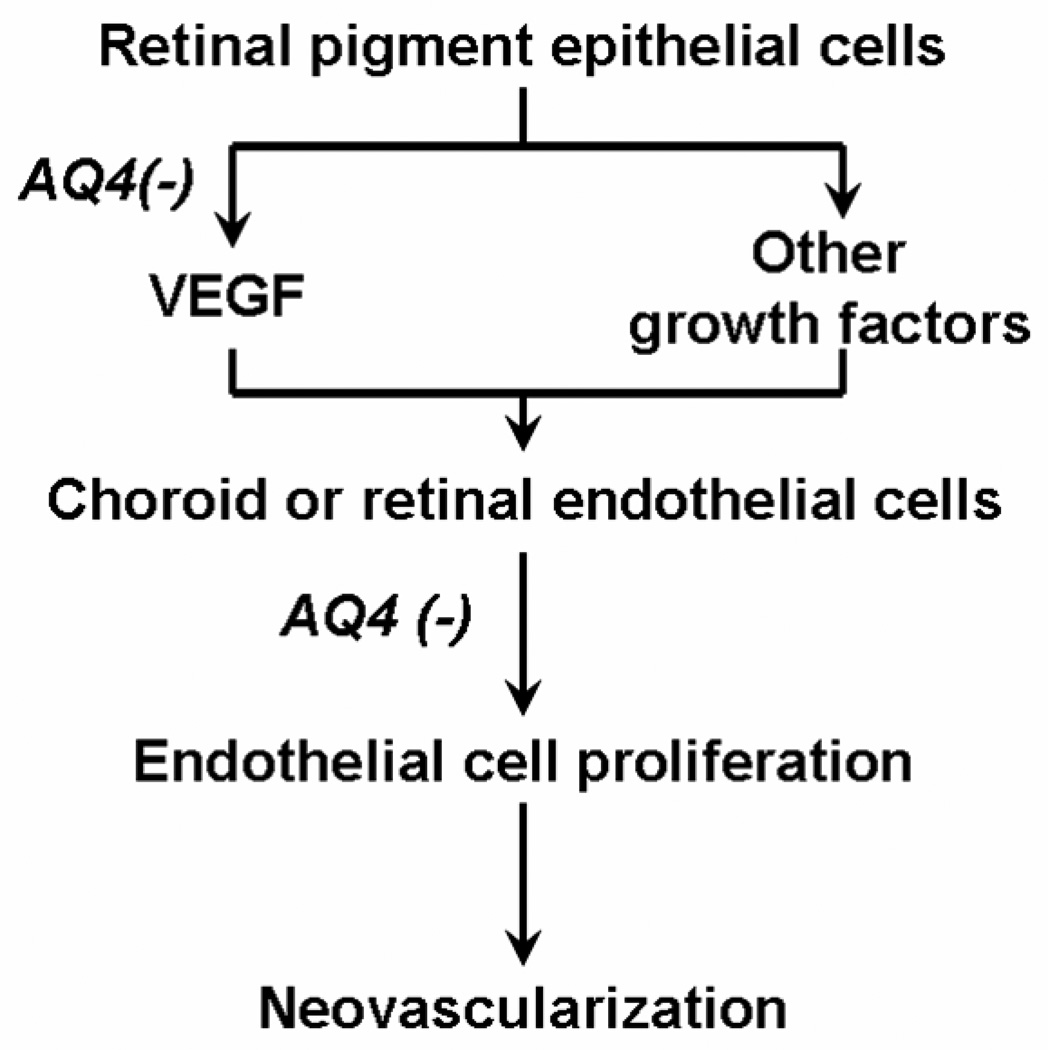
In conclusion, this study is the first to demonstrate that AQ4, a topoisomerase II inhibitor, exerts antiproliferative and anti-VEGF effects in retinal cells (Fig. 9). The antiproliferative and VEGF secretion inhibition is not dependent on the oxic state of the cells. Thus, even ischemic retinal cells are expected to be affected by AQ4. The clinical use of AQ4 for treating ocular disorders would require thorough toxicity assessment of the drug. However, noteworthy is the fact that no ocular complication has been reported to date for clinically used anthracenedione, mitoxantrone (Schmid et al., 2006). Thus, AQ4 is of potential therapeutic value in treating neovascular and other proliferative disorders of the eye.
Figure 9. Proposed mechanisms for the anti-angiogenic activity of AQ4.
Acknowledgments
This work was primarily supported by Novacea Inc., San Francisco, CA and in part by NIH grants DK064172, EY013842, and EY017045 (through Emory University). We thank Janice Taylor of the Confocal Laser Scanning Microscopy Core Facility at University of Nebraska Medical Center, which is supported by the Nebraska Research Initiative, for providing assistance with confocal microscopy. We thank Dr. Charles Kuszynski and Linda M. Wilkie of Cell Analysis Facility at University of Nebraska Medical Center for assistance with flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akarsu C, Onol M, Hasanreisoglu B. Postoperative 5-fluorouracil versus intraoperative mitomycin C in high-risk glaucoma filtering surgery: extended follow up. Clin. Experiment. Ophthalmol. 2003;31:199–205. doi: 10.1046/j.1442-9071.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- Al-Aswad LA, Huang M, Netland PA. Inhibition of Tenon's fibroblast proliferation and enhancement of filtration surgery in rabbits with cytosine arabinoside. J. Ocul. Pharmacol. Ther. 1999;15:41–49. doi: 10.1089/jop.1999.15.41. [DOI] [PubMed] [Google Scholar]
- Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Induction of vascular endothelial growth factor by 4-hydroxynonenal and its prevention by glutathione precursors in retinal pigment epithelial cells. Eur. J. Pharmacol. 2002;449:213–220. doi: 10.1016/s0014-2999(02)02043-5. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur. J. Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- Bylsma GW, Guymer RH. Treatment of age-related macular degeneration. Clin. Exp. Optom. 2005;88:322–334. doi: 10.1111/j.1444-0938.2005.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Chen CW, Huang HT, Bair JS, Lee CC. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J. Ocul. Pharmacol. 1990;6:175–182. doi: 10.1089/jop.1990.6.175. [DOI] [PubMed] [Google Scholar]
- Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest. Ophthalmol. Vis. Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Frank P, Novak RF. Effects of mitoxantrone and bisantrene on platelet aggregation and prostaglandin/thromboxane biosynthesis in vitro. Anticancer Res. 1986;6:941–947. [PubMed] [Google Scholar]
- Gendron RL, Good WV, Adams LC, Paradis H. Suppressed expression of tubedown-1 in retinal neovascularization of proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2001;42:3000–3007. [PubMed] [Google Scholar]
- Heilmann C, Schonfeld P, Schluter T, Bohnensack R, Behrens-Baumann W. Effect of the cytostatic agent idarubicin on fibroblasts of the human Tenon's capsule compared with mitomycin C. Br. J. Ophthalmol. 1999;83:961–966. doi: 10.1136/bjo.83.8.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, He S, Jin ML, Masiero L, Wiedemann P, Ryan SJ, Kohn EC. Carboxyamido-triazole modulates retinal pigment epithelial and choroidal endothelial cell attachment, migration, proliferation, and MMP-2 secretion of choroidal endothelial cells. Curr. Eye Res. 2005;30:103–113. doi: 10.1080/02713680490894595. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, He S, Wiedemann P. Carboxyamido-triazole inhibits substeps of choroidal neovascularization on retinal pigment epithelial cells and choroidal endothelial cells in vitro. Ophthalmologe. 2004;101:993–997. doi: 10.1007/s00347-003-0874-3. [DOI] [PubMed] [Google Scholar]
- Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem. Biophys. Res. Commun. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Kaven C, Spraul CW, Zavazava N, Lang GK, Lang GE. Thalidomide and prednisolone inhibit growth factor-induced human retinal pigment epithelium cell proliferation in vitro. Ophthalmologica. 2001;215:284–289. doi: 10.1159/000050875. [DOI] [PubMed] [Google Scholar]
- King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem. Biol. Interact. 2005;151:143–149. doi: 10.1016/j.cbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Kawase K, Matsushita H, Minobe M. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch. Ophthalmol. 1991;109:1693–1698. doi: 10.1001/archopht.1991.01080120077030. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Hejmadi MV, McIntyre IA, McAleer JJ, Patterson LH. AQ4N: an alkylaminoanthraquinone N-oxide showing bioreductive potential and positive interaction with radiation in vivo. Br. J. Cancer. 1995;72:76–81. doi: 10.1038/bjc.1995.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Lorelli W, Campochiaro PA. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J. Cell. Biochem. 1999;74:135–143. [PubMed] [Google Scholar]
- Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab. Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest. Ophthalmol. Vis. Sci. 2004;45:617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53:2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- Palmer SS. Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology. 1991;98:317–321. doi: 10.1016/s0161-6420(91)32293-0. [DOI] [PubMed] [Google Scholar]
- Patterson LH. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: a new class of bioreductive agent. Cancer Metastasis Rev. 1993;12:119–134. doi: 10.1007/BF00689805. [DOI] [PubMed] [Google Scholar]
- Polverini PJ, Novak RF. Inhibition of angiogenesis by the antineoplastic agents mitoxantrone and bisantrene. Biochem. Biophys. Res. Commun. 1986;140:901–907. doi: 10.1016/0006-291x(86)90720-5. [DOI] [PubMed] [Google Scholar]
- Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv. Ophthalmol. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Figgitt DP. Mitoxantrone: a review of its use in multiple sclerosis. CNS Drugs. 2004;18:379–396. doi: 10.2165/00023210-200418060-00010. [DOI] [PubMed] [Google Scholar]
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blunt NJ, Desnoyers R, Giles Y, Patterson LH. DNA topoisomerase II-dependent cytotoxicity of alkylaminoanthraquinones and their N-oxides. Cancer. Chemother. Pharmacol. 1997a;39:455–461. doi: 10.1007/s002800050598. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Desnoyers R, Blunt N, Giles Y, Patterson LH, Watson JV. Flow cytometric analysis and confocal imaging of anticancer alkylaminoanthraquinones and their N-oxides in intact human cells using 647-nm krypton laser excitation. Cytometry. 1997b;27:43–53. [PubMed] [Google Scholar]
- Tilleul P, Denis P, Maignen F, Elena PP, Nordmann JP, Leverge R, Rostene W. Effects of different formulations of mitoxantrone (solutions, nanospheres, liposomes) on glaucoma surgery in rabbits. Ophthalmic Res. 1997;29:218–226. doi: 10.1159/000268016. [DOI] [PubMed] [Google Scholar]
- Tomida A, Tsuruo T. Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anticancer Drug Des. 1999;14:169–177. [PubMed] [Google Scholar]
- Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]