SUMMARY
Background:
Pregabalin is a calcium channel α2δ ligand that modifies visceral hypersensitivity in IBS patients. Clinical data for pregabalin in IBS are lacking.
Aim:
To test the efficacy of pregabalin on gastrointestinal symptoms in IBS patients
Methods:
A double-blind, placebo-controlled trial was performed. Adults meeting IBS Rome III criteria with ≥3 pain attacks per month were randomized to pregabalin 225 mg v. placebo twice daily for 12 weeks. Questionnaires were completed weekly. The primary endpoint was average pain Bowel Symptom Scale (BSS) scores weeks 9–12. An intention-to-treat analysis of covariance evaluated treatment effects on quantitative endpoints, adjusting for age and gender. Adequate relief and change in pain score were assessed using a chi-square test.
Results:
85 patients were recruited and randomized. Sample characteristics include: mean age 39.4 (sd=14.6); 73 (86%) female; 37 (44%) IBS-D, 29 (35%) IBS-M, 18 (21%) IBS-C. The pregabalin arm had lower average pain-BSS scores weeks 9–12 (25 v. 42, p=0.008). Compared with placebo, the overall IBS BSS severity score was lower in the pregabalin arm (26 v. 42, p=0.009). Differences were observed for the diarrhoea-BSS and bloating-BSS scores (p=0.049 and 0.016, respectively). No differences between groups were seen for constipation-BSS scores. Adequate relief was not different between the 2 arms (46% v. 36%, p=0.35). 63% pregabalin v. 45% placebo had a change in pain score ≥30 at week 12 from baseline (p=0.10). Post-treatment IBS-QoL scores did not differ between groups.
Conclusion:
This trial suggests that pregabalin may be beneficial for IBS abdominal pain, bloating and diarrhoea.
Keywords: irritable bowel syndrome, abdominal pain, visceral hypersensitivity
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterized by abdominal pain and symptoms of constipation, diarrhoea, or both. The pain is associated or relieved by having a bowel movement and is associated with change in stool consistency or frequency. Several studies have shown that abdominal pain is one of the key drivers of health care seeking.1, 2 Symptoms greatly impact patients’ ability to go to school and/or work3, and health-related quality of life has been shown to be lower in IBS patients.4 Visceral hypersensitivity is also a common feature observed in IBS patients.5 Treatment options for managing IBS-related abdominal pain are available6. Antispasmodics as well as tricyclic antidepressants as neuromodulators are frequently utilized. Newer IBS therapies such as linaclotide, eluxadoline, and a low FODMAP diet are also available.6–8 However, these therapies are not effective for all patients, and patients and providers then struggle to improve pain without resorting to addictive therapies such as opioid drugs, which should almost never be prescribed for IBS pain6.
Pregabalin is a structural derivative of the neurotransmitter, gamma aminobutyric acid. It binds the α2δ subunit on voltage-dependent calcium channels on neurons expressed in the central nervous system. Pregabalin has known analgesic as well as anxiolytic effects. As such, it is FDA-approved for fibromyalgia as well neuropathic pain conditions such as herpetic neuralgia and diabetic neuropathy. Pregabalin has also been used to treat generalized anxiety disorder, and there is literature showing that it may help with other pain conditions such as postoperative pain and chronic pancreatitis.9, 10
Data on pregabalin’s effects on visceral pain and IBS are limited but suggest the drug does reduce gut visceral hypersensitivity.11–13 Because of its known effects on pain, we postulated that pregabalin would decrease bowel symptoms—particularly IBS-related abdominal pain or discomfort. As such, this study was conceived and designed with the aim to collect clinical data on pregabalin’s effects on IBS symptoms.
METHODS
The study was approved by our Institutional Review Board. The trial was registered at clinicaltrials.gov (NCT00977197).
Subjects
Subjects were English-speaking U.S. residents aged 18–70 years who had a clinical diagnosis of IBS, met Rome III criteria for IBS and had at least 3 pain attacks a month of intensity exceeding 50 out of 100 as measured by a 10 cm visual analog scale. Participants were recruited through community, clinical and research advertisement. Participants also had to experience one pain attack exceeding 50/100 in intensity during the 2-week screening period.
Exclusion criteria included concurrent gastrointestinal diagnoses with symptoms that could mimic IBS, severe depression (Hospital Anxiety and Depression Score (HADS) score ≥15), history of psychotic disorders, current or planned pregnancy, lactating, significant medical comorbidities, recent alcohol or substance dependence, and cognitive impairment. In addition, patients who used pregabalin within 30 days or had a known pregabalin allergy were excluded. Also excluded were patients on drugs that could interact with, mimic effects, or exacerbate anticipated side effects (e.g., rosiglitazone, pioglitazone, narcotic medications, anxiolytics, non-narcotic pain medications, mexilitine, dextromethorphan, sleeping pills). IBS-specific medications such as alosetron were not allowed. Participants were asked to refrain from use of over-the-counter medications such as laxatives and antidiarrhoeals but were allowed to continue with documentation of use.
Study Design
The study design was that of a randomized, double-blind, placebo-controlled, two-arm parallel group conducted at a single institution in the Upper Midwest as well as an associated health system site. The recruitment period was March 2010 through October 2014.
Screening Period
At the initial and only study visit, patients were screened for inclusion and exclusion criteria. If eligible, there was a pretreatment 2-week screening period. Patients completed a daily symptom diary that included a rating of abdominal pain. If a patient experienced at least 1 pain attack with intensity equaling or exceeding 50 on a scale of 0–100, the patient was then randomized at week 0.
Randomization and Treatment Phase
A dynamic allocation randomization method14 was used to randomize subjects to one of two treatment arms balanced on symptom subtype (constipation, diarrhoea, mixed) and fibromyalgia status (presence, absence) at week 0. Balancing on IBS subtype was performed to increase the likelihood of comparable distributions between treatment arms. Because Pregabalin is an approved treatment for fibromyalgia and because this condition is a common comorbid condition with IBS, balancing on fibromyalgia status was performed to increase the likelihood of comparable distribution between treatment arms because fibromyalgia can be a common comorbid condition with IBS and we wanted symptom improvement to not be due to imbalanced representation and improvement wellbeing to be attributed to improvement in fibromyalgia symptoms. At the study start, randomization schedules were generated by the statistician, and the schedules were concealed from the study team. At time of subject randomization, the coordinator contacted the statistician with the gender, IBS subtype, and fibromyalgia status and a study subject number was then provided. A prescription with study subject number was faxed to the research pharmacy. A research pharmacist dispensed study drugs in bi-weekly dose packs directly to the patient. Subjects, coordinators, and co-investigators were blinded to treatment arm assignments. Based on the company’s prescribing recommendations for fibromyalgia dosing,15participants randomized to the active treatment group received escalating doses for the first week (75 mg twice daily for 3 days, then 150 mg twice daily for 3 days), 10 weeks of 225 mg twice daily, and tapering doses for week 12 (150 mg twice daily for 3 days, then 75 mg twice daily for 3 days). Participants in the placebo arm received matching placebo. Drug and identical placebo was provided by Pfizer Pharmaceuticals.
Questionnaires
During the treatment phase, patients were asked to complete weekly, self-administered questionnaires. Validated instruments administered weekly included: Bowel Disease Questionnaire (modified for Rome III),16 Bowel Symptom Score, 17 adequate relief of IBS symptoms,18 and IBS quality of life.19 In addition to the weekly questionnaires, the Hospital Anxiety and Depression Scale (HADS) was also included in the study questionnaire biweekly20; the Medical Outcomes Study Sleep Scale was included at baseline, week 4, 8, and 12.21 The Bowel Disease Questionnaire was administered at study entry to evaluate for presence of IBS symptoms and subtype patients into IBS subtypes. The Bowel Symptom Score is a 100 mm visual analog scale for each IBS symptom (overall IBS, pain or discomfort, constipation, diarrhoea, bloating) with range of 0–100. “Did you have adequate relief of your IBS symptoms over the last week?” was asked as a global endpoint. The IBS Quality of Life is a 34-item instrument that yields an overall quality of life score as well as scores for 8 domains (health worry, dysphoria, food avoidance, social reaction, interference with activity, sexual, body image, and relationships). Scores were transformed to a 0–100 point scale with 0 indicating worst quality of life, 100 indicating best quality of life. The HADS is a well-validated 14 item instrument with 7 anxiety items and 7 depression items yielding a score range of 0–21 for anxiety and depression.
Data management
Study data were collected and managed using REDCap electronic data capture tools hosted at Mayo Clinic. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.22 All co-authors had access to the study data and had reviewed and approved the final manuscript
Study endpoints
The a priori primary endpoint for this study was the mean bowel symptom scale score for abdominal pain or discomfort over the last 4 weeks of treatment. Pre-specified secondary endpoints were 1) mean overall and individual (constipation, diarrhoea, bloating) bowel symptom scale scores over the last 4 weeks of treatment; 2) mean bowel symptom scale scores at week 12 of treatment; 3) adequate relief of IBS at least 50% of the last 4 weeks of therapy; 4) ≥30 point change in bowel symptom scale pain or discomfort at week 12 compared to baseline; 5) IBS quality of life scores at week 12. The 30 point reduction in pain or discomfort was selected because it was thought to represent meaningful change, which is consistent with FDA Guidance that was subsequently published.23
Statistical analysis
An intention-to-treat analysis of covariance (ANCOVA) was used to analyze effects of treatment on quantitative endpoints, adjusting for age and gender, and for IBS quality of life scores, baseline values. Missing values were imputed using the overall mean value for quantitative data; responses were assumed to be “no” for the discrete endpoints. Analyses were repeated by IBS subtype (constipation-predominant IBS [IBS-C]; diarrhoea-predominant IBS [IBS-D]; mixed type IBS [IBS-M]). A p-value < 0.05 was considered statistically significant.
Assuming a standard deviation of the pain bowel symptom scale score of 20 (based on baseline values), the study had ≥80% power (α=0.05) to detect the difference in pain bowel symptom scale score of 44 v. 56 (24%) for n=45 versus n=45, 43 v. 56 (26%) for n= 39 versus n=39, 42 v. 56 (29%) for n=34 versus n=34. Assuming a standard deviation of the overall bowel symptom scale score of 23, the study had ≥80% power (α=0.05) to detect the differences in overall bowel symptom scale score of 30 v. 44 (47%) for n=45 versus n=45, 30 v. 45 (50%) for n=40 versus n=40, 30 v. 46 (53%) for n=35 versus n=35.
RESULTS
Recruitment
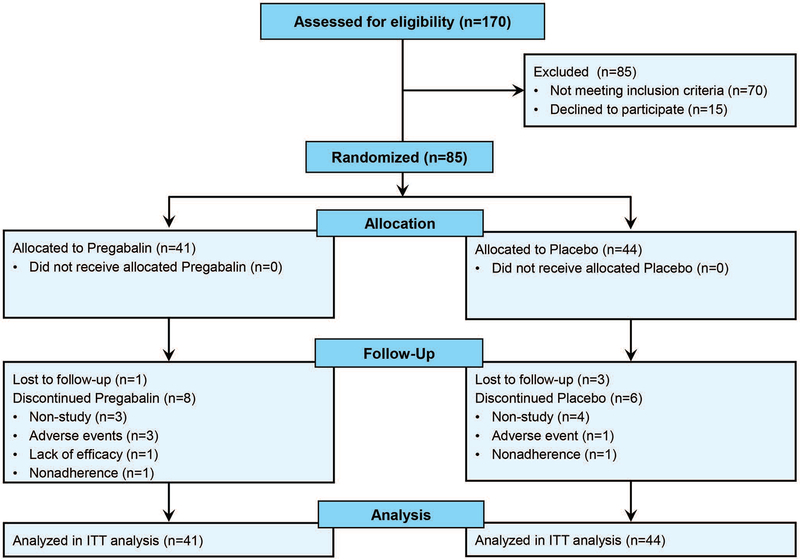
In total, 85 of a goal of 100 eligible patients were recruited and randomized. Recruitment ceased due to slow subject recruitment. 41 were randomized to the pregabalin arm, and 44 were randomized to the placebo arm. 9 subjects per arm dropped out during the study: 7 discontinued for reasons unrelated to the study and 4 discontinued due to adverse events, 4 were lost to follow up, 1 discontinued due to lack of efficacy, and 2 discontinued for non-compliance (Figure 1).
Figure 1.
CONSORT Recruitment + Enrollment Flow Diagram. 85 subjects were eligible for randomization by dynamic allocation balanced for IBS subtype and fibromyalgia status. Both treatment arms had 9 drop outs. Post-treatment data was available from 67 subjects, although all 85 were included in the intent-to-treat analysis.
Sample Characteristics
Sample characteristics for 85 subjects are outlined in Table 1. The mean age was 39.4 (s.d.=14.6), 86% female. IBS subtypes were 38 (45%) diarrhoea-predominant IBS, 29 (35%) mixed IBS, and 18 (21%) constipation-predominant IBS. Mean anxiety and depression scores were 4.7 (sd=3.7) and 5.2 (sd=3.8), respectively. Mean BSS scores for IBS overall were 57 (sd=20). Mean bowel symptom scale scores for pain, constipation, diarrhoea, and bloating were 56 (sd=20), 32 (sd=32), 45 (sd=31), and 56 (sd=24), respectively. The baseline IBS quality of life score was 65 (sd=20). No differences in these characteristics were observed between the two treatment arms.
Table 1.
Baseline sample characteristics
| Pregabalin | Placebo | p-value | |
|---|---|---|---|
| Subjects randomized, n | 41 | 44 | |
| Age, mean (sd) [n=85] | 42.4 (14.2) | 38.3 (15.2) | 0.212 |
| Female, n (%) [n=85] | 36 (88%) | 37 (84%) | 0.759 |
| IBS Type [n=85] | |||
| IBS-Diarrhoea, n (%) | 18 (44%) | 20 (45%) |
0.869 |
| IBS-Mixed, n (%) | 14 (34%) |
15 (35%) |
0.853 |
| IBS-Constipation, n (%) | 9 (22%) |
9 (20%) |
1.000 |
| Fibromyalgia, n (%) [n=85] | 10 (24%) | 7 (16%) | 0.419 |
| HADS Anxiety, mean (sd) [n=74] | 4.7 (3.5) | 4.6 (4.0) | 0.757 |
| HADS Depression, mean (sd) [n=74] | 5.1 (3.3) | 5.3 (4.3) | 0.712 |
| Baseline Overall IBS Severity BSS, mean (sd) [n=73] | 54 (21) |
59 (20) |
0.291 |
| Baseline Pain BSS, mean (sd) [n=74] | 56 (20) | 56 (20) | 0.910 |
| Baseline Diarrhoea BSS, mean (sd) [n=74] | 43 (30) | 46 (32) | 0.638 |
| Baseline Constipation BSS, mean (sd) | 30 (31) | 35 (33) | 0.649 |
| Baseline Bloating BSS, mean (sd) | 56 (20) | 59 (23) | 0.466 |
| Overall IBS QoL score, mean (sd) [n=74] | 66.6 (15.5) | 62.8 (23.1) | 0.733 |
HADS - Hospital Anxiety and Depression Scale, BSS – bowel symptom scale
No differences in characteristics were seen between the treatment arms. Numbers do not consistently equal 85 because patients either withdrew or were lost to follow-up before all baseline data were collected but had been randomized.
Study Outcomes
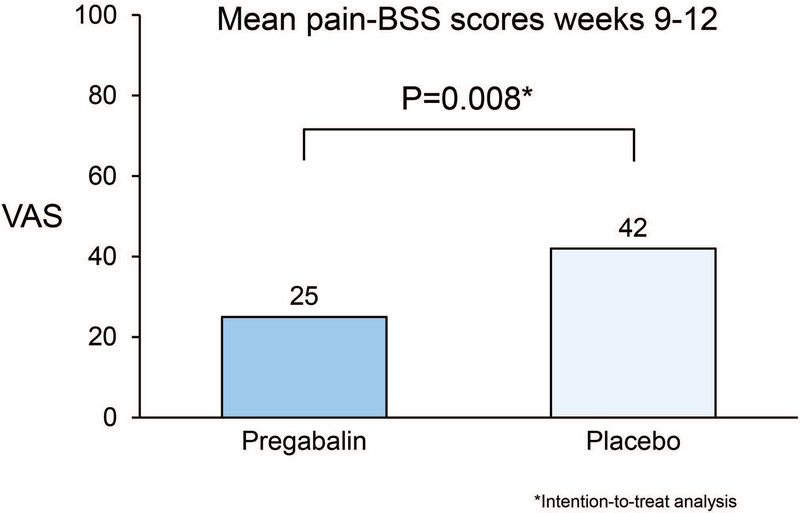
In comparing mean bowel symptoms scale pain or discomfort scores over weeks 9 to 12 (Figure 2), subjects receiving pregabalin reported lower pain scores than those receiving placebo (25 [sd=16] v. 42 [sd=27], p=0.008). Table 2 shows the mean bowel symptoms scale scores at weeks 9–12 and at week 12. In addition to subjects in the active treatment arm having better pain or discomfort bowel symptom scale scores, their overall IBS, diarrhoea, and bloating bowel symptom scale scores were lower than in the placebo arm (p=0.009, 0049, and 0.016, respectively). Findings were similar between the week 9 to 12 data compared with the end of treatment, week 12 BSS scores.
Figure 2.
Mean pain/discomfort Bowel Symptom Scores (BSS) weeks 9–12. Mean abdominal pain scores during the last 4 weeks of treatment were statistically higher in the placebo arm than the pregabalin arm.
Table 2.
Mean Bowel Symptom Scale (BSS) Scores at weeks 9–12 and at week 12
| Per-Protocol (PP) | Intention-to-Treat (ITT) | |||||
|---|---|---|---|---|---|---|
| Pregabalin N=29 |
Placebo N=35 |
PP p-value | Pregabalin N=41 |
Placebo N=44 |
ITT P-value | |
| Mean BSS scores weeks 9–12 (sd) | ||||||
| Pain-BSS | 25 (16) | 42 (27) | 0.013 | 28 (14) | 40 (24) | 0.008* |
| Overall-BSS | 26 (15) | 42 (26) | 0.014 | 29 (13) | 41 (23) | 0.009* |
| Diarrhoea-BSS | 17 (18) | 32 (26) | 0.022 | 19 (16) | 30 (23) | 0.049* |
| Constipation-BSS | 26 (27) | 22 (25) | N.S.. | 25 (22) | 23 (22) | N.S. |
| Bloating-BSS | 29 (23) | 44 (29) | 0.071 | 32 (20) | 42 (26) | 0.016* |
| Mean BSS scores week 12 (sd) | ||||||
| N=29 | N=34 | N=41 | N=44 | |||
| Pain-BSS | 26 (19) | 43 (30) | 0.041 | 29 (16) | 41 (27) | 0.020* |
| Overall-BSS | 28 (20) | 44 (29) | 0.036 | 31 (17) | 43 (25) | 0.024* |
| Diarrhoea-BSS | 17 (18) | 34 (30) | 0.038 | 19 (17) | 32 (26) | 0.030* |
| Constipation-BSS | 26 (30) | 24 (27) | N.S.. | 26 (25) | 24 (23) | N.S. |
| Bloating-BSS | 26 (24) | 45 (32) | 0.053 | 29 (21) | 43 (28) | 0.016* |
Pain, overall IBS, diarrhoea, and bloating scoreswere lower in the active treatment arm for the last 4 weeks of treatment as well as during the last week of treatment.
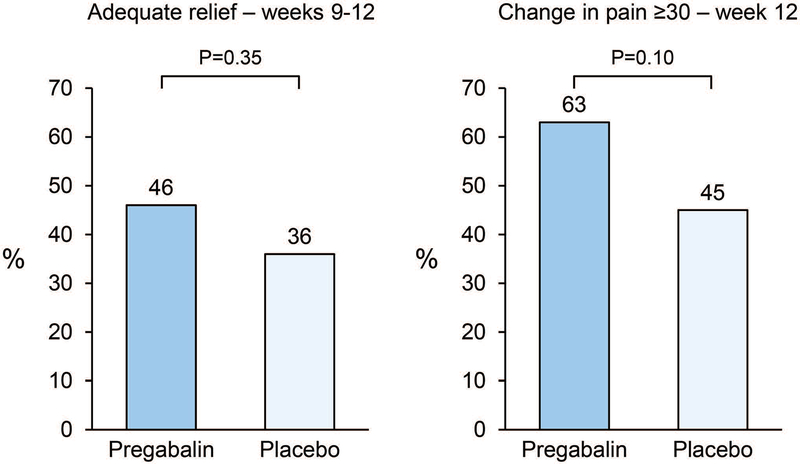
When evaluating adequate relief of IBS symptoms, 46% of patients receiving pregabalin reporting adequate relief for over 50% of weeks 9–12 compared to the 36% of the placebo arm, but this difference was not statistically significantly different (p=0.35) (Figure 3). When comparing the proportion of patients reporting greater than a 30 point change on a 100 point visual analog scale, 63% of pregabalin subjects v. 45% of placebo subjects met this criteria (p=0.10).
Figure 3.
Adequate relief of IBS and change in pain scores ≥30 at week 12.
a. A greater proportion of subjects receiving pregabalin reported adequate relief of IBS compared to subjects receiving placebo (p>0.05)
b. A trend was seen where a greater percentage of the pregabalin treatment arm had at least a 30-point improvement in their abdominal pain scores compared with the placebo arm.
Not adjusted for age and gender
Analyses were repeated by IBS subtype. Mean bowel symptom scale scores at week 9–12, week 12, adequate relief, or proportion reporting at least a 30 point decrease in pain at week 12 did not change for the IBS-C group. For the IBS-D group, pain/discomfort bowel symptom scale scores and overall IBS severity scores were lower in the pregabalin arm (23.0 (13.2 sd) v. 38.3 (27.2 sd), p=0.068; and 22.4 (11.7 sd) v. 38.4 (26.6 sd), p=0.052, respectively). However, no differences were seen for proportion reporting adequate relief (56% v. 47%, p=0.62) or proportion reporting at least 30 point decrease in pain (67% v. 53%, p=0.38). For the mixed-type IBS subjects, mean pain/discomfort bowel symptom scale scores were lower in the pregabalin arm (27.4 (21.3 sd) v. 48.3 (24.7 sd), p=0.093), and a greater proportion of patients in the pregabalin arm reported adequate relief of IBS symptoms for more than 50% of the last 4 weeks of treatment and a greater than 30 point pain score decrease (50% v. 20%, p=0.13 and 64% v. 27%, p=0.042, respectively).
IBS quality of life scores at week 12 are shown in Table 4. Overall IBS quality of life mean scores did not differ at week 12 between the pregabalin and placebo arm. Of the 8 domains, only the sexual domain was different between the two treatments arms (p=0.07). The other 7 domains—dysphoria, interference, body image, health worry, food avoidance, social reaction, relationships—were similar between the two groups.
Table 4.
Mean IBS Quality of Life scores at week 12 (sd)
| Pregabalin | Placebo | ITT p-value | |
|---|---|---|---|
| Overall | 69 (18) | 64 (25) | N.S. |
| Dysphoria | 72 (22) | 65 (29) | N.S. |
| Interference | 65 (20) | 61 (29) | N.S. |
| Body image | 69 (21) | 68 (26) | N.S. |
| Health worry | 72 (24) | 68 (22) | N.S. |
| Food avoidance | 47 (26) | 49 (34) | N.S. |
| Social reaction | 73 (25) | 65 (28) | N.S. |
| Sexual | 76 (27) | 67 (31) | N.S. |
| Relationships | 75 (19) | 71 (26) | N.S. |
IBS-related quality of life—overall as well as the eight domains—did not differ between the two treatment arms at the end of treatment.
Other Outcomes
Besides the primary and secondary outcomes, several other outcomes were evaluated. Anxiety and depression scales decreased slightly at week 12 compared to week 0, but did not differ between the two treatment arms (p=0.83 and p=0.55, respectively). Sleep scores did not change or changed nominally from baseline to week 12. For example, the Medical Outcomes Study Sleep Scale Sleep Problems Index 1 scores were 40.1 at baseline and 36.44 at week 12 in the pregabalin arm, and 43.3 at baseline and 41.9 at week 12 in the placebo arm. The Sleep Problems Index 2 scores were 42.1at baseline and 36.9 at week 12 in the pregabalin arm and 45.1 at baseline and 42.8 at week 12 in the placebo arm
Adverse Events
Adverse events were collected from all individuals randomized. Adverse events were common in both treatment groups. 28 (68%) of the pregabalin arm reported a side effect; 24 (55%) of the placebo arm reported a side effect (p=0.19). The more common side effects are summarized in Table 3. Gastrointestinal symptoms were common in both treatment arms, but did not differ between active and placebo arm, except for constipation which was slightly more common in the pregabalin arm (p=0.10). As anticipated, neurological symptoms—such as blurred vision, dizziness, altered sensorium—were more common in the pregabalin arm than the placebo arm. One subject died for reasons unrelated to the study.
Table 3.
Common adverse events in the treatment arms
| Pregabalin | Placebo | p-value | |
|---|---|---|---|
| Total | 28 (68%) | 24 (55%) | N.S. |
| Diarrhoea | 6 (15%) | 7 (16%) | N.S. |
| Abdominal pain | 13 (32%) | 13 (30%) | N.S. |
| Upset stomach | 4 (10%) | 1 (2%) | N.S. |
| Constipation | 9 (22%) | 4 (9%) | N.S. |
| Nausea | 6 (15%) | 2 (5%) | N.S. |
| Fullness | 2 (5%) | 0 (0%) | N.S. |
| Blurred vision | 6 (15%) | 1 (2%) | 0.05 |
| Dizzy | 13 (32%) | 2 (5%) | 0.01 |
| High or tipsy | 4 (10%) | 0 (0%) | 0.05 |
Side effects were comparable between the pregabalin and placebo arms except blurred vision, dizziness, and feeling high/tipsy which were more common in the active treatment arm.
DISCUSSION
This clinical trial evaluated pregabalin’s effect on IBS symptoms in IBS patients and suggests that pregabalin has beneficial effects on overall IBS symptoms as well as IBS-related abdominal pain, diarrhoea, and bloating. Importantly, for our primary outcome evaluating its effect on abdominal pain and discomfort, patients receiving pregabalin reported lower pain scores than those receiving placebo (25 v. 42, p=0.008). One of our secondary endpoints of a trend with a decrease in pain scores of at least 30 points also may support its efficacy on IBS-related abdominal pain (p=0.10). The onset of symptom relief was rapid within the first week of treatment (data not shown) and persisted throughout the 12 weeks of treatment.
Pregabalin alters visceral sensation in animal models and in patients with IBS, and this may explain its mechanism of action. In the guinea pig, intrinsic primary afferent neurons express α2δ1, and pregabalin appeared to bind these neurons.24 In rats, pregabalin appeared to decrease viscerosomatic and autonomic responses to colorectal distension.11 In colonic mouse models of post inflammatory visceral hypersensitivity, pregabalin significantly reduced colonic distention induced sensation.25 Iturrino, et al. showed that a single 200mg dose of pregabalin in healthy volunteers resulted in a 25% reduction in gas and pain sensation ratings in healthy volunteers.13
The first data in IBS patients was collected in a study by Houghton, et al.12 In this study, 26 IBS patients underwent rectal barostat testing before and after 3 weeks of pregabalin or placebo. Patients receiving pregabalin reported increased sensory thresholds for first sensation, desire to defecate, and pain. Furthermore, increased rectal compliance was also seen. A trend for lower average daily pain scores in the pregabalin v. placebo arm was seen (p=0.068). However, pregabalin’s impact on other IBS symptoms was not collected. More recently, Iturrino studied the effects of a single 200mg dose on symptoms and colonic sensation thresholds and compliance in 18 patients with IBS-C and observed no effects on these outcomes.26 We believe our study results complement the findings of these previous studies, but enhancements and strengths of our study include that our study sample size was larger, treatment duration was longer, our study focused on IBS symptoms rather than GI physiology, and our study sample included all IBS subtypes.
The maximal benefit appeared to be for diarrhoea-predominant IBS and mixed-typed IBS, but not constipation-predominant IBS in the subgroup analysis. In the sample overall, pregabalin did not appear to impact constipation scores. However, our constipation-predominant IBS subgroup was the smallest of the three groups, so it is conceivable that an effect would have been observed had a larger group been included. However, this lack of effect may be a real finding in that constipation is a known side effect of pregabalin, and Iturrino et al’s small study of IBS-C patients also found no apparent positive benefit.26 However, our study did not attempt to actively treat constipation, and it is conceivable that had their bowel regimen been more actively managed with stool softeners or laxatives, that a positive effect on pain could have been observed. Another promising observation was the benefit in mixed-IBS; no FDA-approved drugs are available for this IBS subtype.
The negative findings of our study include adequate relief of IBS symptoms, ≥30 point decrease in pain scores, depression and anxiety scores, as well as IBS-related quality of life. There was a greater proportion of pregabalin patients reporting adequate relief of IBS symptoms compared to placebo patients (46% v. 36%, p=0.35) but this was not statistically significant. Similarly, a larger percentage of pregabalin subjects experienced a ≥30 point decrease in abdominal pain (p=0.10). The study although not large was adequately powered for the primary outcome but not the secondary outcomes. Although pregabalin is an anxiolytic, it did not impact depression or anxiety scores in our study sample, but we cannot exclude a floor effect with our subjects reporting relatively low anxiety and depression scores. Regarding the IBS quality of life scores, it is not clear why scores did not improve compared to baseline. Overall IBS quality of life scores only decreased 4 points from baseline in the pregabalin arm, and by 1 point in the placebo arm. The possibilities include that pregabalin does not impact quality of life, or that IBS quality of life may be relatively stable and may require longer treatment to positively impact quality of life, or that there was a floor effect, or adverse effects may have negated any gain in quality of life. Two recent phase 3 eluxadoline clinical trials did demonstrate improvement in IBS quality of life, but the trials were larger (n=2427 adults) and longer (26 or 52 weeks) and focused solely on IBS-D patients.7 A floor effect is a possibility in that our patients reported moderate quality of life scores at baseline. However, based on these results, it is not clear that pregabalin will improve quality of life.
Emphasizing the limitations of this study is important. This study sample size from this single center trial was not large— 85 were randomized. We had hoped to recruit 100 subjects, but recruitment was slow and the study was terminated early. Our study intentionally targeted patients with moderate-severe pain to decrease the likelihood of a floor effect. However, for that same reason, we found it difficult to recruit patients in pain seeking immediate symptom relief. This study also did not utilize the FDA outcomes for IBS. This study was conceived and designed prior to the FDA guidance document.23 As such, although we can provide data on patients reporting a 30 point reduction in pain from baseline (66% v. 45%, p=0.10), we cannot provide data on improvement in number of complete, spontaneous bowel movements in our constipation patients nor can we provide data on the change in days with fewer bowel movements without Bristol 6 or 7 consistency stools. Based on our recruitment study, results should be generalizable to community IBS patients as well as referral-level IBS patients; it’s applicability to those with mild or severe pain is not known.
Abdominal pain is often cited as a major driver for patients with IBS to seek medical care.27 Treatment options for IBS-related pain include antispasmodics, peppermint oil,28 probiotics,29 and antidepressants.30 A systematic review of IBS treatments also suggests that linaclotide has beneficial effects on IBS pain.6 Lubiprostone decreases pain, bloating, and improves bowel habit in those with IBS with constipation.31 Eluxadoline was more effective than placebo in reduction of abdominal pain and improvement in stool consistency.7, 32 For IBS with diarrhoea, rifaximin may improve IBS.33 However, these therapies are very specific to IBS subtype. Newer IBS therapies for IBS also provide promise for IBS pain management. Dietary interventions such as the low FODMAP diet show promise. Halmos, et al. performed a randomized trial comparing the effects of a low FODMAP diet compared to a traditional Australian diet and observed that patients of all IBS subtypes had lower overall symptom scores on the low fermentable carbohydrate diet including pain.34 However, firm conclusions on the benefits of this diet cannot be made due to the paucity of unbiased randomized trials.35 Nonetheless, many patient find incomplete symptom relief with conventional interventions. Because this trial shows efficacy in patients with moderate-severe pain, we speculate that pregabalin may be useful to non-constipated IBS patients who fail other conventional therapies.
Our study provides novel and important data suggesting a positive effect of pregabalin on IBS symptoms, particularly for mixed-type IBS and diarrhoea-predominant IBS patients. A large multicenter trial of IBS-D or IBS-M patients utilizing the FDA outcome should be pursued as pregabalin shows promise, and treatment options for IBS-related pain and mixed bowel habit IBS remain limited.
Acknowledgements:
Special thanks to Lori R. Anderson for her assistance in submitting this manuscript.
Grant Support: This study was an investigator-initiated study (IIS) funded by Pfizer Pharmaceuticals. This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
ClinicalTrials.gov #NCT00977197
Footnotes
Conflict of Interest: None to declare
Disclosures: This study was an investigator-initiated study (IIS) funded by Pfizer Pharmaceuticals. Grant funding supported study-related patient costs and remuneration, personnel time, data analysis, and administrative costs and fees. Beyond funding and supplying drug, Pfizer was not involved with study execution, analysis, or interpretation.
REFERENCES
- 1.Koloski NA, Talley NJ, Boyce PM. Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol 2001;96:1340–9. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Boyce PM, Jones M. Predictors of health care seeking for irritable bowel syndrome: a population based study. Gut 1997;41:394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569–80. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–60. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;303:G775–85. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Quigley EM, Task Force on the Management of Functional Bowel D. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109 Suppl 1:S2–26; quiz S27. [DOI] [PubMed] [Google Scholar]
- 7.Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, Davenport JM, McIntyre G, Lopez R, Turner L, Covington PS. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016;374:242–53. [DOI] [PubMed] [Google Scholar]
- 8.Varju P, Farkas N, Hegyi P, Garami A, Szabo I, Illes A, Solymar M, Vincze A, Balasko M, Par G, Bajor J, Szucs A, Huszar O, Pecsi D, Czimmer J. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS One 2017;12:e0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SS, Wu Z, Zhang LC, Zhang Z, Chen RP, Huang YH, Chen H. Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: a meta-analysis. Acta Anaesthesiol Scand 2015;59:147–59. [DOI] [PubMed] [Google Scholar]
- 10.Gurusamy KS, Lusuku C, Davidson BR. Pregabalin for decreasing pancreatic pain in chronic pancreatitis. Cochrane Database Syst Rev 2016;2:CD011522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravnefjord A, Brusberg M, Larsson H, Lindstrom E, Martinez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol 2008;155:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut 2007;56:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iturrino J, Camilleri M, Busciglio I, Burton D, Zinsmeister AR. Effect of the alpha2delta ligand, pregabalin, on colonic sensory and motor functions in healthy adults. Am J Physiol Gastrointest Liver Physiol 2011;301:G377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therneau TM. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials 1993;14:98–108. [DOI] [PubMed] [Google Scholar]
- 15.sLyrica Prescribing Information. http://labeling.pfizer.com/showlabeling.aspx?id=561, 2018.
- 16.Talley NJ, Phillips SF, Melton J 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med 1989;111:671–4. [DOI] [PubMed] [Google Scholar]
- 17.Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA 1998;280:1585–9. [DOI] [PubMed] [Google Scholar]
- 18.Mangel AW, Hahn BA, Heath AT, Northcutt AR, Kong S, Dukes GE, McSorley D. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res 1998;26:76–81. [DOI] [PubMed] [Google Scholar]
- 19.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998;43:400–11. [DOI] [PubMed] [Google Scholar]
- 20.Burt T, IsHak WW. Outcome measurement in mood disorders In: IsHak WW, Burt T, Sederer LI, eds. Outcome measurement in pyschiatry: A critical review. Washington, D.C.: American Psychiatric Publishing, Inc., 2002:155–190. [Google Scholar]
- 21.Hays RD, Stewart AL Sleep measures In: Stewart AL, Ware JE, ed. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press, 1992:235–259. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. Guidance for Industry: Irritable Bowel syndrome - Clinical Evaluation of Drugs for Treatment, http://www.fda.gov/downloads/drugs/guidances/ucm205269.pdf, 2012.
- 24.Needham K, Bron R, Hunne B, Nguyen TV, Turner K, Nash M, Furness JB. Identification of subunits of voltage-gated calcium channels and actions of pregabalin on intrinsic primary afferent neurons in the guinea-pig ileum. Neurogastroenterol Motil 2010;22:e301–8. [DOI] [PubMed] [Google Scholar]
- 25.Meleine M, Boudieu L, Gelot A, Muller E, Lashermes A, Matricon J, Silberberg C, Theodorou V, Eschalier A, Ardid D, Carvalho FA. Comparative effects of alpha2delta-1 ligands in mouse models of colonic hypersensitivity. World J Gastroenterol 2016;22:7111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iturrino J, Camilleri M, Busciglio I, Burton D, Zinsmeister AR. Pilot trial: pregabalin on colonic sensorimotor functions in irritable bowel syndrome. Dig Liver Dis 2014;46:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am 2005;34:189–204. [DOI] [PubMed] [Google Scholar]
- 28.Chumpitazi BP, Kearns GL, Shulman RJ. Review article: the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther 2018;47:738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hungin APS, Mitchell CR, Whorwell P, Mulligan C, Cole O, Agreus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Seifert B, Wensaas KA, Winchester C, de Wit N, European Society for Primary Care G. Systematic review: probiotics in the management of lower gastrointestinal symptoms - an updated evidence-based international consensus. Aliment Pharmacol Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med 2017;376:2566–2578. [DOI] [PubMed] [Google Scholar]
- 31.Chang L, Chey WD, Drossman D, Losch-Beridon T, Wang M, Lichtlen P, Mareya S. Effects of baseline abdominal pain and bloating on response to lubiprostone in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2016;44:1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chey WD, Dove LS, Andrae DA, Covington PS. Early response predicts a sustained response to eluxadoline in patients with irritable bowel syndrome with diarrhoea in two Phase 3 studies. Aliment Pharmacol Ther 2017;45:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pimentel M Review article: potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2016;43 Suppl 1:37–49. [DOI] [PubMed] [Google Scholar]
- 34.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75 e5. [DOI] [PubMed] [Google Scholar]
- 35.Krogsgaard LR, Lyngesen M, Bytzer P. Systematic review: quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment Pharmacol Ther 2017;45:1506–1513. [DOI] [PubMed] [Google Scholar]