Abstract
Mutations in the gene SCAPER (S-phase CyclinA Associated Protein residing in the Endoplasmic Reticulum) have recently been identified as causing syndromic autosomal recessive retinitis pigmentosa with the extraocular manifestations of intellectual disability and attention-deficit/hyperactivity disorder. We present the case of an 11-year-old boy that presented to our clinic with the complaint of decreased night vision. Clinical presentation, family history, and diagnostic imaging were congruent with the diagnosis of autosomal recessive retinitis pigmentosa. Genetic testing of the patient and both parents via whole-exome sequencing revealed the homozygous mutation c.2023–2A>G in SCAPER. Unique to our patient’s presentation is the absence of intellectual disability and attention-deficit/hyperactivity disorder, suggesting that SCAPER-associated retinitis pigmentosa can also present without systemic manifestations.
Keywords: SCAPER, retinitis pigmentosa, autosomal recessive, syndromic disorder
Background
Retinitis pigmentosa (RP) refers to a group of inherited retinal disorders caused by the degeneration of rod photoreceptors followed by degeneration of the cones (Hartong, Berson, & Dryja, 2006; Jauregui et al., 2018). The disease is characterized by symptoms of nyctalopia, followed by progressive visual field constriction and ultimately blindness (Hamel, 2006; Jauregui et al., 2018). The incidence of RP is approximately 1 in 4,000, and is commonly inherited in an autosomal recessive (50–60%), autosomal dominant (30–40%), or X-linked recessive (5–15%) trait (Hartong et al., 2006). In addition, RP is a disease with extensive genetic heterogeneity, as over 140 genes are associated with RP, more than 50 of which are associated with non-syndromic RP (Daiger, Sullivan, & Bowne, 2013). Approximately 20–30% of patients have associated non-ocular disease, accounting for more than 30 different syndromes (Hartong et al., 2006).
SCAPER (S-phase CyclinA Associated Protein residing in the Endoplasmic Reticulum; OMIM#611611) codes for a cyclin A-interacting protein that regulates cell cycle progression by being part of a feedback loop for both G1/S and G2/M phases (Tsang, Wang, Chen, Sanchez, & Dynlacht, 2007). Pathogenic biallelic variants in SCAPER have recently been reported to cause syndromic autosomal recessive RP (arRP) with intellectual disability and attention-deficit/hyperactivity disorder (ADHD) in a total of 4 patients in 3 unrelated families (Tatour et al., 2017). An additional fifth patient from an unrelated family was reported to have an inherited retinal disease (IRD) caused by the variant p.R727X in SCAPER, although detailed clinical information regarding the type of IRD or whether any extra-ocular findings were observed was not provided (Carss et al., 2017).
This study aims to add to the current limited literature on SCAPER-associated RP by reporting a new case caused by a previously reported homozygous canonical splice variant. Furthermore, this study expands the currently-known phenotypic presentation of SCAPER-associated retinopathy to include non-syndromic RP.
Methods
Subjects and ophthalmic evaluation
This study was approved by the Institutional Review Board of Columbia University Medical Center and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from the parents of the patient. Ophthalmic evaluation included measurement of best corrected visual acuity (BCVA), fundus examination and photography after pupillary dilation (>7mm), spectral-domain optical coherence tomography (SD-OCT), and short-wavelength fundus autofluorescence (FAF, 488nm). Full-field electroretinography (ff-ERG) was performed using DTL recording electrodes and Ganzfeld stimulation according to standards from the International Society for Clinical Electrophysiology of Vision (ISCEV) (McCulloch et al., 2015a, 2015b).
DNA analyses
DNA was isolated from peripheral blood from the proband and both parents for whole exome sequencing (WES). WES was performed by using SureSelectXT Human All Exon V5+UTRs (Agilent Technologies) capture and HiSeq2500 (Illumina) sequencing technology. Sequence reads obtained were analyzed for the presence of pathogenic mutations by alignment to the human genome reference sequence (GRCh37/hg19) using the NextGENe software (Softgenetics) and our own analytical pipeline at the Laboratory of Personalized Genomic Medicine at Columbia University (Wang et al., 2016). Identified variants were assessed for clinical phenotypic match and American College of Medical Genetics and Genomics (ACMG) guidelines for the interpretation of sequence variants (Richards et al., 2015).
Structural modeling
The coiled-coil domain (residues 395–770) and putative transmembrane helix (residues 1049–1064) of human SCAPER were modeled using MUSTER (Wu & Zhang, 2008). The zinc-finger/C2H2 domain (793–867) was modeled using the matrin-2 zinc finger domain (PDB 1ZU1; 39% sequence identity) as a template in MODELLER 9.14 (Webb & Sali, 2016). The remaining primary sequence was predicted to be intrinsically-disordered in Pfam 31.0 (Finn et al., 2014). PyMOL generated all structural figures (DeLano, 2002).
Clinical Report
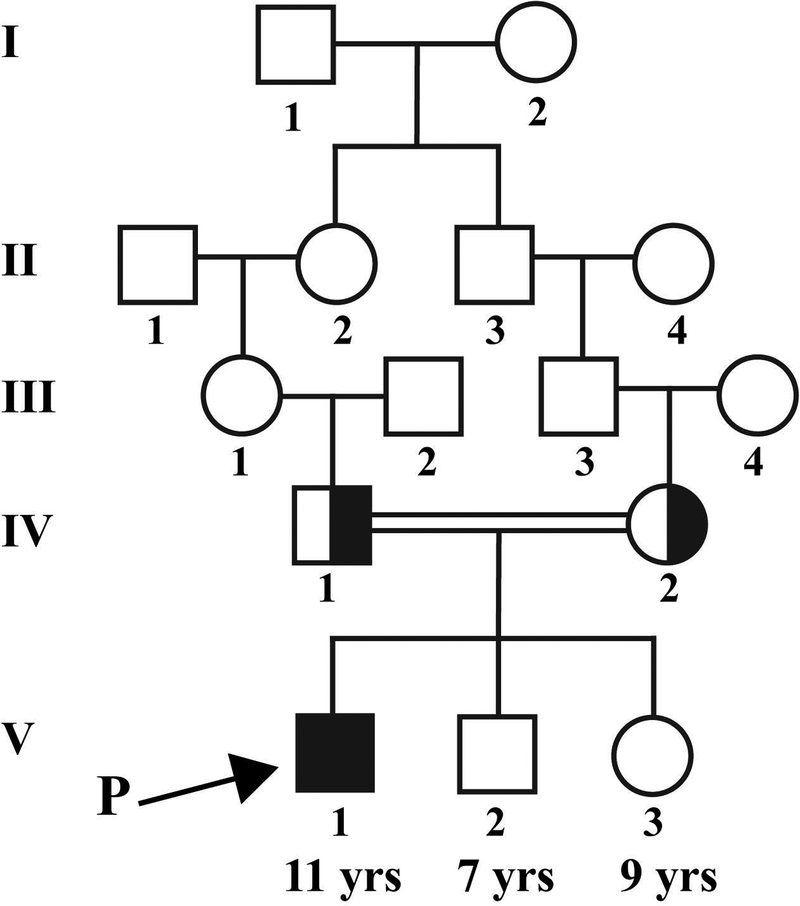
An 11-year-old boy was referred to our clinic for a diagnosis of RP. The parents noticed that he had night vision problems around 2 years ago. Medical and ocular history were otherwise non-significant, including an absence of intellectual disability and ADHD. Per the parents, there has not been any indications that suggest intellectual disability or ADHD either at school, home, or in other settings. Collateral clinical records from the pediatrician indicated that all developmental milestones had been met in time and that there has not been any signs or symptoms suggesting intellectual disability or ADHD. The family is of Jordanian Arab descent and family history is significant for consanguinity, as the patient’s paternal grandmother and maternal grandfather are first cousins (Figure 1). There are no other similarly affected individuals in the family.
Figure 1. Pedigree segregating the SCAPER variant of the proband presented.
The arrow indicates the proband described in this report. Family history is significant for consanguinity, as the proband’s paternal grandmother and maternal grandfather are first cousins. The asymptomatic younger siblings did not undergo clinical examination or genetic testing.
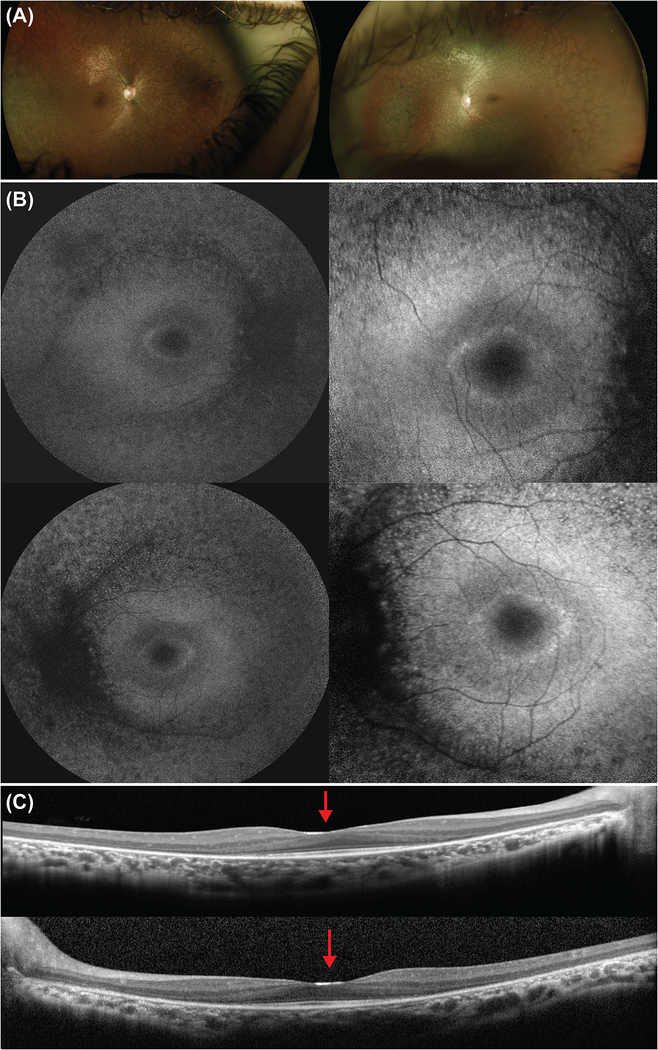
On examination, BCVA was 20/40 on the right and 20/25 on the left eye. Dilated fundus examination revealed a pale optic disc and no cystoid macular edema bilaterally. Bilateral bone-spicule intraretinal pigment migration was observed, mainly in the superior and nasal fields, with peripheral retinal atrophy (Figure 2).
Figure 2. Multimodal imaging of the proband at presentation.
(a) Color fundus images of the right and left eye, respectively, showed attenuated vessels, pale optic disc, peripheral retina atrophy, and bone-spicule intraretinal pigment migration. (b) Fundus autofluorescence images of the right (top) and left (bottom) eye demonstrated peripheral atrophy and the presence of a hyperautofluorescent ring on the foveal area. The ring is seen in more detail with the 30-degree images (right column). (c) Spectral-domain optical coherence tomography scan through the fovea revealed peripheral thinning of the retina. The ellipsoid zone was disrupted peripherally and conserved in the foveal area. In addition, the foveal border is enlarged and flattened, with a shallow foveal pit (red arrow).
Macular SD-OCT scans revealed peripheral thinning of the retina, affecting mainly the outer nuclear layer. In addition, disruption of the ellipsoid zone (EZ) line was observed in the periphery, while it was conserved in the foveal area. The foveal border was flattened and enlarged. On FAF, peripheral atrophy was observed along with the presence of a hyperautofluorescent ring in the foveal area commonly observed in RP patients. Scotopic rod-specific and maximal responses on ff-ERG were undetectable in both eyes. Photopic 30 Hz-flicker amplitudes were markedly subnormal bilaterally.
Genetic testing via WES revealed a homozygous canonical splice variant c.2023–2A>G in the gene SCAPER (Tatour et al., 2017). WES results from both parents confirmed that each one is a heterozygous carrier for this variant. The c.2023–2A>G variant is absent from gnomAD, ExAC, and the NHLBI Exome variant server, suggesting that it is not a common benign variant present in the populations represented in those databases. Other rare variants identified in WES analysis were excluded based on clinical assessment of phenotypic fit or were benign or likely benign based on ACMG guidelines for the interpretation of sequence variants (Richards et al., 2015).
Discussion
This study presents the first reported case of pathogenic biallelic variants in the gene SCAPER as manifesting with RP without syndromic findings.
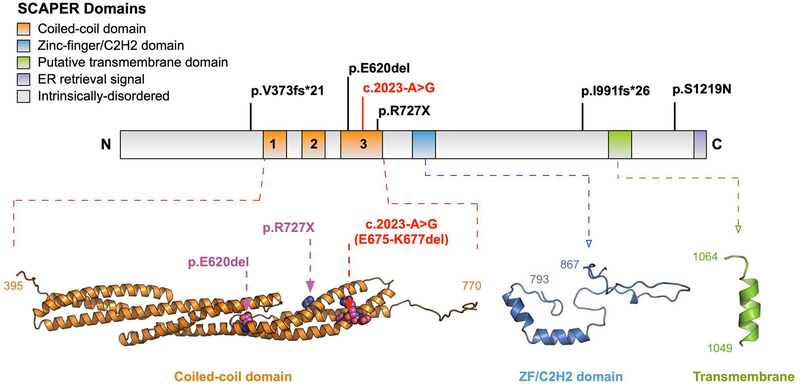
The SCAPER protein consists of three putative coiled-coil domains, which are critical for its interaction with cyclin-A/Cdk2, as well as a zinc finger domain, a transmembrane domain, and a C-terminal endoplasmic retrieval signal (Figure 3) (Tsang et al., 2007). The patient described in this study was found to be homozygous for a canonical splice variant c.2023–2A>G, which has been previously described in pair of siblings from a consanguineous union (Tatour et al., 2017). Furthermore, splicing assays have demonstrated that the c.2023–2A>G variant exposes a cryptic splice acceptor site in exon 19 that is predicted to lead to the deletion of 3 amino acids (E675-K677) within the third putative coiled-coil domain (Figure 3) (Tatour et al., 2017). A single amino acid deletion (E620del) has also been described within this domain in a patient with SCAPER-associated arRP, suggesting a critical structural or functional role for this region of the protein (Figure 3) (Tatour et al., 2017).
Figure 3. Schematic of the SCAPER protein demonstrating the different domains and the location of all known pathogenic variants identified in SCAPER relative to the putative domains.
The canonical splice variant (c.2023-A>G) marked in red was identified in our patient and has also been previously reported in two siblings. This variant is predicted to delete 3 residues (E675-K677) in the coiled-coil domain. The p.E620del and p.R727X variants are also located in this region. The p.V373fs*21, p.I991fs*26, and p.S1219N variants are located in predicted unstructured regions of the protein. No known variants are in the putative zinc-finger/C2H2 and transmembrane domains.
The ocular findings of RP in our patient are congruent to those reported in the literature, where the reported patients had symptom onset during the early teenage years. In particular, the reported siblings with the same homozygous c.2023–2A>G mutation in SCAPER were of Israeli Muslim Arab decent and had an onset of visual symptoms between the ages of 12–13 years, with non-detectable scotopic and photopic ERG responses by 15 years of age. The absence of extra-ocular manifestations in our patient is interesting, given the mild intellectual disability and attention-deficit/hyperactivity disorder observed in the siblings homozygous for the same variant in Tatour et al., 2017. It is possible that the c.2023–2A>G variant leads to a retina specific phenotype and the extra-ocular manifestations are independent in the previously reported patients, who are also from a consanguineous union. We also cannot exclude the possibility that genetic modifiers affect extra-occular manifestations of SCAPER related disease, as there does seem to be variability in both the presence of developmental delay and the severity of intellectual disability in previously reported patients.
Of note, our patient has two younger siblings, 7 and 9 years old (Figure 1). Given that the reported patients with SCAPER-associated RP presented on their early teenage years, it was suggested to the parents for the two younger siblings to undergo genetic testing. Since they are currently asymptomatic, the parents refused genetic testing or clinical examination.
SD-OCT scans in our patient revealed an enlarged and flattened foveal border, a finding also observed in the patients reported in the literature (Tatour et al., 2017). Nevertheless, previous studies have reported significant variability in foveal pit morphology based on race and ethnic backgrounds (Wagner-Schuman et al., 2011). Furthermore, the shallow foveal pit observed on these patients might be the result of severe attenuation of the outer nuclear layer causing loss of the macular volume. Thus, larger cohorts of patients are needed to investigate whether the morphologic foveal changes observed are a feature associated with SCAPER RP.
Though we report the first non-syndromic manifestation of SCAPER-associated RP, this phenomenon is well-established for other genes, which can also cause RP with syndromic or non-syndromic findings. The gene USH2A, for example, is one of the most common causes of non-syndromic arRP, while it can also manifest as Usher syndrome type 2 (Daiger, Bowne, & Sullivan, 2007; Hartong et al., 2006; Pierrache et al., 2016; Sengillo et al., 2017). Other genes that behave similarly include BBS1 and CLRN1, which in addition to causing non-syndromic arRP, can also manifest as Bardet-Biedl or Usher syndrome type 3, respectively (Estrada-Cuzcano et al., 2012; Khan et al., 2011).
Because the retinal phenotype of null SCAPER mutations in mouse models is not congenital but rather presents later in life, it has been suggested that SCAPER does not play a role in the development of photoreceptors, but rather in their function and/or maintenance (Tatour et al., 2017). Given that SCAPER-associated RP can present with a syndromic, as previously reported, or non-syndromic phenotype, as we report, knowing the exact role and function of SCAPER in the retina and the brain could help elucidate the mechanisms behind the two different phenotypic presentations.
Acknowledgements:
Sincere appreciation and thanks are extended to the family described for their willingness to participate.
Funding: The Jonas Children’s Vision Care and Bernard & Shirlee Brown Glaucoma Laboratory are supported by the National Institutes of Health [P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437], National Cancer Institute Core [5P30CA013696], the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA. VM is supported by NIH grants [K08EY020530, R01EY024665, R01EY025225, R01EY024698, R21AG050437, P30EY026877], Doris Duke Charitable Foundation Grant 2013103, and Research to Prevent Blindness (RPB). GV is supported by NIH grants [F30EYE027986 and T32GM007337]. R. J. is supported by the RPB medical student eye research fellowship.
Footnotes
Ethics approval and consent to participate: All study procedures were defined and patient consent was obtained as outlined by the protocol #AAAR0284 approved by the Institutional Review Board at Columbia University Medical Center. The study adhered to the tenets of the Declaration of Helsinki.
Consent for publication: Consent was obtained from the parents as the proband is a minor.
Availability of data and materials: For reasons of privacy, the clinical imaging presented is not publicly available.
Conflict of Interest: The authors declare no competing interests.
References
- Carss KJ, Arno G, Erwood M, Stephens J, Sanchis-Juan A, Hull S, … Raymond FL (2017). Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am J Hum Genet, 100(1), 75–90. doi: 10.1016/j.ajhg.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger SP, Bowne SJ, & Sullivan LS (2007). Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol, 125(2), 151–158. doi: 10.1001/archopht.125.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger SP, Sullivan LS, & Bowne SJ (2013). Genes and mutations causing retinitis pigmentosa. Clin Genet, 84(2), 132–141. doi: 10.1111/cge.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL (2002). The PyMOL molecular graphics system. http://www.pymol.org/. Retrieved from http://www.pymol.org/
- Estrada-Cuzcano A, Koenekoop RK, Senechal A, De Baere EB, de Ravel T, Banfi S, … Klevering BJ (2012). BBS1 mutations in a wide spectrum of phenotypes ranging from nonsyndromic retinitis pigmentosa to Bardet-Biedl syndrome. Arch Ophthalmol, 130(11), 1425–1432. doi: 10.1001/archophthalmol.2012.2434 [DOI] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, … Punta M (2014). Pfam: the protein families database. Nucleic Acids Res, 42(Database issue), D222–230. doi: 10.1093/nar/gkt1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C (2006). Retinitis pigmentosa. Orphanet J Rare Dis, 1, 40. doi: 10.1186/1750-1172-1-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, & Dryja TP (2006). Retinitis pigmentosa. Lancet, 368(9549), 1795–1809. doi: 10.1016/S0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- Jauregui R, Cho GY, Takahashi VKL, Takiuti JT, Bassuk AG, Mahajan VB, & Tsang SH (2018). Caring for Hereditary Childhood Retinal Blindness. Asia Pac J Ophthalmol (Phila). doi: 10.22608/APO.201851 [DOI] [PubMed] [Google Scholar]
- Khan MI, Kersten FF, Azam M, Collin RW, Hussain A, Shah ST, … den Hollander AI (2011). CLRN1 mutations cause nonsyndromic retinitis pigmentosa. Ophthalmology, 118(7), 1444–1448. doi: 10.1016/j.ophtha.2010.10.047 [DOI] [PubMed] [Google Scholar]
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, & Bach M (2015a). Erratum to: ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol, 131(1), 81–83. doi: 10.1007/s10633-015-9504-z [DOI] [PubMed] [Google Scholar]
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, & Bach M (2015b). ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol, 130(1), 1–12. doi: 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- Pierrache LH, Hartel BP, van Wijk E, Meester-Smoor MA, Cremers FP, de Baere E, … Klaver CC (2016). Visual Prognosis in USH2A-Associated Retinitis Pigmentosa Is Worse for Patients with Usher Syndrome Type IIa Than for Those with Nonsyndromic Retinitis Pigmentosa. Ophthalmology, 123(5), 1151–1160. doi: 10.1016/j.ophtha.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, … Committee, A. L. Q. A. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengillo JD, Cabral T, Schuerch K, Duong J, Lee W, Boudreault K, …Tsang SH (2017). Electroretinography Reveals Difference in Cone Function between Syndromic and Nonsyndromic USH2A Patients. Sci Rep, 7(1), 11170. doi: 10.1038/s41598-017-11679-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatour Y, Sanchez-Navarro I, Chervinsky E, Hakonarson H, Gawi H, Tahsin-Swafiri S, … Ben-Yosef T (2017). Mutations in SCAPER cause autosomal recessive retinitis pigmentosa with intellectual disability. J Med Genet. doi: 10.1136/jmedgenet-2017-104632 [DOI] [PubMed] [Google Scholar]
- Tsang WY, Wang L, Chen Z, Sanchez I, & Dynlacht BD (2007). SCAPER, a novel cyclin A-interacting protein that regulates cell cycle progression. J Cell Biol, 178(4), 621–633. doi: 10.1083/jcb.200701166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman M, Dubis AM, Nordgren RN, Lei Y, Odell D, Chiao H, … Carroll J (2011). Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci, 52(1), 625–634. doi: 10.1167/iovs.10-5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lichter-Konecki U, Anyane-Yeboa K, Shaw JE, Lu JT, Ostlund C, … Worman HJ (2016). A mutation abolishing the ZMPSTE24 cleavage site in prelamin A causes a progeroid disorder. J Cell Sci, 129(10), 1975–1980. doi: 10.1242/jcs.187302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, & Sali A (2016). Comparative Protein Structure Modeling Using MODELLER. Curr Protoc Protein Sci, 86, 2 9 1–2 9 37. doi: 10.1002/cpps.20 [DOI] [PubMed] [Google Scholar]
- Wu S, & Zhang Y (2008). MUSTER: Improving protein sequence profile-profile alignments by using multiple sources of structure information. Proteins, 72(2), 547–556. doi: 10.1002/prot.21945 [DOI] [PMC free article] [PubMed] [Google Scholar]