INTRODUCTION
While advancements in left ventricular assist devices (LVAD) have led to improved survival1–5, complications associated with longterm exposure to continuous-flow (CF) circulatory support, such as gastrointestinal bleeding, pump thrombosis and stroke result in significant morbidity. Strokes affect 10% of patients in the first year of support alone.6
Management algorithms for CF-LVAD patients suffering from stroke have been published previously.7 In this article, we summarize the clinical burden of strokes placed in context of other complications, comorbidities, and medication-effects that work together in an almost synergistic fashion to increase the risk of stroke. Physiologic (mal)adaptations to continuous-flow circulatory support are reviewed with an emphasis on the arterial baroreceptor reflex, neurohumoral axis and implications on blood pressure control. Cerebral perfusion and autoregulatory processes in the setting of heart failure with reduced ejection fraction (HFrEF), hypertension and CF-LVAD support are discussed. Finally, we highlight important areas of future research that will advance our understanding of the physiology of this unique patient population, and pave the way for novel management strategies to prevent strokes in CF-LVAD patients.
CLINICAL OUTCOMES IN THE SETTING OF CF-LVADs
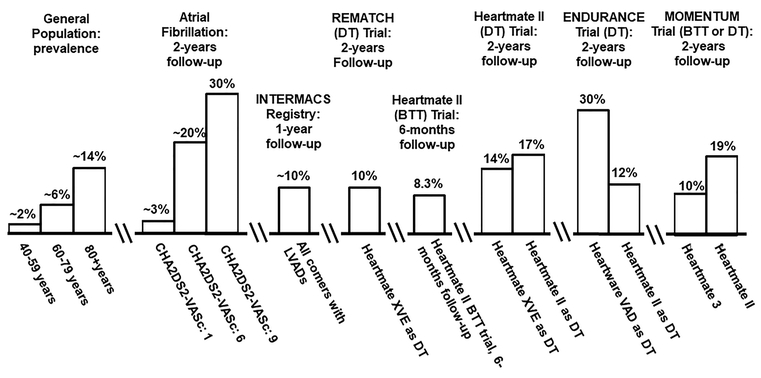
The rate of adverse events associated with CF-LVAD use – specifically, strokes – is high (figure 1), as 10% of individuals are affected by stroke in the first year of support alone.6, 9
Figure 1: Stroke Incidence in Major LVAD Trials.
CF-LVAD patients experience a high-stroke rate in comparison to other populations. Stroke rate among the general population derived from AHA Heart Disease and Stroke Statistics-2017 update.8 Two-year stroke rate among individuals with atrial fibrillation stratified by a CHA2DS2-VASc score of 1, 6 and 9 for reference. BTT: bridge-to-transplant; DT: destination therapy.
Furthermore, according to the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), between 6–24 months of support, stroke remains the primary cause of death.9 The stroke rate associated with pulsatile LVADs (now historical and replaced by CF-LVADs), was 4.35 times that of patients managed medically over two years.10 In the original trials evaluating the first CF-LVAD (the “Heartmate II”), the rate of disabling stroke was similar among individuals managed with the Heartmate II CF-LVAD v. pulsatile devices (17% v. 14%, respectively, P=0.56) over two years.2 The Heartware VAD (“HVAD”), a newer CF-LVAD currently in use, was associated with a high stroke rate compared to the. Heartmate II (29.7% v. 12.1%, P<0.001) over two-years.4 Finally, the newest CF-LVAD, the “Heartmate 3”, was found to have a much lower stroke rate than the Heartmate II device (10.1% v. 19.2%, P=0.02) over a two-year period.11 Thus, refinement of device technology has led to a reduction in the incidence of stroke among these patients compared to older pumps.
The INTERMACS registry defines ischemic stroke as a new acute neurologic deficit (or acute encephalopathy or seizures in children <6 months) of any duration associated with acute infarction on imaging corresponding anatomically to the clinical deficit.12 Acute symptomatic intracranial hemorrhage is defined as a new acute neurologic deficit (or acute encephalopathy or seizures in children <6 months) attributable to intracranial hemorrhage.12 Strokes among CF-LVAD patients may be either hemorrhagic or ischemic.13 According to the INTERMACS registry, there is a slight predominance of ischemic over hemorrhagic (51% v. 49%, respectively).6 In one large population-based analysis of 1813 patients, the annual incidence of ischemic and hemorrhagic strokes were 5.5% and 3.1%, respectively.14
Associations Between Stroke and Other Comorbidities During CF-LVAD Support
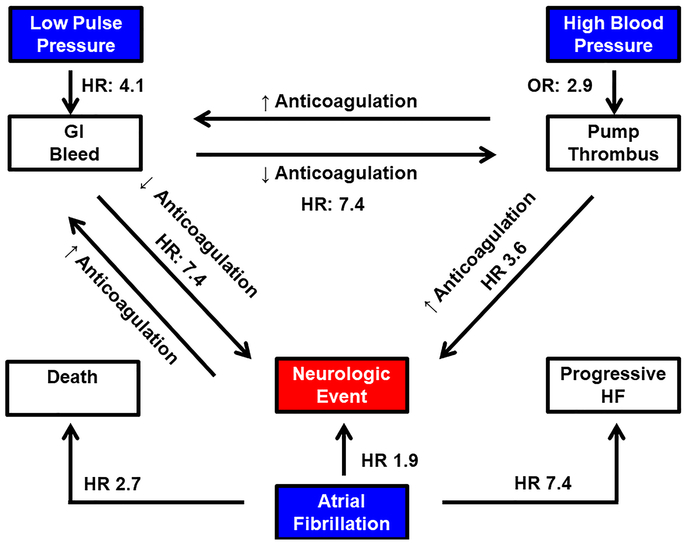
As conceptualized in figure 2, neurologic events are often precipitated by other adverse events commonly encountered with CF-LVAD support, such as nonsurgical/gastrointestinal bleeding, which affects almost one-third of CF-LVAD patients at 1 year15, or pump thrombosis, which impacts ~5% of patients at 1 year and 8% after two years.16 There is an inverse relationship between the degree of pulsatility and the rate of nonsurgical bleeding17, and the rate of thromboembolic events increases dramatically following a bleeding event18, likely related to a reduction in strength of anticoagulation. Uncontrolled blood pressure is associated with an increased risk of pump thrombosis.19, 20 The mechanism underlying this association between hypertension and pump thrombosis remains unclear, but may be related to an increase in shearing forces of blood products as they traverse the pump. After formation of a pump thrombus, the risk of a hemorrhagic event approximately triples likely due, at least in part, to an increase in anticoagulation intensity.16 The risk of thrombosis is greatest when mean arterial pressure exceeds 90mmHg.19 To minimize the risk of pump thrombosis related to hypertension, the International Society of Heart Lung Transplantation (ISHLT) recommends that pharmacologic therapy be implemented to maintain a MAP < 80mmHg.21
Figure 2: Life with a CF-LVAD: “Between a Rock and Hard Place”.
Minimally pulsatile/nonpulsatile flow increases the risk of nonsurgical bleeding by four-fold.17 Subsequent reductions in anticoagulation following a bleeding event are associated with more than a seven-fold increase in pump thrombosis and/or thromboembolic event.18 Uncontrolled blood pressure nearly triples the odds of pump thrombosis formation, which in turn, more than triples risk of a neurologic event.16 Atrial fibrillation doubles the risk of a neurologic event, and significantly increases the risk of progressive heart failure and death27, 28. GI: gastrointestinal; HF: heart failure; HR: hazard ratio; OR: odds ratio.
Device-related infections may affect up to one fourth of CF-LVAD patients.22, 23 Bloodstream infections increase the stroke risk, possibly as a result of formation of mycotic aneurysms from bacterial seeding.24 Finally, CF-LVAD use has been associated with an acquired von Willebrand Syndrome due to cleavage of large multimers by the metalloprotease ADAMTS-13, which predisposes these patients to bleeding.25
At least one fourth of patients with HFrEF have concomitant atrial fibrillation (AF) at the time of diagnosis.26 The presence of AF in the setting of LVAD support significantly impacts longterm outcomes, increasing the risk of progressive HF by 7-fold, and approximately doubling the risk of neurologic events and death.27, 28 The increase in risk of thromboembolic events is not necessarily due to inadequate anticoagulation. In one series, CF-LVAD patients with AF had higher international normalized ratios at the time of the event than patients without AF (2.70±0.94 v. 1.54±0.34, P=0.003) and for the four weeks preceding the event (2.33±0.65 v. 1.57±0.31, P=0.006). Thus, AF, substantially increases the risk of stroke.
EXTRACRANIAL IMPLICATIONS OF CONTINUOUS-FLOW CIRCULATORY SUPPORT
Arterial Baroreceptors and the Neurohumoral Axis
Animal models have demonstrated that sympathetic neural activity (SNA) is regulated on a beat-to-beat basis by pulsatile distension of arterial baroreceptors in concert with the normal cardiac cycle, such that expansion of the receptors (e.g. during systole) leads to a reduction in sympathetic tone, while recoiling of the receptors (e.g. during diastole, or in instances of hypotension) leads to an upregulation of sympathetic activity.29–32 This inverse relationship between pulsatility and sympathetic tone also exists in humans, as demonstrated by microneurography studies to quantify muscle SNA (MSNA) levels in patients with CF-LVADs, and pulsatile pumps.33, 34 The degree of sympathetic activation associated with CF-LVAD support is quite profound, as CF-LVAD patients have supine levels of sympathetic activity that exceed levels observed in normal individuals in an upright position (norepinephrine 536±333pg/ml for CF-LVAD patients lying supine v. 341±131pg/ml for healthy controls at a 60 degree head-up tilt position).34 This degree of neurohumoral activation appears to be achieved through unloading of the arterial baroreceptors as a result of a reduction in pulsatily.33, 34
Blood Pressure Considerations in the Setting of CF-LVAD Support
The development of hypertension among CF-LVAD patients is multifactorial. First, sympathetic overdrive increases total peripheral resistance (TPR) and contributes to development/worsening of clinical hypertension.35 In addition, diastolic BP is higher among CF-LVAD patients than normal individuals33, 34, 36 since pump flow is continuous and not gated to the cardiac cycle. The net effect is an increase in mean arterial pressure (MAP) and reduced pulse pressure.
Hypertensive individuals have greater blood pressure variability (BPV) than normotensive persons37, and the risk of end-organ damage38 and cardiovascular-related mortality rise in proportion to BPV.39 Spontaneous oscillations in BP occur as a result of respirations (“high-frequency” oscillations occurring at approximately 0.2Hz in humans) and fluctuations in sympathetic tone (“low-frequency” oscillations occurring at 0.05–0.2Hz).40 These sympathetic-mediated oscillations are responses to random perturbations of the cardiovascular system that would otherwise disrupt normal homeostasis.41 For example, reductions in central blood volume (through postural changes) activate low-frequency oscillations in sympathetic tone to maintain arterial perfusion.42 These spontaneous low-frequency BP oscillations also stimulate endogenous nitric-oxide release and contribute to normal end-organ function.43, 44 While low-frequency oscillations in sympathetic activity may be preserved in HFrEF45, it is unknown whether they occur following implantation of a CF-LVAD.
INTRACRANIAL CONSIDERATIONS OF CONTINUOUS-FLOW CIRCULATORY SUPPORT
Cerebral Autoregulation
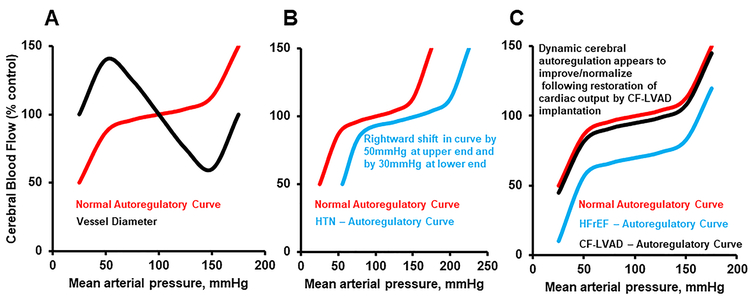
Autoregulatory processes ensure that cerebral perfusion is maintained amidst dynamic fluctuations in arterial perfusion pressure (figure 3). Animal models46 and human studies47, 48 have demonstrated that dynamic autoregulation operates within a period of several seconds, to ensure that cerebral blood flow (CBF) is maintained across a spectrum of perfusion pressures. The mechanism is multifactorial and includes both myogenic/vascular and neurogenic components.46, 49 The myogenic arm is intrinsic to vascular smooth muscle cells, and causes vessel diameter to constrict and dilate in response to increases and decreases in perfusion pressure.50 The neurogenic component refers to the very rich supply of sympathetic nerve fibers to cerebral blood vessels51, which mediate changes in vessel diameter in response to fluctuations in cerebral perfusion pressure.
Figure 3: Cerebral Autoregulatory Curves in Health and Disease.
(A): normal autoregulatory curve demonstrating change in cerebral blood flow (red) and cerebral vessel diameter (black) in response to changes in arterial perfusion pressure. (B): rightward shift in autoregulatory curve (blue) in the setting of chronic hypertension. (C): downward shift in autoregulatory curve in setting of HFrEF (blue), and improvement/normalization following CF-LVAD implantation (black).
Influence of Hypertension on Cerebral Autoregulation
Because CF-LVAD patients are predisposed to uncontrolled blood pressure, it is important to consider the implications of hypertension on the autoregulatory curve. As demonstrated in figure 3, chronic hypertension has two primary effects on cerebral autoregulation: 1) a rightward shift in the upper end of the autoregulatory plateau52; and 2) a reduction in maximal dilator capacity of the cerebral vasculature.53, 54 Animal models have demonstrated that this rightward shift may be as much as 50mmHg, and results from arteriolar constriction and hypertrophy of both large and small cerebral arterioles (according to the Law of Laplace), with a resultant increase in vascular resistance.54 This rightward shift protects the blood brain barrier55 and prevents vessels from overdistension in the setting of hypertension54, ensuring that CBF remains (relatively) unchanged amidst elevated blood pressure levels that would otherwise result in passive vasodilatation in normotensive persons.52 At the lower end of the autoregulatory curve, reductions in CBF may occur at higher levels of arterial pressure due to impaired cerebral vasodilatation in the setting of vessel hypertrophy.52 Hypertensive animal models have demonstrated that the lower-end of the autoregulatory curve may be rightward shifted by up to 30mmHg.54
The second major effect of hypertension on autoregulation is a reduction in maximal vasodilatory capacity of cerebral arterioles.52 In animal models, cerebrovascular resistance is higher among hypertensive compared to normotensive animals during iatrogenic seizures that force cerebral vessels to maximally dilate.53 The result is a blunted increase in CBF.52 The mechanism, is at least in part, related to vessel wall hypertrophy with an increase in cerebrovascular resistance.52, 56 Additionally, hypertension compromises the vasodilatory response to vasoactive substances such as acetylcholine, adenosine 5’-diphosphate (ADP) and bradykinin.57–59 It has also been suggested that platelets may initiate a vasoconstrictor response in the presence of chronic hypertension, thereby predisposing patients to ischemia and stroke.52
Influence of HFrEF on Cerebral Autoregulation
It is well established that HFrEF is associated with an increased risk of stroke.60–62 Functional limitations, specifically related to abnormalities in dynamic cerebral autoregulation, occur among individuals with HFrEF, which may result from a downward shift in the autoregulatory curve (figure 3).63, 64 Using transfer function analysis to determine the impact of BP fluctuations on CBF, HFrEF patients had an impaired autoregulatory index (a measure of the degree of change in middle cerebral arterial velocity [MCAV] for any change in MAP)63, indicating that dynamic cerebral autoregulation was significantly reduced.
Cerebral Autoregulation following CF-LVAD Implantation
There are no studies evaluating changes in cerebral autoregulation prior to and following CF-LVAD implantation. However, two studies have demonstrated that static65 and dynamic48 cerebral autoregulation are normal among CF-LVAD patients. In cat models, it was shown that sympathetic stimulation attenuates the increase in cerebral blood flow66, and reduces the degree of disruption in the blood brain barrier that otherwise results from sudden increases in arterial pressure.67 Thus, the heightened sympathetic tone among CF-LVAD patients might play a protective role. Given the strong association between uncontrolled BP and stroke68, these data collectively reinforce the importance of BP control in this population to minimize the risk of adverse cerebrovascular events.
Microembolic Events During CF-LVAD Support
Transcranial Doppler (TCD) has been used to quantify the burden of cerebral microemboli among patients with valve prostheses, intracardiac shunts and during cardiac procedures/surgeries69, through detection of “microembolic signals” (MES). Among patients supported with earlier devices that are no longer in use (both pulsatile and CF-LVADs), a high prevalence of MES was reported.70, 71 Specifically, patients with pulsatile LVADs experienced, on average, 2.3±9.2 MES per 30-minute monitoring period.70 Among CF-LVAD patients, 35% experienced cerebral microemboli, with a mean count of 81±443 MES per hour.71 Interestingly, the MES burden with the CF-LVADs declined with supplemental oxygen.71, 72 Within the confines of this limited experience, it was suggested that microemboli from pulsatile pumps were solid, while those from CF-LVADs are predominantly gaseous and formed through cavitation.73 However, solid and gaseous microemboli alike can be detrimental to brain structure and function.74 For example, among individuals who underwent heart valve replacement with mechanical or biologic prostheses, the odds ratio for stroke, transient ischemic attack or amaurosis fugax was increased among individuals with solid microemboli on TCD assessed one year following surgery.75 Similarly, during carotid stenting and endarterectomy, perioperative solid and gaseous microemboli alike were associated with ipsilateral ischemic strokes and/or new ipsilateral lesions on diffusion-weighted cerebral MRI.74
CONCLUSION AND FUTURE RESEARCH DIRECTIONS
There remain several areas of uncertainty that must be addressed in order to reduce the stroke risk in this population and improve outcomes. First, while survival among HFrEF patients is closely related to the degree of sympathetic overactivity76, 77, the extent to which sympathetic overactivity contributes to outcomes in the CF-LVAD population is unclear. In addition, the degree to which the autonomic nervous system exerts control over BPV and spontaneous oscillations in blood pressure is unknown, since the (denervated) CF-LVAD has no role in the baroreceptor feedback loop. In the setting of CF-LVAD support, the absence of low-frequency BP oscillations may increase the risk of end-organ dysfunction.43, 44
Studies in animals78 and humans79–81 have repeatedly found that cerebral perfusion is reduced among HFrEF patients. The magnitude of impairment in CBF is proportional to B-type natriuretic peptide levels82, New York Heart Association functional classification82, 83, and ejection fraction.83 While there are no studies directly evaluating CBF prior to and following LVAD implantation, our group previously found that supine resting MCAV, among both CF-LVAD and pulsatile LVAD patients, was comparable to healthy controls.48 This finding indirectly suggests that CBF, which is reduced in the setting of HFrEF, normalizes following CF-LVAD implantation, at least under resting conditions – however, longitudinal studies that assess patients prior to and following CF-LVAD implantation, are necessary to formally determine the degree improvement in CBF both at rest and with activity. Finally, regarding microemboli among CF-LVAD patients, there are no data regarding MES burden among patients supported by devices that are currently in use.
In conclusion, the rate of neurologic complications remains unacceptably high following CF-LVAD implantation and is a major source of morbidity and mortality. Several factors account for the high stroke rate, including patient comorbidities, medication-effects and pump thrombosis. However, minimally/entirely nonpulsatile flow imparts subtle yet consequential effects on autonomic processes and feedback loops, such as the arterial baroreceptor reflex pathway, which contribute to hypertension, which in turn, may adversely affect autoregulatory processes and disrupt cerebral homeostasis. Future research should focus on determining the extent to which abnormalities in autonomic reflexes contribute to stroke in HFrEF and CF-LVAD populations, define the microemboli burden among CF-LVAD devices currently in use, and finally, work towards development of biologically sensitive devices that are less disruptive to cardiac and cerebrovascular reflexes, with the ultimate goal of reducing stroke burden and preserving neurocognitive function.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Cornwell is supported by an NIH/NHLBI Mentored Patient-Oriented Research Career Development Award (#1K23HLI32048–01),the Susie and Kurt Lochmiller Distinguished Heart Transplant Fund, and the Clinical Translational Science Institute at the University of Colorado Anschutz Medical Campus. Dr. Ambardekar is supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program. Dr. Pal is supported by Medtronic Inc. Dr. Tarumi is supported by an NIH/NHLBI Pathway to Independence Award (5K99HL133449). Dr. Aaronson receives institutional contracted research support from Medtronic and Abbott, and is also a consultant for Medtronic.
Footnotes
DISCLOSURES AND CONFLICTS OF INTEREST: Dr. Pal acknowledges research support from Medtronic. Dr. Aaronson acknowledges research support from Medtronic, Abbott, and is a consultant for Medtronic.
CITATIONS:
- 1.Miller LW, Pagani FD, Russell SD, Ranjit J, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. New England Journal of Medicine. 2007:885–896 [DOI] [PubMed] [Google Scholar]
- 2.Slaughter M, Rogers J, Milano C, Russell S, Conte J, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. New England Journal of Medicine. 2009;361:2241–2251 [DOI] [PubMed] [Google Scholar]
- 3.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200 [DOI] [PubMed] [Google Scholar]
- 4.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. The New England journal of medicine. 2017;376:451–460 [DOI] [PubMed] [Google Scholar]
- 5.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr., Colombo PC, et al. A fully magnetically levitated circulatory pump for advanced heart failure. The New England journal of medicine. 2017; 376:440–450 [DOI] [PubMed] [Google Scholar]
- 6.Acharya D, Loyaga-Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, et al. Intermacs analysis of stroke during support with continuous-flow left ventricular assist devices: Risk factors and outcomes. JACC Heart Fail. 2017;5:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: Risk factors, diagnosis, and treatment. J Heart Lung Transplant. 2014;33:878–887 [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual intermacs report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086 [DOI] [PubMed] [Google Scholar]
- 10.Rose EA GA, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P. Long-term use of a left ventricular assist device for end-stage heart failure. New England Journal of Medicine. 2001;345:1435–1443 [DOI] [PubMed] [Google Scholar]
- 11.Mehra MR, Goldstein DJ, Uriel N, Cleveland JC, Yuzefpolskaya M, Salerno C, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. New England Journal of Medicine. 2018; 378: 1386–1395 [DOI] [PubMed] [Google Scholar]
- 12.INTERMACS. Intermacs user’s guide 2014. Available at: Https://www.Uab.Edu/medicine/intermacs/intermacs-documents. Accessed november 26, 2018.
- 13.Nassif ME, LaRue SJ, Raymer DS, Novak E, Vader JM, Ewarld GA, et al. Relationship between anticoagulation intensity and thrombotic or bleeding outcomes among outpatients with continuous-flow left ventricular assist devices. Circulation Heart Failure. 2016;9:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh NS, Cool J, Karas MG, Boehme AK, Kamel H. Stroke risk and mortality in patients with ventricular assist devices. Stroke. 2016;47:2702–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: Results from the roadmap study. Journal of the American College of Cardiology. 2015;66:1747–1761 [DOI] [PubMed] [Google Scholar]
- 16.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, et al. Interagency registry for mechanically assisted circulatory support (intermacs) analysis of pump thrombosis in the heartmate ii left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22 [DOI] [PubMed] [Google Scholar]
- 17.Wever-Pinzon O, Selzman CH, Drakos SG, Saidi A, Stoddard GJ, Gilbert EM, et al. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device heartmate ii. Circulation. Heart failure. 2013;6:517–526 [DOI] [PubMed] [Google Scholar]
- 18.Stulak JM, Lee D, Haft JW, Romano MA, Cowger JA, Park SJ, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014;33:60–64 [DOI] [PubMed] [Google Scholar]
- 19.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, et al. An analysis of pump thrombus events in patients in the heartware advance bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34 [DOI] [PubMed] [Google Scholar]
- 20.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. The New England journal of medicine. 2014;370:33–40 [DOI] [PubMed] [Google Scholar]
- 21.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 international society for heart and lung transplantation guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant. 2013;32:157–187 [DOI] [PubMed] [Google Scholar]
- 22.Gordon RJ, Weinberg AD, Pagani FD, Slaughter MS, Pappas PS, Naka Y, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Horo JC, Abu Saleh OM, Stulak JM, Wilhelm MP, Baddour LM, Rizwan Sohail M. Left ventricular assist device infections: A systematic review. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2018;64:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal A, Gupta A, Kumar S, Baumblatt JA, Pauwaa S, Gallagher C, et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO journal. 2012;58:509–513 [DOI] [PubMed] [Google Scholar]
- 25.Harvey L, Holley C, Roy SS, Eckman P, Cogswell R, Liao K, et al. Stroke after left ventricular assist device implantation: Outcomes in the continuous-flow era. Ann Thorac Surg. 2015;100:535–541 [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925 [DOI] [PubMed] [Google Scholar]
- 27.Stulak JM, Deo S, Schirger J, Aaronson KD, Park SJ, Joyce LD, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg. 2013;96:2161–2167 [DOI] [PubMed] [Google Scholar]
- 28.Enriquez AD, Calenda B, Gandhi PU, Nair AP, Anyanwu AC, Pinney SP. Clinical impact of atrial fibrillation in patients with the heartmate ii left ventricular assist device. Journal of the American College of Cardiology. 2014;64:1883–1890 [DOI] [PubMed] [Google Scholar]
- 29.Chapleau MW, Heesch CM, Abboud FM. Prevention or attenuation of baroreceptor resetting by pulsatility during elevated pressure. Hypertension. 1987;9:III137–III137 [DOI] [PubMed] [Google Scholar]
- 30.Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs [published erratum appears in circ res 1988 jul;63(1):272]. Circulation Research. 1987;61:648–658 [DOI] [PubMed] [Google Scholar]
- 31.Chapleau MW, Hajduczok G, Abboud FM. Pulsatile activation of baroreceptors causes central facilitation of baroreflex. American Journal of Physiology - Heart and Circulatory Physiology. 1989;256:H1735–H1741 [DOI] [PubMed] [Google Scholar]
- 32.Chapleau MW, Abboud FM. Determinants of sensitization of carotid baroreceptors by pulsatile pressure in dogs. Circulation Research. 1989;65:566–577 [DOI] [PubMed] [Google Scholar]
- 33.Cornwell WK 3rd, Tarumi T, Stickford A, Lawley J, Roberts M, Parker R, et al. Restoration of pulsatile flow reduces sympathetic nerve activity among individuals with continuous-flow left ventricular assist devices. Circulation. 2015;132:2316–2322 [DOI] [PubMed] [Google Scholar]
- 34.Markham DW, Fu Q, Palmer MD, Drazner MH, Meyer DM, Bethea BT, et al. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circulation. Heart failure. 2013;6:293–299 [DOI] [PubMed] [Google Scholar]
- 35.Grassi G Assessment of sympathetic cardiovascular drive in human hypertension: Achievements and perspectives. Hypertension. 2009;54:690–697 [DOI] [PubMed] [Google Scholar]
- 36.Castagna F, Stohr EJ, Pinsino A, Cockcroft JR, Willey J, Reshad Garan A, et al. The unique blood pressures and pulsatility of lvad patients: Current challenges and future opportunities. Current hypertension reports. 2017;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circulation Research. 1983;53:96–104 [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. Journal of Hypertension. 1987;5:93–98 [DOI] [PubMed] [Google Scholar]
- 39.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities- the ohasama study. Hypertension. 2000;36:901–906 [DOI] [PubMed] [Google Scholar]
- 40.Japundzic N, Grichois ML, Zitoun P, Laude D, JL E. Spectral analysis of blood pressure and heart rate in conscious rats- effects of autonomic blockers. 30 1990:91–100 [DOI] [PubMed] [Google Scholar]
- 41.Julien C The enigma of mayer waves: Facts and models. Cardiovascular research. 2006;70:12–21 [DOI] [PubMed] [Google Scholar]
- 42.Hammer PE, JP S. Resonance in a mathematical model of baroreflex control: Arterial blood pressure waves accompanying postural stress. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288:R1637–R1648 [DOI] [PubMed] [Google Scholar]
- 43.Nafz B, Wagner CD, PB P. Endogenous nitric oxide buffers blood pressure variability between 0.2 and 0.6 hz in the conscious rat. American Journal of Physiology Heart and Circulatory Physiology. 1997;272:H632–H637 [DOI] [PubMed] [Google Scholar]
- 44.Nafz B, Stegemann J, Bestle MH, Richter N, Seeliger E, Schimke I, et al. Antihypertensive effect of 0.1-hz blood pressure oscillations to the kidney, circulation 2000. CIrculation. 2000;101: 553–557. [DOI] [PubMed] [Google Scholar]
- 45.Butler GC, Ando SI, Floras JS. Fractal component of variability of heart rate and systolic blood pressure in congestive heart failure. Clinical Science. 1997;92:543–550 [DOI] [PubMed] [Google Scholar]
- 46.Symon L, Held K, Dorsch NWC. A study of regional autoregulation in the cerebra circulation to increased perfusion pressure in normocapnia and hypercapnia. Stroke. 1973;4:139–147 [DOI] [PubMed] [Google Scholar]
- 47.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Hypertension. 1989;20: 45–52. [DOI] [PubMed] [Google Scholar]
- 48.Cornwell WK 3rd, Tarumi T, Aengevaeren VL, Ayers C, Divanji P, Fu Q, et al. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Heart Lung Transplant. 2014;33:1295–1303 [DOI] [PubMed] [Google Scholar]
- 49.Edvinsson L, Krause DN. Cerebral blood flow and metabolism. 2nd Edition Philadelphia, PA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 50.Folklow B Description of the myogenic hypothesis. Circulation: research. 1964;15:279–287 [PubMed] [Google Scholar]
- 51.Bill A, Linder J. Sympaathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiologica Scandinavica. 1976;96:114–121 [DOI] [PubMed] [Google Scholar]
- 52.Faraci FM, Baumbach GL, DD. H. Cerebral circulation: Humoral regulation and effects of chronic hypertension. Journal of the American Society of Nephrology 1990;1:53–57 [DOI] [PubMed] [Google Scholar]
- 53.Sadoshima S, Bisija D.W., Heistad DD. Mechanisms of protection against stroke in stroke-prone spontaneously hypertensive rats. American Journal of Physiology 1983;244:H406–H412 [DOI] [PubMed] [Google Scholar]
- 54.Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419 [DOI] [PubMed] [Google Scholar]
- 55.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall:Lumen ratio of cerebral vessels. Hypertension. 1980;2 [DOI] [PubMed] [Google Scholar]
- 56.Johansson BB, Nilsson B. Cerebral vasomotor reactivity in normotensive and spontaneously hypertensive rats. Stroke. 1979;10:572–576 [DOI] [PubMed] [Google Scholar]
- 57.Mayhan WG, Faraci FM, Heistad DD. Impairment of endothelium-dependent responses of cerebral arterioles in chronic hypertension. American Journal of Physiology. 1987;253:H1435–H1440 [DOI] [PubMed] [Google Scholar]
- 58.Yang ST, Mayhan WG, Faraci FM, Heistad DD. Endothelium-dependent responses of cerebral blood vessels during chronic hypertension. Hypertension. 1991;17: 612–618 [DOI] [PubMed] [Google Scholar]
- 59.Mayhan WG, Faraci FM, Heistad DD. Responses of cerebral arterioles to adenosine 5’-diphosphate, serotonin, and the thromboxane analogue u-46619 during chronic hypertension. Hypertension. 1988;12(6): 556–561 [DOI] [PubMed] [Google Scholar]
- 60.Haeusler K, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982 [DOI] [PubMed] [Google Scholar]
- 61.de Bruijn R, Portegies M, Leening M, Bos MJ, Hofman A, van der Lugt A, et al. Subclinical cardiac dysfunction increases the risk of stroke and dementia. The rotterdam study. Neurology. 2015;84:833–840 [DOI] [PubMed] [Google Scholar]
- 62.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: Heart disease and stroke statistics−−2012 update: A report from the american heart association. Circulation. 2012;125:188–197 [DOI] [PubMed] [Google Scholar]
- 63.Caldas JR, Panerai RB, Haunton VJ, Almeida JP, Ferreira GS, Camara L, et al. Cerebral blood flow autoregulation in ischemic heart failure. American journal of physiology. Regulatory, integrative and comparative physiology. 2017;312:R108–R113 [DOI] [PubMed] [Google Scholar]
- 64.Cornwell WK 3rd, Levine BD. Patients with heart failure with reduced ejection fraction have exaggerated reductions in cerebral blood flow during upright posture. JACC Heart Fail. 2015;3:176–179 [DOI] [PubMed] [Google Scholar]
- 65.Ono M, Joshi B, Brady K, Easley RB, Kibler K, Conte J, et al. Cerebral blood flow autoregulation is preserved after continuous-flow left ventricular assist device implantation. Journal of cardiothoracic and vascular anesthesia. 2012;26:1022–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busija DW, Heistad DD, Marcus ML. Effects of sympathetic nerves on cerebral vessels during acute, moderate increases in arterial pressure in dogs and cats. Circ Res. 1980;46:696–702 [DOI] [PubMed] [Google Scholar]
- 67.Heistad DD, Marcus ML. Effect of sympathetic stimulation on permeability of the blood-brain barrier to albumin during acute hypertension in cats. Circ Res. 1979;45:331–338 [DOI] [PubMed] [Google Scholar]
- 68.Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, et al. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circulation. Heart failure. 2015;8:551–556 [DOI] [PubMed] [Google Scholar]
- 69.Dittrich R, Ringelstein EB. Occurrence and clinical impact of microembolic signals during or after cardiosurgical procedures. Stroke. 2008;39:503–511 [DOI] [PubMed] [Google Scholar]
- 70.Nabavi DG, Stockmann J, Schmid C, Schneider M, Hammel D, Scheld HH, et al. Doppler microembolic load predicts risk of thromboembolic complications in novacor patients. The Journal of Thoracic and Cardiovascular Surgery. 2003;126:160–167 [DOI] [PubMed] [Google Scholar]
- 71.Thoennissen NH, Schneider M, Allroggen A, Ritter M, Dittrich R, Schmid C, et al. High level of cerebral microembolization in patients supported with the debakey left ventricular assist device. J Thorac Cardiovasc Surg. 2005;130:1159–1166 [DOI] [PubMed] [Google Scholar]
- 72.Thoennissen NH, Allroggen A, Dittrich R, Ritter M, Schmid C, Scheld HH, et al. Can doppler time domain analysis of microembolic signals discriminate between gaseous and solid microemboli in patients with left ventricular assist devices? Neurological Research. 2003;27:780–784 [DOI] [PubMed] [Google Scholar]
- 73.Droste DW, Hansberg T, Kemeny V, Hammel D, Schulte-Altedorneburg G, Nabavi DG, et al. Oxygen inhalation can differentiate gaseous from nongaseous microemboli detected by transcranial doppler ultrasound. Stroke. 1997;28:2453–2456 [DOI] [PubMed] [Google Scholar]
- 74.Skjelland M, Krohg-Sorensen K, Tennoe B, Bakke SJ, Brucher R, Russell D. Cerebral microemboli and brain injury during carotid artery endarterectomy and stenting. Stroke. 2009;40:230–234 [DOI] [PubMed] [Google Scholar]
- 75.Skjelland M, Michelsen A, Brosstad F, Svennevig JL, Brucher R, Russell D. Solid cerebral microemboli and cerebrovascular symptoms in patients with prosthetic heart valves. Stroke. 2008;39:1159–1164 [DOI] [PubMed] [Google Scholar]
- 76.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with congestive heart failure. New England Journal of Medicine. 1984;311:819–823 [DOI] [PubMed] [Google Scholar]
- 77.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. International journal of cardiology. 2009;135:302–307 [DOI] [PubMed] [Google Scholar]
- 78.Caparas S, Clair M, Krombach SR, Hendrick JW, Houch WV, Kribbs SB, et al. Brain blood flow patterns after the development of congestive heart failure: Effects of treadmill exercise. Critical Care Medicine. 2000;28:209–214 [DOI] [PubMed] [Google Scholar]
- 79.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533 [DOI] [PubMed] [Google Scholar]
- 80.Rajagopalan B, Raine A, Cooper R, J L. Changes in cerebral blood flow in patients with severe congestive cardiac failure before and after captopril American Journal of Medicine. 1984;76:86–90 [DOI] [PubMed] [Google Scholar]
- 81.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Transcranial doppler blood flow assessment in patients with mild heart failure: Correlates with neuroimaging and cognitive performance. Congest Heart Fail. 2008;14:61–65 [DOI] [PubMed] [Google Scholar]
- 82.Choi BR, Kim JS, Yang YJ, Park KM, Lee CW, Kim YH, et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. The American journal of cardiology. 2006;97:1365–1369 [DOI] [PubMed] [Google Scholar]
- 83.Loncar G, Bozic B, Lepic T, Dimkovic S, Prodanovic N, Radojicic Z, et al. Relationship of reduced cerebral blood flow and heart failure severity in elderly males. Aging Male. 2011;14:59–65 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.