Abstract
Variation in development mediates phenotypic differences observed in evolution and disease. Although the mechanisms underlying phenotypic variation are still largely unknown, recent research suggests that variation in developmental processes may play a key role. Developmental processes mediate genotype-phenotype relationships and consequently play an important role regulating phenotypes. In this review, we provide an example of how shared and interacting developmental processes may explain convergence of phenotypes in spliceosomopathies and ribosomopathies. These data also suggest a shared pathway to disease treatment. We then discuss three major mechanisms that contribute to variation in developmental processes: genetic background (gene-gene interactions), gene-environment interactions, and developmental stochasticity. Finally, we comment on evolutionary alterations to developmental processes, and the evolution of disease buffering mechanisms.
Keywords: morphological variation, craniofacial anomalies, evolution of development, genotype-phenotype relationships, ribosomopathies, spliceosomopathies
Introduction:
Variation in development underlies phenotypic differences in evolution and disease. Despite the importance of developmental variation, mechanisms underlying its generation are still poorly understood. In the post-genomic area, significant research in developmental biology has been devoted to understanding gene function. In such studies, discussion of phenotypic variation is typically minimized in order to better reveal causal genotype-phenotype relationships. Similarly, candidate gene and genome-wide association studies have been commonly employed to correlate genetic information with disease phenotypes (Liu et al., 2012; Yu et al., 2017). However, as more of these studies are reported, it is becoming increasingly clear that genotype-phenotype relationships are complex, and that phenotypes associated with loss of function mutations can be quite variable (Hallgrimsson et al., 2009; Parsons et al., 2008). Recent research has explicitly focused on variation as key to understanding genotype-phenotype relationships. In particular, the role of development in mediating phenotypic variance has helped explain variation in phenotypic penetrance (Green et al., 2017; Young et al., 2014).
With very few exceptions, mutations affecting individual genes do not have a predictable phenotypic outcome. While some cellular diseases, such as sickle cell anemia, exhibit high penetrance, similar precise genotype-phenotype correlations are rarely observed in complex morphological structures. In fact, the presence of disease-causing mutations in healthy subjects indicates that incomplete penetrance of Mendelian disorders may be more common than expected (Chen et al., 2016). This is especially true for craniofacial phenotypes, which involve particularly complex genotype-phenotype relationships (Hallgrimsson et al., 2014). Craniofacial development involves many genes of small effect (Porto et al., 2016), which work together in sophisticated protein complexes that regulate developmental processes, and facial phenotypes result from the summation of many hierarchical developmental processes (Fish, 2016; Hallgrimsson et al., 2009; Porto et al., 2016).
Identifying developmental processes as central mediators of genotype-phenotype relationships has several implications for disease diagnosis and treatment. First, mutations in genes that contribute to related developmental processes are predicted to share disease phenotypes. Second, identification of specific developmental processes disrupted by disease mutations may provide avenues for therapeutic treatment at the metabolic, rather than genetic, level. To provide an example of how developmental processes mediate genotype-phenotype relationships, we first review recent research on spliceosomopathies and ribosomopathies suggesting that the pathogenesis of these diseases may be related through interacting developmental processes upstream of protein synthesis. Facial development requires many such developmental processes, therefore, understanding why and how developmental processes vary is important to understanding variation in the penetrance and/or severity of disease mutations. We describe mechanisms contributing to variation in developmental processes, including gene-gene interactions, gene-environment interactions, and developmental stochasticity. Finally, we comment on evolutionary alterations to developmental processes, and the evolution of disease buffering mechanisms.
Developmental processes linking spliceosomopathies and ribosomopathies
Spliceosomopathies
Spliceosomopathies are caused by defects in the splicing machinery. Pre-mRNA splicing is the molecular process that ensures the ligation of exons encoded by a specific gene (Will and Luhrmann, 2011). This highly orchestrated process is mediated by a large ribonucleoprotein (RNP) complex called the spliceosome. A nascent mRNA molecule can contain constitutive as well as variable exons. The difference between these two types of exons lies in the frequency at which they are included in the mature mRNA. While constitutive exons are always a part of the mature mRNA, the inclusion of variable exons depends on different spatiotemporal contexts (Kriventseva et al., 2003). Alternative splicing is the primary mechanism used by our cells to produce different versions (isoforms) of a specific mRNA molecule in order to expand the transcriptomic repertoire and increase proteomic diversity (Nilsen and Graveley, 2010). The expression of certain, alternatively spliced, isoforms is important during tissue development (Baralle and Giudice, 2017). Therefore, the de-regulation of factors that promote splicing results in numerous human diseases.
Several human congenital disorders classified as mandibulofacial (MFD) or acrofacial dysostoses (AFD) are linked to mutations in genes coding for splicing factors. MFDs are a group of congenital diseases that arise from the abnormal development of the first and second pharyngeal arches whereas AFDs are a subdivision of MFDs that also involve limb defects (Wieczorek, 2013). Mutations in genes known to code for splicing factors such as SF3B4, EFTUD2, TXNL4A, EIF4A3, or SNRPB have recently been identified as disease-causing genes in individuals with MFDs or AFDs (Lehalle et al., 2015). Mutations in SF3B4 or EFTUD2 are commonly found in patients diagnosed with Nager syndrome or Mandibulofacial Dysostosis Guion-Almeida type (MFDGA), respectively (Bernier et al., 2012; Lines et al., 2012).
Most spliceosomopathies share similar craniofacial phenotypes (Table 2). Nager syndrome is characterized by a facial phenotype that includes malar and mandibular hypoplasia, down-slanting palpebral fissures, external ear defects, and cleft palate (Bernier et al., 2012; Czeschik et al., 2013; Petit et al., 2014). The most common limb defects associated with Nager syndrome, which contribute to its classification as an AFD, include pre-axial limb defects, primarily hypoplastic or absent thumbs (Bernier et al., 2012; Czeschik et al., 2013; Petit et al., 2014). Patients diagnosed with MFDGA have a facial phenotype that overlaps with that of Nager syndrome but also involves microcephaly. Additionally, individuals with MFDGA occasionally exhibit pre-axial limb defects such as proximally placed thumbs or polydactyly of thumbs (Guion-Almeida et al., 2006; Lines et al., 2012).
Table 2:
List of genes associated with spliceosomopathies and ribosomopathies, their disease phenotypes, and known molecular function.
| Gene | Syndrome | Human Phenotype | References | Mutation | Function of Gene Product |
|---|---|---|---|---|---|
| SF3B4 | 1. Nager Syndrome 2. Rodriguez Syndrome |
Craniofacial – malar and mandibular hypoplasia, down-slanting palpebral fissures, external ear defects and cleft palate. Limb – primarily hypoplastic or absent thumbs. |
- Bernier et al., 2012 - Petit et al., 2013 - Champion-Arnaud and Reed, 2004 |
Frameshift or nonsense resulting in haploinsufficiency. | Member of SF3B complex of U2 snRNP involved in tethering U2 complex to branch site in pre-mRNA. |
| EFTUD2 | Mandibulofacial Dysostosis, Guion-Almeida Type (MFDGA) |
Craniofacial – overlaps with Nager syndrome and includes microcephaly. Limb – occasionally involves defects such as proximally placed thumbs and polydactyly of thumbs. |
- Lines et al., 2012 - Fabrizio et al., 1997 |
Frameshift, nonsense, missense, deletions resulting in haploinsufficiency. | Encodes the spliceosomal GTPase U5–116kD, a member of the U5 snRNP. |
| TXNL4A | Burn-Mckeow Syndrome |
Craniofacial – cleft lip and/or palate, short palpebral fissure, coloboma of the lower eyelids, prominent nasal bridge, and choanal atresia. Other – heart defects, large protruding ears. |
- Wieczorek et al., 2014 - Reuter et al., 1999 |
Heterozygous deletion in the promoter region of TXNL4A. | Yeast ortholog of TXNL4A (Dib1) encodes essential component of the U4/U6-U5 tri-snRNP complex |
| EIF4A3 | Richieri-Costa-Pereira Syndrome |
Craniofacial – midline cleft mandible, cleft palate, glossoptosis, and micrognathia. Limb – limb reductions and clubbed feet. |
- Favaro et al., 2014 - Andreou and Klostermeier 2013 |
Expansion of 18–20 nucleotide motifs in the 5’ UTR of EIF4A3. | Member of the exon junction complex (EJC). Anchors the EJC to the RNA. |
| SNRNPB | Cerebro-costo-mandibular Syndrome (CCMS) |
Craniofacial – cleft palate, glossoptosis, and micrognathia. Rib defects – posterior gaps and missing ribs. |
- Tooley et al., 2016 - Lynch et al., 2014 - Bacrot et al., 2014 - Will and Lührmann, 2011 |
SNRPB codes for three splice variants, one containing an alternative exon that contains a premature stop and functions to auto-regulate protein levels. In CCMS, mutation in the splicing silencer region of the alternative exon increase its inclusion and result in lower levels of SmB and SmB’. | SNRNPB-encoded SmB and SmB’ are splicing isoforms of one of the seven Sm proteins found in each snRNP. |
| CHD7 FAM172A | CHARGE Syndrome |
Coloboma of the eye, Heart defects, Atresia of choanae, Retardation of growth and development, Genital abnormalities, and Ear anomalies. Craniofacial – temporal bone anomalies and cleft lip and/or cleft palate. |
- Zentner et al., 2010 - Vissers et al., 2004 - Bajpai et al., 2010 - Schulz et al., 2014 |
Nonsense, missense, and single-copy 8q12 deletions of CHD7. | CHD7 is a chromatin remodeling factor that regulates the expression of key genes in the NCC GRN. |
| TCOF1, POLR1C, POLR1D, DDX2 | Treacher Collins Syndrome (TCS) |
Craniofacial – down-slanting palpebral fissures, micrognathia, facial bone hypoplasia, and cleft palate. Other – external ear defects, inner ear, and lower eyelid anomalies. |
- Fazen et al., 1967 - Phelps et al., 1981 - Edwards et al., 1997 - Dauwerse et al., 2011 - Valdez et al., 2004 - Gonzales et al., 2005 |
Mutations in the TCOF1 gene include splice site, missense, and nonsense mutations as well as insertions and deletions. | TCOF1, along with other factors, plays an important role in rDNA transcription and rRNA processing. |
| Ribosomal protein genes including: RPS19, RPS26, RPS27, RPL5, RPL11, GATA1 | Diamond-Blackfan anemia (DBA) |
Craniofacial – resemble defects observed in TCS. Limb – thumb abnormalities. Other – anemia caused by decrease of erythroid precursors. |
- Delaporta et al., 2014 - Kim et al., 2012 - Gazda et al., 2008 - Willing et al., 1999 - Fylgare et al., 2007 - Choesmel et al., 2007 - Doherty et al., 2010 - Sankaran et al., 2012 |
Mutations in RPS19 include nonsense, missense, frameshift, and splice site mutations as well as deletions. | RPS19 is required for 18S rRNA synthesis and 40S ribosomal subunit maturation. |
SF3B4 codes for a splicing factor (known as SF3B4, SAP49, or SF3B49) which is one of the seven proteins that make up the SF3B complex of the U2 snRNP (Will and Luhrmann, 2011). SF3B4 binds upstream of the branch site in nascent mRNA molecules and interacts with other factors, especially SAP145, to tether the U2 snRNP to the branch site (Champion-Arnaud and Reed, 1994). In Xenopus, knock-down of Sf3b4 phenocopies the craniofacial defects of human patients with Nager Syndrome (Devotta et al., 2016). Functional studies in Xenopus showed that Sf3b4 is required for the formation and survival of neural crest cells (NCCs) and causes facial defects due to a reduction in progenitors. Devotta and colleagues (2016) found that some genes involved in NCC development were down regulated, however, they did not find evidence to support the hypothesis that the down-regulation of these genes was associated with defective splicing. In contrast, a recent study by Marques and colleagues (2016) in human tissue showed that mRNA splicing is impaired in chondrocytes of fetuses with Rodriguez syndrome, which is a more severe and lethal form of Nager syndrome that is also caused by mutations in SF3B4. SF3B4 has also been reported to bind to BMPR-IA and inhibit BMP-mediated osteochondral cell differentiation, which may also contribute to skeletal defects (Nishanian and Waldman, 2004; Watanabe et al., 2007).
MFDGA is associated with mutations in EFTUD2, which encodes the spliceosomal GTPase U5–116kD that is part of the U5 snRNP (Fabrizio et al., 1997; Lines et al., 2012). The function of EFTUD2 has been less well studied, however, the S. cerevisiae homolog of U5–116kD, Snu114p, is involved in spliceosome activation by regulating the dissociation of the U4 and U6 RNAs (Bartels et al., 2002). Further, EFTUD2 interacts with SF3B4 in the human spliceosome (Hegele et al., 2012). Together, these data suggest that disease phenotypes associated with Nager Syndrome and MFDGA are caused by defective splicing.
Charge syndrome (Fam and Chd7)
CHARGE syndrome is a human congenital disorder that involves a combination of phenotypes emphasized in the acronym for CHARGE, which is coloboma of the eye, heart defects, atresia of choanae, retardation of growth and development, genital abnormalities, and ear anomalies (Pagon et al., 1981). CHARGE syndrome is associated with mutations in CHD7 (chromodomain helicase DNA-binding protein 7) which are thought to result in haploinsufficiency (Vissers et al., 2004). CHD7 is known to regulate the expression of important genes in the NCC gene regulatory network and has been identified as one of the chromatin factors that might affect the outcome of splicing (Bajpai et al., 2010; Schulz et al., 2014). Although loss of function mutations in CHD7 are associated with CHARGE, up to 30% of patients with the disease do not test positive for CHD7 mutations (Zentner et al., 2010).
A recent publication by Belanger and colleagues (2018) showed that the pathogenic mechanism that underlies CHARGE syndrome consists of the dysregulation of co-transcriptional alternative splicing. They used the Toupee mouse line as a model for investigating CHD7 mutation-negative CHARGE. The Toupee line was generated by insertion of a tyrosinase minigene into the FVB/N genetic background in a locus that controls NCC development (Pilon, 2016). ToupeeTg/Tg NCCs migrate more slowly than those of wild-type mice, and they show a significant decrease in proliferation and an increase in apoptosis (Belanger et al., 2018). The transgene insertion site of the Toupee line was determined to be the last intron of Fam172a which is a highly conserved gene between mouse and human. Fam172a is ubiquitously expressed during development of wild-type embryos and down-regulated in ToupeeTg/Tg embryos. Double-immunofluorescence and coimmunoprecipitation experiments revealed that Fam172a interacts with Argonaute 2 (Ago2) in the nucleus. Argonaute proteins, specifically AGO1 and AGO2, are involved in the regulation of alternative splicing by coupling chromatin structure to RNA polymerase (Pol) II elongation (Ameyar-Zazoua et al., 2012). Further, they showed that among the binding partners of Fam172a there is an enrichment for chromatin proteins and splicing factors. Exogenous Fam172a was found to promote the interaction between Chd7 and Ago2. Furthermore, transcriptome analysis of ToupeeTg/Tg NCCs revealed that 30% of all aberrantly spliced transcripts correspond to genes that are also affected during transcription. Collectively, this data supports the hypothesis that Fam172a, Ago2, and Chd7 interact in a complex with chromatin to regulate alternative splicing (Belanger et al., 2018).
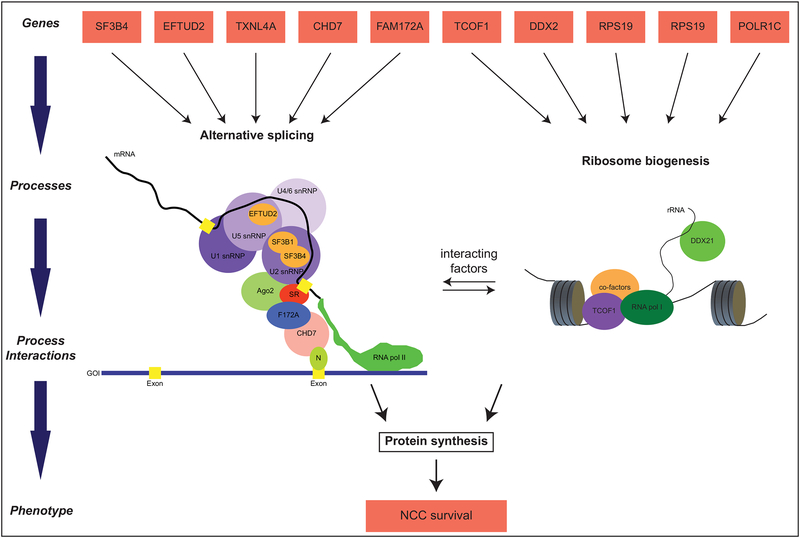
The results of Bélager and colleagues (2018) are intriguing because they suggest that CHARGE syndrome may be a spliceosomopathy. To test their hypothesis, they treated lymphoblastic cell lines (LCLs) derived from patients with CHD7 mutation-negative CHARGE with rapamycin. Rapamycin is known to suppress the TOR pathway which, when unperturbed, promotes ribosome biogenesis by upregulating ribosomal gene expression (Li et al., 2014; Martin et al., 2004). Rapamycin treatment causes a decrease in the expression of ribosomal protein genes, which in turn results in an increase in the number of available splicing factors that can promote the splicing of other pre-mRNAs (Munding et al., 2013). When LCLs from CHARGE patients were treated with rapamycin, the defective splicing of four genes expressed was rescued (Belanger et al., 2018). Taken together, these results suggest that the dysregulation of co-transcriptional alternative splicing is the pathogenic mechanism that underlies both CHD7 mutation-negative and CHD7 mutation-positive cases of CHARGE (Belanger et al., 2018). Further, these data suggest that alternative splicing and ribosome biogenesis are interacting developmental processes (Fig. 1).
Figure 1: Developmental processes integrating ribosomopathies and spliceosomopathies.
Relationships between genes and traits, shown in red boxes, are modeled to illustrate complexity and show processes integrating ribosomopathies and spliceosomopathies. Alternative splicing (left) and ribosome biogenesis (right) are two connected molecular processes upstream of protein synthesis. The spliceosomal small nuclear ribonucleoproteins (snRNPs), shown in purple, catalyze the splicing of exons, shown in yellow, in nascent mRNA molecules. Splicing factors associated with developmental defects are depicted as orange ovals inside their corresponding snRNPs. Other factors that link transcription and splicing are shown interacting with the spliceosome and RNA polymerase II. In the left panel, RNA polymerase I is shown synthesizing a strand of rRNA while interacting with TCOF1 and other co-factors. Both these molecular processes lead to protein synthesis, which is crucial for neural crest cell survival.
Ribosomopathies
Ribosomopathies are caused by defects in ribosome biogenesis. Ribosomes are large RNP complexes composed of 4 ribosomal RNAs (rRNAs) and at least 80 RNA binding proteins that translate spliced mRNA into protein (Liu and Ellis, 2006). Ribosome biogenesis involves all three RNA polymerases and hundreds of other proteins involved in rRNA maturation and assembly into small and large subunits. This process is temporally and spatially separated, with Pol I-mediated transcription of rRNA in the nucleolus, and Pol II-mediated transcription of ribosomal protein genes in the nucleoplasm (Russo and Russo, 2017). Pol III synthesis of 5S rRNA also occurs in the nucleoplasm. Maturation of ribosomes is completed in the cytoplasm after nuclear export (Henras et al., 2015). Thus, ribosome biogenesis consists of a hierarchal series of processes upstream of protein synthesis (Fig. 1).
A number of syndromes are characterized as ribosomopathies, most notably Treacher Collins Syndrome (TCS). TCS is characterized by midfacial hypoplasia, micrognathia with or without cleft palate, underdeveloped external ears and inner ear anomalies with hearing loss, coloboma, and downward slanting eyes (Terrazas et al., 2017). TCS is caused predominantly by mutations in TCOF1, with mutations in POLR1D and POLR1C associated with some TCOF1 mutation-negative cases (Terrazas et al., 2017). TCOF1 is a nucleolar phosphoprotein implicated in Pol I transcription of rRNA (Larsen et al., 2014). In mice, heterozygous mutations in Tcof1 cause massive apoptosis of pre-migratory NCCs, leading to craniofacial hypoplasia that phenocopies TCS malformations in human patients (Dixon et al., 2006). Craniofacial defects caused by Tcof1 haploinsufficiency can be rescued by inhibition or down-regulation of p53, which reduces NCC apoptosis (Jones et al., 2008).
POLR1C and POLR1D are subunits of RNA Pol I and III, which mediate rRNA transcription. Similar to Tcof1 mutations in mice, polr1c and polr1d loss-of-function in zebrafish reduces ribosome biogenesis, causes NCC apoptosis, and generates defects of the facial skeleton suggestive of TCS. Additionally, the facial cartilage defects can mostly be rescued by inhibiting p53 (Noack Watt et al., 2016). These data suggest a similar pathogenic mechanism for TCOF1, POLR1D, and POLR1C. Recent work suggests that a shared mechanism in TCS may be Pol I transcriptional stress, which causes loss of DDX21 from chromatin and its re-localization from the nucleolus to the nucleoplasm (Calo et al., 2015). DDX21 is a DEAD-box RNA helicase involved in both nucleolar Pol I transcription of rRNA and subsequent Pol II-mediated transcription of ribosomal proteins in the nucleoplasm (Calo et al., 2015). Reduction of POLR1D or TCOF1 causes DDX21 re-localization to the nuceloplasm, and preventing DDX21 loss from the nucleolus rescues craniofacial defects in tcof1 deficient Xenopus embryos. Further, knock-down of DDX21 alone induces NCC apoptosis and TCS-like craniofacial defects (Calo et al., 2018).
As with spliceosomopathies, some ribosomopathies affect the craniofacial complex, while others have more widespread effects including axial and limb defects (Yelick and Trainor, 2015). Interestingly, DDX21 dysfunction is also observed upon knock-down of genes associated with other ribosomopathies, such as Diamond-Blackfan anemia (Calo et al., 2018). Taken together, these data suggest that p53 activation and DDX21 re-localization downstream of rDNA damage caused by Pol I transcriptional stress may be a common mediator of ribosomopathies (Calo et al., 2018).
Alternative splicing and ribosomal biogenesis interact upstream of protein synthesis
Studies in multiple model systems indicate that ribosomopathies share a pathogenic mechanism, which is p53 mediated cell death (Calo et al., 2018; Mills and Green, 2017). Similarly, studies in Xenopus, zebrafish, and mouse models also reveal that haploinsufficiency of genes coding for splicing factors ultimately results in increased cell death (Belanger et al., 2018; Devotta et al., 2016; Lei et al., 2016). Additionally, CHD7 deficiency causes an increase in p53 activation in mouse NCCs and human fibrobalsts (Van Nostrand et al., 2014). CHD7 is also reported to negatively regulate p53 expression by binding its promoter which could be one of the mechanisms that explains how low levels of CHD7 result in an upregulation of p53 expression (Van Nostrand et al., 2014). This suggests that NCC death could also be a pathogenic mechanism underlying CHARGE syndrome.
In a recent study, Zhang and colleagues describe how the ribosomal proteins Rpl22 and Rpl22-Like1 (Rpl22l1) perform extra-ribosomal functions by acting in the nucleoplasm to regulate the splicing of Smad2 in Xenopus embryos (Zhang et al., 2017). In this case ribosomal and splicing factors interact to control morphogenesis, further suggesting that these two molecular processes are linked.
The convergence on a similar cellular outcome, such as pre-migratory NCC death, might explain why ribosomopathies and spliceosomopaties have overlapping craniofacial phenotypes. But why should a deficit in a general regulator of cell function have cell-type-specific effects? NCC development involves multiple developmental processes, including induction, specification, epithelial-mesenchymal transition (EMT), delamination, and migration (Sauka-Spengler and Bronner-Fraser, 2008). EMT, in particular, involves significant changes in cell polarity and adhesion, which requires significant changes in gene expression, protein turnover, and metabolic inputs (Kalluri and Weinberg, 2009). It is at this stage that the survival and proliferation of NCCs appear to be particularly sensitive to perturbation, which may imply a high demand for protein synthesis in these cells. However, rescue of NCC survival by down-regulation of p53 in Tcof-deficient mice occurs independently of ribosome biogenesis (Jones et al., 2008). This suggests that deficits in ribosome biogenesis can be compensated for in NCCs, as they are in other cell types.
Recently, Calo and colleagues (2018) addressed the specific question of why mutations affecting the ribosome biogenesis pathway particularly impact NCCs. They showed that NCCs are sensitized to p53 stabilization and are 2-fold more likely than other embryonic cell types to undergo apoptosis when treated with a p53 stabilizing drug. When impaired, molecular processes such as alternative splicing and ribosome biogenesis result in an increase of p53 expression and activation (Allende-Vega et al., 2013; Dixon et al., 2006). In normal development, NCC express high levels of p53 relative to other cells (Calo et al., 2018; Rinon et al., 2011). High levels of p53 may be necessary to regulate NCC proliferation during EMT (Rinon et al., 2011). Thus, the complex development of NCCs, involving high p53 expression, may explain their susceptibility to defects in general regulators of cell function.
Diseases caused by interacting developmental processes may share a metabolic treatment
Patients with AFDs or MFDs are diagnosed based on a series of phenotypes that overlap and can be subtly different (Green et al., 2013). For example, Diamond-Blackfan anemia with Mandibulofacial Dysostosis, a ribosomopathy, involves phenotypes that are also seen in MFDGA, a spliceosomopathy (Gripp et al., 2014). Often times, patients who are diagnosed with one syndrome are later found to harbor mutations linked to a different syndrome that is phenotypically similar to the first one (Bernier et al., 2012; Gordon et al., 2012; Vincent et al., 2016). The absence of precise genotype-phenotype correlations may be due to the fact that the different mutations occur in genes functioning in the same and/or closely interacting developmental processes. Individual differences in genetic background, environmental influences, or developmental stochasticity, as described below, could also contribute to variability in phenotypes, thus further complicating genotype-phenotype correlations. However, this complication could be a benefit to disease treatment rather than an obstacle. Therapies that attempt to restore protein function due to loss-of-function mutations have achieved little success (Dietz, 2010). A promising alternative is to focus on modifiers that buffer or compensate for reductions in protein function (Chen et al., 2016). Increased understanding of how genes relate to developmental processes will be an important step to facilitate this therapeutic alternative.
Variation in developmental processes
In the above discussion, we have described how many genes contribute to a single developmental process, and how multiple developmental processes contribute to a single cellular phenotype (NCC survival). This can explain how mutations in seemingly unrelated genes can cause broadly similar phenotypes. However, disease causing mutations also exhibit variation in penetrance and severity, which may also be explained by considering phenotypes from the perspective of developmental processes. Therefore, understanding why and how developmental processes vary is important to understanding phenotypic variation. Factors contributing to variation in developmental processes include gene-gene interactions, gene-environment interactions, and developmental stochasticity.
Gene-gene interactions
Developmental processes rely on the interaction of many different gene products. Therefore, phenotypic outcomes of mutations affecting a target gene may be modified by its genetic background, that is, the genotype of all the other genes it interacts with in the regulation of a particular process. Such interactions between a target gene and its modifiers are referred to as epistasis. Epistatic interactions can affect the dominance, penetrance, expressivity, and pleiotropy of a mutation (Mackay, 2014; Nadeau, 2001; Riordan and Nadeau, 2017). Dominance modifiers cause heterozygotes to develop disease phenotypes similar to homozygous mutants.
Penetrance modifiers affect the frequency, but not severity, of disease. For example, Pfeiffer syndrome is an autosomal dominant disorder caused by mutations in FGF receptors (FGFRs) that is characterized by craniosynostosis, and other dysmorphic facial features, including bulging eyes, a high forehead, mid-facial hypoplasia, and micrognathia (Chokdeemboon et al., 2013). Several genetic variants of FGFR2 can cause Pfeiffer syndrome, however, only one mutation in FGFR1, the missense p.P252R alteration, has been reported in association with Pfeiffer syndrome (Muenke et al., 1994). However, the presence of the p.P252R FGFR1 mutation is also found in healthy individuals (Chen et al., 2016). The genetic study of Chen and colleagues (2016) found at least 8 rare, deleterious mutations in healthy individuals, indicating the capacity of certain genetic backgrounds to buffer the effects of disease-causing mutations.
Expressivity modifiers determine the severity of mutations. For example, a recent study in mice revealed the variable impact of craniofacial defects caused by mutations in Sprouty genes (Percival et al., 2017). In particular, the FVB/NJ background was found to be more robust to Sprouty mutations than either 129X1/SvJ or C57BL/6J. This study also revealed that modifier genes can also change the direction of the effect, as the same loss of function mutation in Spry1 caused opposite effects on craniofacial shape in two different inbred backgrounds (Percival et al., 2017). Finally, pleiotropy modifiers determine the number of features that are affected by a mutation. Thus, genetic modifiers can also impact the spatial and temporal context of mutations (Mackay, 2014).
In most cases, the underlying mechanisms modifiying penotypic output of target gene mutations are unknown. However, some interesting examples have been elucidated. For example, craniosynostosis, the premature ossification of cranial sutures, can result from epistatic interactions between multiple members of the osteogenic differentiation pathway downstream of BMP signaling (Timberlake et al., 2018). The BMP signaling cascade involves multiple activators and inhibitors upstream of osteogenic differentiation (Fig 2A). In a recent study investigating genetic causes of non-syndromic craniosyostosis, mutations in SMAD6, an inhibitor of BMP signaling, were identified (Timberlake et al., 2016). The causative SMAD6 mutations were often found in an unaffected parent, reflective of their incomplete penetrance. An earlier genome-wide association study had identified a craniosynostosis “risk allele” of BMP2. This risk allele harbors several single nucleotide polymorphisms associated with craniosynostosis within a non-coding region downstream BMP2 (Justice et al., 2012). Predicted transcription-factor binding sites in this region suggest it may be a cranial specific regulatory region increasing BMP2 expression. Notably, this BMP2 variant is common and only very rarely causes craniosynostosis on its own (Komatsu and Mishina, 2016). However, when the BMP2 risk allele occurs in association with SMAD6 mutations, craniosynostosis occurs 100% of the time (Timberlake et al., 2016). Similarly, a child with a severe case of craniosynostosis was found to have an SMAD6 mutation (inherited from an unaffected parent) and a de novo TCF12 mutation (Timberlake et al., 2018). TCF12 heterodimerizes with TWIST1 to transcriptionally repress osteogenic genes downstream of BMP signaling (Fig. 2A).
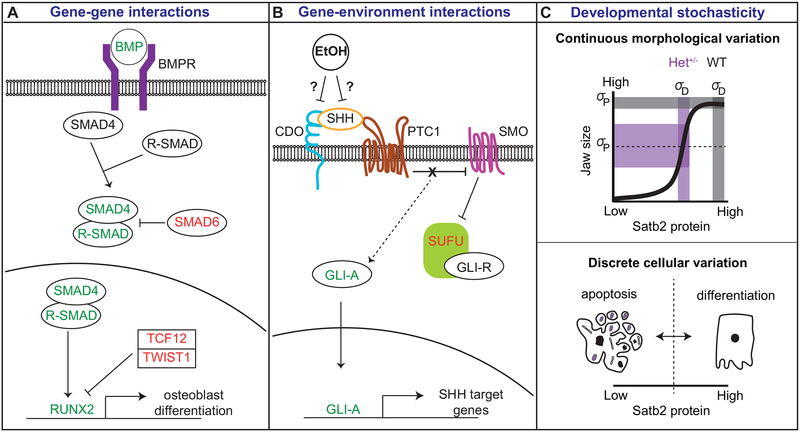
Figure 2: Mechanisms regulating variation in developmental processes.
Factors contributing to variation in developmental processes include A) gene-gene interactions, B) gene-environment interactions, and C) developmental stochasticity. A) Gene-gene interactions are modeled by BMP signaling in osteogenesis. Proteins in red are negative regulators of the pathway; proteins in green are positive regulators. Heterozygous mutations in SMAD6, a negative regulator of the BMP pathway, may be buffered if they occur in isolation. However, if they occur in a “risk allele” background in which BMP levels are increased or a second negative regulator (e.g., TCF12) is decreased, disease phenotypes are observed. Image modeled after Timberlake et al. 2018. B) Gene-environment interactions are modeled by ethanol (EtOH) influences on SHH signaling. EtOH may exacerbate mutations in CDO, a SHH co-receptor, by negatively interacting with SHH binding to its receptors. Image modeled after Kahn et al. 2016. C) Developmental stochasticity is modeled by Satb2-mediated variation in jaw size. Satb2 protein levels have a non-linear relationship with jaw size, where wild-type and homozygous mutant individuals exhibit little population variance in size (grey shaded rectangles). However, heterozygous mutants are highly variable in size, encompassing the range of variation between wild-type and mutant (purple shaded rectangles). This continuous morphological variation (upper panel) can be explained by discrete cellular variation (lower panel). Satb2+/− cells are predicted to generate Satb2 protein levels that are at or near the threshold for Satb2 activation. Those cells that meet or surpass the threshold will proliferate and differentiate into osteoblasts; those cells that fall below the threshold will undergo apoptosis. Thus, random variation in the degree of heterogeneity in cell fate between individuals can explain variation in jaw size. Dotted lines indicate threshold for protein activity.
Thus, the developmental process of suture ossification can be modified by several different types of genetic modifications, many of which involve multiple “hits.” Allelic variation at the BMP2 locus among normal populations causes background differences in BMP2 expression. In an allelic background where BMP2 is high, a mutation reducing expression of a BMP inhibitor causes craniosynostosis. In this example, genetic alterations contributing to these epistatic interactions reduce gene expression through changes to cis-regulatory DNA sequences or through loss of function mutations within the protein coding sequence.
Genetic background may also explain inherited differences in methylation of regulatory elements, which can affect gene expression and disease penetrance. Zebrafish mutants in mef2ca exhibit variation in ectopic bone formation in their hyoid skeleton (Nichols et al., 2016). The ectopic bone results from a switch in cell fate from ligament to bone. mef2c is a MADS domain-containing transcription factor regulating skeletal development (Miller et al., 2007). Null mef2ca mutants exhibit low penetrance of ligament-to-bone transition However, the mef2cab1086 mutant allele, which is predicted to form a truncated protein with deleterious activity, exhibits variable fate switching (Nichols et al., 2016). Penetrance of fate switching in mef2cab1086 mutants is heritable and strains with high penetrance express high levels of mutant transcript while low penetrance strains have low levels of expression. Further, levels of mef2cab1086 are associated with differences in methylation of an upstream transposable element that is thought to regulate its expression. Thus, the mef2cab1086 allele mediates cell fate changes in a Mendelian-like manner, yet it exhibits variable penetrance due to differences in epigenetic-mediated expression levels.
In isolation, methylation differences would be considered epigenetic modifications. However, Nichols and colleagues (2016) argue that an as yet unidentified genetic variant is ultimately responsible for the inherited difference in methylation. Differences in methylation at risk loci for cleft lip and/or palate have also been associated with variation in penetrance (Alvizi et al., 2017). These data suggest that allelic differences in genes regulating methylation could be an under-appreciated mechanism contributing to variation in phenotypic penetrance.
Gene-environment interactions
Gene-environment interactions may be defined where one allele (polymorphism) responds differently to an external factor (Durham et al., 2017). In humans, external factors including maternal nutritional status, diabetes/obesity-related conditions, and exposure to medications and/or environmental toxins are known to affect the incidence of craniofacial disease (Zhu et al., 2009). The exact molecular mechanisms by which environmental factors influence phenotypes are not well established and may be difficult to study in human populations. However, some insights on teratogenic mechanisms have been elucidated from studies in animal models, especially the effects of ethanol on holoprosencephaly (Fig. 2B).
Holoprosencephaly (HPE) is a highly variable congenital anomaly characterized by defects in midline patterning. Mutations in gene members of the SHH pathway are implicated in HPE, however, up to one-third of mutation carriers do not exhibit a clinical phenotype, and mutations found in many HPE patients are inherited from unaffected parents (Roessler and Muenke, 2010; Solomon et al., 2012). Genetic modifiers, including the SHH co-receptors BOC and GAS1, contribute to the complex etiology of HPE (Hong et al., 2017; Seppala et al., 2014). However, environmental factors are also implicated. In particular, ethanol is an HPE-inducing teratogen, and studies of prenatal ethanol exposure in animal models have consistently shown that ethanol contributes to HPE by disrupting SHH signaling (Ahlgren et al., 2002; Hong and Krauss, 2012, 2017; Li et al., 2007).
In mice, homozygous mutations in Cdo, another Shh co-receptor, produce HPE phenotypes with background-specific phenotypic severity (Hong and Krauss, 2012). In the 129S6 background, Cdo−/− mice display low penetrance of HPE that can be exacerbated by either genetic or environmental factors. For example, the additional loss of one allele of Shh or Boc causes severe HPE phenotypes (Tenzen et al., 2006). Similarly, ethanol exposure exacerbates defects in midline patterning and SHH signaling in 129S6 Cdo−/− mice (Hong and Krauss, 2012; Kahn et al., 2017). The precise mechanism by which ethanol perturbs SHH signaling remains unknown. However, it has been hypothesized that ethanol directly inhibits CDO activity (Kahn et al., 2017). It has also been hypothesized that ethanol reduces SHH signaling by blocking cholesterol modification of SHH (Li et al., 2007). These data suggest that individuals with mutations in genes involved in SHH signaling may be particularly susceptible to embryonic ethanol exposure (Hong and Krauss, 2012).
Developmental stochasticity
Embryogenesis is the process by which a single cell generates all the cellular and tissue diversity within an organism from the same, shared genome. Despite this tremendous power to generate variation in gene expression and cell fate, development typically produces robust phenotypes (Waddington, 1942). A variety of mechanisms have evolved to limit phenotypic variation and/or direct it within a developmental structure, such as a body plan. Nevertheless, random variation in developmental processes can cause subtle phenotypic variation, which can be observed in isogenic populations such as genetically identical, inbred littermate mice (Hallgrimsson et al., 2009; Parsons et al., 2008).
Studies in bacteria and yeast have shown that gene expression is noisy and that clonal populations of cells exhibit substantial molecular variation (Eldar et al., 2009; Eldar and Elowitz, 2010). Such noise appears to be essential to many cellular activities, including diversification of cell fates as well as adaptive evolution (Oates, 2011). Molecular variation in isogenic cells may also be influenced by cell cycle state or differences in location related to other cells, signaling molecules, or extra-cellular matrix. Under normal developmental conditions, such molecular variation is typically buffered by tissue-level processes, producing only subtle craniofacial variation (Thornhill and Moller, 1997). However, molecular noise may be especially relevant to phenotypic variation in the context of genetic mutations.
Cell fate decisions are mediated, in part, by transcription factor expression and loss of function mutations in key transcription factors can cause alterations to cell fate decisions. For example, in the developing zebrafish head, barx1 regulates the decision to become a joint cell versus a cartilage cell v(Nichols et al., 2013), and mef2ca controls ligament versus bone cell fate decisions (Nichols et al., 2016). A threshold model has been proposed to explain these alternative cell fate decisions, where the binary choice between cell fates depends upon whether or not sufficient protein levels are achieved to activate one cell fate over a default cell fate (Nichols et al., 2016; Oates, 2011). Therefore, mutations that reduce protein levels such that they are at or near the threshold will produce heterogeneity in cell fate decisions as stochastic variation results in some cells reaching the threshold and others not. Variation in disease severity is the consequence of tissue-level responses to heterogeneity in single cell behavior (Oates, 2011). Thus, developmental stochasticity may explain both discrete cellular variation and continuous morphological variation (Fig. 3C).
For example, mice with mutations in Satb2 exhibit continuous variation in jaw size (Fish et al., 2011). While complete loss of Satb2 function causes extreme micrognathia and cleft palate, Satb2+/− mice exhibit a variable reduction in jaw size. Notably, dentary length of Satb2+/− mice encompasses the range of variation between wild-type and homozygous mutants. Reduction in jaw size upon loss of Satb2 is associated with apoptosis of NCC progenitors (Britanova et al., 2006). That is, Satb2 activation is required for NCC survival and differentiation. This implies that the average cellular protein level in heterozygous mice is near the threshold for Satb2 activation (Fig. 2C, upper panel). Thus, in Satb2+/− mice, minor random variation in Satb2 protein levels leads to cellular heterogeneity in Satb2 network activation, and cells failing to activate the Satb2 network undergo apoptosis (Fig. 2C, lower panel). Variation in jaw size in heterozygous mice is therefore associated with inter-individual variation in the number of cells that undergo apoptosis.
The threshold model results in a non-linear relationship between genotype and phenotype, which has previously been predicted to explain high levels of morphological variation in disease models (Hallgrimsson et al., 2009; Marcucio et al., 2011; Young et al., 2010). Evidence for this non-linear model in craniofacial disease was recently presented for mutations affecting Fgf8, a critical regulator of facial development (Green et al., 2017). In mice, reduction in Fgf8 mRNA does not affect facial shape until Fgf8 levels drop below 40% of wild-type expression. Importantly, molecular variance exhibited by mutant individuals is similar to that observed in wild-type individuals. That is, increased phenotypic variance in mutant individuals is not due to an increase in molecular noise, but rather due to the average levels of protein being at or near the threshold for activation (Fig. 2C, upper panel).
Evolution of developmental processes
Much of our discussion so far has focused on how alterations to developmental processes contribute to phenotypic variation in disease. It is worth considering if and how alterations to developmental processes underlying vertebrate diversification are similar or different from those occurring in disease processes. As an example, we will consider evolutionary alterations to splicing patterns. Finally, we briefly discuss the evolution of mechanisms buffering phenotypic variation.
Splicing alterations in evolution and disease
Splicing patterns have rapidly diverged in vertebrate evolution, and likely had a more important role in species-specific phenotypes than do alterations to overall gene expression levels (Barbosa-Morais et al., 2012). In a comparison of the jaws of six different species of cichlids, differences in splicing were found to be much higher than overall gene expression differences, suggesting that alterations to splicing may facilitate rapid divergence (Singh et al., 2017). Increased alternative splicing has contributed to extensive proteomic diversity in mammals, and especially primates, relative to other clades (Barbosa-Morais et al., 2012; Gueroussov et al., 2017). For example, exon skipping is more common in human embryos compared to mouse, contributing to approximately double the number of isoforms generated per orthologous gene (Chen et al., 2017).
Species-specific changes to splicing are mostly cis-directed, occuring as changes to splice recognition sites, however, evolution of trans-acting RNPs also played a critical role in generating proteome diversity (Barbosa-Morais et al., 2012). In particular, “nucleic acid binding” genes were found to be among the most frequently associated genes with species-classifying splicing events. Notably, cis-mediated species-specific differences in splicing of RNPs preferentially affect disordered regions rather than nucleic acid binding domains (Barbosa-Morais et al., 2012; Gueroussov et al., 2017). Disordered domains lack stable 3D structures. Instead they undergo induced fit structural changes and, therefore, are flexible mediators of protein-protein interactions (Tompa et al., 2015). Thus, mammalian-specific splicing events generate an increase in RNP diversity by retaining nuclei acid binding domians, but alternatively including disordered domains (Gueroussov et al., 2017). In turn, RNP diversity contributes to increased complexity of splicing through variation in the formation of high-order protein assembiles on pre-mRNA (Fig. 3).
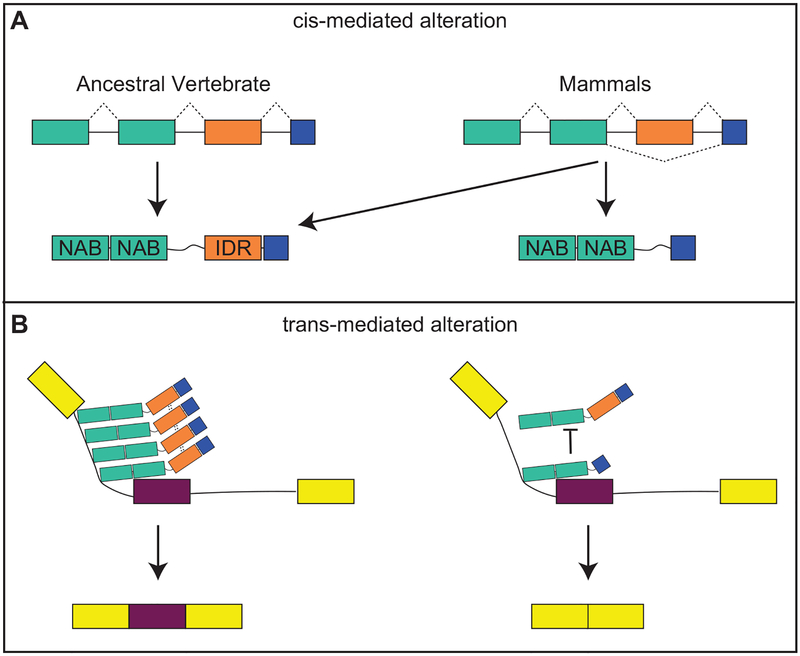
Figure 3: Mechanisms contributing to the evolution of splicing patterns.
Both A) cis-mediated and B) trans-mediated alterations contribute to the evolution of splicing patterns. A) Mutations affecting splice recognition sites contribute to increased exon skipping in mammals. Such mutations are enriched around exons containing intrinsic disordered regions (IDR) and under-represented around nuclei acid binding domains (NAB). In this example, mammals are able to produce two protein isoforms from the same mRNA, one containing and IDR and one lacking the IDR. B) IDRs contribute to protein-protein interactions. A protein complex assembled among IDR-containing RNPs may promote exon inclusion (left). In the absence of IDRs, such complexes are not formed and exon skipping occurs (right). Image modeled after Gueroussov et al. 2017.
Mutations associated with spliceosomopathies are mostly thought to result in haploinsufficiency of the affected gene (see Table 2 and references therein). In most cases, the mutations occur in the protein coding region, but do so in a manner that is not thought to produce a functional protein, but rather reduce overall protein levels (e.g., Marques et al., 2016). Similarly, tissue-specific splicing decisions often result from differences in levels or activity of splicing factors (Grosso et al., 2008). Tissue-specific splicing can be mediated by tissue-specific expression of spliceosome-associated RNPs or by alterations to the levels of core U snRNPs (Grosso et al., 2008; Pacheco et al., 2006). Interestingly, reductions in levels of RNPs occurring as a consequence of a disease-causing mutation cause regulated shifts in splicing profiles. For example, knock-down of Rpl22l1 causes mis-splicing of smad2. In zebrafish, mis-splicing of smad2 is mediated by exon 9 skipping; In mice, exons 7 and 8 are skipped. The resulting smaller mRNAs do not produce protein, leading to overall reduction in smad2 levels which subsequently contribute to defects in gastrulation (Zhang et al., 2017). Notably, loss of Rpl22l1 function does not increase variation or randomize splicing outcomes. Rather, a complete shift from exon inclusion to exon skipping occurs in the absence of Rpl22l1.
Several other recent investigations of disease-causing mutations in splicing factors have shown that dysregulation of splicing occurs as a shift in splicing patterns (e,g, from exon inclusion to exon skipping) in a limited set of genes, rather than global disruption to splicing. For example, mice with heterozygous mutations in Chd7 exhibit multiple splicing alterations, including exon skipping, retained introns, and alternative splice sites, however, only 227 splicing events were modulated in these mutants (Belanger et al., 2018). Therefore, the disease mechanism could be characterized as a shift from one regulated state to another regulated state. Thus, both the specific molecular mechanism (changes in splicing factor levels) and outcome (regulated shift in splicing patterns) occur in evolutionary and disease processes.
Evolution of buffering mechanisms
Cellular outcomes are often determined by multiple regulatory inputs (e.g., Fig. 2A). Such complexity in gene regulatory networks buffers transcriptional noise and can also often buffer alterations to transcription levels caused by a single mutation. In particular, the development of complex structures such as the craniofacial complex is robust to most heterozygous mutations (Loewe and Hill, 2010). However, mutations in some genes (most of those discussed above), generate variable disease phenotypes in the heterozygous state. This suggests that some genes are more susceptible to perturbation than others.
Several recently described disease models propose that proteins typically have a threshold level for activation with a range in which normal function occurs, which can be modeled as a non-linear curve (Fish, 2016; Green et al., 2017; Nichols et al., 2016; Young et al., 2010). This non-linear model explains both increased phenotypic variance in disease resulting from genetic mutations that decrease protein levels, as well as why different genes may have different susceptibility to heterozygous mutations based on the position of the curves along the x-axis (Fig. 4A). The presence of proteins that have a lower threshold requirement for maintaining normal phenotypic outcomes may reflect the evolution of buffering mechanisms.
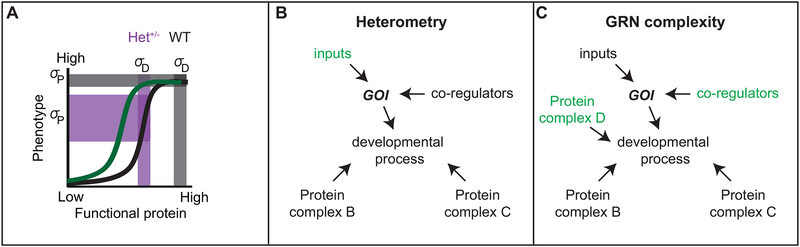
Figure 4: Evolution of buffering mechanisms.
A) Non-linear model of genotype-phenotype relationships, where genotype is represented as functional protein produced by a gene (x-axis). Protein level variance is represented by the vertical bars; horizontal bars represent variation in phenotype. Note that, based on the threshold model, the same variance in protein levels has a significantly different effects on phenotype depending on protein levels relative to the threshold, where dark grey is wild-type (WT) and light gret is mutant (Het+/−). Either heterometry or increases in GRN complexity may shift the position of a threshold value in a non-linear genotype-phenotype curve (black to green). B) Alterations to inputs regulating a gene of interest (GOI) may affect its levels (heterometry). C) Alterations to the number of co-regulators of protein complexes regulating a developmental process can increase gene regulatory network (GRN) complexity.
At least two possible mechanisms could contribute to buffering genetic mutations. Genetic alterations that increase the input on a gene of interest (increase positive regulation), may increase robustness to a loss of one allele (Fig. 4B). Similarly, alterations to other genes in the same gene regulatory network may buffer reductions in any one particular gene (Fig. 4C). The contribution of many genes to a single process may therefore be a mechanism for developmental robustness. Further, the complexity of genetic interactions regulating developmental processes explains the poor genotype-phenotype correlations of many diseases, where phenotypic defects are not the result of a single mutation, but rather result from the combination of several genes with additive effects (Manolio and Collins, 2009).
Conclusion
Recent research has described how developmental processes are key mediators of genotype-phenotype relationships. Multiple genes contribute to the regulation of relatively fewer developmental processes and phenotypic outcomes ultimately derive from the orchestration and interaction of these developmental processes. We have described how individuals carrying mutations in genes involved in mRNA splicing and ribosome biogenesis have similar craniofacial disease phenotypes. Based on recent investigations into the pathogenesis of these diseases, we argue that alternative splicing and ribosome biogenesis are related processes acting upstream of protein synthesis. Because these two processes utilize some of the same molecular resources, they have similar metabolic profiles. In particular, craniofacial defects associated with both spliceosomopathies and ribosomopathies result, at least in part, from apoptosis of pre-migratory NCC. These data are particularly relevant to clinical treatments and precision medicine. Understanding disease through affected developmental processes has the potential to focus treatments on cellular and metabolic outcomes of processes rather than attempting to treat each genetic mutation individually. Finally, disease and evolutionary phenotypes may result from similar alterations to developmental processes. Therefore, further investigation into how and why developmental processes vary will have significant impact on both disease and evolutionary mechanisms.
Table 1:
List of abbreviations used in the manuscript.
| Abbreviation | Description |
|---|---|
| AFD | acrofacial dysostosis |
| EMT | epithelial-mesenchyal transition |
| HPE | Holoprosencephaly |
| IDR | intrinsic disordered region |
| LCLs | Lymphoblastic cell lines |
| MFD | mandibulofacial dysostosis |
| MFDGA | Mandibulofacial Dysostosis Guion-Almeida type |
| NCC | neural crest cell |
| POL | polymerase |
| RNP | ribonucleoprotein |
| rRNA | ribosomal RNA |
| TCS | Treacher Collins Syndrome |
Acknowledgements
We would like to thank our collaborators, Rebecca Green, Benedikt Hallgrimsson, and Ralph Marcucio, for ongoing discussions that contributed to ideas presented in this review. We also thank Evelyn Schwager and two anonymous reviewers for comments on previous versions of this manuscript. This work was supported by the National Institutes of Health [R15 DE026611–01].
References:
- Ahlgren SC, Thakur V, Bronner-Fraser M. 2002. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci U S A 99: 10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende-Vega N, Dayal S, Agarwala U, Sparks A, Bourdon JC, Saville MK. 2013. p53 is activated in response to disruption of the pre-mRNA splicing machinery. Oncogene 32: 1–14. [DOI] [PubMed] [Google Scholar]
- Alvizi L, Ke X, Brito LA, Seselgyte R, Moore GE, Stanier P, Passos-Bueno MR. 2017. Differential methylation is associated with non-syndromic cleft lip and palate and contributes to penetrance effects. Sci Rep 7: 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, Morozova N, Fenouil R, Descostes N, Andrau JC, Mathieu J, Hamiche A, Ait-Si-Ali S, Muchardt C, Batsche E, Harel-Bellan A. 2012. Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol 19: 998–1004. [DOI] [PubMed] [Google Scholar]
- Andreou AZ, Klostermeier D. 2013. The DEAD-box helicase eIF4A: paradigm or the odd one out? RNA Biol 10: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacrot S, Doyard M, Huber C, Alibeu O, Feldhahn N, Lehalle D, Lacombe D, Marlin S, Nitschke P, Petit F, Vazquez MP, Munnich A, Cormier-Daire V. 2015. Mutations in SNRPB, encoding components of the core splicing machinery, cause cerebro-costo-mandibular syndrome. Hum Mutat 36: 187–190. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J. 2010. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle FE, Giudice J. 2017. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, Slobodeniuc V, Kutter C, Watt S, Colak R, Kim T, Misquitta-Ali CM, Wilson MD, Kim PM, Odom DT, Frey BJ, Blencowe BJ. 2012. The evolutionary landscape of alternative splicing in vertebrate species. Science 338: 1587–1593. [DOI] [PubMed] [Google Scholar]
- Bartels C, Klatt C, Luhrmann R, Fabrizio P. 2002. The ribosomal translocase homologue Snu114p is involved in unwinding U4/U6 RNA during activation of the spliceosome. EMBO Rep 3: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger C, Berube-Simard FA, Leduc E, Bernas G, Campeau PM, Lalani SR, Martin DM, Bielas S, Moccia A, Srivastava A, Silversides DW, Pilon N. 2018. Dysregulation of cotranscriptional alternative splicing underlies CHARGE syndrome. Proc Natl Acad Sci U S A 115: E620–E629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier FP, Caluseriu O, Ng S, Schwartzentruber J, Buckingham KJ, Innes AM, Jabs EW, Innis JW, Schuette JL, Gorski JL, Byers PH, Andelfinger G, Siu V, Lauzon J, Fernandez BA, McMillin M, Scott RH, Racher H, Consortium FC, Majewski J, Nickerson DA, Shendure J, Bamshad MJ, Parboosingh JS. 2012. Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am J Hum Genet 90: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, Tarabykin V. 2006. Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. Am J Hum Genet 79: 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Flynn RA, Martin L, Spitale RC, Chang HY, Wysocka J. 2015. RNA helicase DDX21 coordinates transcription and ribosomal RNA processing. Nature 518: 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Gu B, Bowen ME, Aryan F, Zalc A, Liang J, Flynn RA, Swigut T, Chang HY, Attardi LD, Wysocka J. 2018. Tissue-selective effects of nucleolar stress and rDNA damage in developmental disorders. Nature 554: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion-Arnaud P, Reed R. 1994. The prespliceosome components SAP 49 and SAP 145 interact in a complex implicated in tethering U2 snRNP to the branch site. Genes Dev 8: 1974–1983. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen J, Yang J, Chen L, Qu X, Shi C, Ning B, Shi L, Tong W, Zhao Y, Zhang M, Shi T. 2017. Significant variations in alternative splicing patterns and expression profiles between human-mouse orthologs in early embryos. Sci China Life Sci 60: 178–188. [DOI] [PubMed] [Google Scholar]
- Chen R, Shi L, Hakenberg J, Naughton B, Sklar P, Zhang J, Zhou H, Tian L, Prakash O, Lemire M, Sleiman P, Cheng WY, Chen W, Shah H, Shen Y, Fromer M, Omberg L, Deardorff MA, Zackai E, Bobe JR, Levin E, Hudson TJ, Groop L, Wang J, Hakonarson H, Wojcicki A, Diaz GA, Edelmann L, Schadt EE, Friend SH. 2016. Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nat Biotechnol 34: 531–538. [DOI] [PubMed] [Google Scholar]
- Chokdeemboon C, Mahatumarat C, Rojvachiranonda N, Tongkobpetch S, Suphapeetiporn K, Shotelersuk V. 2013. FGFR1 and FGFR2 mutations in Pfeiffer syndrome. J Craniofac Surg 24: 150–152. [DOI] [PubMed] [Google Scholar]
- Choesmel V, Bacqueville D, Rouquette J, Noaillac-Depeyre J, Fribourg S, Cretien A, Leblanc T, Tchernia G, Da Costa L, Gleizes PE. 2007. Impaired ribosome biogenesis in Diamond-Blackfan anemia. Blood 109: 1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeschik JC, Voigt C, Alanay Y, Albrecht B, Avci S, Fitzpatrick D, Goudie DR, Hehr U, Hoogeboom AJ, Kayserili H, Simsek-Kiper PO, Klein-Hitpass L, Kuechler A, Lopez-Gonzalez V, Martin M, Rahmann S, Schweiger B, Splitt M, Wollnik B, Ludecke HJ, Zeschnigk M, Wieczorek D. 2013. Clinical and mutation data in 12 patients with the clinical diagnosis of Nager syndrome. Hum Genet 132: 885–898. [DOI] [PubMed] [Google Scholar]
- Dauwerse JG, Dixon J, Seland S, Ruivenkamp CA, van Haeringen A, Hoefsloot LH, Peters DJ, Boers AC, Daumer-Haas C, Maiwald R, Zweier C, Kerr B, Cobo AM, Toral JF, Hoogeboom AJ, Lohmann DR, Hehr U, Dixon MJ, Breuning MH, Wieczorek D. 2011. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet 43: 20–22. [DOI] [PubMed] [Google Scholar]
- Delaporta P, Sofocleous C, Stiakaki E, Polychronopoulou S, Economou M, Kossiva L, Kostaridou S, Kattamis A. 2014. Clinical phenotype and genetic analysis of RPS19, RPL5, and RPL11 genes in Greek patients with Diamond Blackfan Anemia. Pediatr Blood Cancer 61: 2249–2255. [DOI] [PubMed] [Google Scholar]
- Devotta A, Juraver-Geslin H, Gonzalez JA, Hong CS, Saint-Jeannet JP. 2016. Sf3b4-depleted Xenopus embryos: A model to study the pathogenesis of craniofacial defects in Nager syndrome. Dev Biol 415: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz HC. 2010. New therapeutic approaches to mendelian disorders. N Engl J Med 363: 852–863. [DOI] [PubMed] [Google Scholar]
- Dixon J, Jones NC, Sandell LL, Jayasinghe SM, Crane J, Rey JP, Dixon MJ, Trainor PA. 2006. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci U S A 103: 13403–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty L, Sheen MR, Vlachos A, Choesmel V, O’Donohue MF, Clinton C, Schneider HE, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Glader B, Arceci RJ, Farrar JE, Atsidaftos E, Lipton JM, Gleizes PE, Gazda HT. 2010. Ribosomal protein genes RPS10 and RPS26 are commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet 86: 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham EL, Howie RN, Cray JJ. 2017. Gene/environment interactions in craniosynostosis: A brief review. Orthod Craniofac Res 20 Suppl 1: 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SJ, Gladwin AJ, Dixon MJ. 1997. The mutational spectrum in Treacher Collins syndrome reveals a predominance of mutations that create a premature-termination codon. Am J Hum Genet 60: 515–524. [PMC free article] [PubMed] [Google Scholar]
- Eldar A, Chary VK, Xenopoulos P, Fontes ME, Loson OC, Dworkin J, Piggot PJ, Elowitz MB. 2009. Partial penetrance facilitates developmental evolution in bacteria. Nature 460: 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar A, Elowitz MB. 2010. Functional roles for noise in genetic circuits. Nature 467: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Laggerbauer B, Lauber J, Lane WS, Luhrmann R. 1997. An evolutionarily conserved U5 snRNP-specific protein is a GTP-binding factor closely related to the ribosomal translocase EF-2. EMBO J 16: 4092–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro FP, Alvizi L, Zechi-Ceide RM, Bertola D, Felix TM, de Souza J, Raskin S, Twigg SR, Weiner AM, Armas P, Margarit E, Calcaterra NB, Andersen GR, McGowan SJ, Wilkie AO, Richieri-Costa A, de Almeida ML, Passos-Bueno MR. 2014. A noncoding expansion in EIF4A3 causes Richieri-Costa-Pereira syndrome, a craniofacial disorder associated with limb defects. Am J Hum Genet 94: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazen LE, Elmore J, Nadler HL. 1967. Mandibulo-facial dysostosis. (Treacher-Collins syndrome). Am J Dis Child 113: 405–410. [DOI] [PubMed] [Google Scholar]
- Fish JL. 2016. Developmental mechanisms underlying variation in craniofacial disease and evolution. Dev Biol 415: 188–197. [DOI] [PubMed] [Google Scholar]
- Fish JL, Villmoare B, Kobernick K, Compagnucci C, Britanova O, Tarabykin V, Depew MJ. 2011. Satb2, modularity, and the evolvability of the vertebrate jaw. Evol Dev 13: 549–564. [DOI] [PubMed] [Google Scholar]
- Flygare J, Aspesi A, Bailey JC, Miyake K, Caffrey JM, Karlsson S, Ellis SR. 2007. Human RPS19, the gene mutated in Diamond-Blackfan anemia, encodes a ribosomal protein required for the maturation of 40S ribosomal subunits. Blood 109: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazda HT, Sheen MR, Vlachos A, Choesmel V, O’Donohue MF, Schneider H, Darras N, Hasman C, Sieff CA, Newburger PE, Ball SE, Niewiadomska E, Matysiak M, Zaucha JM, Glader B, Niemeyer C, Meerpohl JJ, Atsidaftos E, Lipton JM, Gleizes PE, Beggs AH. 2008. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet 83: 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales B, Henning D, So RB, Dixon J, Dixon MJ, Valdez BC. 2005. The Treacher Collins syndrome (TCOF1) gene product is involved in pre-rRNA methylation. Hum Mol Genet 14: 2035–2043. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Petit F, Oufadem M, Decaestecker C, Jourdain AS, Andrieux J, Malan V, Alessandri JL, Baujat G, Baumann C, Boute-Benejean O, Caumes R, Delobel B, Dieterich K, Gaillard D, Gonzales M, Lacombe D, Escande F, Manouvrier-Hanu S, Marlin S, Mathieu-Dramard M, Mehta SG, Simonic I, Munnich A, Vekemans M, Porchet N, de Pontual L, Sarnacki S, Attie-Bitach T, Lyonnet S, Holder-Espinasse M, Amiel J. 2012. EFTUD2 haploinsufficiency leads to syndromic oesophageal atresia. J Med Genet 49: 737–746. [DOI] [PubMed] [Google Scholar]
- Green B, Nikkhah D, Cobb AR, Dunaway DJ. 2013. Craniofacial disorders that have phenotypic overlap with Treacher Collins syndrome. J Plast Reconstr Aesthet Surg 66: e234–235. [DOI] [PubMed] [Google Scholar]
- Green RM, Fish JL, Young NM, Smith FJ, Roberts B, Dolan K, Choi I, Leach CL, Gordon P, Cheverud JM, Roseman CC, Williams TJ, Marcucio RS, Hallgrimsson B. 2017. Developmental nonlinearity drives phenotypic robustness. Nat Commun 8: 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Curry C, Olney AH, Sandoval C, Fisher J, Chong JX, Genomics UWCfM, Pilchman L, Sahraoui R, Stabley DL, Sol-Church K. 2014. Diamond-Blackfan anemia with mandibulofacial dystostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. Am J Med Genet A 164A: 2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso AR, Gomes AQ, Barbosa-Morais NL, Caldeira S, Thorne NP, Grech G, von Lindern M, Carmo-Fonseca M. 2008. Tissue-specific splicing factor gene expression signatures. Nucleic Acids Res 36: 4823–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueroussov S, Weatheritt RJ, O’Hanlon D, Lin ZY, Narula A, Gingras AC, Blencowe BJ. 2017. Regulatory Expansion in Mammals of Multivalent hnRNP Assemblies that Globally Control Alternative Splicing. Cell 170: 324–339 e323. [DOI] [PubMed] [Google Scholar]
- Guion-Almeida ML, Zechi-Ceide RM, Vendramini S, Tabith Junior A. 2006. A new syndrome with growth and mental retardation, mandibulofacial dysostosis, microcephaly, and cleft palate. Clin Dysmorphol 15: 171–174. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. 2009. Deciphering the Palimpsest: Studying the Relationship Between Morphological Integration and Phenotypic Covariation. Evol Biol 36: 355–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Mio W, Marcucio RS, Spritz R. 2014. Let’s face it--complex traits are just not that simple. PLoS Genet 10: e1004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele A, Kamburov A, Grossmann A, Sourlis C, Wowro S, Weimann M, Will CL, Pena V, Luhrmann R, Stelzl U. 2012. Dynamic protein-protein interaction wiring of the human spliceosome. Mol Cell 45: 567–580. [DOI] [PubMed] [Google Scholar]
- Henras AK, Plisson-Chastang C, O’Donohue MF, Chakraborty A, Gleizes PE. 2015. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip Rev RNA 6: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Krauss RS. 2012. Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice. PLoS Genet 8: e1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Krauss RS. 2017. Ethanol itself is a holoprosencephaly-inducing teratogen. PLoS One 12: e0176440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Srivastava K, Kim S, Allen BL, Leahy DJ, Hu P, Roessler E, Krauss RS, Muenke M. 2017. BOC is a modifier gene in holoprosencephaly. Hum Mutat 38: 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, Dixon MJ, Trainor PA. 2008. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med 14: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice CM, Yagnik G, Kim Y, Peter I, Jabs EW, Erazo M, Ye X, Ainehsazan E, Shi L, Cunningham ML, Kimonis V, Roscioli T, Wall SA, Wilkie AO, Stoler J, Richtsmeier JT, Heuze Y, Sanchez-Lara PA, Buckley MF, Druschel CM, Mills JL, Caggana M, Romitti PA, Kay DM, Senders C, Taub PJ, Klein OD, Boggan J, Zwienenberg-Lee M, Naydenov C, Kim J, Wilson AF, Boyadjiev SA. 2012. A genome-wide association study identifies susceptibility loci for nonsyndromic sagittal craniosynostosis near BMP2 and within BBS9. Nat Genet 44: 1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BM, Corman TS, Lovelace K, Hong M, Krauss RS, Epstein DJ. 2017. Prenatal ethanol exposure in mice phenocopies Cdon mutation by impeding Shh function in the etiology of optic nerve hypoplasia. Dis Model Mech 10: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Ahn HS, Back HJ, Cho B, Choi EJ, Chung NG, Hwang PH, Jeoung DC, Kang HJ, Kim H, Ko KN, Koo HH, Kook H, Lee KC, Lim HJ, Lim YT, Lyu CJ, Park JE, Park KD, Park SK, Ryu KH, Seo JJ, Shin HY, Sung KW, Yoo ES. 2012. Clinical and hematologic manifestations in patients with Diamond Blackfan anemia in Korea. Korean J Hematol 47: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Mishina Y. 2016. An epistatic explanation. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriventseva EV, Koch I, Apweiler R, Vingron M, Bork P, Gelfand MS, Sunyaev S. 2003. Increase of functional diversity by alternative splicing. Trends Genet 19: 124–128. [DOI] [PubMed] [Google Scholar]
- Larsen DH, Hari F, Clapperton JA, Gwerder M, Gutsche K, Altmeyer M, Jungmichel S, Toledo LI, Fink D, Rask MB, Grofte M, Lukas C, Nielsen ML, Smerdon SJ, Lukas J, Stucki M. 2014. The NBS1-Treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat Cell Biol 16: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehalle D, Wieczorek D, Zechi-Ceide RM, Passos-Bueno MR, Lyonnet S, Amiel J, Gordon CT. 2015. A review of craniofacial disorders caused by spliceosomal defects. Clin Genet 88: 405–415. [DOI] [PubMed] [Google Scholar]
- Lei Q, Li C, Zuo Z, Huang C, Cheng H, Zhou R. 2016. Evolutionary Insights into RNA trans-Splicing in Vertebrates. Genome Biol Evol 8: 562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kim SG, Blenis J. 2014. Rapamycin: one drug, many effects. Cell Metab 19: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Yang HT, Zdanowicz M, Sicklick JK, Qi Y, Camp TJ, Diehl AM. 2007. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest 87: 231–240. [DOI] [PubMed] [Google Scholar]
- Lines MA, Huang L, Schwartzentruber J, Douglas SL, Lynch DC, Beaulieu C, Guion-Almeida ML, Zechi-Ceide RM, Gener B, Gillessen-Kaesbach G, Nava C, Baujat G, Horn D, Kini U, Caliebe A, Alanay Y, Utine GE, Lev D, Kohlhase J, Grix AW, Lohmann DR, Hehr U, Bohm D, Consortium FC, Majewski J, Bulman DE, Wieczorek D, Boycott KM. 2012. Haploinsufficiency of a spliceosomal GTPase encoded by EFTUD2 causes mandibulofacial dysostosis with microcephaly. Am J Hum Genet 90: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, van der Lijn F, Schurmann C, Zhu G, Chakravarty MM, Hysi PG, Wollstein A, Lao O, de Bruijne M, Ikram MA, van der Lugt A, Rivadeneira F, Uitterlinden AG, Hofman A, Niessen WJ, Homuth G, de Zubicaray G, McMahon KL, Thompson PM, Daboul A, Puls R, Hegenscheid K, Bevan L, Pausova Z, Medland SE, Montgomery GW, Wright MJ, Wicking C, Boehringer S, Spector TD, Paus T, Martin NG, Biffar R, Kayser M. 2012. A genome-wide association study identifies five loci influencing facial morphology in Europeans. PLoS Genet 8: e1002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, Ellis SR. 2006. Ribosomes and marrow failure: coincidental association or molecular paradigm? Blood 107: 4583–4588. [DOI] [PubMed] [Google Scholar]
- Loewe L, Hill WG. 2010. The population genetics of mutations: good, bad and indifferent. Philos Trans R Soc Lond B Biol Sci 365: 1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DC, Revil T, Schwartzentruber J, Bhoj EJ, Innes AM, Lamont RE, Lemire EG, Chodirker BN, Taylor JP, Zackai EH, McLeod DR, Kirk EP, Hoover-Fong J, Fleming L, Savarirayan R, Care4Rare C, Majewski J, Jerome-Majewska LA, Parboosingh JS, Bernier FP. 2014. Disrupted auto-regulation of the spliceosomal gene SNRPB causes cerebro-costo-mandibular syndrome. Nat Commun 5: 4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC. 2014. Epistasis and Quantitative Traits: Using Model Organisms to Study Gene-Gene Interactions. Nature reviews. Genetics 15: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Collins FS. 2009. The HapMap and genome-wide association studies in diagnosis and therapy. Annu Rev Med 60: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Young NM, Hu D, Hallgrimsson B. 2011. Mechanisms that underlie co-variation of the brain and face. Genesis 49: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Tenney J, Duran I, Martin J, Nevarez L, Pogue R, Krakow D, Cohn DH, Li B. 2016. Altered mRNA Splicing, Chondrocyte Gene Expression and Abnormal Skeletal Development due to SF3B4 Mutations in Rodriguez Acrofacial Dysostosis. PLoS Genet 12: e1006307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DE, Soulard A, Hall MN. 2004. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell 119: 969–979. [DOI] [PubMed] [Google Scholar]
- Miller CT, Swartz ME, Khuu PA, Walker MB, Eberhart JK, Kimmel CB. 2007. mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev Biol 308: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EW, Green R. 2017. Ribosomopathies: There’s strength in numbers. Science 358. [DOI] [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, Robin NH, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, et al. 1994. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet 8: 269–274. [DOI] [PubMed] [Google Scholar]
- Munding EM, Shiue L, Katzman S, Donohue JP, Ares M Jr. 2013. Competition between pre-mRNAs for the splicing machinery drives global regulation of splicing. Mol Cell 51: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JH. 2001. Modifier genes in mice and humans. Nature Reviews Genetics 2: 165. [DOI] [PubMed] [Google Scholar]
- Nichols JT, Blanco-Sanchez B, Brooks EP, Parthasarathy R, Dowd J, Subramanian A, Nachtrab G, Poss KD, Schilling TF, Kimmel CB. 2016. Ligament versus bone cell identity in the zebrafish hyoid skeleton is regulated by mef2ca. Development 143: 4430–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JT, Pan L, Moens CB, Kimmel CB. 2013. barx1 represses joints and promotes cartilage in the craniofacial skeleton. Development 140: 2765–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TW, Graveley BR. 2010. Expansion of the eukaryotic proteome by alternative splicing. Nature 463: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanian TG, Waldman T. 2004. Interaction of the BMPR-IA tumor suppressor with a developmentally relevant splicing factor. Biochem Biophys Res Commun 323: 91–97. [DOI] [PubMed] [Google Scholar]
- Noack Watt KE, Achilleos A, Neben CL, Merrill AE, Trainor PA. 2016. The Roles of RNA Polymerase I and III Subunits Polr1c and Polr1d in Craniofacial Development and in Zebrafish Models of Treacher Collins Syndrome. PLoS Genet 12: e1006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates AC. 2011. What’s all the noise about developmental stochasticity? Development 138: 601–607. [DOI] [PubMed] [Google Scholar]
- Pacheco TR, Moita LF, Gomes AQ, Hacohen N, Carmo-Fonseca M. 2006. RNA interference knockdown of hU2AF35 impairs cell cycle progression and modulates alternative splicing of Cdc25 transcripts. Mol Biol Cell 17: 4187–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagon RA, Graham JM Jr., Zonana J, Yong SL. 1981. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr 99: 223–227. [DOI] [PubMed] [Google Scholar]
- Parsons TE, Kristensen E, Hornung L, Diewert VM, Boyd SK, German RZ, Hallgrimsson B. 2008. Phenotypic variability and craniofacial dysmorphology: increased shape variance in a mouse model for cleft lip. J Anat 212: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival CJ, Marangoni P, Tapaltsyan V, Klein O, Hallgrímsson B. 2017. The Interaction of Genetic Background and Mutational Effects in Regulation of Mouse Craniofacial Shape. G3: Genes|Genomes|Genetics 7: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit F, Escande F, Jourdain AS, Porchet N, Amiel J, Doray B, Delrue MA, Flori E, Kim CA, Marlin S, Robertson SP, Manouvrier-Hanu S, Holder-Espinasse M. 2014. Nager syndrome: confirmation of SF3B4 haploinsufficiency as the major cause. Clin Genet 86: 246–251. [DOI] [PubMed] [Google Scholar]
- Phelps PD, Poswillo D, Lloyd GA. 1981. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome). Clin Otolaryngol Allied Sci 6: 15–28. [DOI] [PubMed] [Google Scholar]
- Reuter K, Nottrott S, Fabrizio P, Luhrmann R, Ficner R. 1999. Identification, characterization and crystal structure analysis of the human spliceosomal U5 snRNP-specific 15 kD protein. J Mol Biol 294: 515–525. [DOI] [PubMed] [Google Scholar]
- Pilon N 2016. Pigmentation-based insertional mutagenesis is a simple and potent screening approach for identifying neurocristopathy-associated genes in mice. Rare Dis 4: e1156287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto A, Schmelter R, VandeBerg JL, Marroig G, Cheverud JM. 2016. Evolution of the Genotype-to-Phenotype Map and the Cost of Pleiotropy in Mammals. Genetics 204: 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinon A, Molchadsky A, Nathan E, Yovel G, Rotter V, Sarig R, Tzahor E. 2011. p53 coordinates cranial neural crest cell growth and epithelial-mesenchymal transition/delamination processes. Development 138: 1827–1838. [DOI] [PubMed] [Google Scholar]
- Riordan JD, Nadeau JH. 2017. From Peas to Disease: Modifier Genes, Network Resilience, and the Genetics of Health. Am J Hum Genet 101: 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Muenke M. 2010. The molecular genetics of holoprosencephaly. Am J Med Genet C Semin Med Genet 154C: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Russo G. 2017. Ribosomal Proteins Control or Bypass p53 during Nucleolar Stress. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Ghazvinian R, Do R, Thiru P, Vergilio JA, Beggs AH, Sieff CA, Orkin SH, Nathan DG, Lander ES, Gazda HT. 2012. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest 122: 2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. 2008. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol 9: 557–568. [DOI] [PubMed] [Google Scholar]
- Schulz Y, Wehner P, Opitz L, Salinas-Riester G, Bongers EM, van Ravenswaaij-Arts CM, Wincent J, Schoumans J, Kohlhase J, Borchers A, Pauli S. 2014. CHD7, the gene mutated in CHARGE syndrome, regulates genes involved in neural crest cell guidance. Hum Genet 133: 997–1009. [DOI] [PubMed] [Google Scholar]
- Seppala M, Xavier GM, Fan CM, Cobourne MT. 2014. Boc modifies the spectrum of holoprosencephaly in the absence of Gas1 function. Biol Open 3: 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Borger C, More H, Sturmbauer C. 2017. The Role of Alternative Splicing and Differential Gene Expression in Cichlid Adaptive Radiation. Genome Biol Evol 9: 2764–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Bear KA, Wyllie A, Keaton AA, Dubourg C, David V, Mercier S, Odent S, Hehr U, Paulussen A, Clegg NJ, Delgado MR, Bale SJ, Lacbawan F, Ardinger HH, Aylsworth AS, Bhengu NL, Braddock S, Brookhyser K, Burton B, Gaspar H, Grix A, Horovitz D, Kanetzke E, Kayserili H, Lev D, Nikkel SM, Norton M, Roberts R, Saal H, Schaefer GB, Schneider A, Smith EK, Sowry E, Spence MA, Shalev SA, Steiner CE, Thompson EM, Winder TL, Balog JZ, Hadley DW, Zhou N, Pineda-Alvarez DE, Roessler E, Muenke M. 2012. Genotypic and phenotypic analysis of 396 individuals with mutations in Sonic Hedgehog. J Med Genet 49: 473–479. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell 10: 647–656. [DOI] [PubMed] [Google Scholar]
- Terrazas K, Dixon J, Trainor PA, Dixon MJ. 2017. Rare syndromes of the head and face: mandibulofacial and acrofacial dysostoses. Wiley Interdiscip Rev Dev Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Moller AP. 1997. Developmental stability, disease and medicine. Biol Rev Camb Philos Soc 72: 497–548. [DOI] [PubMed] [Google Scholar]
- Timberlake AT, Choi J, Zaidi S, Lu Q, Nelson-Williams C, Brooks ED, Bilguvar K, Tikhonova I, Mane S, Yang JF, Sawh-Martinez R, Persing S, Zellner EG, Loring E, Chuang C, Galm A, Hashim PW, Steinbacher DM, DiLuna ML, Duncan CC, Pelphrey KA, Zhao H, Persing JA, Lifton RP. 2016. Two locus inheritance of non-syndromic midline craniosynostosis via rare SMAD6 and common BMP2 alleles. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake AT, Wu R, Nelson-Williams C, Furey CG, Hildebrand KI, Elton SW, Wood JS, Persing JA, Lifton RP. 2018. Co-occurrence of frameshift mutations in SMAD6 and TCF12 in a child with complex craniosynostosis. Hum Genome Var 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley M, Lynch D, Bernier F, Parboosingh J, Bhoj E, Zackai E, Calder A, Itasaki N, Wakeling E, Scott R, Lees M, Clayton-Smith J, Blyth M, Morton J, Shears D, Kini U, Homfray T, Clarke A, Barnicoat A, Wallis C, Hewitson R, Offiah A, Saunders M, Langton-Hewer S, Hilliard T, Davis P, Smithson S. 2016. Cerebro-costo-mandibular syndrome: Clinical, radiological, and genetic findings. Am J Med Genet A 170A: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Tompa P, Schad E, Tantos A, Kalmar L. 2015. Intrinsically disordered proteins: emerging interaction specialists. Curr Opin Struct Biol 35: 49–59. [DOI] [PubMed] [Google Scholar]
- Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. 2004. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci U S A 101: 10709–10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nostrand JL, Brady CA, Jung H, Fuentes DR, Kozak MM, Johnson TM, Lin CY, Lin CJ, Swiderski DL, Vogel H, Bernstein JA, Attie-Bitach T, Chang CP, Wysocka J, Martin DM, Attardi LD. 2014. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature 514: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M, Genevieve D, Ostertag A, Marlin S, Lacombe D, Martin-Coignard D, Coubes C, David A, Lyonnet S, Vilain C, Dieux-Coeslier A, Manouvrier S, Isidor B, Jacquemont ML, Julia S, Layet V, Naudion S, Odent S, Pasquier L, Pelras S, Philip N, Pierquin G, Prieur F, Aboussair N, Attie-Bitach T, Baujat G, Blanchet P, Blanchet C, Dollfus H, Doray B, Schaefer E, Edery P, Giuliano F, Goldenberg A, Goizet C, Guichet A, Herlin C, Lambert L, Leheup B, Martinovic J, Mercier S, Mignot C, Moutard ML, Perez MJ, Pinson L, Puechberty J, Willems M, Randrianaivo H, Szakszon K, Toutain A, Verloes A, Vigneron J, Sanchez E, Sarda P, Laplanche JL, Collet C. 2016. Treacher Collins syndrome: a clinical and molecular study based on a large series of patients. Genet Med 18: 49–56. [DOI] [PubMed] [Google Scholar]
- Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. 2004. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36: 955–957. [DOI] [PubMed] [Google Scholar]
- Waddington CH. 1942. Canalization of development and the inheritance of acquired characteristics. Nature 150:563–565. [Google Scholar]
- Watanabe H, Shionyu M, Kimura T, Kimata K, Watanabe H. 2007. Splicing factor 3b subunit 4 binds BMPR-IA and inhibits osteochondral cell differentiation. J Biol Chem 282: 20728–20738. [DOI] [PubMed] [Google Scholar]
- Wieczorek D 2013. Human facial dysostoses. Clin Genet 83: 499–510. [DOI] [PubMed] [Google Scholar]
- Wieczorek D, Newman WG, Wieland T, Berulava T, Kaffe M, Falkenstein D, Beetz C, Graf E, Schwarzmayr T, Douzgou S, Clayton-Smith J, Daly SB, Williams SG, Bhaskar SS, Urquhart JE, Anderson B, O’Sullivan J, Boute O, Gundlach J, Czeschik JC, van Essen AJ, Hazan F, Park S, Hing A, Kuechler A, Lohmann DR, Ludwig KU, Mangold E, Steenpass L, Zeschnigk M, Lemke JR, Lourenco CM, Hehr U, Prott EC, Waldenberger M, Bohmer AC, Horsthemke B, O’Keefe RT, Meitinger T, Burn J, Ludecke HJ, Strom TM. 2014. Compound heterozygosity of low-frequency promoter deletions and rare loss-of-function mutations in TXNL4A causes Burn-McKeown syndrome. Am J Hum Genet 95: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CL, Luhrmann R. 2011. Spliceosome structure and function. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig TN, Draptchinskaia N, Dianzani I, Ball S, Niemeyer C, Ramenghi U, Orfali K, Gustavsson P, Garelli E, Brusco A, Tiemann C, Perignon JL, Bouchier C, Cicchiello L, Dahl N, Mohandas N, Tchernia G. 1999. Mutations in ribosomal protein S19 gene and diamond blackfan anemia: wide variations in phenotypic expression. Blood 94: 4294–4306. [PubMed] [Google Scholar]
- Yelick PC, Trainor PA. 2015. Ribosomopathies: Global process, tissue specific defects. Rare Dis 3: e1025185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. 2010. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development 137: 3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrimsson B, Marcucio RS. 2014. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development 141: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Zuo X, He M, Gao J, Fu Y, Qin C, Meng L, Wang W, Song Y, Cheng Y, Zhou F, Chen G, Zheng X, Wang X, Liang B, Zhu Z, Fu X, Sheng Y, Hao J, Liu Z, Yan H, Mangold E, Ruczinski I, Liu J, Marazita ML, Ludwig KU, Beaty TH, Zhang X, Sun L, Bian Z. 2017. Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nat Commun 8: 14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Layman WS, Martin DM, Scacheri PC. 2010. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A 152A: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, O’Leary MN, Peri S, Wang M, Zha J, Melov S, Kappes DJ, Feng Q, Rhodes J, Amieux PS, Morris DR, Kennedy BK, Wiest DL. 2017. Ribosomal Proteins Rpl22 and Rpl22l1 Control Morphogenesis by Regulating Pre-mRNA Splicing. Cell Rep 18: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kartiko S, Finnell RH. 2009. Importance of gene-environment interactions in the etiology of selected birth defects. Clin Genet 75: 409–423. [DOI] [PubMed] [Google Scholar]