Abstract
Cross-coupling chemistry is widely applied to carbon-carbon bond formation in the synthesis of medicines, agrochemicals, and other functional materials. Recently, single-electron-induced variants of this reaction class have proven particularly useful in the formation of C(sp2)–C(sp3) linkages, although certain compound classes have remained a challenge. Here, we report the use of sulfones to activate the alkyl coupling partner in nickel-catalyzed radical cross-coupling with aryl zinc reagents. This method’s tolerance of fluoroalkyl substituents proved particularly advantageous for the streamlined preparation of pharmaceutically oriented fluorinated scaffolds that previously required multiple steps, toxic reagents, and nonmodular retrosynthetic blueprints. Five specific sulfone reagents facilitate the rapid assembly of a vast set of compounds, many of which contain challenging fluorination patterns.
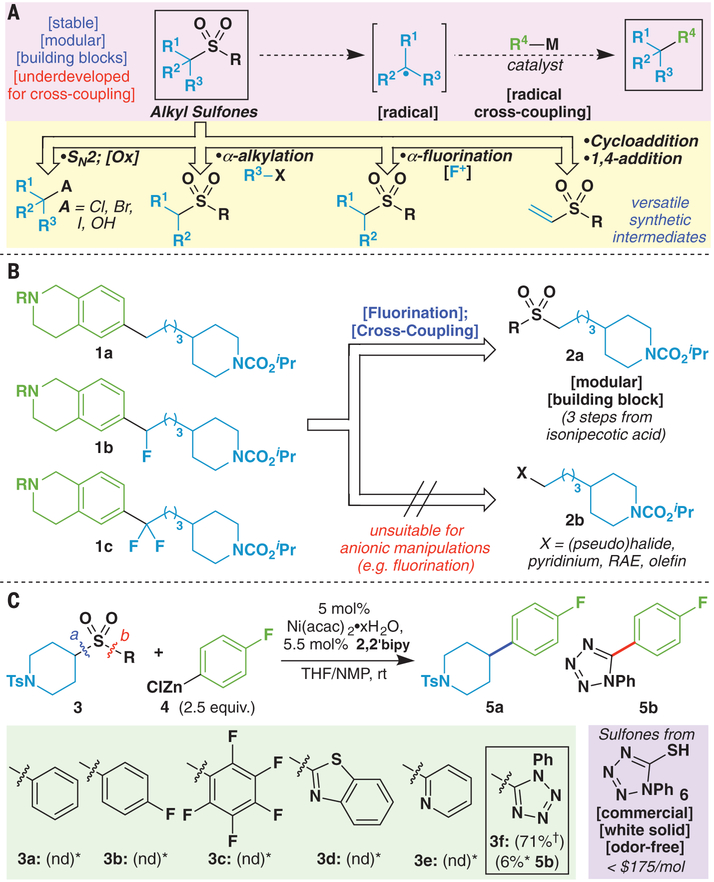
Cross-coupling proceeding through a single-electron transfer pathway to forge new carbon-carbon (C-C) bonds is complementary to the robust two-electron coupling paradigm of the venerable Heck, Suzuki, and Negishi reactions (1). In recent years, metal-catalyzed radical cross-coupling (RCC) has proven effective for forging bonds to sp3-hybridized secondary and tertiary carbon centers that remain challenging for the more widely applied two-electron palladium (Pd) catalysis (2). To realize the full potential of RCC strategies, functional groups more versatile than alkyl halides (3–8) are needed to expand the range of compatible coupling partners (Fig. 1A). In this regard, alkyl carboxylic acids (3, 4), alkylboron (5) and alkylsilicon (6) derivatives, olefins (7), alkyl pyridinium salts (derived from amines) (8), and alkyl dihydropyridines (9) have been identified as useful agents in RCC. Here, we show that readily available sulfones (specifically bearing the 1-phenyl-1H-tetrazol-5-yl group) can be directly used as an orthogonal redox-active functional group for RCC-based synthesis. The sulfones present three specific advantages. First, they tolerate powerful anionic chemistry (α-alkylation and fluorination) for functional elaboration before the cross-coupling event (Fig. 1, A and B) (10,11); second, when attached to unsaturated systems, they enable facile cycloadditions and Michael/Giese-type additions (12); and last, their stability and crystallinity allow for convenient manipulation.
Fig. 1. Sulfone cross-coupling.
(A) Alkyl sulfone: Modular electrophile for radical cross-coupling. Alkyl sulfones represent a promising and underexplored functional group for one-electron cross-coupling processes because of their distinct reactivity. (B) Alkyl sulfones: Ideal building blocks afford modular approach to druglike compounds. Described is the potential to achieve modular synthesis of fluorine-containing C(sp3)–rich architectures. (C) Initial investigations and optimization: Sulfones as radical cross-coupling electrophiles. Described is the identification of a redox-active sulfone and conditions in order to achieve selective cross-coupling under Ni-catalysis. *Yields determined by 19F nuclear magnetic resonance (NMR) with 1-fluoronapthalene as an internal standard. †Isolated yield. 2,2′-bipy, 2,2′-bipyridine; THF, tetrahydrofuran; NMP, 1-methylpyrrolidin-2-one; nd, not detected with 19F NMR.
The literature is replete with examples of sulfones serving a variety of different roles in synthesis, from facilitating olefin formation and cycloadditions to their use in two-electron cross-couplings. In the latter regard, the independent work of the Crudden (13) and Li (14) research groups has stood out for their use of benzylic phenyl sulfones in a variety of Pd- and nickel (Ni)–catalyzed desulfonylative cross-coupling methodologies. The first systematic study of the use of an unactivated alkyl sulfone for RCC emerged in 2013 from the Denmark laboratory’s report of iron (Fe)–catalyzed Kumada cross-coupling to forge C(sp2)–C(sp3) bonds (15). These elegant studies pointed to the improvements needed for mainstream adoption of this chemistry. An ongoing medicinal chemistry project at Pfizer could have used this reaction, but the limited scope prevented its wide adoption [beyond the use of phenyl-magnesium bromide (PhMgBr)]. Thus, first forays into this area centered on identifying a suitably substituted sulfone that would be susceptible to reduction under mild (for example, low-valent Ni) reaction conditions to generate an alkyl radical and unreactive sulfinate anion (4). We evaluated a variety of sulfones differing in relative reduction potential, electronegativity, and size under Ni-catalyzed Negishi-type conditions (Fig. 1C). In the case of aryl sulfone 3a, used previously by Denmark, starting material was recovered with only traces of formation of the desired product 5a; sulfones 3b to 3e performed similarly. A primary breakthrough was the discovery that a redox-active phenyl-tetrazole (PT) sulfone 3f was distinctly capable of delivering the desired RCC product 5a (71% isolated yield). As outlined in tables S1 to S8, a variety of conditions were surveyed, and Ni proved an effective catalyst, whereas Fe did not; concurrent with our report, difluoro-methyl 2-pyridyl sulfone was found to be a competent coupling partner under Fe catalysis (16). The use of a bipyridine-type ligand was also imperative because performing the reaction in the absence of ligand resulted in exclusive formation of the biaryl by-product; without the Ni precatalyst and bipyridine ligand, no reaction occurred.
From a pragmatic perspective, PT-sulfones can be easily derived from alcohols or alkyl halides by using Mitsunobu conditions or SN2 displacement with inexpensive and odor-free thiol 6 followed by straightforward oxidation in order to produce stable products that are often crystalline (17). As shown below, the ability of PT-sulfones to enable modular synthesis strategies inspired the development of five reagents (two of which are new chemical entities).
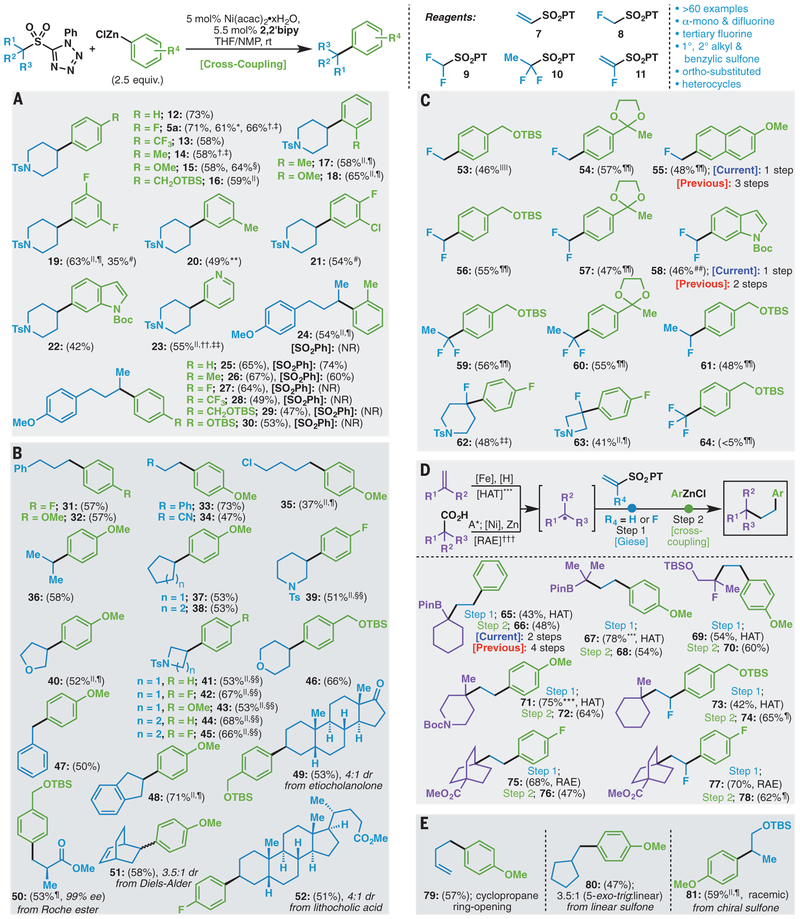
The robustness of the optimized reaction conditions for the desulfonylative RCC reaction is illustrated in the synthesis of more than 60 products (Fig. 2, A to E). The scope of this methodology was initially evaluated with a variety of aryl zinc reagents (Fig. 2A). PT-sulfone 3f reacted with 13 different organozinc reagents to produce a series of arylated piperidine derivatives (5a and 12 to 23). Ortho-substituted arylzinc reagents could be used under the reaction conditions (17 and 18), and potential electrophilic coupling partners such as aryl chlorides were also tolerated on the arylzinc reagent (21). Both electron-rich and electron-deficient heterocyclic organozinc reagents proved competent coupling partners in this reaction manifold, as exemplified by indole 22 and pyridine 23. To assess the disclosed method relative to the prior state of the art in the cross-coupling of unactivated alkyl sulfones, we conducted a direct comparison of substrates (24 to 30) previously evaluated under Fe catalysis. The conditions reported here compared favorably in all cases to literature-reported yields and allowed access to compounds that were previously inaccessible via a desulfonylative cross-coupling route (15). Arylzinc reagents can also be accessed through lithium-halogen exchange/transmetallation or magnesium (Mg)–halogen exchange/transmetallation and successfully used under the reaction conditions in yields comparable with those of arylzinc reagents derived via Mg insertion (71 versus 66% isolated yield for 5a, for example).
Fig. 2. Scope of the Ni-catalyzed cross-coupling of redox-active PT-sulfones.
Experimental details are provided in the supplementary materials. (A) Aryl zinc reagents. (B) Sulfones. (C) Alkylfluorine sulfones. (D) Successive coupling. (E) Mechanistic studies. *2.5 mmol scale. †Arylzinc reagent prepared by means of lithium-halogen exchange with n-BuLi/transmetallation with ZnCl2. ‡10 mole % (mol %) Ni(acac)2•xH2O, 11% 2,2′-bipy. §0.5 mmol scale. ∥60°C. ¶20 mol % Ni(acac)2•xH2O, 22 mol % 2,2′-bipy, 3.0 equivalent arylzinc reagent. #Arylzinc reagent prepared by means of magnesium-halogen exchange with iPrMgCl·LiCl/transmetallation with ZnCl2. **Arylzinc reagent was prepared by using commercial solution of ZnCl2 in 2-MeTHF. ††N,N′-dimethylformamide (DMF) instead of NMP. ††20 mol % Ni(acac)2•xH2O, 40 mol % 2,2′-bipy, 3.0 equivalent arylzinc reagent. §§30 mol % Ni(acac)2•xH2O, 33 mol % 2,2′-bipy, 4.0 equivalent arylzinc reagent. ∥∥30 mol % Ni(acac)2•xH2O, 60 mol % bathophenanthroline, 3.0 equivalent arylzinc reagent. ¶¶20 mol % Ni(acac)2•xH2O, 40 mol % bathophenanthroline, 3.0 equivalent arylzinc reagent. ##1.0 equivalent Ni(acac)2•xH2O, 1.1 equivalent bathophenanthroline, 6.0 equivalent arylzinc reagent. ***Experimental details and preparation are available in (27). †††Experimental details are available in (28). rt, room temperature; 2,2′-bipy, 2,2′-bipyridine; THF, tetrahydrofuran; NMP, 1-methylpyrrolidin-2-one; NR, no reaction; HAT, hydrogen atom transfer; A*, activation with NHPI; RAE, redox-active ester; DMF, N,N-dimethylformamide.
Desulfonylative RCC is demonstrated in Fig. 2B as a means to synthesize a broad range of compounds from 18 different readily accessible sulfones. Primary (31 to 35, 47, and 50), secondary (acylic and cyclic; 36 to 46, 48, 49, 51, and 52), and benzylic (47) arylated products could be accessed from the corresponding sulfone. Alkyl chlorides (35) were tolerated under the reaction conditions despite their propensity to engage in single-electron chemistry under Ni catalysis, establishing the orthogonality of these two alkyl electrophiles. Moreover, desulfonylative RCC provides a straightforward means to access A-ring–modified steroids (49 and 52). As a testament to the mild reaction conditions, Roche ester-derived 47 was successfully synthesized with no erosion of enantiomeric excess. Because of the well-studied reactivity of vinyl sulfones in cycloaddition chemistry, 7 was treated with cyclo-hexadiene in a Diels-Alder reaction and subsequently cross-coupled to afford [2.2.2]-bicycle 51. Compound 7 is known to react with dienes under mild and more selective conditions than those of acrylates or the corresponding phenyl vinyl sulfone (12).
The primary advantage of this chemistry lies in its ability to simplify the retrosynthetic analysis of complex sp3-rich organofluorine building blocks so that the C–C bond–forming disconnection used is the same regardless of fluorine content. Whereas methods to install fluoroalkyl groups via cross-coupling chemistry exist, they are limited by the lack of facile access to the corresponding fluoroalkyl electrophiles (a full listing is provided in fig. S24) (18–20). In the case of simple mono-fluoromethyl or difluoromethyl groups, many reagents are difficult to handle (for example, gaseous) or require additional steps to remove superfluous functionality (21). It is in this context that α-fluoroalkyl sulfones were evaluated; these are well regarded as stable reagents used in synthetic organic chemistry for a variety of applications (22), such as the installation of fluoroalkenes from carbonyl-containing compounds (23) as well as reacting as radical precursors under photoinduced electron transfer conditions (24). Redox-active α-fluoro-PT-sulfones were thus investigated in order to install fluorinated groups onto arenes by means of RCC (Fig. 2C). By using bathophenanthroline as the ligand with a 1:2 ratio of Ni precatalyst to ligand, Negishi-type arylation of mono- and difluorinated sulfones could be achieved.
Fluoromethyl reagent 8 and difluoromethyl reagent 9 were prepared in a straightforward manner according to literature procedures from inexpensive CFH2Cl and CF2HCl, respectively (25). Difluoroethyl reagent 10 was accessed through anionic functionalization [deprotonation and quenching with N-fluorobenzenesulfonimide (NFSI)] of the parent ethyl PT-sulfone. Using other corresponding alkyl radical precursors (alkyl halides, alkyl carboxylic acids, alkylboron and alkylsilicon derivatives, olefins, alkyl pyridinium salts, and alkyl dihydropyridines), this anionic manipulation would not be possible; thus, redox-active PT-sulfones offer a distinct advantage to more traditional alkyl electrophiles in that they engender a modular solution for the installation of monofluoro- and difluoroalkyl groups onto an aryl group from a single sulfone starting material. These reagents were successfully used to access mono- and difluoroalkyl arenes (53 to 55, 56–58, 59, and 60) and could be used to shorten synthetic routes to compounds found in the literature (55 and 58) (21, 25). Although CH2F and CF2H moieties can be installed from the corresponding halides, such procedures are inconvenient because they often require an excess of gaseous reagents. The synthesis of additional tertiary fluorides (62 and 63) was similarly achieved, presenting a useful alternative to traditional tertiary fluoride synthesis from the corresponding tertiary alcohol and treatment with diethylaminosulfur trifluoride (DAST; a highly toxic and dangerous reagent), a transformation that often proceeds in low yield (26). A current limitation is that the trifluoromethyl group cannot be readily installed with this method (64).
Reagents such as 7 and 11 open up distinct possibilities for stepwise, successive RCC chemistry because the PT-sulfone is known to enhance the rate of Giese-type radical additions (12). A variety of olefin- and carboxylic acid-derived radicals could be smoothly intercepted with 7 or 11 followed by desulfonylative RCC in order to generate structures that in some cases would be challenging to otherwise procure (27, 28). For example, 66 is prepared in the literature by using an elegant deborylative cyclization (29); the current modular approach starts with commercial cyclohexenyl boronic ester followed by olefin RCC and then desulfonylative RCC. Similarly, quaternary-center-bearing substrates 72, 74, 76, and 78 are easily produced from either readily available acids or olefins through concurrent RCC chemistry, thus obviating the need for either deoxyfluorination or vexing cross-coupling challenges. Last, the success of this process points to the orthogonality of redox-active PT-sulfones both in the context of reductive chemistry (olefin HAT and acid Giese) and known radical precursors (boronic esters).
Mechanistically (fig. S9), alkyl radicals are posited intermediates under the reaction conditions, as evidenced by radical probe substrates 79 (cyclopropane ring-opening), 80 (5-exo-trig cyclization), and 81 (racemic product from a chiral sulfone), as shown in Fig. 2E (30).
In collaboration with Asymchem, 7, 8, 9, and 10 have been prepared on a large scale (a graphical procedure is provided in the supplementary materials).
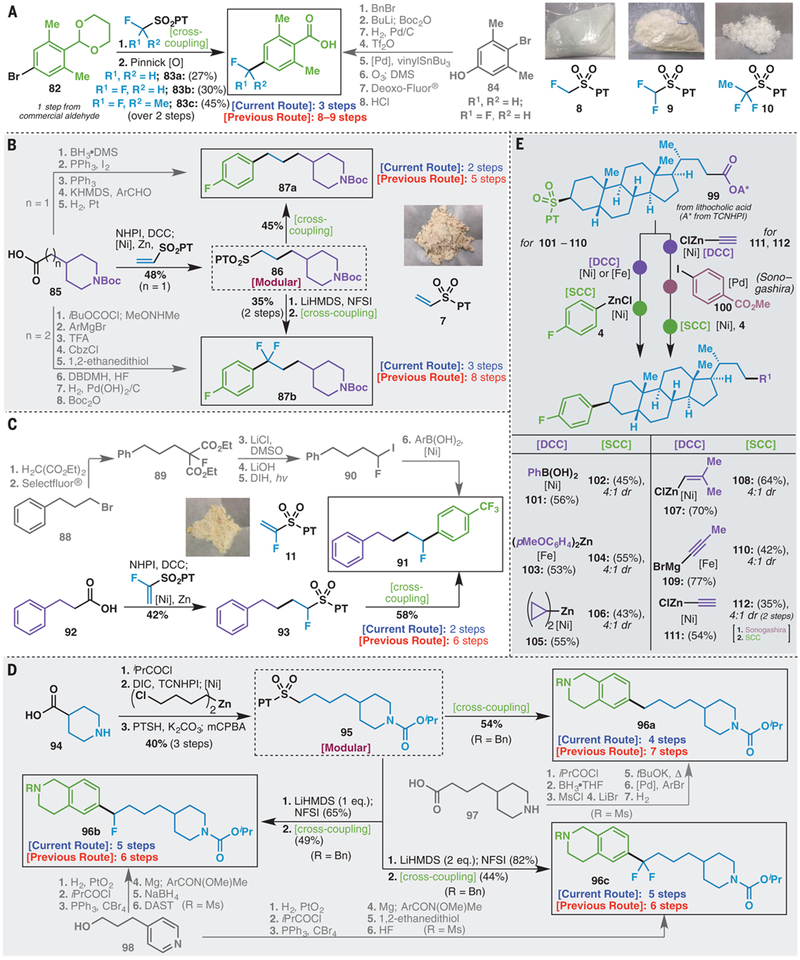
As illustrated in Fig. 3, the strategic impact of this chemistry shines in the preparation of complex, medicinally oriented building blocks that contain varying levels of fluorination. Traditionally, retrosynthetic analysis of these targets has centered around the installation of the fluorine atom; carbonyl chemistry and deoxyfluorination are therefore of prime importance. Moreover, such routes typically rely on highly toxic and dangerous reagents (such as DAST). Shown in Fig. 3, A to D, are examples from modern medicinal chemistry where this challenge is vividly displayed and contrasted with an RCC approach. In such contexts, fluorine atoms are often incorporated into scaffolds so as to alter solubility, efficacy, or metabolic properties. The often empirical nature of structure-activity relationships requires the targeting of multiple fluorinated variants as bioisosteric replacements (31), and such case studies are widespread in the medicinal chemistry literature. For instance (Fig. 3A), Schering scientists targeted building blocks 83a and 83b, differing only in the substitution at a single carbon (containing one or two fluorine atoms) (32). To access these simple structures, an eight-step sequence was devised from phenol 84; only two of these steps are strategic and produce C–C bonds. In contrast, acetal 82 could be directly coupled with reagent 8, 9, or 10 to deliver 83a and 83b and a new analog 83c after simple oxidation (the acetal is deprotected during the RCC workup with 1M HCl), thus avoiding tin-based reagents, ozonolysis, and deoxyfluorination steps.
Fig. 3. Simplification of synthesis using sulfone-cross-coupling logic.
(A) Fluorinated bioisosteres: A simple, modular approach using bench-stable sulfones (8 to 10). (B) Redox-active sulfones for rapid, late-stage divergent analog access. (C) Fluorine embedded, successive radical cross-coupling. (D) Reimagining retrosynthesis in medicinal chemistry: Simplified access to a popular set of alkyl- and alkyl-fluoro compounds. (E) Chemoselective, sequential C–C cross-coupling. Experimental details are provided in the supplementary materials. [DCC], decarboxylative cross-coupling; [SCC], desulfonylative cross-coupling; DBDMH, 1,3-dibromo-5,5-dimethylhydantoin.
A case study from a recent Merck campaign demonstrates the power of a concurrent RCC strategy for modularly generating C(sp3)–rich scaffolds (Fig. 3B) such as 87a and 87b (33, 34). In the original preparation, the absence or presence of fluorine atoms dictates the starting materials and methods used. In the preparation of the unfluorinated compound (five steps), an aryl aldehyde is used in concert with a Wittig reaction to forge the central C–C bond followed by hydrogenation to furnish 87a. To access the latter, fluorine-substituted analog (87b, eight steps), a different route is required because the difluorinated carbon must be installed via deoxyfluorination of an aryl ketone, thus requiring a different starting material, protecting group swaps owing to harsh reagents (Boc → Cbz → Boc), thiols, and toxic HF. Using RCC-based logic, the same starting material can be used to make both 87a and 87b by using acid 85 (n = 1 CH2 unit in the alkyl chain) in two successive RCC steps, the first of which uses the redox-active N-hydroxyphthalimide (NHPI) ester derived from the acid in a Giese-type addition to PT-sulfone reagent 7 to yield 86. In the case of 87a, redox-active PT-sulfone 86 is directly subjected to RCC to furnish 87a, whereas for 87b, anionic α-fluorination followed by RCC delivers 87b. Both routes proceed in a fraction of the steps previously required and obviate the need for superfluous steps and/or toxic reagents.
A recent (2017) patent from the Shanghai Institute of Organic Chemistry (Fig. 3C) serves as a good example for the advantage of using the redox-active PT-sulfone over an alkyl halide for achieving the modular synthesis of a simple fluorinated scaffold (35). In the reported approach to 91 (six steps), an alkyl bromide is homologated with malonate and fluorinated with Selectfluor to produce a diester, which in turn undergoes decarboxylation and Hunsdiecker iodination to furnish a geminal-dihalide suitable for Ni-catalyzed Suzuki coupling. Alternatively, hydrocinnamic acid 92 could be homologated via the corresponding redox-active ester by using reagent 11 through Giese addition followed by RCC in order to deliver 91 in only two steps. In such a case, the fluorine atom in 91 was handled from a planning perspective as though it were any other substituent (methyl, aryl, or hydrogen), circumventing the fluorine-specific logic normally required.
The fourth case study (Fig. 3D), drawn from the patent literature (Novartis), demonstrates the advantage of a modular approach that diverges from the same intermediate even when the overall step count is similar (11). Targets 96a to 96c differ only in the presence or absence of benzylic fluorine atoms (0, 1, or 2), yet their preparation is guided not by the carbon skeleton (which comprises >90% of the molecular weight of 96b and 96c) but rather by fluorine atom incorporation. Whereas desfluoro analog 96a is prepared from acid 97 via a Heck/hydrogenation sequence, mono- and difluoro analogs 96b and 96c require a pyridine-based starting material (98), which must be saturated, converted to a Grignard reagent, and added into an aryl subunit to afford a carbonyl group that serves as a gateway to introduce fluorine through deoxyfluorination. A very different blueprint emerges when using RCC. Thus, simple isonipecotic acid can be subjected to decarboxylative alkyl-alkyl RCC with an alkyl-chloride–bearing zinc reagent followed by SN2 displacement with 6 and oxidation to afford 95, from which 96a to 96c can all be accessed. Similarly, direct RCC, α-fluorination/RCC, or α-difluorination/RCC affords 96a, 96b, or 96c, respectively. Thus, the aforementioned case studies (Fig. 3, A to D) point to disconnection strategies that retain focus on the carbon skeleton during synthesis planning rather than allowing a single fluorine atom to completely alter the logic used.
In a final demonstration of the potential of RCC in synthesis (Fig. 3E), lithocholic acid was converted to a compound containing both a redox-active ester and sulfone, and controlled sequential RCC was demonstrated. Decarboxylative cross-coupling (DCC) proceeded selectively to afford products of Suzuki arylation (101) (36), Negishi arylation (103) (37), alkylation (105) (38), alkenylation (107) (39), and alkynylation (109 and 111) (40). Subsequently, those products underwent clean SCC arylation, delivering an array of useful diversity (102, 104, 106, 108, and 110). In the case of alkyne 111, a classic two-electron cross-coupling (Sonogashira) could be conducted in an orthogonal manner before the final SCC to deliver 112. Clearly, the choreography of both one- and two-electron–based cross-coupling protocols holds great potential not only for modular scaffold design but also for emerging programmed automated synthesis paradigms. Competition experiments suggest that the general order of reactivity in RCC chemistry correlates to the following qualitative trend: Cl/Br < SO2PT < NHPI/TCNHPI [experiments run under SCC conditions with primary alkyl systems (fig. S1)].
It is worth reflecting on the complementarity of the sulfone and decarboxylative cross-coupling processes. The decarboxylative manifold has a distinct advantage in that the starting materials are ubiquitous, whereas the sulfone system stands out on the basis of privileged reactivity preceding the cross-coupling event and circumvention of prefunctionalization steps (ester hydrolysis and/or activation). Reagents 7 and 11, stable crystalline solids, are illustrative of the practical advantage of PT-sulfones over analogous carboxy congeners that are not only toxic but also highly volatile. For example, the carboxy analog of reagent 8 (monofluoroacetic acid) is toxic, and the carboxy analog of reagent 9 does not currently participate in RCC. Anionic α-fluorination of esters is rare, and the common route to such α-substituted mono- and difluorinated systems involves α-oxidation and deoxyfluorination. In addition, crystalline and easily handled redox-active PT-sulfones can be used in concert with other single-electron and two-electron cross-coupling steps so as to enable another dimension of modular synthesis planning. Although there are some low-yielding substrates, most cross-couplings attempted reliably produced the desired cross-coupled product. The mass balance of reactions includes recovered starting material and biaryl products resulting from cleavage of bond b (Fig. 1C). The safety profiles (thermal onset, friction, and shock sensitivity) of reagents 8 and 9 have been evaluated (table S20) and were found to be nonhazardous.
Supplementary Material
ACKNOWLEDGMENTS
We thank D.-H. Huang and L. Pasternack (TSRI) for assistance with NMR spectroscopy; J. Chen (TSRI Automated Synthesis Facility); A. Rheingold, C. E. Moore, and M. A. Galella (University of California, San Diego) for x-ray crystallographic analysis; J. J. Sabatini (U.S. Army Research Laboratory) for friction sensitivity testing of compounds 8 and 9; and J. E. Spangler (Pfizer) and E.-X. Zhang (Asymchem) for helpful discussions.
Funding: Financial support for this work was provided by Pfizer and the National Institutes of Health (grant GM-118176). Vividion Therapeutics supported a predoctoral fellowship to R.R.M., the U.S. Department of Defense supported a predoctoral fellowship to J.T.E. (National Defense Science and Engineering Graduate Fellowship Program), the Innovation Fund Denmark supported a predoctoral fellowship to M.M.K. (grant 4135-00085B), and Nankai University supported C.B.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: Experimental procedures, frequently asked questions, optimization data, 1H NMR spectra, 13C NMR spectra, and mass spectrometry data are available in the supplementary materials. Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1590142–1590144, 1819895, and 1819896.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Yan M, Lo JC, Edwards JT, Baran PS, J. Am. Chem. Soc 138, 12692–12714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu GC, ACS Cent Sci 3, 692–700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo Z et al. , Science 345, 437–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornella J et al. , J. Am. Chem. Soc 138, 2174–2177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellis JC, Primer DN, Molander GA, Science 345, 433–436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouffroy M, Primer DN, Molander GA, J. Am. Chem. Soc 138, 475–478 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green SA, Matos JLM, Yagi A, Shenvi RA, J. Am. Chem. Soc 138, 12779–12782 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Basch CH, Liao J, Xu J, Piane JJ, Watson MP, J. Am. Chem. Soc 139, 5313–5316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima K, Nojima S, Nishibayashi Y, Angew. Chem. Int. Ed 55, 14106–14110 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Simpkins NS, Sulphones in Organic Synthesis (Pergamon, 1993). [Google Scholar]
- 11.Alper PB et al. , U.S. patent US20100022515 A1 (2010). [Google Scholar]
- 12.Rodrigo E, Alonso I, García Ruano JL, Cid MB, J. Org. Chem 81, 10887–10899 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ariki ZT, Maekawa Y, Nambo M, Crudden CM, J. Am. Chem. Soc 140, 78–81 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Wu J-C et al. , Angew. Chem. Int. Ed 51, 9909–9913 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Denmark SE, Cresswell AJ, J. Org. Chem 78, 12593–12628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao W et al. , J. Am. Chem. Soc 140, 880–883 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Taylor RT et al. , in e-EROS Encyclopedia of Reagents for Organic Synthesis, Fuchs P, Bode J, Charette A, Rovis T, Eds. (Wiley, 2015), pp. 1–4. [Google Scholar]
- 18.Xiao Y-L, Min Q-Q, Xu C, Wang R-W, Zhang X, Angew. Chem. Int. Ed 55, 5837–5841 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Sheng J, Ni H-Q, Liu G, Li Y, Wang X-S, Org. Lett 19, 4480–4483 (2017). [DOI] [PubMed] [Google Scholar]
- 20.An L, Xiao Y-L, Min Q-Q, Zhang X, Angew. Chem. Int. Ed 54, 9079–9083 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y et al. , ACS Catal 3, 631–634 (2013). [Google Scholar]
- 22.Prakash GKS, Hu J, Acc. Chem. Res 40, 921–930 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Huang W, Zhu L, Hu J, Org. Lett 12, 1444–1447 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Rong J et al. , Angew. Chem. Int. Ed 55, 2743–2747 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Woolridge EM, Rokita SE, Tetrahedron Lett 30, 6117–6120 (1989). [Google Scholar]
- 26.Sun D et al. , Bioorg. Med. Chem. Lett 19, 1522–1527 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Lo JC et al. , J. Am. Chem. Soc 139, 2484–2503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin T et al. , Angew. Chem. Int. Ed 56, 260–265 (2017). [Google Scholar]
- 29.Hong K, Liu X, Morken JP, J. Am. Chem. Soc 136, 10581–10584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tasker SZ, Standley EA, Jamison TF, Nature 509, 299–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meanwell NA, J. Med. Chem 54, 2529–2591 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Baroudy BM et al. , U.S. patent US6391865 B1 (2002). [Google Scholar]
- 33.Caldwell C et al. , U.S. patent US6265434 B1 (2001). [Google Scholar]
- 34.Lynch CL et al. , Bioorg. Med. Chem. Lett 13, 119–123 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, An L, Chinese patent CN106278847A (2017). [Google Scholar]
- 36.Wang J et al. , Angew. Chem. Int. Ed 55, 9676–9679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toriyama F et al. , J. Am. Chem. Soc 138, 11132–11135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin T et al. , Science 352, 801–805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards JT et al. , Nature 545, 213–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JM et al. , Angew. Chem. Int. Ed 56, 11906–11910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.