Abstract
Background:
Intraductal papillary mucinous neoplasms (IPMN) are currently managed based on imaging characteristics and cyst fluid sampling. This study was designed to determine if MUC13, a glycoprotein aberrantly overexpressed in pancreatic adenocarcinoma, might aid in distinguishing high-risk lesions (high grade dysplasia/invasive disease) from low-grade lesions.
Methods:
MUC13 immunohistochemical staining was performed on surgically resected formalin-fixed tissue specimens from 49 IPMNs and 23 non-mucinous cysts (16 SCA, 3 benign cysts, 4 chronic pancreatitis/pseudocyst). Membranous MUC13 expression was measured by H-score, which quantifies staining intensity and the percentage of cells involved (range 0–300).
Results:
MUC13 expression was detected in all IPMNs and was significantly greater than in non-mucinous cysts (median 210 vs 40, p<0.001). MUC13 expression was similar among main (n=26), branch (n=15), and mixed (n=8) duct lesions (median 210, 200, 225, respectively). The highest expression was observed in tumors with intestinal and pancreatobiliary histologic features (both median 225) and the lowest in gastric type lesions (median 200). MUC13 expression was significantly greater in high-risk lesions (n=21) compared to those with low-grade dysplasia (n=28) (median 250 vs 195, p<0.001).
Conclusion:
MUC13 expression was significantly greater in high-risk IPMNs in this analysis. The preoperative assessment of MUC13 in cyst fluid samples warrants further investigation.
Keywords: IPMN, mucin, pancreatic cyst, biomarker
Introduction
The identification of pancreatic cysts is increasing with the growing use of cross-sectional imaging.[1–3] Intraductal papillary mucinous neoplasms (IPMN) represent a group of mucin-producing cystic lesions with varying degrees of malignant potential, ranging from low-grade dysplasia to invasive carcinoma.[4] At present, management of these lesions is based on clinical guidelines largely reliant on imaging characteristics and the sampling of cyst fluid.[5–7] However, the capacity of current modalities to predict high-grade dysplasia or invasive disease remains imperfect.[8–11] As such, a significant portion of patients harboring more indolent lesions may undergo a potentially unnecessary pancreatectomy with its inherent morbidity and mortality. Likewise, falsely negative results upon interrogation of cyst fluid may lead to delayed recognition and resection of high-risk lesions. Therefore, the identification of additional biomarkers associated with aggressive cell behavior in these tumors would be of great value in clinical decision making.[12,13]
Mucins are high molecular weight glycoproteins found in mucus that play an important role in diverse biological functions, such as differentiation, cell adhesion, immune response, and cell signaling. Altered mucin expression can be seen in a variety of diseases, including cancer.[14] Published work from our group suggest that a transmembrane mucin, MUC13, is aberrantly overexpressed in pancreatic ductal adenocarcinoma (PDAC) and its ectopic expression leads to increased tumor growth and invasion.[15–18] We have observed MUC13 expression to be greatest along the apical membrane of ductal cells. In addition, we have found that MUC13 can be detected in PDAC precursor lesions such as pancreatic intraepithelial neoplasms (PanINs) as well as PDAC but exhibits low or undetectable expression in normal pancreatic tissue.[17,18]
The purpose of this study was to assess the expression of MUC13 in IPMN ductal and histologic subtypes, as well as other non-mucinous cystic neoplasms. Furthermore, we intended to correlate this information with known pathologic prognostic factors for IPMN. Based on our previous results demonstrating an association with PDAC and its precursor lesions, we hypothesize that MUC13 may serve as a potential marker of invasive disease within IPMNs.
Materials and Methods
Study population and specimens
We retrospectively reviewed a prospectively collected database of pancreatectomies performed at University of Tennessee Health Science Center affiliated hospitals (Methodist Le Bonheur Healthcare system, Baptist Memorial Hospital) from 1998 – 2016, and patients with a surgically resected, pathologically confirmed IPMN were identified. Baseline demographic characteristics were collected including age, race, sex, and BMI along with tumor clinicopathologic data. IPMN histologic subtype (gastric, intestinal, pancreatobiliary, oncocytic), the extent of ductal involvement (main duct, branch duct, mixed), and the size of the pathologic specimen were recorded. For classification purposes, cases were grouped based on the greatest degree of dysplasia present within the specimen and a “high risk” lesion was defined as having high-grade dysplasia (HGD) and/or an invasive carcinoma component. Pathologic specimens were reviewed and representative formalin-fixed paraffin-embedded (FFPE) tissue blocks were serially sectioned at 4 μm. Previously, we have observed greater variability for MUC13 expression near the center of pancreatic lesions, likely related to a degree of necrosis. Therefore, an effort was made to select FFPE tissue sections from the leading edges of lesions. Hematoxylin and eosin (H&E) stained slides were reviewed by an experienced pancreatic pathologist (K.T.P.) blinded to the results of the original pathologic report and classified according to the degree of dysplasia present. For comparison purposes, we also identified 23 patients having undergone resection of a serous cystadenoma (n = 16) or other lesion which may have been preoperatively diagnosed as an IPMN but ultimately were found to be a benign process (lymphoepithelial cyst [n = 1], benign pancreatic retention cyst [n = 2], chronic pancreatitis with ductal dilation or pseudocyst [n = 4]). This study was approved by the University of Tennessee Health Science Center and Baptist Memorial Healthcare institutional review boards.
Immunohistochemistry for MUC13 expression
Immunohistochemical staining was performed using a commercially available kit (Biocare Medical, CA, USA) as described previously.[17] Briefly, tissue sections were deparaffinized with xylene, rehydrated in graded alcohol and underwent blocking of endogenous peroxidase activity. Heated antigen retrieval was performed followed by incubation with monoclonal MUC13 antibody overnight. Slides were then stained with Biocare’s DAB chromogen and counterstained with hematoxylin. Finally, tissues were again dehydrated in graded alcohol followed by xylene and remounted.
Immunohistochemical scoring
Slides were digitized using an Aperio scanner (Leica Biosystems) and digital images were reviewed individually using Aperio Imagescope (Leica Biosystems). Membranous immunohistochemical staining intensity was assessed using the modified histochemical score (H-score), as previously described.[19–21] The scoring system includes an evaluation of both the percentage and intensity of stained target cells. Scores are assigned on a 0 to 3 scale corresponding to negative, faint, moderate, and strong staining, respectively. The percentage of positively stained cells at each intensity was estimated and the products of the intensity and percentage were summed. Possible H-scores ranged from 0 to 300.
Statistical analysis
Continuous variables were summarized as medians with interquartile ranges (IQR) and compared using the Wilcoxon Rank Sum test or Kruskall-Wallis test. Categorical variables were summarized with relative frequencies and analyzed using the chi square test. All hypotheses testing was two-sided and a p value of < 0.05 was considered indicative of statistical significance. Statistical analysis was performed using IBM SPSS Statistics version 24 (IBM Corp.).
Results
A total of 49 patients with an IPMN were identified with FFPE tissue available for analysis. Baseline characteristics for the group along with 23 patients with benign non-mucinous cysts are shown in Table 1. There was no significant difference in age or gender between the groups and more than half of the patients with IPMN were female. A greater proportion of the IPMN group were white compared to the non-mucinous cyst group (72.0% vs 43.5%, p = 0.019). Most (66.7%) IPMNs were found in the proximal portion of the gland, while a majority of nonmucinous lesions were located in the distal pancreas (73.9%). Three patients (5.9%) had IPMNs located diffusely throughout the gland. Among the 49 IPMN specimens, 26 (53.1%) were classified as main duct, 16 (30.6%) side branch, and 8 (16.3%) were mixed duct variants. With regard to histologic subtype, 23 (46.9%) were gastric, 13 (26.5%) intestinal, 11 (22.4%) were pancreatobiliary, and 1 (2.0%) was oncocytic. In one sample, the histologic subtype was unable to be definitively determined due to replacement with invasive carcinoma.
Table 1:
Baseline characteristics for patients with IPMN (n = 49) and non-mucinous cysts (n = 23).
| Characteristic | IPMN (n = 49) | Non-mucinous cyst* (n = 23) | p value |
|---|---|---|---|
| Age, median (IQR) | 65 (60–71) | 63 (58–72) | 0.248 |
| Gender, n (%) | 0.515 | ||
| Male | 21 (42.9) | 8 (34.8) | |
| Female | 28(57.1) | 15(65.2) | |
| Race, n (%) | 0.009 | ||
| White | 36 (75.0) | 10(43.5) | |
| Black | 12(25.0) | 13(56.5) | |
| Lesion location, n (%) | 0.001 | ||
| Head/neck/uncinate | 32(65.3) | 6(26.1) | |
| Body/tail | 14(28.6) | 17(73.9) | |
| Diffuse/multifocal | 3(6.1) | 0 (0.0) |
IPMN – intraductal papillary mucinous neoplasm; IQR – interquartile range;
includes serous cystadenoma (SCA), lymphoepithelial cyst, pancreatic inclusion cyst, chronic pancreatitis with pseudocyst.
MUC13 expression in IPMNs
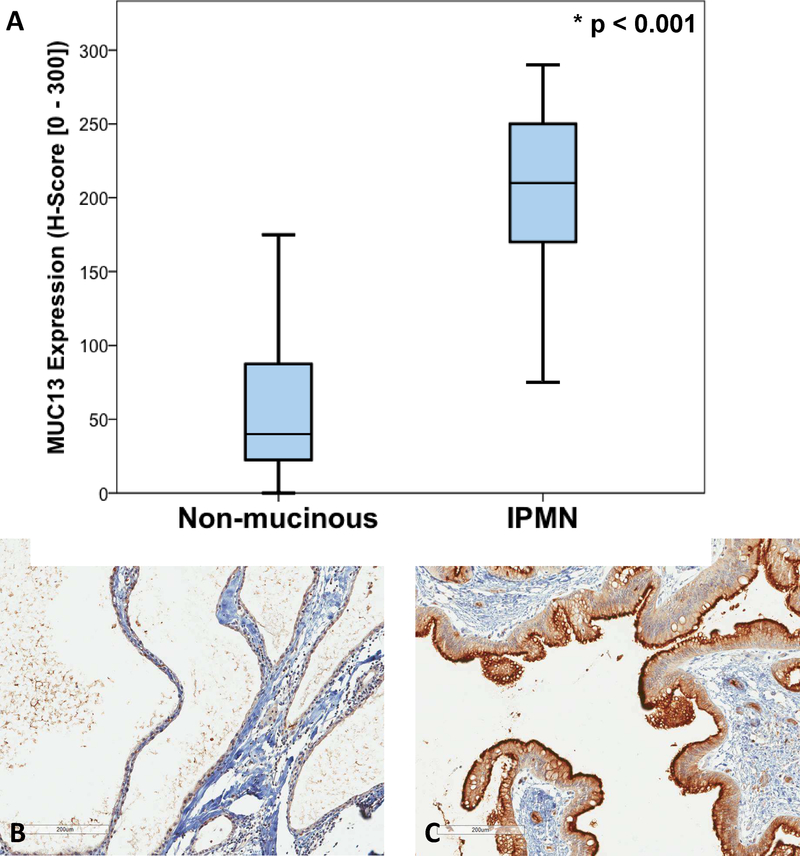
Upon assessment of MUC13 immunoreactivity, all 49 (100%) IPMN specimens displayed MUC13 membranous expression. Among IPMN specimens, the median H-score was 210 (IQR 170 – 250), which was significantly greater than that of the non-mucinous control group (median H-score 40 [IQR 20 – 90], p < 0.001) (Figure 1A). Representative MUC13 immunohistochemical staining for serous cystadenoma and IPMN specimens are shown in Figure 1B and 1C, respectively. Staining of IPMNs usually involved dense apical membrane expression with varying degrees of cytoplasmic staining while serous and other non-mucinous cysts typically displayed only faint membranous staining.
Figure 1:
MUC13 staining in non-mucinous cystic lesions and IPMNs. A. Comparison of MUC 13 immunohistochemical expression in non-mucinous cystic lesions (median 40, IQR 20 – 90) vs IPMN (median 210, IQR 170 – 250, p < 0.001). Representative images of MUC13 immunohistochemical staining for a serous cystic lesion (non-mucinous) (B) and an IPMN (C). (10X magnification).
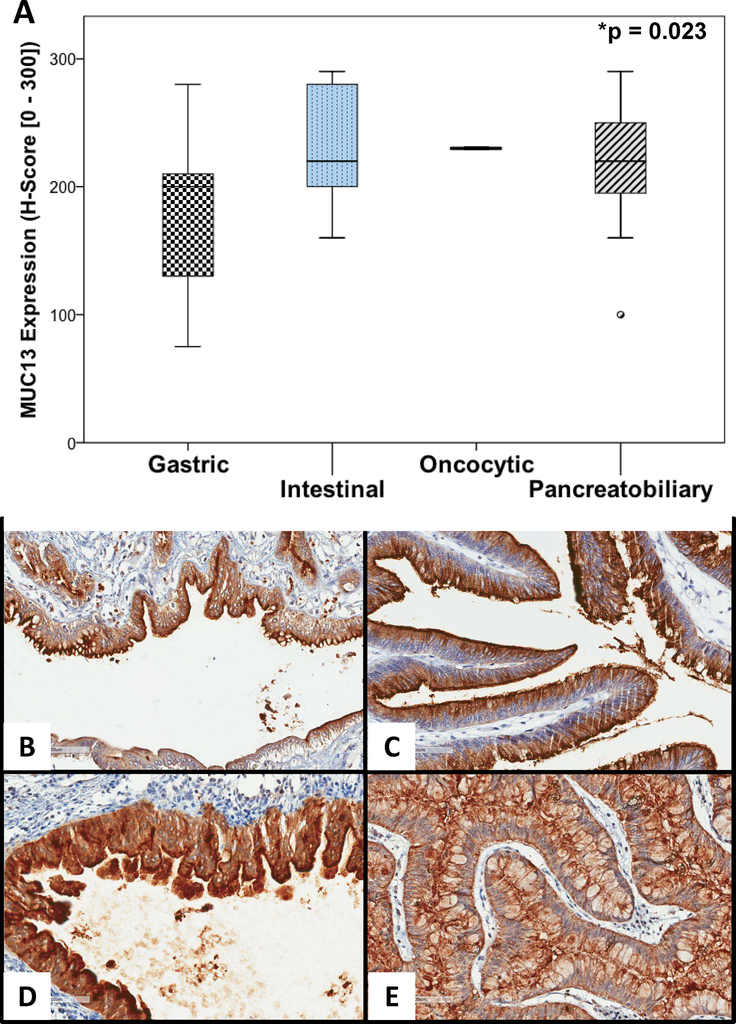
On examination by ductal involvement, no significant difference in MUC13 expression was observed between main duct (median 210, IQR 170 – 252.5), branch duct (median 200 IQR 120 – 230), or mixed duct (median 225, IQR 195 – 272.5) subtypes (p = 0.362). Specimens with intestinal and pancreatobiliary histologic features displayed greater MUC13 expression (median 220 for both) than the gastric subtype (median 200) (p = 0.023) (See Figure 2A). Only one specimen was classified as oncocytic (H-score 230), limiting the ability to calculate summary descriptive statistics for this group. Representative immunohistochemical staining patterns for the different subtypes are displayed in Figure 2B – E.
Figure 2:
MUC13 expression for IPMN histologic subtypes. Comparison of median MUC13 immunohistochemical staining intensity among the four IPMN histologic subtypes (A) with representative images of MUC13 staining patterns in gastric (B), intestinal (C), oncocytic (D), and pancreatobiliary (E) subtypes. (20X magnification).
Comparison of high and low-risk IPMNs
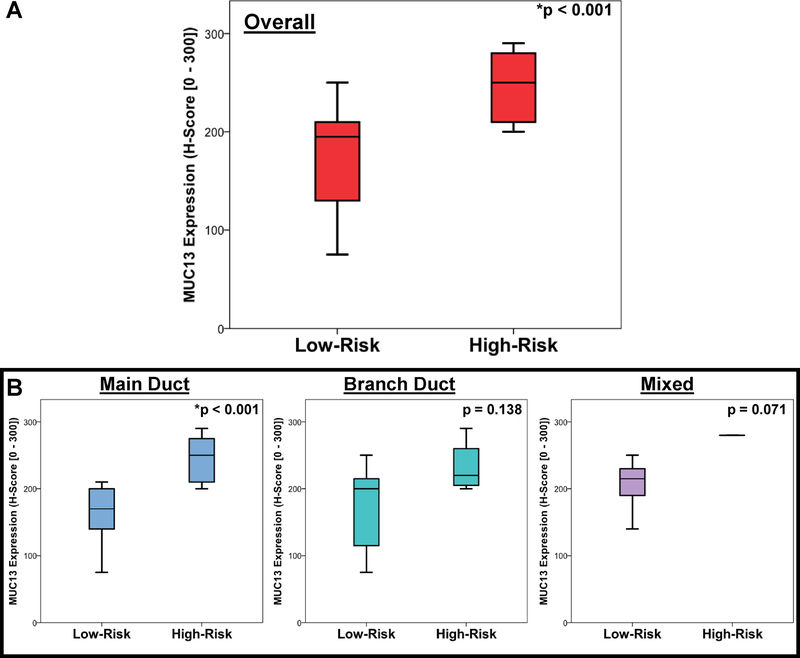
After review, 28 (57.1%) IPMNs were classified as low-risk, and 21 (42.9%) were considered high-risk (10 – HGD, 11 – invasive component). MUC13 expression was noted to be significantly greater among high-risk lesions (median 250 vs 195, p < 0.001) (Figure 3A). Upon sub-stratification, this trend held true for main, branch, and mixed duct variants, although this difference was only statistically significant for main duct IPMNs (p < 0.001) (Figure 3B).
Figure 3:
MUC13 immunohistochemical expression in invasive or high-grade IPMNs compared to low-risk IPMNs. (A) Comparison in all lesions combined (High-risk lesions, median 250 [IQR 210 – 280] vs low risk lesions, median 195 [IQR 125 – 210], p < 0.001) and (B) stratified by pattern of ductal involvement.
On comparison of low- and high-risk tumors (Table 2), no difference was observed with regard to patient demographics or tumor location within the gland. Other than MUC13 expression, only tumor size was significantly associated with high-risk lesions (median 2.7 cm vs 1.7 cm, p = 0.029). We did note that a greater proportion of high-risk tumors involved the main pancreatic duct, although this did not achieve statistical significance.
Table 2:
Comparison between high-risk and low-risk IPMN lesions.
| Characteristic | Low-risk lesion (n = 28) | High-risk lesion* (n = 21) | p value |
|---|---|---|---|
| Age, median (IQR) | 64(61–71) | 70 (64 – 73) | 0.346 |
| Gender, n (%) | 1.000 | ||
| Male | 12 (42.9) | 9 (42.9) | |
| Female | 16(57.1) | 12(57.1) | |
| Race, n (%) | 0.401 | ||
| White | 19 (70.4) | 17(81.0) | |
| Black | 8 (29.6) | 4(19.0) | |
| Tumor location, n (%) | 0.736 | ||
| Proximal (head/neck/uncinate) | 17 (60.7) | 15 (71.4) | |
| Distal (body/tail) | 9(32.1) | 5 (23.8) | |
| Diffuse/multifocal | 2(7.1) | 1 (4.8) | |
| Tumor size (cm), median (IQR) | 1.7(1.5–2.6) | 2.7(1.8–4.1) | 0.029 |
| Ductal involvement, n (%) | 0.083 | ||
| Main duct | 11 (39.3) | 15 (71.4) | |
| Branch duct | 11 (39.3) | 4(19.0) | |
| Mixed | 6(21.4) | 2 (9.5) | |
| Histologic subtype, n (%) | 0.126 | ||
| Intestinal | 5 (17.9) | 8(38.1) | |
| Gastric | 17 (60.7) | 6 (28.6) | |
| Oncocytic | 1 (3.6) | 0 (0.0) | |
| Pancreatobiliary | 5 (17.9) | 6 (28.6) | |
| Indeterminate | 0 (0.0) | 1 (4.8) | |
| MUC13 expression, median (IQR) | 212.5 (120–255) | 270(210–290) | 0.031 |
High grade dysplasia or invasive component found on final pathology; IQR – interquartile range
Discussion
Currently, the accurate detection of high-grade dysplasia and/or invasive carcinoma (high-risk lesions) in mucinous pancreatic cysts remains imperfect. To our knowledge, this is the first evaluation of MUC13 in IPMNs, and highlights its potential as a biomarker for high-risk IPMNs. Our analysis revealed MUC13 to be highly expressed in IPMNs compared to nonmucinous cysts and showed greater rates of expression among high-risk lesions. Among the pathologic features examined in this study, only MUC13 expression and tumor size were associated with high-risk IPMNs.
Recently a variety of biomarkers have been investigated in the evaluation of pancreatic cysts.[22–27] Cyst fluid CEA, the only routinely utilized marker to distinguish mucinous cysts, has an estimated accuracy near 80%.[28,29] While cyst fluid CEA may be beneficial in differentiating mucinous pancreatic cysts, it has not been effective in predicting the degree of dysplasia or invasive cancer within a lesion.[30] Several studies have demonstrated increased accuracy for mucinous pancreatic cyst differentiation through the detection of glycosylated variants of mucins, specifically MUC5AC.[24,25,31] Our previous studies of the role of MUC13 in PDAC led us to consider its use for distinguishing high-risk pathologic features in IPMNs as we have previously noted high levels of its expression in PDAC tissues. In addition, when comparing PanIN I to PanIN II/III lesions we have noted an increase in MUC13 expression on immunohistochemical analysis which suggests it may have a role in malignant transformation.[17,18] Thus, in a similar vein, MUC13 assessment may be helpful in distinguishing low-grade dysplasia from high-grade dysplasia and invasive disease among IPMNs. In the current study we noted significantly greater MUC13 expression among high-risk main duct IPMNs compared to their low-grade counterparts. However, most of these patients are referred for resection based solely on their pattern of ductal involvement. Decisions regarding branch duct IPMNs on the other hand are much less clear, as current algorithms and biomarkers have been unable to determine optimal resection criteria. There appeared to be a trend toward greater MUC13 expression among high-risk side branch variants in our analysis but this did not achieve statistical significance. In the future, we hope to examine this subgroup further in a larger sample of specimens to determine if this observation is significant.
Previous reports have explored additional mucins in high-risk IPMNs with varying results.[30] In an immunohistochemical analysis of 51 IPMN tissues, Luttges et al. found that all IPMNs were positive for MUC5 and a majority were also positive for MUC2 while at the same time displaying very little MUC1 expression.[32] These authors also observed uniform expression of MUC1 and MUC5 among 35 examined ductal adenocarcinoma tissues. Nakamura et al. also observed MUC2 expression in IMPNs based on IHC, but found that only 18/50 tissues studied were positive for its expression.[33] However, they did note that all tissues with MUC2 expression (100%) were from main duct type lesions whereas 21 of 32 (65%) IPMNs negative for MUC2 expression were branch duct variants. Accordingly, the incidence of carcinoma was significantly higher among MUC2 positive IPMNs (89% vs 19%, p < 0.001). In another report from Japan, Yonezawa et al studied the immunohistochemical expression patterns for MUC1 and MUC2 among 46 invasive ductal carcinomas and 16 IPMNs.[34] In this report, almost all invasive carcinomas expressed MUC1 (>95%), whereas MUC2 staining was not seen in any invasive carcinoma tissues. Furthermore, the authors noted that MUC1 expression was low in the non-invasive growth areas of IPMN tissues, yet positive in the invasive growth areas leading them to conclude that the invasive regions of IPMNs acquire a MUC1 expression pattern similar to invasive carcinoma. In a study of cyst fluid mucin expression by enzyme-linked immunosorbent assay (ELISA), Maker et al. found MUC1 expression to be very low among 40 resected IPMNs, with no correlation between MUC1 level and degree of dysplasia present.[35] However, the authors did note significantly greater levels of MUC2 and MUC4 expression in high-risk cysts (HGD or invasion). Although multiple studies have described the differential expression of mucins in IPMN tissue and cyst fluid, no consensus exists as to how best utilize them in the assessment and stratification of pancreatic cysts. Ultimately, the most accurate analysis of pancreatic cysts will likely involve an assay of multiple molecular targets and biomarkers.[30]
The current study is limited by its retrospective nature and relatively small sample size. In addition, the semi-quantitative analysis of immunohistochemical staining via H-score is somewhat subjective in nature. This method is thoroughly described in the literature, reproducible, and familiar to most trained pathologists.[36,37] In addition, we are currently examining the reliability and validity of digital image analysis as a more objective means of assessing MUC13 immunohistochemical expression.
At the onset of this study, we had hoped to correlate MUC13 expression with other factors commonly assessed preoperatively in IPMNs such as cyst fluid CEA, cytology, amylase, and imaging characteristics such as mural nodularity. Unfortunately, this data was not available in a significant percentage of patients. We recognize the importance of assessing MUC13 expression in association with other risk factors and plan to assess these relationships prospectively. Finally, we were only able to assess the tissue expression of mucin in this study, whereas ideally, we would like to measure MUC13 expression in cyst fluid aspirates, as is currently done for CEA, amylase, and cytologic analysis. At present, we are prospectively accumulating and analyzing these specimens.
Conclusion
MUC13 immunohistochemical staining was significantly greater in IPMN tissue compared to non-mucinous cyst controls in this analysis. Among IPMNs, high-risk main duct lesions displayed greater MUC13 expression compared to low-risk main duct IPMNs, suggesting that MUC13 may be able to distinguish these lesions preoperatively. Further studies, including assessment of larger numbers of side branch IPMNs and prospective evaluation of pancreatic cyst fluid aspirates are warranted to investigate the role of MUC13 in mucinous pancreatic cysts.
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (R01 CA206069, CA204552, CA210192, CA142736) (S.C.C.) along with the University of Tennessee Health Science Center College of Pharmacy (2016) and Dean’s Seed/Instrument Grants (2017) (S.C.C.). The authors would also like to acknowledge the continued financial support of the Herb Kosten Foundation and a generous donation from the Dermon family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None declared.
Authors have nothing to disclose.
References:
- [1].McDonald JM, Williard W, Mais D, Beitler A. The incidence of intraductal papillary mucinous tumors of the pancreas. Curr Surg 2000;57:610–4. doi: 10.1016/S0149-7944(00)00392-5. [DOI] [PubMed] [Google Scholar]
- [2].Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Winter JM, Cameron JL, Lillemoe KD, Campbell KA, Chang D, Riall TS, et al. Periampullary and Pancreatic Incidentaloma: A Single Institution’s Experience With an Increasingly Common Diagnosis. Ann Surg 2006;243:673–83. doi: 10.1097/01.sla.0000216763.27673.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary mucinous neoplasms of the pancreas. Cancer 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- [5].Tanaka M, Chari S, Adsay V, Carlos Castillo F-D, Falconi M, Shimizu M, et al. International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- [6].Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang J-Y, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- [7].Vege SS, Ziring B, Jain R, Moayyedi P, Adams MA, Dorn SD, et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- [8].Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, et al. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol 2001;96:1429–34. doi:S0002927001023577 [pii]\r10.1111/j.1572-0241.2001.03794.x. [DOI] [PubMed] [Google Scholar]
- [9].Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol 2008;15:3187–92. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- [10].Wilson GC, Maithel SK, Bentrem D, Abbott DE, Weber S, Cho C, et al. Are the Current Guidelines for the Surgical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas Adequate? A Multi-Institutional Study. J Am Coll Surg 2017;224:461–9. doi: 10.1016/j.jamcollsurg.2016.12.031. [DOI] [PubMed] [Google Scholar]
- [11].Wong J, Weber J, A. Centeno B, Vignesh S, Harris CL, Klapman JB, et al. High-Grade Dysplasia and Adenocarcinoma Are Frequent in Side-Branch Intraductal Papillary Mucinous Neoplasm Measuring Less than 3 cm on Endoscopic Ultrasound. J Gastrointest Surg 2013;17:78–85. doi: 10.1007/s11605-012-2017-0. [DOI] [PubMed] [Google Scholar]
- [12].Allen PJ, D’Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, et al. A Selective Approach to the Resection of Cystic Lesions of the Pancreas. Ann Surg 2006;124:237–47. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fong ZV, Fernández-del Castillo C. Intraductal Papillary Mucinous Neoplasm of the Pancreas. Surg Clin North Am 2016;96:1431–45. doi: 10.1016/j.suc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- [14].Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khan S, Sikander M, Ebeling MC, Ganju A, Kumari S, Yallapu MM, et al. MUC13 interaction with receptor tyrosine kinase HER2 drives pancreatic ductal adenocarcinoma progression. Oncogene 2016;36:1–10. doi: 10.1038/onc.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chauhan SC, Ebeling MC, Maher DM, Koch MD, Watanabe A, Aburatani H, et al. MUC13 Mucin Augments Pancreatic Tumorigenesis. Mol Cancer Ther 2012;11:24–33. doi: 10.1158/1535-7163.MCT-11-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, et al. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget 2014;5:7599–609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khan S, Zafar N, Khan SS, Setua S, Behrman SW, Stiles ZE, et al. Clinical significance of MUC13 in pancreatic ductal adenocarcinoma. HPB 2018:1–10. doi: 10.1016/j.hpb.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aloysius MM, Zaitoun AM, Awad S, Ilyas M, Rowlands BJ, Lobo DN. Mucins and CD56 as markers of tumour invasion and prognosis in periampullary cancer. Br J Surg 2010;97:1269–78. doi: 10.1002/bjs.7107. [DOI] [PubMed] [Google Scholar]
- [20].Ansari D, Urey C, Gundewar C, Bauden MP, Andersson R. Comparison of MUC4 expression in primary pancreatic cancer and paired lymph node metastases. Scand J Gastroenterol 2013;48:1183–7. doi: 10.3109/00365521.2013.832368. [DOI] [PubMed] [Google Scholar]
- [21].McCarty KS, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res 1986;46:4244–8. [PubMed] [Google Scholar]
- [22].Ivry SL, Sharib JM, Dominguez DA, Roy N, Hatcher SE, Yip-Schneider MT, et al. Global Protease Activity Profiling Provides Differential Diagnosis of Pancreatic Cysts. Clin Cancer Res 2017;23:4865–74. doi: 10.1158/1078-0432.CCR-16-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zikos T, Pham K, Bowen R, Chen AM, Banerjee S, Friedland S, et al. Cyst Fluid Glucose is Rapidly Feasible and Accurate in Diagnosing Mucinous Pancreatic Cysts. Am J Gastroenterol 2015;110:909–14. doi: 10.1038/ajg.2015.148. [DOI] [PubMed] [Google Scholar]
- [24].Cao Z, Maupin K, Curnutte B, Fallon B, Feasley CL, Brouhard E, et al. Specific Glycoforms of MUC5AC and Endorepellin Accurately Distinguish Mucinous from Nonmucinous Pancreatic Cysts. Mol Cell Proteomics 2013;12:2724–34. doi: 10.1074/mcp.M113.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sinha J, Cao Z, Dai J, Tang H, Partyka K, Hostetter G, et al. A Gastric Glycoform of MUC5AC Is a Biomarker of Mucinous Cysts of the Pancreas. PLoS One 2016;11:e0167070. doi: 10.1371/journal.pone.0167070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carr RA, Yip-Schneider MT, Dolejs S, Hancock BA, Wu H, Radovich M, et al. Pancreatic Cyst Fluid Vascular Endothelial Growth Factor A and Carcinoembryonic Antigen: A Highly Accurate Test for the Diagnosis of Serous Cystic Neoplasm. J Am Coll Surg 2017;225:93–100. doi: 10.1016/j.jamcollsurg.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maker AV, Katabi N, Qin L-X, Klimstra DS, Schattner M, Brennan MF, et al. Cyst Fluid Interleukin-1 (IL1 ) Levels Predict the Risk of Carcinoma in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin Cancer Res 2011;17:1502–8. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- [29].Cizginer S, Turner B, Bilge a R, Karaca C, Pitman MB, Brugge WR. Cyst Fluid Carcinoembryonic Antigen Is an Accurate Diagnostic Marker of Pancreatic Mucinous Cysts. Pancreas 2011;40:1024–8. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- [30].Maker AV, Carrara S, Jamieson NB, Pelaez-Luna M, Lennon AM, Dal Molin M, et al. Cyst Fluid Biomarkers for Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Critical Review from the International Expert Meeting on Pancreatic Branch-Duct-Intraductal Papillary Mucinous Neoplasms. J Am Coll Surg 2015;220:243–53. doi: 10.1016/j.jamcollsurg.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Haab BB, Porter A, Yue T, Li L, Scheiman J, Anderson MA, et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann Surg 2010;251:937–45. doi: 10.1097/SLA.0b013e3181d7738d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lüttges J, Zamboni G, Longnecker D, Klöppel G. The Immunohistochemical Mucin Expression Pattern Distinguishes Different Types of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Determines Their Relationship to Mucinous Noncystic Carcinoma and Ductal Adenocarcinoma. Am J Surg Pathol 2001;25:942–8. doi: 10.1097/00000478-200107000-00014. [DOI] [PubMed] [Google Scholar]
- [33].Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol 2002;197:201–10. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]
- [34].Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, et al. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int 1998;48:319–22. [DOI] [PubMed] [Google Scholar]
- [35].Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McCarty KS, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- [37].Kasajima A, Papotti M, Ito W, Brizzi MP, La Salvia A, Rapa I, et al. High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum Pathol 2017. doi: 10.1016/j.humpath.2017.11.008 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]