Abstract
Tissue engineering holds great promise in regenerative medicine. However, the field of tissue engineering faces a myriad of difficulties. A major challenge is the necessity to integrate vascular networks into bioengineered constructs to enable physiological functions including adequate oxygenation, nutrient delivery, and removal of waste products. The last two decades have seen remarkable progress in our collective effort to bioengineer human-specific vascular networks. Studies have included both in vitro and in vivo investigations, and multiple methodologies have found varying degrees of success. What most approaches to bioengineer human vascular networks have in common, however, is the synergistic use of both (1) endothelial cells (ECs)—the cells used to line the lumen of the vascular structures and (2) perivascular cells—usually used to support EC function and provide perivascular stability to the networks. Here, we have highlighted trends in the use of various cellular sources over the last two decades of vascular network bioengineering research. To this end, we comprehensively reviewed all life science and biomedical publications available at the MEDLINE database up to 2018. Emphasis was put on selective studies that definitively used human ECs and were specifically related to bioengineering vascular networks. To facilitate this analysis, all papers were stratified by publication year and then analyzed according to their use of EC and perivascular cell types. This study provides an illustrating discussion on how each alternative source of cells has come to be used in the field. Our intention was to reveal trends and to provide new insights into the trajectory of vascular network bioengineering with regard to cellular sources.
Keywords: Vascularization, Endothelial progenitor cells, iPS cells, Stem cells, Mesenchymal cells, Hydrogel, Angiogenesis, Vasculogenesis
Introduction
Tissue engineering holds great promise in regenerative medicine as a means to generate competent replacement tissues with therapeutic potential. Over the last two decades, the original notion of simply combining primary cells into biocompatible scaffolds to generate surrogate tissues has matured considerably. Advances include the advent of a variety of stem cell sources, the development of novel biomaterials, and a much deeper understanding of the mechanisms regulating interactions between cells and scaffolds. Nevertheless, despite remarkable pre-clinical progress, most tissue engineering efforts still remain mainly empirical. Indeed, translation of tissue engineering products into clinical practice has yet to occur at a meaningful pace, and currently only a handful of engineered tissues have achieved some degree of clinical success.
The field of tissue engineering faces a myriad of difficulties. At the forefront of these challenges is the necessity to integrate complex three-dimensional (3D) vascular networks into bioengineered constructs to enable adequate oxygenation, nutrient delivery, and removal of waste products upon implantation [1]. Strategies to ensure appropriate vascularization have included the delivery of angiogenic factors to promote the ingrowth of pre-existing host microvessels [2–4]. However, studies have consistently shown that the ingrowth of angiogenic sprouts is likely insufficient, and that to achieve rapid and complete vascularization of thick engineered tissues, constructs would need some kind of built-in vasculature [5]. Over the last two decades, researchers have resorted to exploiting the inherent blood vessel-forming ability of primary endothelial cells (ECs) in an effort to incorporate such built-in vascular networks. Currently, consensus still holds in that bioengineering vascular networks remains a priority in tissue engineering and in that the use of ECs is central to this effort.
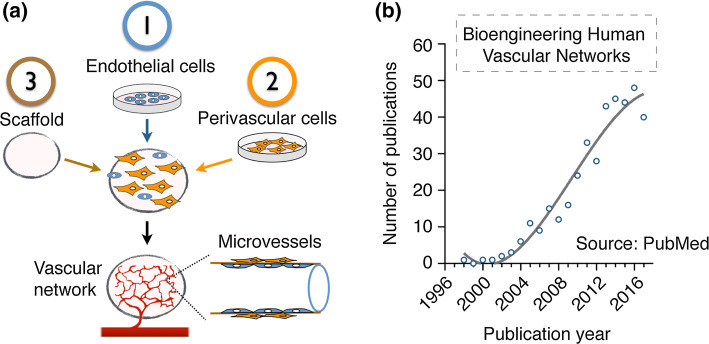
The pursuit of bioengineered human vascular networks is a relatively recent area of research, with the first studies carried out in the late 1990’s. Over these two decades, studies have included both in vitro investigations as well as in vivo xenograft models (mainly using immunodeficient mice as recipients), and multiple methodologies have found varying degrees of success. Despite broad diversity, most approaches have in common the use of the following key elements: (1) human ECs—used to line the lumen of the bioengineered vascular structures; (2) human perivascular cells—used to support EC function and/or provide perivascular stability to the networks; and (3) a scaffold—this provides a physical space for the cells to interact and for the vascular network to develop (Fig. 1a). The goal of this review is to highlight and discuss trends in the use of cellular sources over the last two decades of vascular network bioengineering research. This review, however, does not discuss the myriad fabrication processes by which researchers have approached the generation of vascular networks. For example, we did not analyze whether studies have favored processes based on spontaneous cellular self-assembly or if they have resorted to methods to endothelialize engineered microchannels. Also, we acknowledge there have been remarkable efforts in the lymphatic system as well, including bioengineering human lymphatic vessels that have been proven functional in vitro and in vivo [6, 7]. However, we did focus this review only on blood vascular bioengineering and thus the second vascular system, the lymphatic system, was not discussed. For simplicity, we have structured our discussion into two distinct sections corresponding to sources of human ECs and perivascular cells.
Fig. 1.
Bioengineering human microvascular networks. a Schematic depicting the key elements that are common to most approaches in human vascular network bioengineering: (1) human ECs, (2) human perivascular cells and (3) the scaffold. b Number of publications per year pertinent to bioengineering human microvascular networks. Publications were identified from the MEDLINE database using the PubMed search engine and included all papers up to the year 2018. All potentially relevant publications were individually reviewed to confirm suitability. Emphasis was put on identifying studies that definitively used human ECs and that were specifically related to bioengineering vascular networks. A total of 782 publications were pre-identified as potentially relevant, from which 371 were confirmed suitable according to our criteria
One of the main objectives of this review is to reveal trends and anticipate future directions. To this end, we accounted for all available publications in the field, regardless of the perceived importance and influence of any individual study. The methodology followed was based on a comprehensive examination of all life science and biomedical publications available at the MEDLINE database. We reviewed all available publications up to 2018. To facilitate this task, we used the PubMed search engine and introduced several search filters that could pre-identify all potentially relevant publications. Each pre-selected publication was then individually reviewed to confirm suitability. Emphasis was put on selective studies that definitively used human ECs (from any source) and that were specifically related to bioengineering vascular networks. Thus, studies that used ECs from non-human sources or that focused on tissue engineering single vascular grafts or conduits were not selected for further analysis. In addition, we restricted our search to studies published in English and excluded review papers. Our PubMed search identified 782 publications as potentially relevant, from which 371 were deemed suitable according to our criteria. All papers were stratified by publication year (Fig. 1b) and then analyzed according to their use of ECs (Fig. 2) and perivascular cells (Fig. 3).
Fig. 2.
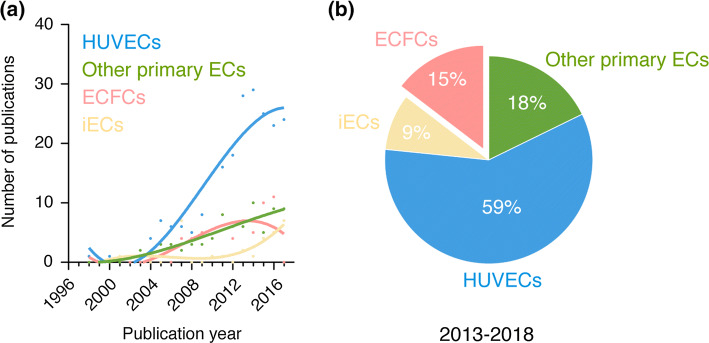
Sources of human endothelial cells in vascular network bioengineering. a Number of publications per year for each source of human ECs found in all the studies analyzed. Sources of human ECs were divided into four groups corresponding to (1) HUVECs, (2) other primary ECs, (3) ECFCs, and (4) pluripotent stem cell-derived ECs (iECs). b Percentage of studies for each source of human ECs over the period 2013–2018
Fig. 3.
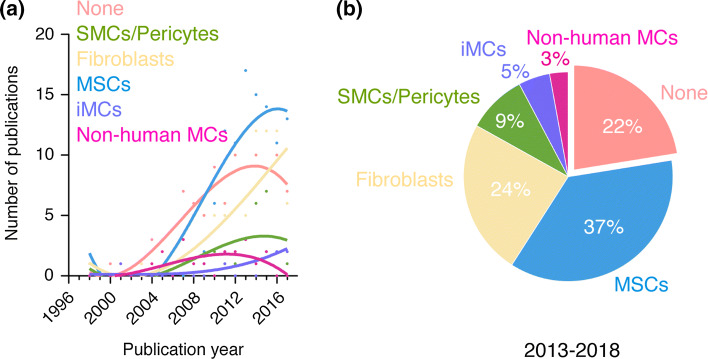
Sources of perivascular cells in human vascular network bioengineering. a Number of publications per year for each source of perivascular cells found in all the studies analyzed. Sources of perivascular cells were divided into six groups corresponding to (1) no perivascular cells (none), (2) SMCs and pericytes, (3) fibroblasts, (4) MSCs, (5) pluripotent stem cell-derived perivascular cells (iMCs), and (6) non-human perivascular cells (non-human MCs). b Percentage of studies for each source of perivascular cells over the period 2013–2018
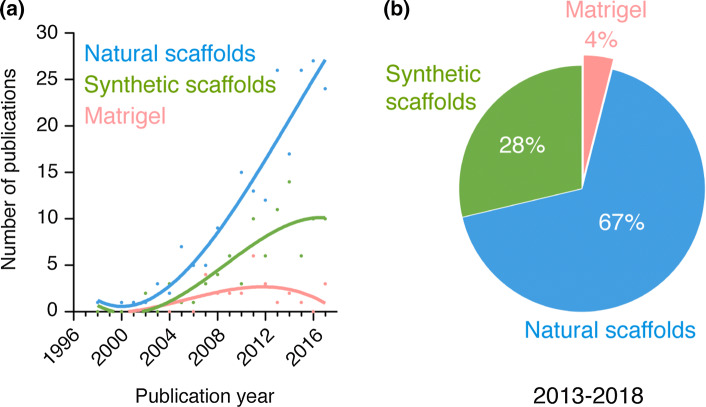
Our discussion is focused on trends and directions for each alternative source of cells. The other key component, the scaffold, was out of the scope and thus was not discussed in this review. Nevertheless, to give a historical perspective on the types of materials that have been favored over the years in this area of research, we classified all papers by three major classes of scaffolds including natural scaffolds, synthetic scaffolds, and Matrigel (Fig. 4). Our analysis revealed that natural scaffolds (i.e., scaffolds based on naturally occurring polymers such as collagen, gelatin and fibrin) remain the most prevalent choice in the field (Fig. 4a). Over the period 2013–2018, 67% of all the studies analyzed used natural scaffolds to support the formation of vascular networks, whereas 28% used synthetic scaffolds, and 4% Matrigel. Notwithstanding the central role played by the scaffolds, they likely had little influence on the choices made by investigators with regard to the sources of cells. Thus, we believe the trends revealed by our analysis are largely independent of the type of scaffold used. Further details and discussion about biomaterials and scaffolds can be found elsewhere [8–13].
Fig. 4.
Classification of scaffold in human vascular network bioengineering. a Number of publications per year for the different kind of scaffolds found in all the studies analyzed. For simplicity, scaffolds were divided only into three major categories corresponding to (1) natural scaffolds, (2) synthetic scaffolds, and (3) matrigel. b Percentage of studies for each scaffold category over the period 2013–2018
Sources of endothelial cells in human vascular network bioengineering
Human umbilical vein endothelial cells (HUVECs)
For decades, the study of human endothelial biology was primarily conducted with mature ECs obtained from living human vasculature. Among all sources, the successful isolation of ECs from human umbilical veins in the early 1970’s was of singular importance and provided unprecedented access to cultures of human ECs in laboratories around the world [14]. Indeed, HUVECs rapidly became a staple tool in vascular biology research with a dominant presence in the field to date.
The first efforts in vascular network bioengineering came in the late 1990’s. By then, HUVECs had been studied in culture for well over two decades, and thus there was a wealth of knowledge that positioned these cells as the preferred option. In 1998, Black et al. used HUVECs for the reconstruction of a human capillary-like network in a tissue-engineered skin equivalent [15]. This study was one of the first in vitro demonstrations of bioengineering human microvessels. In 2000, Schechner et al. used HUVECs in a proof-of-concept study that demonstrated the feasibility of engrafting a bioengineered human vascular network in vivo [16]. This landmark study established the conditions for assembling HUVECs into a capillary network within a three-dimensional (3D) hydrogel, and demonstrated that this bioengineered human vascular network was able to connect with the host circulatory system and to undergo remodeling into complex microvessels upon surgical implantation into severe combined immuno-deficient (SCID) mice. This paper became very influential in the field; the approach of assembling HUVECs in 3D hydrogels was adopted by numerous groups and remains the base of many investigations to date. It is worth noting that in this original study, HUVECs were genetically modified to overexpress the caspase-resistant Bcl-2 protein in an effort to delay apoptosis and enhance cell survival and proliferation. Nevertheless, subsequent studies demonstrated that the need for genetic manipulations could be by-passed. In 2004, Koike et al. showed that networks of long-lasting human blood vessels could be bioengineered in mice by co-implantation of non-modified HUVECs and perivascular precursors (murine embryonic 10T1/2 cell line) [17]. These networks were proven stable and functional for up to 1 year in vivo. Of note, the study by Koike et al. was the first to illustrate the importance of adding perivascular cells for lasting in vivo engraftment.
These early efforts on vascular network bioengineering using HUVECs were critical proof-of-concept studies and collectively demonstrated that pre-assembled human microvessels transplanted into mice were able to connect with host vessels. The approach of bioengineering pre-assembled vascular structures ahead of implantation in a 3D hydrogel has been used in many subsequent studies in the field of tissue engineering research, including efforts to vascularized engineered muscle [18], bone [19], and myocardial tissues [20].
HUVECs are widely used by the global vascular biology community. This includes the subfield of vascular network bioengineering. Examination of all relevant publications available in PubMed in this particular area of research revealed that the use of HUVECs has been and continues to be dominant since the early 2000’s (Fig. 2a). Moreover, the prevailing presence of HUVECs appears to have increased over the last few years; 59% of all the studies analyzed over the period 2013–2018 used HUVECs as the source of human ECs (Fig. 2b). Thus, despite the advent of alternative stem/progenitor cell sources, HUVECs remain as the preferred choice of human ECs in bioengineering to date. The reasons for this prevalence are multiple, but they all essentially stem from the fact that HUVECs have been studied for well over four decades, which is far more than any other source of human ECs. Over the years, the accumulated knowledge on HUVECs have conferred an advantage over other options and positioned these cells as the preferred choice for the development of protocols and standardized assays in vascular biology and angiogenesis research. The establishment of standard assays that call for HUVECs has in turn perpetuated the need for these cells, and their availability is now widespread at both research laboratories and commercial companies. In addition, HUVECs are isolated from discarded umbilical cord tissue, which are abundant, using simple techniques at relatively low costs.
In terms of future directions, the trends observed in current studies do not suggest a foreseeable decline in the use of HUVECs in vascular network bioengineering. Thus, HUVECs will likely remain a popular option in this field for years to come. Nevertheless, a number of advances could eventually produce a decline in the prevalence of HUVECs. For example, the heterogeneity of ECs continues to be a subject of intensive investigation and mounting evidence indicates that the endothelium regulates multiple regenerative processes in an organ/tissue-specific manner. Hence, it is conceivable that forthcoming efforts will focus on bioengineering vascular beds with organ-specific ECs, which might become possible by educating stem cell-derived ECs with competent tissue-specific properties. Also, HUVECs have limited life-span in culture and their use poses limitations with regard to clinical translation in an autologous setting. Hence, other sources of ECs derived from either progenitor cells or from pluripotent stem cells may very well gain advantage over HUVECs in coming years.
Other primary human endothelial cells
The successful isolation and culture of HUVECs from umbilical cords prompted the search for additional sources of human ECs. Indeed, in the decade following the isolation of HUVECs, ECs were derived from a variety of primary human tissues, including small diameter veins and the microvasculature of tissues such as skin [21] and adipose [22]. Soon after, studies demonstrated that irrespective of the origin within the vasculature, ECs from other mature vessels display a similar ability to assemble into capillary-like structures in culture like that displayed by HUVECs. Thus, by the early 2000’s, a number of alternative human ECs had been extensively studied by the vascular biology community and were readily available for the incoming bioengineering efforts.
Besides HUVECs, one of the most studied sources of primary human ECs has been the dermal microvascular endothelial cells (HDMECs). As with HUVECs, the majority of the studies on vascular network bioengineering with HDMECs have been conducted in vitro. Nevertheless, some of the early proof-of-concept demonstrations were carried out in vivo using immunodeficient mouse models. In 2001, Nör et al. used HDMECs embedded in Matrigel and transplanted in poly-l-lactic acid (PLLA) sponges into SCID mice. This study demonstrated HDMECs organized into functional microvessels that were evident from 7 to 10 days after implantation and formed functional anastomoses with the mouse vasculature, thus containing mouse blood cells in their lumens [23]. The study also showed that the human vessels became invested by perivascular smooth muscle actin-expressing mouse cells at 21 days after implantation, a sign of vessel stability. In 2002, Peters et al. also used HDMECs—this time in poly(lactic-co-glycolic acid) (PLGA) matrices that contained VEGF—to engineer human vascular networks into SCID mice [24]. The HDMEC-lined vessels organized into immature structures within 3 days and were fully functional after 14 days. HDMECs (mainly derived from discarded juvenile foreskin) continue to be used with certain regularity in vascular network bioengineering studies to date.
Another widespread source of human ECs is the white adipose tissue. The appeal of adipose tissue is that fat is plenty and readily available in adults. Biopsies of adipose tissue can be obtained with minimal intervention in an ambulatory setting and, in principle, this source could provide a more practical alternative to obtain large amounts of ECs for autologous therapies than HUVECs (umbilical cord) or HDMECs (foreskin). Certainly, ECs can be isolated and cultured from the stromal vascular fraction of human adipose tissues and efforts in bioengineering vascular networks with adipose tissue-derived ECs have been pursued over the last decade [25–29]. Collectively, studies with immunodeficient mouse models have demonstrated that human white adipose tissue is a dependable source of ECs with robust ability to form functional blood vessels in vivo.
Although less prevalent than skin and adipose tissue, other sources of human ECs have been proposed for vascular network bioengineering. A few recent examples include those derived from the omentum [30], aorta [31], coronary arteries [32], brain [33], cardiac [34, 35], and lung [36] microvasculatures.
Together, the use of alternative sources of primary human ECs in vascular network bioengineering has experienced a progressive increase in presence that is in line with the overall trend in the number of publications in this area of research over the years (Fig. 2a). Over the period 2013–2018, 18% of all the studies analyzed used alternative (not HUVECs) sources of primary human ECs (Fig. 2b). However, the gap between the number of studies that used HUVECs and those that used other ECs has widened over the last decade (Fig. 2a), which indicates a decline in the prevalence for these cells. This could simply be due to the inherent limitations affecting most primary cells with regard to their clinical translational potential (i.e., morbidity associated with their derivation, diminished proliferative and regenerative capacity in elder patients, short life-span of the cells in culture). In any case, the decline in the prevalence of primary ECs is likely to continue over the next decade, as efforts with progenitor and stem cell-derived ECs continue to grow. A caveat could be a resurgence in the collective appreciation for tissue specificity and the importance of how ECs regulate stem cell activities and regenerative processes in a tissue-specific manner. Thus, forthcoming efforts to bioengineer tissue-specific vascular beds (e.g., brain-, bone-, myocardium-specific vasculatures) will probably also include the use of primary tissue-specific ECs.
Human endothelial progenitor cells
For decades, obtaining human ECs involved harvesting them from healthy living blood vessels. However, it was widely recognized that this approach lacked broad clinical future due to the morbidity produced by collecting healthy tissues and to the fact that mature primary ECs displayed limited replicative capacity in culture. These limitations instigated widespread interest in finding alternative sources of autologous human ECs that might be less invasive and more replicative, including stem and progenitor cell sources [37].
One such alternative arose in the late 1990’s with the discovery of a subset of progenitor cells that circulate in human peripheral blood and differentiate in culture into bona fide ECs [38]. Certainly, for clinical applications, the identification of endothelial progenitor cells in circulation represented a promising opportunity to non-invasively obtain the required endothelial population. Nowadays, these cells are more commonly known as endothelial colony-forming cells (ECFCs), and thus this is the term we have used herein. However, it is worth noting that for the most part of the last two decades, there has been a general lack of agreement regarding nomenclature, and multiple terms have been indiscriminately used in the literature, including endothelial progenitor cells (EPCs), blood outgrowth ECs (BOECs), and the aforementioned ECFCs. Even more confusing, some of the terms (most notably “EPCs”) have often referred to subsets of circulating cells with no direct endothelial identity. Nevertheless, consensus is mounting in recent years as reflected by a 2017 statement on nomenclature published by multiple leading laboratories in the field [39].
Despite the ambiguous terminology, human ECFCs have been extensively characterized over the last 18 years and are now reasonably well understood. The robust endothelial phenotype of ECFCs has been confirmed repeatedly [40, 41]. Human ECFCs do express all the usual EC markers (e.g., VE-Cadherin, CD31, vWF), uptake low-density lipoproteins (e.g., Ac-LDL), and bind to specific lectins with high affinity (e.g., Ulex europaeus agglutinin 1, UEA-1), all expected characteristics of ECs. Human ECFCs’ ability to form functional vascular networks has also been repeatedly demonstrated in vivo [41–43]. Equally important, ECFCs maintain this robust endothelial identity through prolonged periods in culture, indicative of phenotypic stability [40].
The first use of human ECFCs in vascular network bioengineering was reported by Wu et al. [44]. In this study, cord blood-derived ECFCs were embedded in vitro into three-dimensional (3D) polyglycolic acid-poly-l-lactic acid (PGA-PLLA) scaffolds together with human smooth muscle cells (SMCs) and formed human microvessels that were uniformly present throughout the construct. Thus, this proof-of-concept study indicated that human ECFCs are well suited for creating microvascular networks within tissue-engineered constructs. In 2005, Sieminski et al. also used a 3D (type I rat tail collagen hydrogel) in vitro model and demonstrated that human ECFCs exhibited superior vascular network-forming ability relative to vessel-derived endothelial cells (including HUVECs), which was attributed to an increased force generation by the ECFCs [42]. In 2007, Fuchs et al. co-cultured human peripheral blood-derived ECFC with human osteoblasts in vitro and demonstrated that ECFCs formed highly organized microvessel-like structures that were more robust than those formed by HUVECs [45].
The ability of ECFCs to form robust vascular networks has been also demonstrated in vivo. In 2006, Shepherd et al. introduced the idea of repopulating decellularized tissues using human ECFCs [46]. Specifically, decellularized human skin substitutes were repopulated with cord blood-derived ECFCs and the grafts were then transplanted in vivo onto mice for 21 days. ECFCs were shown to integrate into the graft, forming perfused vessels that connected with incoming host vessels. This was one of the first in vivo demonstrations of using human ECFCs in the context of human vascular network bioengineering.
The capacity of human ECFCs to self-assemble into perfused vascular networks in vivo was further demonstrated in two independent studies published in 2007. In one of these studies, carried out by Melero-Martin et al., human ECFCs and human saphenous vein SMCs were embedded in Matrigel and injected subcutaneously into immunodeficient nude mice. One week later, examination of the implants revealed an extensive network of lumenal structures that were unequivocally lined by the human ECFCs and contained murine erythrocytes, which indicated formation of functional anastomoses with the host vasculature [40]. Of note, this study established feasibility of using both umbilical cord blood and adult peripheral blood as possible sources of ECFCs, although later in 2008, Au et al. reported that only the vessels formed by cord blood-derived ECFCs appeared to be sufficiently long-lasting [43]. Also, in 2007, Yoder et al. reported the ability of human ECFCs to form a perfused network of blood vessels after implantation into NOD/SCID mice using a collagen/fibronectin hydrogel construct [41]. This study was important in many respects but mainly because it refuted a potential myeloid origin of ECFCs. In addition, it illustrated for the first time that human ECFCs could actually form vascular networks in vivo without the support of exogenous perivascular cells. Nevertheless, it is worth noting that despite the ability of implants with only ECFCs, multiple studies have subsequently established that the microvascular density achieved by ECFCs without the use of mural cells (e.g., SMCs, MSCs) is notably inferior to that achieved with perivascular support [42, 47].
Short after these initial demonstrations, other significant in vivo studies with human ECFCs ensued. In 2008, Melero-Martin et al. engineered robust and long-lasting vascular networks using a combination of human ECFCs and mesenchymal stem cells (MSCs) that were originated from either cord blood or adult bone marrow [48], a significant development considering the prominent role that MSCs later acquired in this field. Soon after, similar results were reported with human adipose tissue-derived MSCs [49] and dermal fibroblasts [47] as the supporting cells. In 2009, Fuchs et al. demonstrated the use of human ECFCs for vascularization of engineered bone tissue constructs in vivo [50]. In 2011, Kang et al. demonstrated that vascular networks bioengineered with human ECFCs in mice could be explanted and reconnected into secondary mice, re-establishing perfusion, a feature that may extend the potential applications of this cell-based technology for transplantable large tissue-engineered constructs [51].
Collectively, the number of demonstrations has steadily continued to grow since 2004 and nowadays a growing number of laboratories routinely use human ECFCs for their bioengineering efforts (Fig. 2a). Over the period 2013–2018, 15% of all the studies analyzed used ECFCs as their source of human ECs (Fig. 2b). This prevalence though is still far behind from that of HUVECs (59%). In fact, as with other primary ECs, the gap between the number of studies that used HUVECs and those that used ECFCs has widen over the last decade (Fig. 2a). This somewhat slow incorporation of ECFCs in vascular network bioengineering studies may seem surprising. After all, ECFCs constitute an autologous source of primary ECs that can be derived by non-invasive means (i.e., blood draw), have a stable phenotype, and have robust proliferative and blood vessel-forming abilities [52]. Moreover, ECFCs are widely accessible (certainly to laboratories in the vicinity of medical centers and hospitals) and nowadays even commercially available. So why then are ECFCs not more prevalent? One simple explanation could be that there has not been enough time for everyone to embrace these cells yet—much of the time since their discovery has been spent in issues surrounding their definition, nomenclature, and possible origin rather than in promoting their widespread use. But there likely are other reasons as well.
One concern is the low frequency of ECFCs in adults. Indeed, ECFCs comprise a very small subpopulation of circulating cells in human adult peripheral blood—about 0.05–0.2 cells/ml, which is approximately 15-fold lower than in umbilical cord blood [53]. This low frequency—together with the lack of a unique set of distinctive cellular markers—has made the isolation of adult ECFCs very challenging [54]. In addition, there have been concerns regarding variability among donors and a number of studies have recognized the absence of ECFCs in a substantial proportion of healthy and non-healthy (e.g., patients with coronary artery disease and age-related macular degeneration) adult subjects [55–57]. Unfortunately, the mechanism by which ECFCs are mobilized into circulation, and how this process is modulated with age, in health and disease, is not currently known. Therefore, concerns derived from the low occurrence of ECFCs in adults are likely to remain in coming years.
As consensus mounts with regard to their identity, the prospect of ECFCs is likely to improve over the next few years. It is important to note that umbilical cord blood-derived ECFCs do not suffer from the same challenges as adult ECFCs and thus a distinction should be made between this source and adult peripheral blood. Most notably, cord blood ECFCs are significantly more frequent and their life-span in culture is demonstrably superior to that of adult ECFCs. Hence, in coming years, cord blood ECFCs will continue to be embraced by bioengineering laboratories to a higher degree than adult ECFCs. Nevertheless, because in general most patients would not have access to their own umbilical blood, the use of cord blood-derived ECFCs poses a limitation in terms of developing autologous cell therapies. This limitation might be circumvented by the establishment of cell banks where major human leukocyte antigen (HLA) haplotypes can be matched to reduce immunogenicity. These future banks would, for example, store enough samples of cord blood-derived ECFCs to cover a large part of the HLA diversity found in the general population. Altogether, forthcoming efforts in human vascular network bioengineering will probably continue to include the use of ECFCs.
Human endothelial cells derived from pluripotent stem cells
Over the last two decades, the search for alternative sources of autologous ECs have included those derived from human pluripotent stem cells [37]. Indeed, the excitement of using human embryonic stem cells (ESCs) in regenerative medicine has existed since they were first isolated in culture from the inner cell mass of human blastocysts [58]. Human ESCs could provide an unlimited number of pluripotent cells, which could subsequently generate sufficient ECs for any vascular cell therapy. In principle, patient-specific ESCs could be derived by therapeutic cloning from pre-implantation stage embryos produced by somatic cell nuclear transfer [59]. However, in practice, the use of human embryos poses ethical concerns that remain unresolved. In addition, harnessing the full therapeutic potential of ESCs might be challenging and would require methodologies for large expansion of ESCs as well as a better understanding of the mechanisms controlling their differentiation.
Despite uncertain clinical potential, feasibility of human ESC-derived ECs in vascular network bioengineering was demonstrated in early pre-clinical studies in mice. One of the initial in vivo proof-of-concept studies was reported by Levenberg et al. [60]. In this study, CD31 + ECs were derived from embryoid bodies (EBs) that were formed by an approved human ESC line (H9 clone). These ESC-derived ECs were shown to form perfused human-specific microvessels 7–14 days after implantation into SCID mice. A few years later, in 2007, Wang et al. differentiated human ESCs into ECs using a scalable two-dimensional method. After transplantation into SCID mice, the ESC-derived ECs formed a robust network of blood vessels that integrated into the host circulatory system and was functional for up to 150 days [61]. Subsequent studies demonstrated the ability of human ESC-derived ECs to facilitate vascularization of implanted tissue-engineered constructs. These studies included subcutaneous implants with cells seeded onto porous poly(2-hydroxyethyl methacrylate) scaffolds as well as collagen gel constructs containing human ESC-derived ECs and implanted into infarcted nude rat hearts. In both cases, the human ECs formed robust networks of patent vessels filled with host blood cells [62, 63]. Collectively, these studies established ESC as an alternative option for human endothelial cells in vascular network bioengineering.
The use of human ESC as a source of ECs in bioengineering has diminished over the years. Undoubtedly, this is mainly due to the advent of human induced pluripotent stem cells (iPSCs) and their consolidation as a viable alternative to ESCs. Indeed, the discovery of methods to convert somatic human cells into iPSCs through expression of a defined set of transcription factors created another possibility of producing patient-specific ECs for regenerative medicine [64–66]. As with ESCs, iPSCs could potentially provide an unlimited number of ECs for vascular therapies and bioengineering purposes. However, unlike ESCs, iPSCs do not pose major ethical concerns. Moreover, autologous ECs obtained from iPSCs would avoid allogenic immune rejection, which was another concern when considering ESCs [67]. Thus, excitement surrounding the potential of iPSC-derived ECs is widely shared.
The first demonstrations of human iPSC-derived ECs used methods similar to those previously established with human ESCs. In 2009, Taura et al. used iPSCs generated from human skin fibroblasts; the iPSCs were then differentiated into ECs in the presence of a murine bone marrow-derived OP9 stromal cell line and exogenous VEGF [68]. As with ESCs, the mechanism by which human iPSCs differentiate into ECs involved the generation of intermediate TRA1-60-/Flk-1 + precursors, and the efficiency of obtaining ECs from iPSCs was comparable to that of ESC-derived ECs [68]. Perhaps more importantly, studies have shown iPSC-derived ECs can display proper vascular function in vivo. For example, in 2011, Rufaihah et al. demonstrated that transplantations of human iPSC-derived ECs into ischemic hind limbs of immunodeficient mice were successfully incorporated into the host vasculature and significantly accelerated improvement in local blood flow [69]. Over the last few years, there has been an increasing number of encouraging studies using human iPSC-derived ECs. Despite rapid progress, several hurdles still remain before iPSC-derived cells become a clinical reality, including the uncertainty about their potential tumorigenicity, the long-term consequences of potential genetic and epigenetic alternations, as well as issues regarding their immunogenicity [67, 70]. Nevertheless, it should be noted that the methods to obtain iPSCs and to differentiate them into ECs have both evolved considerably in recent years.
In the context of bioengineering, the ability of human iPSC-derived ECs to form perfused vascular networks in vivo was first demonstrated in two independent studies published in 2013. In one of these studies, carried out by Samuel et al., human iPSC-derived ECs generated from healthy donors were shown to form stable functional blood vessels in vivo, lasting for 280 days in SCID mice [71]. Of note, in this study, ECs were transplanted in combination with mesenchymal progenitor cells (MPCs), which were also derived from human iPSCs. Furthermore, the study demonstrated that blood vessels can also be generated in vivo with human ECs and MPCs obtained from type 1 diabetic patient-derived hiPSCs, suggesting feasibility for future clinical translation. Similarly, Kusuma et al. demonstrated that ECs and pericytes, both also derived from human iPSCs, can self-organize to form bioengineered vascular networks that survived implantation into nude mice, integrated with the host vasculature, and established blood flow [72]. Collectively, these studies were critical proof-of-concept in vascular network bioengineering.
Nowadays, protocols to derive ECs from human iPSC are rapidly being incorporated into laboratories around the world and the number of studies that use human iPSC-derived ECs to bioengineer vascular networks has increased accordingly (Fig. 2a). Nevertheless, the occurrence of studies with pluripotent stem cells as the source of ECs is still low. Indeed, we found that over the period 2013–2018, only 9% of all the studies analyzed used human pluripotent cells (either ESCs or iPSCs) as their source of human ECs (Fig. 2b). This low prevalence in vascular network bioengineering is likely due to fact that the arrival of human ESCs and iPSCs has occurred only recently. Indeed, work with human iPSC-derived ECs has had less than a decade to mature, and differentiation methods and protocols continue to develop. Nevertheless, in the last few years there has been a noticeable increase in the use of pluripotent stem cells as the source of human ECs (Fig. 2a), a trend that is likely to continue in coming years. The prospect of an increased prevalence is consistent with the potential advantages that human iPSCs could bring to the field.
First, the unlimited and rapid growth potential of pluripotent stem cells provides a clear advantage over primary ECs, which certainly have a limited life-span in culture. This is not to say that iPSC-derived ECs have unlimited growth potential. In fact, one pressing challenge in the field continues to be the inability to expand iPSC-derived ECs robustly. From a translational standpoint, one could envision performing cell expansion at the iPSC level, before their differentiation into ECs, which would eliminate the need for massive EC expansion.
A second advantage is the ease at which autologous, patient-specific ECs can be generated from iPSCs. As discussed earlier, some of the most robust primary human ECs (namely, HUVECs and cord blood-derived ECFCs) are not suitable candidates in an autologous setting because patients generally have no access to their own umbilical cords. Meanwhile, recent advances in iPSC technologies have made it feasible—and increasingly more affordable—to derive iPSCs from virtually any individual with no added morbidity and regardless of age and health.
Last, iPSC-derived ECs may also become a useful tool in the study of tissue specificity. Mounting evidence indicates that the endothelium is not a monolith, and ECs regulate multiple processes in a tissue-specific manner [73–77]. For example, tissue-specific EC-derived factors stimulate self-renewal and in situ expansion of stem cells residing in lung, liver, bone, and neural tissues, contributing to the regeneration of these tissues upon injury [58–62]. Thus, to recapitulate the full complexity of human tissues, bioengineers may need to use tissue-specific primary ECs. However, as pointed out earlier, obtaining human ECs routinely from primary tissues is not trivial. Meanwhile, emerging evidence indicates that iPSC-derived ECs resemble highly plastic immature ECs and, therefore, could be susceptible to acquire tissue specificity upon exposure to tissue microenvironmental cues [78–80]. For example, Lippmann et al. showed that iPSC-derived ECs acquired blood–brain barrier (BBB) properties (including well-organized tight junctions, appropriate expression of nutrient transporters, and polarized efflux transporter activity) when co-cultured with astrocytes [81]. Nolan et al. demonstrated that following engraftment into liver and kidney, immature ECs underwent a process of in vivo education, acquiring structural and phenotypic attributes of the native tissue-resident ECs (i.e., liver ECs and glomeruli ECs, respectively) [77]. Accordingly, iPSC-derived ECs may be susceptible to undergo a process of tissue-specific education/maturation, which could in principle be harnessed by bioengineers to recapitulate EC heterogeneity.
A safety concern for human iPSCs is tumorigenicity—i.e., the formation of teratomas and/or malignant neoplasms [82]. This is certainly a concern that is specific to iPSC-derived cells and that is not present in primary ECs and ECFCs. To minimize this risk, emphasis should be put on having adequate control over the differentiation process and effective purification steps to eliminate possible undifferentiated cells. In addition, iPSC lines should be routinely screened for the presence of unwanted mutations. Overall, mounting evidence in animal studies has so far indicated a good safety profile with pluripotent stem cell derivatives. Nevertheless, tumorigenicity should remain an issue to consider.
Taken all together, we foresee iPSCs as perhaps the source of human ECs with the most potential, and thus we would expect a marked increase in the prevalence of vascular network bioengineering studies that include human iPSC-derived ECs in the coming years.
Sources of perivascular cells in human vascular network bioengineering
Bioengineering vascular networks with no perivascular cell support
Our collective understanding of the heterotypic interactions between endothelial and perivascular cells precedes the advent of studies on vascular network bioengineering. Indeed, for over three decades, studies have substantiated the molecular pathways involved in the maturation of nascent vasculature via the recruitment of perivascular mural cells and their role in stabilizing the endothelium [83–85]. Consequently, it is not entirely surprising that current efforts in vasculature bioengineering often include the use of perivascular cells as a means to support EC stability. Nevertheless, some of the initial approaches in bioengineering were aimed at harnessing the inherent ability of ECs without explicit inclusion of perivascular cells in the constructs. After all, ECs can form vascular networks in the absence of mural cells, and the recruitment of supporting perivascular cells and subsequent stabilization could occur upon implantation of the constructs into the host. Also, using an additional cell type significantly complicates future clinical translation of EC-based therapies and thus researchers have often resisted multicellular approaches.
The majority of the studies that do not explicitly use any source of perivascular cells in their methods are conducted in vitro. Nevertheless, one of the earliest reports in which human vascular networks were bioengineered with no perivascular cell support was the seminal study by Schechner et al. [16]. In that study, HUVECs were suspended alone, with no perivascular cells, in collagen/fibronectin gels wherein they first formed tubular structures in vitro within 20 h. The cell-laden constructs were then surgically implanted into the abdominal wall of severe combined immunodeficient (SCID) mice and human EC-lined vessels were detected in the implants by 30 days. Although the constructs lacked initial perivascular cell support, the study found that HUVECs needed to be transduced with the anti-apoptotic gene Bcl-2 to achieve meaningful survival, long enough to recruit host perivascular support. This, however, is one of the few studies in which human vascular networks were engrafted in vivo with no perivascular cells. Indeed, consensus holds in that meaningful engraftment of bioengineered vascular networks in vivo requires mural cell participation.
The number of studies in human vascular network bioengineering that lack perivascular cell support has experienced a progressive increase that is in line with the overall trend in this area of research (Fig. 3a). Over the period 2013–2018, 22% of all the studies analyzed did not explicitly use perivascular cells (Fig. 3b). This high prevalence may appear surprising given that vascular networks lacking perivascular cells have been consistently shown to display inefficient engraftment and poor stability in vivo. However, it is important to note that the majority of these studies were conducted in vitro, where, unlike in vivo, human ECs can self-assemble into vessels without the need for perivascular coverage. In any case, collectively, the most prevalent option in the field remains the supply of perivascular cells and future studies are likely to continue this trend.
Human primary smooth muscle cells and pericytes
Vascular smooth muscle cells (SMCs) and pericytes are collectively referred to as perivascular cells or mural cells and are adluminal cells that reside in close contact with ECs along the vasculature. Pericytes surround microvessels and capillaries, whereas SMCs contribute to the vascular wall of larger vessels; both are important for vascular development and stability [83, 85]. Perivascular cells are involved in the formation of the vasculature and respond to a variety of EC-derived factors such as platelet-derived growth factor B (PDGF-B) in a paracrine manner [86]. Vessels with perivascular coverage transit to a quiescent status that is characterized by cessation of EC proliferation, insensitivity to angiogenic stimulus, and decreased permeability. Indeed, vessels without proper perivascular coverage tend to regress over time [87].
Given the critical role of perivascular cells on the formation and stability of the vasculature, the use of primary SMCs in vascular network bioengineering was a natural choice that was examined in early studies. In 2004, Wu et al. showed that a combination of human ECFCs and mature human saphenous vein-derived SMCs was able to self-assemble into microvessel-like structures in vitro when co-seeded on a biodegradable scaffold of polyglycolic acid/poly-l-lactic acid (PGA/PLLA) [44]. Importantly, this study showed that in the absence of SMCs, ECs were not able to assemble into stable luminal structures and thus suggested that a two-cell system composed of ECs and SMCs could prove more robust and efficient for building vascular networks. In 2007, Melero-Martin et al. conducted in vivo studies and demonstrated that human ECFCs and saphenous vein-derived SMCs that were combined as a single-cell suspension in Matrigel formed vascular networks in 7 days after subcutaneous implantation into immunodeficient athymic nude mice [40]. Evaluation of implants at 1 week revealed an extensive network of human-specific luminal structures containing erythrocytes, indicating formation of functional anastomoses with the host vasculature. Of note, perfused human lumens were surrounded by α-SMA + perivascular cells, which contributed to the long-term stabilization of vessels; implants containing only ECFCs or SMCs did not yield human vessels. In subsequent years, studies from Jordan Pober’s group examined differences between using pericytes and SMCs in the context of vascular bioengineering. These studies showed that the use of human pericytes preferentially favored the formation of capillary-like structures, whereas SMCs promoted arteriole formation [88, 89]. Hence, both perivascular cell types may have distinctive uses.
These early efforts on vascular network bioengineering using human primary SMCs or pericytes were proof-of-concept studies and collectively demonstrated perivascular cells as critical supporting elements both in vitro and in vivo. Indeed, this two-cell system soon became the standard in the field of vascular network bioengineering, although the source of perivascular cells varies between studies. Overall, the prevalence of human primary SMCs/pericytes as the choice of perivascular cells has not grown in line with the overall trend in this area of research (Fig. 3a). Over the period 2013–2018, only 9% of all the studies analyzed use human primary SMCs or pericytes as perivascular cells (Fig. 3b). This low prevalence can be mainly attributed to the fact that SMCs/pericytes are primary cells and thus have inherent limitations that hinder their translational potential, including morbidity associated with their derivation (for example, from the saphenous vein), diminished proliferative and regenerative capacity in elder patients, and short life-span of the cells in culture. Moreover, the early advent of alternative sources of perivascular cells (most notably fibroblast and MSCs) with equal supporting capacity but with less intrinsic limitations, progressively rendered the use of SMCs/pericytes as a less preferable option. As a result, the use of primary human SMCs/pericytes soon became limited to few studies that were generally more focused on understanding mural cell biology than on actual bioengineering, and this trend is likely to continue in years to come. Nevertheless, efforts to understand the role of perivascular cells in a bioengineering setting are still ongoing. This includes characterization of mural functions in bioengineered vessels and efforts to determine the extent to which perivascular cells from alternative sources can robustly support the formation of new vasculature and best recapitulate the functions of actual primary SMCs and pericytes.
Human fibroblasts
Fibroblasts are spindle-shaped cells present in the stromal compartment of all connective tissues where, among other functions, they contribute to maintain the structural integrity of the tissues [90]. Fibroblasts are thus ubiquitous and, not surprisingly, were among the first human primary cells successfully adapted to grow in tissue culture in the 1960’s [91]. In research, human fibroblasts are commonly isolated from a variety of tissues, most notably juvenile foreskin and adult dermal skin. Once in culture, fibroblasts can grow with ease and thereby can be expanded to large numbers. In an autologous setting, the isolation of fibroblasts only requires a small biopsy of tissue, which could be obtained by low invasive means; thus, fibroblasts are widely considered to have broad translational potential. Indeed, human fibroblasts rapidly became a popular choice in many areas of regenerative medicine research, including the generation of iPSCs and direct reprogramming [64, 92].
Fibroblasts are interstitial cells and, therefore, are not perivascular cells per se. However, fibroblasts do have a close relationship with the vascular system. For example, fibroblasts produce a variety of cytokines and growth factors with well-established proangiogenic properties such as VEGF and bFGF [83, 84]. Consequently, it is not surprising that fibroblasts were central to the establishment of many in vitro angiogenesis model. They also had an early prominent role in the area of vascular network bioengineering. Indeed, one of the first studies in the field by Black et al. used human fibroblasts as supporting cells [15]. In this study, Black et al. developed a vascular-like network inside tissue-engineered skin to improve graft vascularization. These pre-vascularized skin grafts were assembled by three human cell types—HUVECs, dermal fibroblasts, and keratinocytes—co-cultured in a 3-D collagen gel. In this context, fibroblasts were shown to provide essential support and to promote spontaneous formation of capillary-like structures by the HUVECs [15]. Similar approaches soon became popular in vitro models to prevascularize grafts, especially in the field of dermal tissue engineering [6, 93, 94].
A decade after the Black et al. [15] study, in 2009, Chen et al. performed the first in vivo proof-of-concept study with fibroblasts supporting a bioengineered human vascular network [47]. This study pre-vascularized HUVECs in fibrin-based constructs containing human dermal fibroblasts, and subcutaneously implanted them into the dorsal surface of immunodeficient mice. The study demonstrated that the presence of fibroblasts accelerated the formation of functional anastomoses between the bioengineered vessels and the host vasculature following implantation. Similarly, in 2010, Hendrickx et al. demonstrated that human dermal fibroblasts mediated the formation of a robust vascular network by ECFCs in an in vivo skin wound-healing model [95]. Of note, this study showed that fibroblasts served as a major source of a plethora of trophic factors (including VEGF-A, PIGF, angiopoietin-1, MCP-1, bFGF, and MMP1) that collectively supported the formation of blood vessels. Collectively, these studies established that human fibroblasts can facilitate the formation of bioengineered vascular networks through providing a proangiogenic and anti-apoptotic environment that assists ECs. Nevertheless, it is worth mentioning that although the supporting function of fibroblasts for EC engraftment is well documented, their contribution to the actual perivascular coverage of the vessels remains unclear. Further research is warranted to elucidate the stability of vessels engineered with the support of fibroblasts as well as the fate of the fibroblasts themselves within the grafts.
Currently, fibroblasts continue to be widely used in tissue engineering and regenerative medicine research. The reasons for this high prevalence are multiple and include widespread availability and easy, somewhat inexpensive cell culture conditions. In vascular network bioengineering, examination of all relevant publications available in PubMed revealed that the use of human fibroblasts continues to grow since the early 2000’s (Fig. 3a). Moreover, this prevalent use of fibroblasts appears to have increased over the last few years; over the period 2013–2018, 24% of all studies analyzed used human fibroblasts as the source of supporting cells (Fig. 3b), choice that is only second to the use of MSCs. Despite this popularity, a few concerns with the use of fibroblasts remain to be addressed. These include the issue of fibroblast heterogeneity, which is a subject of active investigation [96]. Indeed, fibroblast is a broad term that may encompass many subtypes of different stromal cells, but we currently lack a decisive cell marker for each of these cells [97]. Depending on their localization within the tissues and on various other conditions, fibroblasts could exhibit considerable variation in morphology, size, and shape, suggesting the existence of discrete cellular subsets [98]. Moreover, due to the lack of definite markers, fibroblasts and MSCs are difficult to distinguish in culture on the basis of cell morphology, and thus it is conceivable that some cultures of fibroblasts contain certain percentages of MSCs. Equally, some claimed MSC cultures do in fact include differentiated fibroblasts (which lack multilineage differentiation potential) [99, 100]. Therefore, a certain degree of uncertainty remains a concern in studies using fibroblasts or MSCs as supporting perivascular cells. In addition, the long-term durability of vessels bioengineered with the support of fibroblasts is unclear. Because bona fide fibroblasts should, in principle, not be able to differentiate into smooth muscle cells, the perivascular coverage of the newly formed vessels may solely rely on the ingrowth of host perivascular cells. However, host perivascular cells may be more or less dysfunctional depending on a possible pathology and/or aging. In any case, these and other questions will probably be answered in coming years, and forthcoming studies will likely continue to use human fibroblasts as cells to support the formation and the engraftment of bioengineered vascular networks.
Human mesenchymal stem cells
Human MSCs were originally identified as adherent cells isolated from bone marrow that have colony-forming ability [101]. MSCs were later found to display multilineage differentiation potential and thus could generate multiple end-stage mesenchymal cell types [102]. Over the years, these cells have been referred to by various names including mesenchymal stromal cells and multipotent stromal cells [103]; however, the term mesenchymal stem cells (MSCs) is currently the most widely used. In 2006, the International Society for Cellular Therapy (ISCT) defined human MSCs as adherent cells capable of undergoing osteogenic, adipogenic, and chondrogenic differentiation, and are positive for cell surface markers CD73, CD90, and CD105, but lack CD11b, CD14, CD34, CD45, CD79a, and HLA-DR expression [104]. Despite these guidelines, the characterization and definition of MSCs remains a challenge [105, 106]. In addition to the bone marrow, MSCs have been found in virtually all other vascularized tissues, primarily as cells residing in perivascular locations and sharing markers similar to pericytes [107]. Moreover, mounting evidence suggests that MSCs’ properties vary depending on the tissue of origin from which they are isolated [108]. In the context of translational research, the most studied sources of MSCs are bone marrow and adipose tissue [109].
Initially, the use of MSCs was focused on their capacity for multilineage differentiation [103]. However, subsequent studies demonstrated that in addition to their inherent progenitor cell potential, MSCs were able to exert other biological functions in a paracrine fashion, through the secretion of trophic factors similar to fibroblasts [110]. This includes the secretion of proangiogenic cytokines and growth factors that have the potential to promote local vascularization, including VEGF, bFGF, and HB-EGF [111]. Due to the fact that MSCs are readily available from tissues such as the bone marrow and adipose, their perivascular origin, and their pro-vascularization properties, MSC usage in vascular network bioengineering was a logical proposition.
The use of human MSCs in vascular network bioengineering can be dated back to the early 2000’s, although at that time the term MSCs was still not as prevalent as is today. In 2003, Borges et al. reported that co-transplantation of human preadipocytes (i.e., adipose tissue-derived MSCs) with HDMECs enabled the early formation of a capillary network in a specially adapted chorioallantoic membrane (CAM) model [112]. In 2004, Wenger et al. formed heterogeneous co-spheroids by mixing human osteoblasts (i.e., bone marrow-derived MSCs) and HUVECs, and demonstrated that the osteoblasts supported the sprouting of HUVECs into extensive capillary networks inside a 3-D collagen matrix [113]. One of the first uses of the term MSCs in vascular network bioengineering was reported by Ghajar et al. [114]. In this study, human MSCs were combined with HUVECs to examine EC sprouting in an in vitro 3-D fibrin matrix model. The addition of MSCs resulted in a significant increase in network formation by the HUVECs, attributed to modulation of proangiogenic factors and the stiffness of the matrix by the MSCs. Furthermore, additional in vitro studies also demonstrated that the contribution of MSCs (from different origins) entailed differentiation into smooth muscle-like cells [115–117]. These cells surrounded the EC-lined lumens and thus served as actual perivascular cells [118].
In vivo demonstrations of the potential of human MSCs in vascular network bioengineering came in the late 2000’s. In 2008, Au et al. showed that human MSCs can serve as a source of perivascular cell precursors [119]. In this study, vessels were bioengineered in vivo using a combination of HUVECs and human bone marrow-derived MSCs. The MSCs were shown to efficiently stabilize the nascent blood vessels in vivo by functioning as perivascular precursor cells, and the vessels remained stable and functional for more than 130 days in SCID mice. The same year, Melero-Martin et al. demonstrated that human bone marrow-derived MSCs were able to support human ECFCs in a similar fashion. In this case, human MSCs were combined with human ECFCs in Matrigel and injected subcutaneously into athymic immunodeficient nude mice. Examination of the implants after 1 week revealed an extensive network of luminal structures that were unequivocally lined by the human ECFCs and contained murine erythrocytes, which indicated formation of functional anastomoses with the host vasculature [48]. Both studies confirmed that MSCs were incorporated as proper perivascular cells, surrounding the human vessels and expressing perivascular markers such as α-SMA. In addition, they illustrated the importance of the two-cell type approach—implanting either ECs or MSCs alone failed to produce meaningful vascularization. Collectively, these studies established feasibility of using human MSCs as a source of perivascular support for bioengineering vascular networks in vivo.
Shortly after these critical demonstrations, other significant in vivo studies with human MSCs ensued. In 2009, Traktuev et al. reported the formation of robust vascular networks using a combination of human ECFCs and adipose tissue-derived MSCs in immunodeficient mice [49]. In 2012, Lin et al. showed that tissue-resident MSCs isolated from four distinct tissues displayed equal capacity to support the formation of human vascular networks in vivo. This demonstrated that the ability to modulate the formation of vasculature is a ubiquitous property of all MSCs, irrespective of their original anatomical location [120]. Furthermore, in 2014, Lin et al. reported that in addition to the support provided by MSCs, ECs themselves serve as mediators for MSC engraftment via paracrine signaling, thus establishing a mechanism for mutual cooperation between both cell types [121]. Trophic factors (most notably PDGF-BB) from ECs were found critical to preserve the perivascular nature and stem cell properties of MSCs in vivo, whereas MSCs that failed to engraft as perivascular cells lost their stemness and became fibroblast-like interstitial cells instead. Therefore, this study suggested that bioengineering vascular networks by co-implanting ECs and MSCs could be a strategy not only to revascularize tissues, but also to enable proper MSCs engraftment in the context of mesenchymal tissue regeneration (e.g., bone and adipose tissue).
Since MSCs were established as possible perivascular partner for ECs, the number of publications that used MSCs for bioengineering vascular networks has soared (Fig. 3a). Indeed, MSCs have rapidly become the most prevalent choice of perivascular cells in the field, especially in studies conducted in vivo. Over the period 2013–2018, 37% of all the publications analyzed used human MSCs as the source of perivascular cells (Fig. 3b) and this prevalence is likely to continue in years to come. There are several intrinsic advantages that make MSCs a preferred perivascular option in vascular network bioengineering. First, autologous human MSCs have robust translational potential, are somewhat abundant, and can be obtained from small adipose tissue and/or bone marrow biopsies by minimally invasive procedures. Second, human MSCs are easy to maintain and expand in culture, and specialized chemical-defined media and supplements are now commercially available for these cells. And third, MSCs are competent adult stem cells and their potential in medicine not only includes their supporting role in the formation of vascular networks but also their ability to regenerate mesenchymal tissues. Collectively, these properties make MSCs particularly appealing in regenerative medicine.
We anticipate a bright prospect for MSCs in the field of vascular network bioengineering. However, there are certain questions that continue to be unresolved. This includes issues about the identity of MSCs and the intrinsic heterogeneity associated with their various tissues of origin. For example, studies have shown that most MSCs have a similar capacity to initially support vascularization in vivo [120]. However, with regards to their multilineage differentiation potential, in vivo studies have often indicated lineage-restricted properties that are related to their tissue of origin [121]. Thus, whether all MSCs possess equal multilineage potential in vivo remains unclear and further studies should identify the differences between the various sources of human MSCs as well as their long-term effects on the vasculature. Certainly, additional insights into the biological attributes of MSCs should result in a more rational use of these cells in vascular network bioengineering.
Human perivascular cells derived from pluripotent stem cells
As with ECs over the last decade, the search for alternative sources of autologous perivascular cells has included those derived from human iPSCs. iPSCs could provide an unlimited number of patient-specific pluripotent cells, which could subsequently generate sufficient perivascular cells for any vascular cell therapy. In practice, the same iPSC lines could be used to generate both ECs and perivascular cells needed for individual patients, simplifying the cell manufacturing process.
Discovering optimal protocols for human perivascular cell differentiation is an active area of investigation. Early methods relied on embryoid body (EB) formation with human ESCs followed by spontaneous differentiation into fibroblast-like stromal cells [122]. Subsequent protocols have adapted a 2-D approach that relies on the transition of human pluripotent stem cells (ESCs or iPSCs) into mesodermal progenitor cells that have the ability to then differentiate into several mesodermal end-stage cell types, including ECs, perivascular cells, and cardiomyocytes [123]. Indeed, studies have shown that human pluripotent stem cells can first be induced to differentiate into an intermediate mesodermal stage by activating Wnt and Activin/Nodal signaling pathways [124]. These intermediates, which express common mesodermal cell markers Flk1 and CD34, can then be differentiated into perivascular cells in the presence of specific growth factors such as PDGF-BB and TGF-β [125].
One of the first demonstrations of human pluripotent stem cell-derived perivascular cells in vascular network bioengineering came from Ferreira et al. [125]. In this study, human ESCs were first grown as EBs for 10 days and then vascular progenitor cells were isolated by virtue of CD34 expression. These CD34+ cells were cultured with PDGF-BB giving rise to smooth muscle-like cells that were characterized by spindle-shaped morphology, expression of smooth muscle cell markers (namely α-SMA, SM myosin heavy chain, calponin, caldesmon, and SM α-22), and the ability to contract and relax in response to agents such as carbachol and atropine. Importantly, this study demonstrated that these human ESC-derived perivascular cells were able to support the formation of functional vascular networks when implanted with ECs into immunodeficient mice.
Validations of competent perivascular cells from human iPSCs in vascular network bioengineering are more recent. In 2013, Samuel et al. derived mesenchymal precursor cells (MPCs) from human iPSCs using a 2-D differentiation protocol and demonstrated that ECs and MPCs derived from the same human iPSC line could successfully form stable functional blood vessels in vivo, lasting for 280 days in SCID mice [71]. Similarly, Kusuma et al. derived a bicellular endothelial/perivascular population from human iPSCs and demonstrated that these cells can self-organize to form microvascular networks in an engineered matrix. Additionally, upon implantation in mice, these engineered human vascular networks integrated with the host vasculature and established blood flow [72]. Together, these studies provided proof that autologous human iPSC-derived vascular precursors can be used for in vivo vascular network bioengineering.
Protocols to derive perivascular cells from human iPSC are rapidly being adopted by laboratories working in the field of tissue engineering and the number of studies that use these cells to bioengineer vascular networks has increased considerably in recent years (Fig. 3a). Nevertheless, the occurrence of studies with pluripotent stem cells as the source of perivascular cells is still low. Over the period 2013–2018, only 5% of all the studies analyzed used human pluripotent cells (either ESCs or iPSCs) as their source of human perivascular cells in vascular network bioengineering (Fig. 3b). As discussed above for ECs, this low prevalence is likely due to fact that protocols to derive perivascular cells from human ESCs and iPSCs have been developed only recently. Moreover, the study of iPSC-derived perivascular cells is often conducted as an addition to iPSC-derived ECs, with cells reported as a byproduct of EC differentiation. Thus, more investigation is still needed to elucidate key uncertainties surrounding the generation of perivascular cells from pluripotent stem cells. This includes questions about heterogeneity and the mechanisms controlling differentiation into different kinds of perivascular cells, from bona fide mature SMCs/pericytes to the more elusive mesenchymal progenitor and stem cells. In any case, over the last few years there has been a noticeable increased in the use of pluripotent stem cells as the source of human perivascular cells (Fig. 3a), a trend that is likely to continue in coming years.
Non-human perivascular cells
Although our analysis focused on human cell sources, it is worth mentioning that some non-human perivascular cells have played a significant historical role in the field of vascular network bioengineering. This is particularly true for the sarcoma cell line 10T1/2, a murine line originally established and characterized by Reznikoff et al. [126]. This line was derived from a line of C3H mouse embryo cells and was shown to have mesodermal differentiation potential similar to that of MSCs. The 10T1/2 cell line was easy to maintain in culture and displayed robust proangiogenic and perivascular properties, and thus rapidly became a staple cell line in vascular biology during the 1990’s [127, 128].
The use of the murine 10T1/2 cell line in human vascular network bioengineering was first reported in the early 2000’s. In 2004, Koike et al. and demonstrated that 10T1/2 cells could serve as mesenchymal precursors and support the engraftment of HUVECs; embedding both cell types in collagen gel led to formation of perfused and long-lasting vascular networks upon implantation into immunodeficient mice [17]. Moreover, this study demonstrated that 10T1/2-derived cells expressed mural-cell markers and were intimately associated with the newly formed vessels, surrounding the HUVEC-lined vessels, which suggested proper perivascular contribution. This was a seminal proof-of-concept report that established the notion of combining two-cell types (endothelial and perivascular) for stable vascular network formation.
After the Koike et al. study, the use 10T1/2 cells became somewhat frequent in the field. In 2005, Levenberg et al. applied a similar approach to engineer a vascularized skeletal muscle tissue by co-culturing human ECs, mouse 10T1/2 cells, and mouse myoblasts on PLLA/PLGA scaffolds [18]. As in the Koike report, this study showed that the presence of mouse 10T1/2 cells was critical, and no proper vessel formation was seen in their absence. Similarly, in 2007, Caspi et al. used 10T1/2 cells in combination with human ECs and ESC-derived cardiomyocytes to form vascularized human cardiac tissue [20]. In 2008, Au et al. used 10T1/2 cells to demonstrate the potential of human ECFCs to form functional and long-lasting vessels in vivo [43]. In 2011, Cheng et al. used HUVECs and 10T1/2 cells to illustrate how engineered blood vessel networks connect to the host vasculature to form anastomoses [129].
Following the success with 10T1/2 cells, other murine embryonic stromal cells were also used, although with much lower prevalence. Collectively, the occurrence of studies with non-human sources of perivascular cells has remained low and the trend suggests a decline over the last few years (Fig. 3a). Over the period 2013–2018, only 3% of all the studies analyzed used non-human perivascular cells (Fig. 3b). The main reason for this decline is likely the limited translational potential of non-human cell sources, including the murine sarcoma cell line 10T1/2. Non-human perivascular cells may continue to be found in human vascular network bioengineering reports; however, their use will likely be restricted to basic proof-of-concept studies with no direct translational relevance. Moreover, the consolidation of human sources of highly proliferative perivascular cells—such as those derived from MSCs and iPSCs—will soon render the use of non-human cells unnecessary, and thus a decline is foreseeable in the use of these cells in years to come.
Summary
Over the last two decades, most studies in the area of human vascular network bioengineering have synergistically combined ECs and supporting perivascular cells. However, the sources used to derive each of these cell types have varied considerably over the years. Here, we have highlighted trends followed by investigators with regard to the use of various cellular sources, from the onset of vascular network bioengineering research to date.
In the case of human ECs, sources have included primary tissues, progenitor cell sources, and, more recently, pluripotent stem cells. Also, efforts towards derivation of human ECs in xeno-free conditions are increasingly more common. For historical reasons, the use of HUVECs as the choice of human ECs has been and continues to be the most prevalent in the field of vascular network bioengineering. Indeed, 59% of all the studies analyzed over the last 5 years used this type of ECs. HUVECs will likely remain a popular option in this field for years to come. Nevertheless, a number of advances could eventually produce a decline in the prevalence of HUVECs. This may include the advent of a new focus on bioengineering organ-specific vascular beds and the consolidation of EC sources with more clinical translational potential such as those derived from either progenitor cells (i.e., ECFCs) or from pluripotent stem cells (iPSCs).
With regard to perivascular cells, sources have also included primary tissues, progenitor cells, and pluripotent stem cells. However, trends in this area have revealed that the choice of perivascular cells has varied more equally between primary cells and progenitor cells. This is reflected in our analysis of the last 5 years where both fibroblasts and MSCs were found as highly prevalent options (24% and 37%, respectively). In addition, a considerable portion of all studies (22%) in the last 5 years proceeded with only ECs and thus did not explicitly include perivascular cells. However, it is important to note that the majority of these studies were conducted in vitro; whereas in vivo, the most prevalent option in the field remains the supply of perivascular cells. Future studies are likely to continue this trend and we should expect a multitude of options for perivascular support. From a translational standpoint, the usage of perivascular progenitor cells (i.e., MSCs) and pluripotent stem cells is likely to gain more influence in the field. In addition, efforts will continue to improve the engraftment of ECs without the explicit use of perivascular cells, which could eventually eliminate the need for multicellular approaches and further facilitate clinical translation.
Acknowledgements
This work was supported by National Institutes of Health Grants R01AR069038, R01HL128452, and R21AI123883 to J. M.-M.
Author contributions
KW, R-ZL, and JMM-M conceived and designed the project, analyzed the data, discussed and edited the results and wrote the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4:160rv12. doi: 10.1126/scitranslmed.3004890. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Investig. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 4.Phelps EA, García AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Said SS, Pickering JG, Mequanint K. Advances in growth factor delivery for therapeutic angiogenesis. J Vasc Res. 2013;50:35–51. doi: 10.1159/000345108. [DOI] [PubMed] [Google Scholar]
- 6.Marino D, Luginbühl J, Scola S, et al. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med. 2014;6:221ra14. doi: 10.1126/scitranslmed.3006894. [DOI] [PubMed] [Google Scholar]
- 7.Maisel K, Sasso MS, Potin L, Swartz MA. Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv Drug Deliv Rev. 2017;114:43–59. doi: 10.1016/j.addr.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen P, Melero Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5:e74–e86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae H, Puranik AS, Gauvin R, et al. Building vascular networks. Sci Transl Med. 2012;4:160ps23. doi: 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y-C, Chen Y-C, Lin R-Z, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22:2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbo JV, Gerecht S. Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res Ther. 2013;4:8. doi: 10.1186/scrt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang WG, Niklason LE. A short discourse on vascular tissue engineering. NPJ Regen Med. 2017 doi: 10.1038/s41536-017-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song H-HG, Rumma RT, Ozaki CK, et al. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimbrone MA, Cotran RS, Folkman MJ. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black AF, Berthod F, L’heureux N, et al. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 16.Schechner JS, Nath AK, Zheng L, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike N, Fukumura D, Gralla O, et al. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 18.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 19.Tsigkou O, Pomerantseva I, Spencer JA, et al. Engineered vascularized bone grafts. Proc Natl Acad Sci USA. 2010;107:3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspi O, Lesman A, Basevitch Y, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 21.Davison PM, Bensch K, Karasek MA. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Investig Dermatol. 1980;75:316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- 22.Kern PA, Knedler A, Eckel RH. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Investig. 1983;71:1822–1829. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nör JE, Peters MC, Christensen JB, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Investig. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 24.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 25.Szöke K, Beckstrøm KJ, Brinchmann JE. Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant. 2012;21:235–250. doi: 10.3727/096368911X580518. [DOI] [PubMed] [Google Scholar]
- 26.Lin R-Z, Moreno-Luna R, Moreno-Luna R, et al. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis. 2013;16:735–744. doi: 10.1007/s10456-013-9350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerqueira MT, Pirraco RP, Martins AR, et al. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 2014;10:3145–3155. doi: 10.1016/j.actbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Klar AS, Guven S, Zimoch J, et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr Surg Int. 2016;32:17–27. doi: 10.1007/s00383-015-3808-7. [DOI] [PubMed] [Google Scholar]
- 29.Freiman A, Shandalov Y, Rozenfeld D, et al. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther. 2016;7:5. doi: 10.1186/s13287-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Komaki M, Hattori H, et al. Therapeutic angiogenesis by implantation of a capillary structure constituted of human adipose tissue microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1300–1306. doi: 10.1161/atvbaha.109.198994. [DOI] [PubMed] [Google Scholar]
- 31.Sasagawa T, Shimizu T, Yamato M, Okano T. Expression profiles of angiogenesis-related proteins in prevascular three-dimensional tissues using cell-sheet engineering. Biomaterials. 2014;35:206–213. doi: 10.1016/j.biomaterials.2013.09.104. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya A, Lin S, Sandig M, Mequanint K. Regulation of vascular smooth muscle cell phenotype in three-dimensional coculture system by Jagged1-selective Notch3 signaling. Tissue Eng Part A. 2014;20:1175–1187. doi: 10.1089/ten.TEA.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herland A, van der Meer AD, FitzGerald EA, et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS One. 2016;11:e0150360. doi: 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valarmathi MT, Fuseler JW, Davis JM, Price RL. A novel human tissue-engineered 3-D functional vascularized cardiac muscle construct. Front Cell Dev Biol. 2017;5:2. doi: 10.3389/fcell.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amano Y, Nishiguchi A, Matsusaki M, et al. Development of vascularized iPSC derived 3D-cardiomyocyte tissues by filtration layer-by-layer technique and their application for pharmaceutical assays. Acta Biomater. 2016;33:110–121. doi: 10.1016/j.actbio.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–132. doi: 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Investig. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina RJ, Barber CL, Sabatier F, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;229:10–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melero-Martin JM, Melero-Martin JM, Khan ZA, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 41.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieminski AL, Hebbel RP, Gooch KJ. Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng. 2005;11:1332–1345. doi: 10.1089/ten.2005.11.1332. [DOI] [PubMed] [Google Scholar]
- 43.Au P, Daheron LM, Duda DG, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577–2588. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 46.Shepherd BR, Enis DR, Wang F, et al. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006;20:1739–1741. doi: 10.1096/fj.05-5682fje. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Aledia AS, Popson SA, et al. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16:585–594. doi: 10.1089/ten.TEA.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melero-Martin JM, De Obaldia ME, De Kang SY, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traktuev DO, Prater DN, Merfeld-Clauss S, et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs S, Ghanaati S, Orth C, et al. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526–534. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 51.Kang K-T, Allen P, Bischoff J. Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood. 2011;118:6718–6721. doi: 10.1182/blood-2011-08-375188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasev D, Koolwijk P, Van Hinsbergh VWM. Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev. 2016;22:371–382. doi: 10.1089/ten.TEB.2016.0050. [DOI] [PubMed] [Google Scholar]
- 53.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 54.Mund JA, Estes ML, Yoder MC, et al. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rignault-Clerc S, Bielmann C, Delodder F, et al. Functional late outgrowth endothelial progenitors isolated from peripheral blood of burned patients. Burns. 2013;39:694–704. doi: 10.1016/j.burns.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Stroncek JD, Grant BS, Brown MA, et al. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473–3486. doi: 10.1089/ten.TEA.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thill M, Strunnikova NV, Berna MJ, et al. Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Investig Ophthalmol Vis Sci. 2008;49:2696–2708. doi: 10.1167/iovs.07-0955. [DOI] [PubMed] [Google Scholar]
- 58.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 59.Wilmut I, Beaujean N, de Sousa PA, et al. Somatic cell nuclear transfer: PubMed—NCBI. Nature. 2002;419:583–587. doi: 10.1038/nature01079. [DOI] [PubMed] [Google Scholar]
- 60.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 62.Nourse MB, Halpin DE, Scatena M, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kraehenbuehl TP, Ferreira LS, Hayward AM, et al. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 66.Park I-H, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 67.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taura D, Sone M, Homma K, et al. Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 69.Rufaihah AJ, Huang NF, Jamé S, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrilleaux B, Knoepfler PS. Inducing iPSCs to escape the dish. Cell Stem Cell. 2011;9:103–111. doi: 10.1016/j.stem.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kusuma S, Gerecht S. Derivation of endothelial cells and pericytes from human pluripotent stem cells. Methods Mol Biol. 2016;1307:213–222. doi: 10.1007/7651_2014_149. [DOI] [PubMed] [Google Scholar]
- 73.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 74.Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci USA. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 77.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White MP, Rufaihah AJ, Liu L, et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31:92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reed DM, Foldes G, Kirkby NS, et al. Morphology and vasoactive hormone profiles from endothelial cells derived from stem cells of different sources. Biochem Biophys Res Commun. 2014;455:172–177. doi: 10.1016/j.bbrc.2014.10.140. [DOI] [PubMed] [Google Scholar]
- 80.Yoder M. Differentiation of pluripotent stem cells into endothelial cells. Curr Opin Hematol. 2015;22:252–257. doi: 10.1097/MOH.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lippmann ES, Azarin SM, Kay JE, et al. Derivation of blood–brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevens KR, Murry CE. Human pluripotent stem cell-derived engineered tissues: clinical considerations. Cell Stem Cell. 2018;22:294–297. doi: 10.1016/j.stem.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 84.Darland DC, D’Amore P. Blood vessel maturation: vascular development comes of age. J Clin Investig. 1999;103:157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folkman MJ, D’Amore P. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/S0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 86.Hellström M, Kalén M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 87.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 88.Shepherd BR, Jay SM, Saltzman WM, et al. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A. 2009;15:165–173. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maier CL, Shepherd BR, Yi T, Pober JS. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17:367–380. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. J Clin Investig. 2018;128:26–35. doi: 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato G. Tissue culture: the unrealized potential. Cytotechnology. 2008;57:111–114. doi: 10.1007/s10616-007-9109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilmut I. The first direct reprogramming of adult human fibroblasts. Cell Stem Cell. 2007;1:593–594. doi: 10.1016/j.stem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Shandalov Y, Egozi D, Koffler J, et al. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci USA. 2014;111:6010–6015. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tonello C, Vindigni V, Zavan B, et al. In vitro reconstruction of an endothelialized skin substitute provided with a microcapillary network using biopolymer scaffolds. FASEB J. 2005;19:1546–1548. doi: 10.1096/fj.05-3804fje. [DOI] [PubMed] [Google Scholar]
- 95.Hendrickx B, Verdonck K, Van den Berge S, et al. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells. 2010;28:1165–1177. doi: 10.1002/stem.445. [DOI] [PubMed] [Google Scholar]
- 96.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 97.Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alt E, Yan Y, Gehmert S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103:197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 100.Denu RA, Nemcek S, Bloom DD, et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 2016;136:85–97. doi: 10.1159/000445096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged: PubMed—NCBI. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 103.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 105.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 106.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 108.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pill K, Hofmann S, Redl H, Holnthoner W. Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: a comparison. Cell Regen (Lond) 2015;4:8. doi: 10.1186/s13619-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 111.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borges J, Mueller MC, Padron NT, et al. Engineered adipose tissue supplied by functional microvessels. Tissue Eng. 2003;9:1263–1270. doi: 10.1089/10763270360728170. [DOI] [PubMed] [Google Scholar]
- 113.Wenger A, Stahl A, Weber H, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;10:1536–1547. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 114.Ghajar CM, Blevins KS, Hughes CCW, et al. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 115.Rohringer S, Hofbauer P, Schneider KH, et al. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014;17:921–933. doi: 10.1007/s10456-014-9439-0. [DOI] [PubMed] [Google Scholar]
- 116.Ghanaati S, Fuchs S, Webber MJ, et al. Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med. 2011;5:e136–e143. doi: 10.1002/term.373. [DOI] [PubMed] [Google Scholar]
- 117.Holnthoner W, Hohenegger K, Husa A-M, et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9:127–136. doi: 10.1002/term.1620. [DOI] [PubMed] [Google Scholar]
- 118.Crisan M, Corselli M, Chen WCW, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin R-Z, Moreno-Luna R, Moreno-Luna R, et al. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin R-Z, Moreno-Luna R, Li D, et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA. 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie C, Ritchie RP, Huang H, et al. Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol. 2011;31:1485–1494. doi: 10.1161/ATVBAHA.110.221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1 + cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Bajpai VK, Mistriotis P, Loh Y-H, et al. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res. 2012;96:391–400. doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferreira LS, Gerecht S, Shieh HF, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 126.Reznikoff CA, Bertram JS, Brankow DW, Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Can Res. 1973;33:3239–3249. [PubMed] [Google Scholar]
- 127.Hirschi KK, Rohovsky SA, D’Amore P. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirschi KK, Rohovsky SA, Beck LH, et al. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.RES.84.3.298. [DOI] [PubMed] [Google Scholar]
- 129.Cheng G, Liao S, Wong HK, et al. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011 doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]