Abstract
Hydrophobic legacy contaminants like dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyls (PCBs) were banned almost half a century ago. While their residues still remain in many environmental compartments, they have undergone extensive aging and likely have lower bioaccessibility (the available fraction) compared to fresh residues. However, risk assessment relies heavily on the use of total chemical concentration, rather than accounting for age-diminished bioaccessibility, likely leading to overestimated risks. In this study, we used 24 h Tenax desorption to measure the potential bioaccessibility of DDTs and PCBs in two sediment cores taken from the Palos Verdes Shelf Superfund site in the Pacific Ocean. The total concentrations of DDTs and PCBs from the core located at the sewage outfall (8C) were as high as 41,000–15,700 μg/kg (dry weight, dw) and 530–2,600 μg/kg dw, respectively, while those from a location 7 km northeast of the outfall (3C) were 2–3 orders of magnitude lower. Bioaccessibility estimated by 24-h Tenax-aided desorption (F24h) decreased in the order of DDD > DDE > DDT for DDT derivatives, and PCB 52 > PCB 70 > PCB 153 for PCB congeners, showing a negative correlation with their log Kow. Due to the extensive aging, F24h values were <20% of the total chemical concentration for most contaminants and <5% for DDT, DDE and PCB 153, suggesting that aging greatly diminished their bioavailability. However, a quantitative relationship between F24h and sediment age along the vertical profile was not found, likely because the contaminant residues had undergone aging before their offsite transport and deposition onto the ocean floor. As the use of man-made chemicals such as DDT and PCBs was discontinued in the U.S. many decades ago, the reduction in their bioavailability due to aging may be universal and should be taken into consideration to avoid overly conservative risk predictions or unnecessary mitigation interventions.
Keywords: Bioavailability, Hydrophobic organic contaminants, Aging effect, Tenax desorption, Legacy contaminants
Capsule:
Although levels of HOCs remain high in the Palos Verdes Shelf sediment, the bioaccessible fractions are extremely low, suggesting limited bioavailability for marine organisms.
INTRODUCTION
The use of many hydrophobic legacy compounds such as DDT and PCBs was phased out in the 1970s, however their residues are still found in soil and sediment in many parts of the world (Anderson et al., 2012; Bettinetti et al., 2016; Zhang et al., 2002). One prominent example is the Palos Verdes Shelf off the coast of Los Angeles, California, where 44 km2 of ocean floor sediment contains approximately 110 tons of DDT and 10 tons of PCBs due to wastewater discharge from a DDT manufacturer and other industries during the 1950–70s (U.S. EPA, 2009). Levels of DDT (and metabolites) up to 200 mg/kg were found at some locations (U.S. EPA, 2009). As these compounds are highly hydrophobic in nature, they have a strong tendency to remain in the bed sediment, where their natural degradation is extremely slow, with reported half lives ranging from 2 to 25 years (Augustijn-Beckers et al., 1994; U.S. EPA, 1989; Luthy et al., 1997; Cornelissen et al. 2005). Currently, the Palos Verdes Shelf is listed as a Superfund site, as the contamination threatens marine wildlife due to potential bioaccumulation of DDT and PCBs. The contamination is also a significant concern to local residents due to potential exposure to these chemicals via fish consumption (U.S. EPA, 2010).
As a contaminant has remained in the sediment (or soil) over decades, it has likely become less bioavailable through a biogeochemical process commonly referred as “aging” (White et al. 1999b). Aging of contaminants may be due to several environmental factors, but the predominant theory is that bioavailability decreases through a reduction in a compound’s bioaccessibility, which includes the freely dissolved and easily desorbed fractions of a contaminant (Alexander, M. 2000). Once the contaminants have reached phase equilibrium, molecules may slowly diffuse into the fine micropores in the sediment and organic matter aggregates, rendering them less accessible for organisms (Ehlers et al., 2003; White et al., 1999b). However, although the effect of aging on contaminant bioavailability is often anticipated, there have been few experimental evaluations of this phenomenon when considering long-term aging in natural sediment, limiting the consideration of aging effect in risk assessment of contaminated sites or evaluation of the need for remediation (White et al. 1999b).
Conventional risk assessment and management of contaminated sites rely almost exclusively on the use of bulk or total contaminant concentration (CT) (Reichenberg et al., 2006; Sormunen et al., 2010). Tenax desorption is a well-established method to quantify bioaccessibility of hydrophobic compounds such as PAHs and PCBs in soil or sediment (Cornelissen et al., 2001; Jia et al., 2014; Nutile et al., 2017; Zhao and Pignatello, 2004). The use of Tenax desorption as a measurement for potential bioaccessibility has been validated with direct bioaccumulation assays in benthic invertebrates such as Neanthes arenaceodentata and Lumbriculus variegatus and the earthworm Eisenia fetida (Jia et al., 2014; You et al., 2006; Wang et al., 2018).
In this study, we aimed to characterize the effect of aging on the potential bioaccessibility of DDTs and PCBs in the bed sediment from the Palos Verdes Shelf by using 24-h Tenax desorption. We hypothesized that due to the long residence time, these contaminant residues have undergone extensive “aging” in the sediment and should exhibit limited bioaccessibility. We further hypothesized that the decrease in bioaccessibility should depend on the sediment age that is related to its burial depth along the sediment profile.
MATERIALS AND METHODS
Chemicals and Sediment Samples
A total of 9 hydrophobic organic compounds (HOCs), including six DDT analogues, i.e., (1,1,1trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)ethane (o,p’-DDT), 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p’-DDT), 1,1-dichloro-2,4-bis-(chlorophenyl)ethylene (o,p’-DDE), 1,1-dichloro-2,2-bis(chlorophenyl)ethylene (p,p’-DDE), 1,1-dichloro-2,4-bis(chlorophenyl)ethane (o,p’-DDD), and 1,1-dichloro-2,2-bis-(chlorophenyl)ethane (p,p’-DDD)), and three PCB congeners, i.e., (2,2’,5,5’-tetrachlorobiphenyl (PCB-52), 2,3’,4’,5-tetrachlorobiphenyl (PCB-70), and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB-153)), were targeted for analysis. Their standards were purchased from AccuStandard (New Haven, CT). Stable isotope labeled analogues 13C- o,p’-DDD and 13C-PCB-153 were purchased from Cambridge Isotope Laboratories (Tewksbury, MA), while deuterated standards PCB-52-d3, PCB-70-d3, p,p’-DDE-d8, o,p’-DDE-d8, p,p’-DDDd8, o,p’-DDT-d8, and p,p’-DDT-d8 were purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). Anhydrous sodium sulfate and Florisil (10–60 mesh) were purchased from Fischer Scientific (Pittsburgh, PA) and Acros Organics, respectively (Morris Plains, NJ). Before use, sodium sulfate was dehydrated at 400 °C and Florisil was activated at 130 °C. Empty 20 mL SPE cartridges were purchased from Supelco (Bellefonte, PA) and all organic solvents were of HPLC grade and were purchased from Fischer Scientific.
Sediments used in this study were taken from the Palos Verdes Shelf Superfund Site, located off the coast of Los Angeles, California. Sediment cores were taken in 2007 from two monitoring stations located on the shelf, and were kindly provided by the Los Angeles County Sanitation District. The 8C core was retrieved at a location at the sewer system outfall and may be considered as the hotspot of contamination. The 3C core was taken further northwest of 8C, about 7 km from 8C (Figure S1 in Supplemental Information, SI). The 8C and 3C sediments were approximately 70 m and 60 m, respectively, below the ocean surface, and the cores were taken using a gravity core sampler.
Upon collection, the sediment cores were sectioned into 2-cm increments, and were stored frozen until analysis. To determine the approximate age of the sediment in each section, aliquots were dated using 210Pb by the constant initial concentration model (CIC) (Liao et al., 2017; Nittrouer et al., 1979).The use of 210Pb dating is common for sediments dated up to 100–150 years old because of the half-life (22.3 years) of the radioactive isotope. Well type intrinsic germanium detectors were used to conduct radiometric measurements of 210Pb and 226Ra via lowbackground gamma counting, with 137Cs activity used as a marker for the peak in atmospheric fallout from 1962 to 1963 (Nittrouer et al., 1979; Schelske et al., 1994). The age of each sediment section was determined using the mean sedimentation rate (MSR), which was calculated by dividing the 210Pb decay constant (−0.031) by the slope of the regression of the natural log of unsupported 210Pb (total 210Pb activity – 226Ra activity) versus the mid-depth cumulative mass in g/cm2 (Nittrouer et al., 1979). Figures of these activities are shown in the SI. For these sediments, age corresponded well with depth in the core (R2 = 0.61 and 0.65 for the 3C and 8C core, respectively, with P < 0.01). The particle size distribution was measured according to Gray et al., 2010. To measure the total organic carbon content (TOC), a small aliquot of each sediment section was placed in aluminum tins, dried at 70 °C for 24 h, and ground to a fine powder. An aliquot of the dried sediment sample was analyzed on a Carbon/Nitrogen Flash Elemental Analyzer EA1112 (CE Elantech, Lakewood, NJ) to determine TOC. Details on particle size distribution and TOC measurement are given in the SI.
Total Chemical Concentration Analysis
To determine the total chemical concentration CT, a 0.5 g aliquot (dry weight basis) of sediment was taken from every other section of the cores and dried by homogenization with sodium sulfate. Each sample was then spiked with stable isotope labeled analogues of DDTs and PCBs as recovery surrogates. The sediment samples were extracted twice using 40 mL of methylene chloride and acetone (1:1, v:v), and the extracts were combined and concentrated to <1 mL. The extracts were then loaded onto a 2 g Florisil cartridge for cleanup. Each sample was eluted with 20 mL of hexane and acetone (9:1, v:v), and the eluate was collected and concentrated to 1 mL. All samples were analyzed in triplicate on a Varian 3800 gas chromatograph equipped with a Varian 1200 triple quadrupole mass spectrometer (GC/MS-MS) for structural identification and quantification of the target analytes.
24-h Tenax Desorption Test
A single interval desorption using Tenax resin was used in this study to estimate the potential bioaccessibility of DDT and PCB residues in the aged sediment cores. Tenax resin beads are often used to measure contaminant bioaccessibility, either via sequential desorption or single interval desorption. Sequential desorption is frequently used to determine desorption rates, and the desorption kinetics may be used to characterize the sorbed compound into rapid, slow, and very slow desorption fractions, shown in the following equation:
where Frapid, Fslow, and Fvs are the rapid, slow, and very slow desorption fractions, respectively (Jia et al. 2014). However, sequential desorption is extremely tedious, time and resource-consuming. Previous studies have shown that a single interval desorption may be used to approximate the rapid desorption fraction, Frapid, and to predict bioaccessibility (Jia et al., 2014; You et al., 2006; Wang et al., 2018). The time used for single-interval Tenax desorption is usually 24 h for DDTs and PCBs, and the derived desorption fraction (F24h) has been shown in several studies to mimic contamination availability for uptake by benthic invertebrates (Cornelissen et al., 2001; Jia et al., 2016; Xu et al., 2008).
To determine F24h, aliquots (1.0 g, dry weight basis) of sediment from 5 fairly similar depths (5, 13, 25, 37, and 49 cm for the 8C core, 9, 17, 25, 37, and 45 cm for the 3C core) along the sediment profile were placed in a 50-mL centrifuge tube and followed by the addition of 0.1 g Tenax resin beads (Scientific Instrument Services, Ringoes, NJ). Artificial seawater (composition details given in the SI) fortified with sodium azide at 200 mg/L to inhibit potential biodegradation was added to the centrifuge tube at a 1:10 (w/w) sediment to water ratio. The sample mixtures were shaken at 60 rpm for 24 h. The sediment slurry was centrifuged at 670 g for 30 min, and the Tenax beads at the surface of the supernatant were collected by filtration into a paper filter and air dried. The Tenax beads were then transferred to a 20 mL glass scintillation vial and extracted with 3 mL of acetone and hexane (1:1, v:v) for 5 minutes using a Fisher Sonic 550 ultrasonic dismembrator. The same extraction step was repeated a total of three consecutive times, the extracts were combined and further concentrated to 1 mL under a stream of nitrogen. All samples were prepared in triplicate and analyzed on the Varian GC/MS-MS.
The bioaccessible fraction given by the 24-h Tenax aided desorption fraction, F24h, was calculated using the following equation
| (1) |
where C24h is the concentration of chemical desorbed from the sediment over 24 h, which equaled to the amount trapped by Tenax divided by the sediment mass, and CT is the total chemical concentration in the sediment (Jia et al., 2016).
Instrumental Analysis
All target analytes were analyzed using a Varian 3800 gas chromatograph (GC) coupled with a Varian 1200 triple quadruple mass spectrometer (MS/MS) (Varian Instruments, Sunnyvale, CA). All samples were injected into the inlet at a temperature of 250 °C in 1 μL aliquots. A DB-5MS ultra inert capillary column measuring 60 m × 0.25 mm × 0.25 μm was used for separation (Agilent, Wilmington, DE). Helium (99.999% purity) was used as the carrier gas at a flow rate of 1 mL/min, while 99.999% argon was used as the collision gas. The GC oven temperature was set initially at 80 °C (held for 1 min), then raised at 15 °C/min to 210 °C, and finally raised at 5 °C/min to 300 °C (held for 15 min). The transfer line temperature was 300 °C and the ionization source temperature was 250 °C. The MS/MS was operated in electron ionization mode at −70 eV with multiple reaction monitoring (MRM). Calibration standards were prepared in nhexane on the same day of analysis.
Quality Assurance and Quality Control (QA/QC)
Several steps were taken to maintain quality assurance and quality control. Blanks were included every 10 samples to check for potential contamination and to ensure that no contamination was present in laboratory materials. Surrogate standards were added to all samples prior to solvent extraction to quantify extraction recoveries. Internal standards were added before injection to correct for instrumental drift during analysis. The limits of detection of the nine analytes were set at a value of three times the background noise, and ranged from 0.05 to 0.5 ng/mL. Calibration standard curves were prepared each day of analysis and were only used when the regression coefficient was greater than 0.99. Statistical significance was determined using Pearson’s correlation coefficient with SigmaPlot 12.0 (Systat Software, San Jose, CA). Gradistat software was used for the particle size distribution analysis (Blott and Pye, 2001).
RESULTS AND DISCUSSION
Total Concentrations and Sediment Profiles of DDTs and PCBs
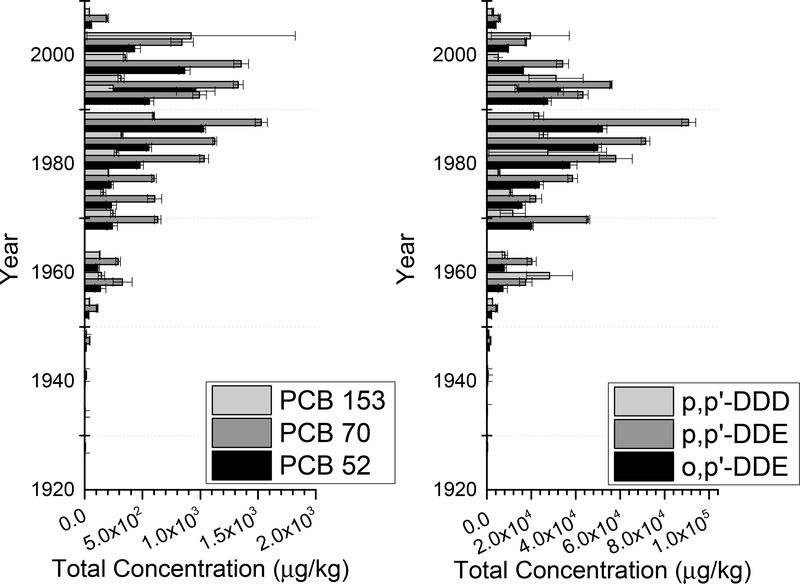
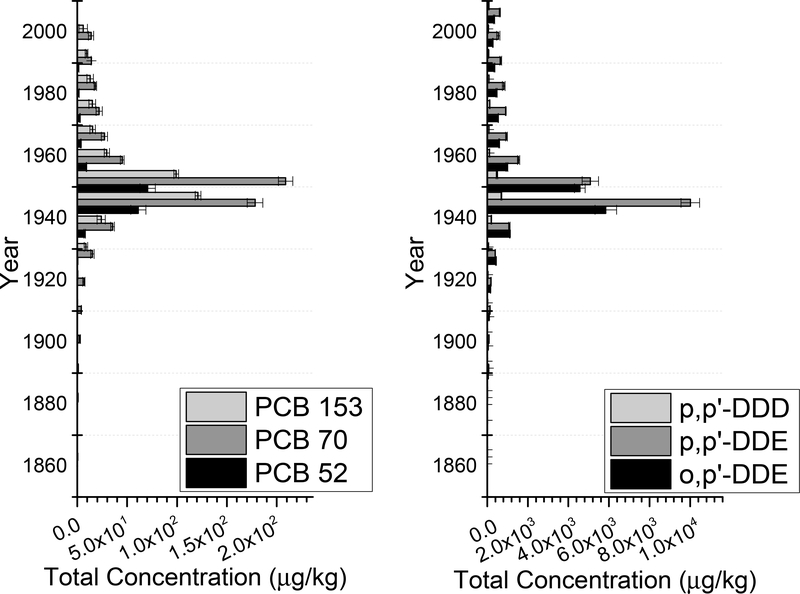
Elevated concentrations were observed in the 8C core for all the targeted analytes as compared to the 3C core (Figures 1 and 2). In the 8C core, the levels ranged from nd to 1530 μg/kg dw for PCB congeners (PCB 52, PCB 70, and 153) with a detection frequency of 96%. Even higher levels were found for DDT derivatives including o,p’-DDE, p,p’-DDE, o,p’-DDD, p,p’-DDD, o,p’-DDT and p,p’-DDT, with concentrations ranging from 108 up to 90,850 μg/kg dw, with a 100% detection frequency. In comparison, in the 3C core, levels of PCBs ranged from nd to 209 μg/kg dw (detection frequency, 74%) and those of DDTs from nd to 10,007 μg/kg dw (detection frequency, 88%). The upper layers of the 8C core had generally higher concentrations than the bottom layers. The peak in concentration was very sharp in the 3C core, with the highest concentrations centering around the 1950s. The vertical distribution of contaminants in the 8C core was less focused, with high levels occurring throughout the top half of the core, spreading from 1970s to 2000s. This could be due to frequent resuspension of sediment at the 8C location because it was located at a sewage outfall, while at the 3C location the sediment was likely less disturbed. However, this resuspension was likely restricted to approximately 0.1 cm depths, so biological processes were likely more prevalent (Wiberg et al., 2002). Out of all DDTs, the compound found at the highest concentrations in both cores was p,p’-DDE, followed by o,p’-DDE, and then p,p’-DDD, which are the main degradates of DDT (Chattopadhyay and Chattopadhyay, 2015). For PCBs, the compound found at the highest concentration was PCB 70 in both cores, followed by either PCB 52 or PCB 153.
Figure 1.
Total concentration profiles of PCBs and DDTs versus sediment age in the 8C core in μg/kg dry weight (d.w.) of sediment. Age corresponds with depth, with the youngest sediment at the top of the Y-axis.
Figure 2.
Total concentration profiles of PCBs and DDTs versus sediment age in the 3C core in μg/kg dry weight (d.w.) of sediment. Age corresponds with depth, with the youngest sediment at the top of the Y-axis.
These results were consistent with earlier studies using samples from the same site, showing elevated levels of DDEs and DDDs (o,p’- and p,p’) as compared to the concentrations of DDTs (U.S. EPA, 2009; Liao et al., 2017; Sanitation Districts of Los Angeles County, 2016). Several previous studies also considered the levels of DDTs and PCBs in sediments from this area, as extensive monitoring has been in place since the 1980s (Sanitation Districts of Los Angeles County, 2016). In a previous study, the geometric mean of total DDTs was 31,300 and 364 μg/kg dw in sediments from the 8C and 3C locations, respectively (Liao et al., 2017). The compound found at the highest concentrations was also p,p’-DDE. Previous studies reported elevated levels of total DDTs and PCBs in the surficial sediment from the 8C location than the 3C location, and the differences were 35–62 times (Sanitation Districts of Los Angeles County, 2016; U.S. EPA, 2009).
The ratio between DDT and its degradates, DDE and DDD, has been used to infer if there are new inputs of technical DDT (Guo et al., 2009). This ratio was calculated using the following equation:
| (2) |
where ∑ DDT is the sum of both o,p’- and p,p’-DDT, ∑ DDE is the sum of both o,p’- and p,p’-DDE, and ∑ DDD is the sum of both o,p’- and p,p’-DDD. A ratio >1 indicates recent inputs of technical DDT, while a ratio <1 suggests degradation and hence aged DDT residues (Guo et al., 2009). The derived values of the ratio are shown in Table 1. The ratios ranged from 0.040–0.287 for the 8C core, and from 0.021–0.104 for the 3C core, suggesting an absence of new DDT input to the shelf and substantial residue aging. A similar ratio was also calculated using data reported in a previous study from the same area (Liao et al., 2017). The ratio for the surficial sediment taken from the 8C location was approximately 0.095, and it was 0.040 for the 3C core. As we anticipated, these ratios were in close agreement with those obtained in this study, even though sediment samples from multiple depths were considered in this study while only sediment samples at the surface were considered in the other study.
Table 1.
Ratios of total DDTs to the sum of its metabolites DDE and DDD and the ratio between DDE and DDD
| Core | Depth (cm) | Year | DDT/(DDE+DDD) | DDE/DDD |
|---|---|---|---|---|
| 8C | 5 | 2002 | 0.29 ± 0.2 | 1.92 ± 1.1 |
| 13 | 1995 | 0.06 ± 0.03 | 2.76 ± 0.8 | |
| 25 | 1984 | 0.04 ± 0.004 | 4.06 ± 0.2 | |
| 37 | 1974 | 0.14 ± 0.02 | 3.19 ±0.3 | |
| 49 | 1962 | 0.11 ± 0.02 | 3.13 ± 0.4 | |
| 3C | 9 | 1991 | 0.07 ± 0.03 | 12.8 ± 0.5 |
| 17 | 1974 | 0.10 ± 0.1 | 10.8 ± 0.7 | |
| 25 | 1959 | 0.02 ± 0.001 | 18.4 ± 0.6 | |
| 37 | 1937 | 0.07 ± 0.1 | 9.46 ± 1.1 | |
| 45 | 1919 | 0.08 ± 0.1 | 9.40 ± 0.5 | |
The ratio of ∑ DDE/∑ DDD has also been used to infer whether the degradation is primarily aerobic or anaerobic transformations, and in places where aerobic degradation is predominant, the ratio of ∑ DDE/∑ DDD is >1; whereas if anaerobic degradation is predominant, the ratio becomes <1 (Da et al., 2014; Wang et al., 2013). In the 8C core, this ratio was calculated to be 1.92 – 4.06, and it ranged from 9.40 to 18.4 in the 3C core. Therefore, it may be concluded that for these sediment sites, aerobic degradation was the predominant degradation pathway for DDT in the marine sediment, which affirmed similar findings in previous studies considering surficial sediments from the Palos Verdes Shelf (Fernandez et al., 2014; Jia et al., 2014).
Bioaccessibility of Aged DDTs and PCBs in Sediment
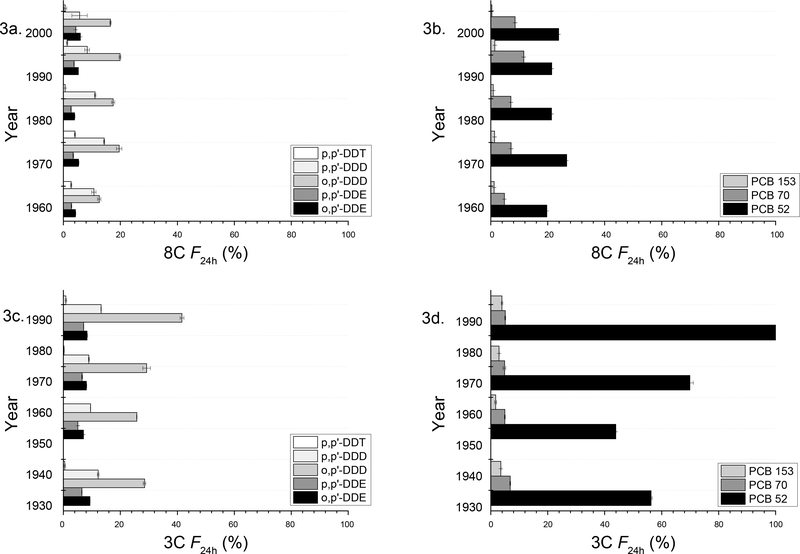
Extraction using Tenax beads has been frequently used to characterize bioaccessibility of hydrophobic contaminants in sediment or soil (Cornelissen et al., 2001; Jia et al., 2014; Jia et al., 2016; Nutile et al., 2017; Sormunen et al., 2010). The fractions derived from the 24 h Tenax desorption, F24h, are shown in Figure 3. The F24h values for ∑ DDTs were nd-19.9% and nd-41.6% for the 8C core and 3C core sediment samples, respectively. For most of the individual compounds, F24h was <20 % of the total sediment concentration, with the exception of only o,p’DDD and PCB 52 in some samples. Among the DDT derivatives, the compound with the highest F24h in both cores was o,p’-DDD (19.9% and 41.6%, in the 8C and 3C core, respectively), and the F24h value decreased in the order of p,p’-DDD (5.67–14.3% in the 8C core; 8.96–13.2% in the 3C core), o,p’-DDE (3.95–5.95% in the 8C core; 8.03–10.1% in the 3C core), p,p’-DDE (2.70–4.36% in the 8C core; 5.09–9.29% in the 3C core), and p,p’-DDT (0.57–4.04% in the 8C core; nd-0.84% in the 3C core). This pattern generally coincided with the hydrophobicity of the DDT analogues. Among the DDT compounds, log Kow was the smallest for o,p’-DDD (5.9), as compared to the other DDT derivatives (log Kow 6.0, 6.0, 6.5, and 6.9 for p,p’-DDD, o,p’-DDE, p,p’-DDE, and p,p’-DDT, respectively) (U.S. Department of Health and Human Services, 2002).
Figure 3.
Average values of the 24-h desorbable fraction of HOCs (F24h) from the 3C and 8C cores versus sediment age. Average values of F24h were calculated using the average 24-h desorbable concentrations (S24h) for each section and dividing that by the average total concentration (CT). Error bars represent the first standard deviation from the mean.
The F24h values for the ∑ PCBs (PCB 52, PCB 70, and PCB 153) ranged from 0.38 to 26.6% and from nd to114.5% for the 8C core and 3C core, respectively. In the 8C core, F24h values were 19.6–26.6% for PCB 52, 4.8–11.6% for PCB 70, and 0.4–1.5% for PCB 153. In the 3C core, F24h values were nd-114.5% for PCB 52, 4.8–6.8% for PCB 70, and 1.7–3.9% for PCB 153. For PCB 52, the exceedance of F24h over 100% was likely due to experimental artifact induced by its very low concentration in the 3C core samples (Figure 2a). For both cores, PCB 52 appeared to be the most accessible, but the F24h value decreased greatly for PCB 70, and further to only a small fraction for PCB 153. This trend also coincided with their relative hydrophobicity as indicated by their log Kow values. The log Kow of PCB 52 (5.8) is significantly smaller than that of PCB 70 (6.2) or PCB 153 (6.9) (Hawker and Connell, 1988; U.S. Department of Health and Human Services, 2002).
Other studies have shown similar trends in bioaccessibility of aged DDT and PCB residues, however these studies only looked at surface sediments, rather than along the sediment profile. In these studies, the bioavailable fraction for o,p’- and p,p’-DDE were also found to be < 20%, and sediment from the 8C location consistently had lower accessibility measurements than surface sediments from the other locations within the shelf (Jia et al. 2014, 2016). When comparing the F24h values in this study between the 8C and 3C cores, the bioaccessible fraction was consistently smaller for both DDTs and PCBs from the 8C core, even though that the overall difference was not statistically significant. This was likely due to the higher organic matter content in the 8C core than that in the 3C core, as organic matter was considered to be the primary domain for binding hydrophobic contaminants (Wang et al., 2018). The TOC in the 8C sediment core was 2.5–2.9 times that in the 3C core for the same depths (Table 2). The difference was likely due to the fact that the 8C core was located at the sewer system outfall, while the 3C core was much further away, meaning that there was a greater amount of organic material being deposited at the outfall and that the organic matter tended to remain close to the discharge point (U.S. EPA, 2009). Therefore, even though the vicinity of outfall point contained the highest levels of DDT and PCB residues, aging, along with the relatively high organic matter content, together rendered the contaminants less bioavailable.
Table 2.
Texture and total organic carbon content (TOC) of sediment samples from cores taken from the Palos Verdes Shelf Superfund site (%)
| Core | Depth (cm) | Year | TOC | Particle Size Distribution |
||
|---|---|---|---|---|---|---|
| Clay | Silt | Sand | ||||
| 8C | 5 | 2002 | 7.1 | 8.9 | 49.2 | 41.9 |
| 13 | 1995 | 9.3 | 10.3 | 58.3 | 31.4 | |
| 25 | 1984 | 10.2 | 9.9 | 63.1 | 27.0 | |
| 37 | 1974 | 7.4 | 7.0 | 48.8 | 44.2 | |
| 49 | 1962 | 6.7 | 9.0 | 56.2 | 34.8 | |
| 3C | 9 | 1991 | 2.9 | 8.1 | 53.1 | 38.7 |
| 17 | 1974 | 3.2 | 8.0 | 50.8 | 41.2 | |
| 25 | 1959 | 3.5 | 10.9 | 60.0 | 29.1 | |
| 37 | 1937 | 2.9 | 7.8 | 44.5 | 47.7 | |
| 45 | 1919 | 2.6 | 6.3 | 43.3 | 50.4 | |
The consistently very small F24h values for the more biologically significant DDT derivatives (i.e., DDT, DDE) and more highly chlorinated PCBs (e.g., PCB153) suggested that due to aging, the majority of these contaminants were irreversibly sequestered in the sediment material. Aging therefore has rendered these contaminants marginally bioavailable to benthic organisms at this site. Although this study focused on how aging influenced relative bioaccessibility, as total concentrations varied between sediment core sections and locations, it is important to note that the actual mass of compound available would also vary. These concentrations are shown in the SI, and generally range between nd-2800 μg/kg for DDTs and PCBs in the 8C core, and nd-104 μg/kg for those in the 3C core. However, assuming F24h is a good proxy for bioaccumulation potential, the use of total sediment concentration would result in overestimation of 10–100 times for DDT and DDE isomers, and 33–100 times for PCB153. The use of bioaccessibility values such as F24h to estimate available concentrations therefore would contribute to improved risk assessment and likely minimize the need for overly protective management practices.
Relationship between Bioaccessibility and Sediment Depth/Age
An effort was further made to explore a quantitative relationship between F24h and sediment depth, as sediment depth corresponds to sediment age. Contrary to our initial assumption, the correlation between F24h and sediment depth and age appeared to be weak in both cores. In the 3C core, F24h values for o,p’-DDD, PCB 52, and PCB 153 showed an apparent negative relationship with sediment age, with R2 values of 0.55, 0.64 and 0.51, respectively, but the correlations were not significant statistically (P > 0.10). In the 8C core, F24h values for p,p’-DDE, p,p’-DDD, p,p’-DDT and PCB 70 also showed some dependence on the sediment age, with R2 values of 0.57, 0.57, 0.53, and 0.62, respectively, but again the correlations were not significant statistically (P = 0.12–0.16). For the rest of compounds, the relationship between F24h and sediment depth or age was even weaker (Figure SI 2).
The absence of a clear dependence of bioaccessibility of the legacy contaminants on sediment depth and age invalidated our second hypothesis and was also in contrast to the findings of several earlier studies. In soil or sediment, bioaccessibility and bioaccumulation of DDT and PAH residues generally diminished with time (Ahmad et al., 2004; Alexander, M., 2000; Kelsey et al., 1997; Hatzinger and Alexander, 1995; Morrison et al., 2000; Shor et al., 2003).When earthworms were exposed to aged and fresh soils contaminated with DDT and its metabolites, soils where the HOC residues had aged for approximately 50 years exhibited the lowest tissue concentrations as compared to soils that had aged 90 days or were freshly treated (Morrison et al., 2000). Aged residues of pyrethroids found in sediment also were resistant to desorption with Tenax beads as compared to fresh residues, implying diminished potential bioavailability (Xu et al. 2008). Even a weakly sorbed pesticide, carbaryl, became less bioavailable as it aged in soils (Ahmad et al., 2004). However, in these studies, a quantitative analysis of the dependence of bioavailability on age of residues was not considered.
Findings from the current study suggested that the relationship between sediment age and the relative bioaccessibility of legacy contaminants may be influenced by factors other than the matrix age alone. Bioaccessibility may be influenced by sediment texture, especially organic carbon content (Table 2). However, the most probable cause for the lack of a quantitative dependence between F24h and sediment depth or age was that DDT and PCB residues on the ocean floor of the Palos Verdes Shelf were transported offsite from terrestrial sources, and the residues may have already undergone extensive aging under terrestrial conditions before their deposition onto the shelf, meaning that the sediment may be younger than the contaminant residue (U.S. EPA, 2010).Therefore, sediment age under such conditions was not indicative of the age of these residues. As discharge and deposition of DDT and PCB residues at this site has continued till today, although at increasingly lower concentrations, even the freshest deposition at the bed surface may be assumed to have aged for several decades under terrestrial conditions.
The conclusion that bioavailability of legacy contaminants does not depend on the age of the matrix, determined by methods such as isotope dating, may be generally true for water bodies contaminated by such persistent pollutants as DDT, PCBs, and PBDEs. The disconnection is due to the fact that the contamination source is usually terrestrial, while only through offsite transport such as runoff and soil erosion the aged contaminants have been moved into their terminal deposition site such as the sediment bed of lakes, estuaries, and coastal waters. Nevertheless, this and other studies together affirmed that aged residues of persistent organic pollutants are much less available as compared to that indicated by the total chemical concentration, and that the diminished bioavailability potential should be considered when evaluating the risk of contamination or designing management practices (Ahmad et al., 2004; Alexander, 2000; Hatzinger and Alexander, 1995; Kelsey et al., 1997; Morrison et al., 2000; Shor et al., 2003; Xu et al. 2008).
Bioaccessibility and Physicochemical Properties
In each core, the derived F24h values were log transformed and further plotted against log Kow values of the individual compounds to establish a semi-empirical model. Data from both cores were used in the simple linear regression analysis and one- way analysis of variance in SigmaPlot to derive the following relationship:
| (3) |
The linear regression (R2= 0.73) was statistically significant with P < 0.001(Figure S4). The semi-empirical Equation 3 was used to predict the bioaccessibility of DDTs and PCBs using their log Kow values by solving the equation for F24h. This equation was capable of predicting F24h values that were within the range of our measured values for most compounds. However, it overestimated the bioaccessibility for o,p’-DDE (Table 3). Out of the DDTs, the compound showing the largest bioaccessibility was o,p’-DDD, which was followed by o,p’-DDE, p,p’-DDD, and then p,p’-DDE. For the PCBs, PCB 52 was the most bioaccessible, followed by PCB 70 and PCB 153. This analysis suggested that hydrophobicity played an important role in the bioaccessibility of aged persistent organic pollutants, with more hydrophobic compounds exhibiting less bioavailability. However, more data are needed to further refine the predictive model using log Kow values for these and other HOCs, as this relationship may only be applicable for sites containing similar compounds that have undergone extensive aging.
Table 3.
Predicted and actual F24h values using Equation 3.
| Compound | log Kow | Predicted F24h (%) | Actual F24h Range (%) |
|---|---|---|---|
| o,p’-DDE | 6.0 | 13 | 3.9–10.1 |
| p,p’-DDE | 6.5 | 3.4 | 2.7–9.3 |
| o,p’-DDD | 5.9 | 18 | 13–42 |
| p,p’-DDD | 6.0 | 12 | 5.7–14 |
| p,p’-DDT | 6.9 | 1.2 | nd-4.0 |
| PCB 52 | 5.8 | 19 | nd-114 |
| PCB 70 | 6.2 | 7.6 | 4.8–12 |
| PCB 153 | 6.9 | 1.2 | 0.4–3.6 |
When examining the relationships between sediment TOC and F24h values of individual compounds, the results were mixed. No significant relationships between F24h and TOC were found for most compounds, with the exception of DDTs in the 3C core. The derived F24h of DDTs in the 3C core was negatively and significantly correlated with the sediment TOC (P= 0.94, p< 0.05), as shown in Table 4. This suggested that for DDTs in the 3C core, higher levels of TOC in the sediment led to potentially lower contaminant bioaccessibility. A weak negative relationship was also found between TOC and F24h of PCBs in the 3C core, even though the relationship was not statistically significant. The inconclusive dependence of F24h on the sediment TOC content implied that not only the quantity, but also the quality, of sediment organic matter affected the potential bioaccessibility of these HOCs. The organic matter in the bed sediment at the Palos Verdes Shelf originated primarily from the discharge of treated municipal wastewater effluent, which is also the source of these contaminants to the shelf (Shon et al., 2006). This concurrent discharge is likely why TOC did not have a clear relationship with bioaccessibility in sediments from this site. The treatment processes at the various facilities likely have undergone progressive changes over the years as well, leading to the deposition of organic matter of different structures and characteristics. In addition, because the organic matter was derived mostly from municipal wastes, the organic matter lacks the aggregate structures typical of natural sediments or soils (Kukkonen et al., 2003). A direct correlation between sediment TOC and bioaccessibility may have also been masked by other factors, such as in situ degradation (You et al., 2006).
Table 4.
Pearson’s linear correlation between total organic carbon (TOC) and the total chemical concentration CT and accessible fraction F24h
| Core | Compounds | P(Total concentrations) | P (F24h) |
|---|---|---|---|
| 8C | ∑DDTs | 0.819**** | 0.344 |
| ∑PCBs | 0.534**** | 0.618 | |
| 3C | ∑DDTs | 0.491*** | −0.94* |
| ∑PCBs | 0.686**** | −0.761 |
p<0.05
p<0.01
p<0.005
p<0.001
Conclusions
Results from this study showed that even though DDTs and PCBs in the marine sediment at the Palos Verdes Shelf remained elevated, due to decades of aging, the potential bioavailability as estimated by Tenax-aided desorption was extremely low, especially for DDT and DDE isomers, and for PCB congeners with a higher degree of chlorination. For DDT, DDE and PCB 153, the bioaccessible fraction was generally predicted to be 5% or less. Aging effect therefore was more pronounced for contaminants with a higher log Kow or stronger hydrophobicity, and log Kow may be used to predict the potential bioavailability of aged legacy contaminants. The finding clearly highlighted the deficiency in the use of the total chemical concentration; for the Palos Verdes Shelf Superfund site, the use of total chemical concentrations would result in an overestimation of risk by a factor of 10 or greater, which may lead to overly protective regulations or unnecessary mitigation practices. The model developed in this study can be improved via the addition of more data from other locations with aged sediment and contaminant residues, as the current model is site specific. An improved model may be useful for sites with these and other hydrophobic contaminants that have undergone extensive aging, as an estimation of bioaccessibility can help prioritize remediation efforts. However, we found that there did not exist a quantitative relationship between the contaminant bioaccessibility and the age of sediment estimated by 210Pb dating on the Palos Verdes Shelf. This was likely caused by a discrepancy between the age of matrix and that of residues, or that sediment matrix age does not always reflect the age of contaminants. For the Palos Verdes Shelf site, even the recent DDT and PCB residues had undergone extensive aging under terrestrial conditions before their deposition and burial in the bed sediment. This observation may be valid for other contaminated sites where the contamination is due to offsite movement of residues; however, evaluation of other contaminated sites is necessary to further validate this assumption. Therefore, the origin of the contaminant residues must be considered to better delineate the underlying causes for age-diminished bioavailability.
Supplementary Material
Highlights:
Total concentrations of DDTs and PCBs remains high on the Palos Verdes Shelf
Aging has greatly diminished bioaccessibility
Bioavailability should be used during risk assessment versus total concentration
ACKNOWLEDGMENTS
This study was funded by the Superfund Research Program of the National Institute of Environmental Health Science via contract 5R01ES024313–04. We thank J. Gully, B. Power and C.L. Tang at the Los Angeles County Sanitation District for collecting the marine sediment core samples at the Palos Verdes Shelf Superfund site. We also thank Dr. Andrew Gray at the University of California, Riverside for access to the Beckman-Coulter LS 13–320 for particle size distribution analysis on our samples.
Footnotes
SUPPORTING INFORMATION
Supporting information including figures and graphs can be found online for free at https://www.journals.elsevier.com/environmental-pollution.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ahmad R, Kookana RS, Megharaj M, Alston AM Aging reduces the bioavailability of even a weakly sorbed pesticide (carbaryl) in soil. Environ. Toxicol. Chem 2004, 23, 2084–2089. [DOI] [PubMed] [Google Scholar]
- 2.Alexander M Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol 2000, 34, 4259–4265. [Google Scholar]
- 3.Anderson M, Conkle J, Gan J Survey of organochlorine pesticides and PCBs in McGrath Lake. University of California, Riverside. State Water Resources Control Board; Agreement No. 09–099-140. 2012. [Google Scholar]
- 4.Augustijn-Beckers PWN; Hornsby AG; Wauchope RD The SCS/ARS/CES pesticide properties database for environmental decision making. Rev. Environ. Contam. Toxicol 1994, 137, 1–82. [PubMed] [Google Scholar]
- 5.Bettinetti R, Quadroni S, Boggio E & Galassi S Recent DDT and PCB contamination in the sediment and biota of the Como Bay (Lake Como, Italy). Sci. Tot. Environ 2016, 542, 404–410. [DOI] [PubMed] [Google Scholar]
- 6.Blott SJ and Pye K GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf 2001, 26, 1237–1248. [Google Scholar]
- 7.Chattopadhyay S and Chattopadhyay D Remediation of DDT and its metabolites in contaminated sediment. Curr. Pollution Rep 2015, 1, 248–264. [Google Scholar]
- 8.Cornelissen G; Rigterink H; ten Hulscher DEM; Vrind BA; van Noort PCM A simple tenax (R) extraction method to determine the availability of sediment-sorbed organic compounds. Environ. Toxicol. Chem 2001, 20, 706–711. [PubMed] [Google Scholar]
- 9.Cornelissen G; Gustafsson O; Bucheli TD; Jonker MTO; Koelmans AA; Van Noort PCM Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol 2005, 39, 6881–6895. [DOI] [PubMed] [Google Scholar]
- 10.Da C; Liu G; Yuan Z Analysis of HCHs and DDTs in a sediment core from the Old Yellow River Estuary, China. Ecotoxicol. Environ. Saf 2014, 100, 171–177. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers LJ; Luthy RG Contaminant bioavailability in soil and sediment. Environ. Sci. Technol 2003, 37, 295A–302A. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez LA; Lao W; Maruya KA; Burgess RM Calculating the diffusive flux of persistent organic pollutants between sediments and the water column on the Palos Verdes Superfund Site using polymeric passive samplers. Environ. Sci. Technol 2014, 48, 3925–3934. [DOI] [PubMed] [Google Scholar]
- 13.Gray AB; Pasternack GB; Watson E. B Hydrogen peroxide treatment effects on the particle size distribution of alluvial and marsh sediments. The Holocene. 2010, 20, 293–301. [Google Scholar]
- 14.Guo Y, Yu HY, Zeng EY Occurrence, source diagnosis, and biological effect assessment of DDT and its metabolites in various environmental compartments of the Pearl River Delta, South China: A review. Environ. Pollut 2009, 157, 1753–1763. [DOI] [PubMed] [Google Scholar]
- 15.Hatzinger PB and Alexander M Effects of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 1995, 29, 537–545. [DOI] [PubMed] [Google Scholar]
- 16.Hawker DW and Connell DW Octanol-water partition coefficients of polychlorinated biphenyl cogeners. Environ. Sci. Technol 1988, 22 382–387. [Google Scholar]
- 17.Jia F; Bao LJ; Crago J; Schlenk D; Gan J Use of isotope dilution method to predict bioavailability of organic pollutants in historically contaminated sediments. Environ. Sci. Technol 2014, 48, 7966–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia F; Liao CY; Xue A; Taylor A; Gan J Comparing different methods for assessing contaminant bioavailability during sediment remediation. Sci. Tot. Environ 2016, 573, 270–277 [DOI] [PubMed] [Google Scholar]
- 19.Kelsey JW, Kottler BD, Alexander M Selective chemical extractants to predict bioavailability of soil–aged organic chemicals. Environ. Sci. Technol 1997, 31, 214–217. [Google Scholar]
- 20.Kukkonen JVK; Landrum PF; Mitra S; Gossiaux DC; Gunnarsson J; Weston D Sediment characteristics affecting desorption kinetics of select PAH and PCB congeners for seven laboratory spiked sediments. Environ. Sci. Technol 2003. 37, 4656–4663. [DOI] [PubMed] [Google Scholar]
- 21.Liao CY; Taylor A; Tang CL; Gully JR; Kenney WF; Brenner M; Gan J Historical record and flux of DDTs and PCBs to the Palos Verdes Shelf Superfund Site, California. Sci. Tot. Environ 2017, 581, 697–704. [DOI] [PubMed] [Google Scholar]
- 22.Luthy RG; Aiken GR; Brusseau ML; Cunningham SD; Gschwend PM; Pignatello JJ; Reinhard M; Traina SJ; Weber WJ; Westall JC Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol 1997, 31, 3341–3347. [Google Scholar]
- 23.Morrison DE, Robertson BK, Alexander M Bioavailability to earthworms of aged DDT, DDE, DDD, and dieldrin in soil. Environ. Sci. Technol 2000, 34, 709–713. [Google Scholar]
- 24.Nittrouer CA; Sternberg RW; Carpenter R; Bennett JT The use of Pb-210 geochronology as a sedimentary tool: application to the Washington Continental Shelf. Mar. Geol 1979, 31, 297–316. [Google Scholar]
- 25.Nutile SA, Harwood AD, Sinche FL, Huff Hartz KE, Landrum PF, Lydy MJ Methodological and Environmental Impacts on Bioaccessibility Estimates Provided by Single-Point Tenax Extractions. Arch. Environ. Contam. Toxicol 2017, 72, 612–621. [DOI] [PubMed] [Google Scholar]
- 26.Reichenberg F; Mayer P Two complementary sides of bioavailability: Accessibility and chemical activity of organic contaminants in sediments and soils. Environ. Toxicol. Chem 2006, 25, 1239–1245. [DOI] [PubMed] [Google Scholar]
- 27.Sanitation Districts of Los Angeles County. 2014–2015 JWPCP biennial receiving water monitoring report. Sanitation Districts of Los Angeles County; Los Angeles, CA, 2016. [Google Scholar]
- 28.Schelske CL; Peplow A; Brenner M; Spencer CN Low-background gamma counting: applications for 210Pb dating of sediments. Journal of Paleolimnology. 1994, 10, 115–128. [Google Scholar]
- 29.Shon HK; Vigneswaran S; Snyder SA Effluent organic matter (EfOM) in wastewater: constituents, effects, and treatment. Environ. Sci. Technol 2006, 36, 327–374. [Google Scholar]
- 30.Shor LM; Rockne KJ; Taghon GL; Young LY; Kosson DS. Desorption kinetics for field-aged polycyclic aromatic hydrocarbons from sediments. Environ. Sci. Technol 2003. 37, 1535–1544. [DOI] [PubMed] [Google Scholar]
- 31.Sormunen AJ, Tuikka AI, Akkanen J, Leppänen MT, Kukkonen JVK Predicting the bioavailability of sediment-associated spiked compounds by using the polyoxymethylene passive sampling and Tenax extraction methods in sediments from three river basins in Europe. Arch. Environ. Contam. Toxicol 2010, 59, 80–90. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. Toxicological profile for DDT, DDE, and DDD. Public Health Service, Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; Atlanta, Georgia: 2002. [Google Scholar]
- 33.U.S. Environmental Protection Agency. Environmental fate and effects division, pesticide environmental fate one line summary: DDT (p, p’); United States Environmental Protection Agency: Washington D. C., 1989. [Google Scholar]
- 34.U. S. Environmental Protection Agency. Interim record of decision: Palos Verdes Shelf operable unit 5 of Montrose Chemical Corporation Superfund Site, Los Angeles County, CA; Region IX, United States Environmental Protection Agency: San Francisco, CA, 2009. [Google Scholar]
- 35.U. S. Environmental Protection Agency. EPA Signs Interim Record of Decision - Remedial Work Begins; Region IX, United States Environmental Protection Agency: San Francisco, CA, 2010. [Google Scholar]
- 36.Wang J; Taylor A; Schlenk D; Gan J Application and validation of isotope dilution method (IDM) for predicting bioavailability of hydrophobic organic contaminants in soil. Environ. Pollut. 2018, 236, 871–877. [DOI] [PubMed] [Google Scholar]
- 37.Wang L; Jia H; Liu X; Sun Y; Yang M; Hong W; Qi H; Li Y Historical contamination and ecological risk of organochlorine pesticides in a sediment core in northeastern Chinese river. Ecotoxicol. Environ. Saf 2013, 93, 112–120. [DOI] [PubMed] [Google Scholar]
- 38.White JC, Hunter M, Nam K, Pignatello JJ, Alexander M. Correlation between biological and physical availabilities of phenanthrene in soils and soil humin in aging experiments. Environ. Toxicol. Chem 1999b, 18, 1720–1737. [Google Scholar]
- 39.Wiberg PL; Drake DE; Harris CK; Noble MA Sediment transport on the Palos Verdes Shelf over seasonal to decadal time scales. Cont. Shelf Res 2002, 22, 987–1004. [Google Scholar]
- 40.Xu Y; Gan J; Wang Z; Spurlock F Effect of aging on desorption kinetics of sediment-associated pyrethroids. Environ. Toxicol. Chem 2008, 27 (6), 1293–1301. [DOI] [PubMed] [Google Scholar]
- 41.You J; Landrum PF; Lydy MJ Comparison of chemical approaches for assessing bioavailability of sediment-associated contaminants. Environ. Sci. Technol 2006, 40, 6348–6353. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Parker A, House A Mai BX, Li XD, Kang YH, Wang ZS. Sedimentary Records of DDT and HCH in the Pearl River Delta, South China. Environ. Sci. Technol 2002, 36, 3671–3677. [DOI] [PubMed] [Google Scholar]
- 43.Zhao D, Pignatello JJ Model-aided characterization of Tenax®-TA for aromatic compound uptake from water. Environ. Toxicol. Chem 2004, 23, 1592–1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.