Abstract
Background and Purpose:
Dietary sodium reduction with concurrent increase in potassium intake is a current public health priority to reduce risk of cardiovascular events. This study explored associations between the spot urine sodium-to-potassium ratio and cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA) longitudinal cohort.
Methods:
The MESA is a prospective cohort study of 6,814 adults from 4 ethnic groups (European-, Asian-, African- and Hispanic-American) with a mean age of 62 (+/−10.2) years and an average of 11.7 (+/−2.2) years of follow-up. Participants were free of clinical cardiovascular disease at baseline. Spot urine sodium and potassium excretion, as a marker of dietary intake, was collected at baseline. The impact of urinary sodium-to-potassium ratio on adjudicated cardiovascular events was assessed using Cox proportional hazards models.
Results:
Only 39% of MESA participants had a urinary sodium-to-potassium ratio ≤1 and these participants experienced only 74 of the 236 strokes. A sodium-to-potassium ratio >1 was associated with a hazard ratio of 1.47 (95% Confidence Interval: 1.07 to 2.00) for risk of stroke, adjusting for age, sex, race, cardiovascular risk factors, socio-demographic characteristics, body size, and kidney function.
Conclusion:
The spot urine sodium-to-potassium ratio (measurable in routine care) is associated with stroke. A urine sodium-to-potassium ratio of ≤1, may be related to a clinically relevant reduction in stroke risk and is a feasible target for health interventions.
Keywords: Sodium, potassium, stroke, sodium-potassium ratio, prospective cohort
INTRODUCTION
Cardiovascular disease is a leading cause of mortality, and hypertension is a known modifiable risk factor1. Dietary sodium reduction and a corresponding increase in potassium intake are the twin mainstays in nutrition guidance to reduce cardiovascular disease risk and control blood pressure2–5. It is universally acknowledged that dietary sodium intakes at population level are too high and potassium levels too low6, 7. Studies based on the National Health and Nutrition Examination Survey (NHANES 2011-2012) reported that adults >20 years consumed a mean of 3,592mg/day sodium and 2,793mg/day potassium8. These consumption estimates for sodium were far above the national guidelines of 2300-2400mg Sodium/person/day2–4, 9. In contrast, consumption estimates for potassium were far below the stated 4,700 mg Potassium/person/day goal10. Some reports show the consumption of sodium and potassium has remained stable over time, suggesting a continued need to address these dietary components7, 11.
Most studies that assess sodium and potassium intake rely on self-reported dietary measures. Yet, these measures are subject to many limitations, including poor recall and inaccurate or incomplete databases12, 13. Urinary excretion of sodium and potassium are good estimates of dietary intake and are not subject to the aforementioned limitations12. Further, using sodium-to-potassium ratios, instead of absolute thresholds, could be useful for formulating dietary guidelines to meet wide ranging energy needs, as suggested by Drewnowski et al14, and the ratio may better reflect CVD risk15–17. A recent meta-analysis of observational studies does support a relationship between dietary sodium-to-potassium ratio and risk of stroke18. The present study adds to the current body of literature by assessing the specific hypothesis that urinary sodium-to-potassium ratio, obtained from spot urines, would predict the risk of subsequent major cardiovascular events in a diverse and multi-ethnic American population.
METHODS
The data used for this project are available through the data coordinating center for the MESA project (https://www.uwchscc.org/ and https://www.mesa-nhlbi.org/). All data requests must be approved by the MESA publications and presentations committee due to concerns from NHLBI about identifiability of individual participants in the full MESA data set.
Sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a large prospective cohort of 6,814 individuals recruited from six field centers (New York, New York; Baltimore, Maryland; Chicago, Illinois; Los Angeles, California; Minneapolis-St. Paul, Minnesota; and Winston-Salem, North Carolina). At enrollment, participants were aged 45-84 years and were selected based on having no clinical CVD events or CVD procedures. By design, four racial/ethnic groups were represented in the selection of participants: European American, African American, Chinese American, and Hispanic American. The study includes five clinical exams to date, which were performed from July 2000 - January 2012. A more detailed description of the study design can be found elsewhere19. The study was conducted under the guidelines of the Declaration of Helsinki and approved by the institutional review boards at each site. Written informed consent of all participants was obtained.
Procedure
Spot urine samples were collected from participants at the time of exam 1 (July 2000 - August 2002). Measures of sodium and potassium excretion were used as a short-term estimate of intake. The amount of sodium excreted in urine is nearly equivalent to intake of dietary sodium9. Researchers estimate that 77-90% of dietary potassium is excreted in the urine and urinary potassium is highly correlated with potassium intake9.
The MESA collected a broad range of covariate data on study participants. MESA participants were asked to come to a morning clinic examination after an overnight fast and were given standard questionnaires to assess a variety of risk factors which included demographic information, smoking, and any medical history of either hypertension or diabetes at the time of the exam. Participants were asked to bring their medications to the clinic to permit a medication inventory and diet was assessed using a food frequency questionnaire. Anthropometric measures and blood pressures were also taken on all study participants at each exam. The amount of physical activity was assessed via questionnaire.
Event Adjudication
MESA identifies possible Myocardial Infarction (MI), heart failure, peripheral vascular disease (PVD), stroke and other events using participant self-report (as part of a broader range of events that are ascertained as primary study endpoints) as its primary means of outcome detection. Participants may report these events at clinic visits, during follow-up telephone calls, or by directly contacting the MESA field center post-event. After each contact, the MESA staff member documents any possible events through both review and abstraction of medical records and death certificates, interviews, and such, to gather as much information as possible about the event. At least two MESA physicians review the case materials for each MI and stroke. Differences in diagnoses between two MESA physician reviewers are resolved by the committees involved. Mean follow-up time for events was over eleven years.
Statistical Analysis
MESA covariates of interest at baseline (2000-2002) were examined using descriptive statistics, including an evaluation of sodium, potassium, and sodium-to-potassium ratio means/proportions by covariates. The primary covariates of interest included the diverse demographics of MESA, namely age, sex, and race/ethnicity.
We examined the associated between sodium-to-potassium ratio and adjudicated cardiovascular events using both a threshold for the sodium-to-potassium ratio of greater than one and sodium-to-potassium ratio as a continuous variable. For outcomes, we considered CVD events (Myocardial infarction, definite angina, stroke, transient ischemic attack, coronary heart disease death), coronary heart disease (MI and angina), heart failure (HF20), peripheral vascular disease (PVD21), and stroke. We considered three levels of adjustment. First, we adjusted for age, sex, and race. Then, for model 2 (our primary model), we also adjusted for diabetes, smoking (current and former), total cholesterol, HDL cholesterol, and treated hypertension. For model 3 we added variables that could have been either confounders or mediators, namely: education, systolic blood pressure, diastolic blood pressure, urine creatinine, hip circumference, body mass index, aspirin use, intentional exercise, and glomerular filtration rate (GFR). Further adjustment was done for dietary energy intake, maximum of common carotid artery intimal medial thickness, and Interleukin 6 (IL6) levels. We excluded participants who consumed fewer than 400 or greater than 6,000 calories per day, as determined by the food frequency questionnaire, because those extremes are implausible estimates of participants’ true energy intake. All associations were estimated by Cox proportional hazards models, with the proportionality of hazards assessed by graphical methods.
Statistical analysis was performed with Stata 11 (StataCorp LP) and SAS 9.1.4. Results were statistically significant at the level of p<0.05.
RESULTS
The mean age of the 6,705 MESA participants with complete data at baseline was 61.2 years (Table 1), with 37% of participants already receiving anti-hypertensive therapy at baseline. The participants averaged a sodium-to-potassium ratio of 1.30 (Table 2), although 39% of participants had a ratio of ≤1 (supplemental Table I).
Table 1.
Demographics of 6705 participants with complete baseline data, Multi-Ethnic Study of Atherosclerosis at baseline, 2000 to 2002
| Characteristic | Mean (sd)/N (%) |
|---|---|
| Age, mean (sd) | 61.2 (10.2) |
| Age Category, n (%) | |
| 45-54 | 1,905 (28.4) |
| 55-64 | 1,853 (27.6) |
| 65-74 | 1,986 (29.7) |
| 75-84 | 955 (14.3) |
| Gender, n (%) | |
| Male | 3160 (47.1) |
| Female | 3545 (52.9) |
| Race/Ethnicity, n (%) | |
| European American | 2,596 (38.7) |
| Chinese American | 799 (11.9) |
| African American | 1,839 (27.4) |
| Hispanic American | 1,471 (21.9) |
| College, n (%) | 2709 (40.4) |
| Smoker, n (%) | 872 (13.0) |
| Ex-smoker, n (%) | 2448 (36.5) |
| Systolic blood pressure, (mmHg), mean (sd) | 126.6 (21.5) |
| Diastolic blood pressure, (mmHg), mean (sd) | 71.9 (10.3) |
| Any anti-hypertensive medication use, n (%) | 2497 (37.3) |
| Diuretic use, n (%) | 1010 (15.1) |
| Aspirin use, n (%) | 1675 (25.0) |
| Statin use, n (%) | 995 (14.9) |
| Body Mass Index, (kg/m2), mean (sd) | 28.3 (5.5) |
| Total cholesterol (mg.dL) | 194.2 (35.7) |
| LDL cholesterol (mg/dL) | 117.3 (31.5) |
| Triglycerides (mg/dL) | 131.4 (88.7) |
| HDL cholesterol (mg/dL) | 51.0 (14.8) |
| eGFR mL/min/1.73 m2, mean (sd) | 78.0 (16.3) |
| Diabetes, n (%) | 837 (12.5) |
Table 2.
Sodium and potassium excretion estimated by spot urine collection baseline demographics, Multi-Ethnic Study of Atherosclerosis, 2000 to 2002
| Sodium [mg/dL (SD)] | Potassium [mg/dL (SD)] | Sodium-to-potassium ratio (SD) | |
|---|---|---|---|
| All | 2366.49 (1169.86) | 2144.24 (1153.33) | 1.30 (0.72) |
| Age Category | |||
| 45-54 | 2379.19 (1197.87) | 2098.23 (1133.31) | 1.31 (0.69) |
| 55-64 | 2379.72 (1199.54) | 2154.18 (1142.47) | 1.29 (0.72) |
| 65-74 | 2336.42 (1130.86) | 2122.75 (1154.57) | 1.30 (0.71) |
| 75-84 | 2401.32 (1218.0) | 2141.99 (1214.43) | 1.33 (0.73) |
| Gender | |||
| Male | 2589.57 (1148.83) | 2279.51 (1128.82) | 1.32 (0.70) |
| Female | 2176.10 (1151.92) | 2032.57 (1164.57) | 1.27 (0.73) |
| Race/Ethnicity | |||
| European American | 2335.88 (1171.91) | 2113.50 (1175.86) | 1.31 (0.74) |
| Chinese American | 2367.55 (1161.8) | 2148.15 (1145.99) | 1.31 (0.75) |
| African American | 2374.21 (1185.11) | 2154.9 (1140.15) | 1.29 (0.70) |
| Hispanic American | 2410.33 (1151.04) | 2183.14 (1133.05) | 1.28 (0.68) |
Higher levels of sodium in the spot urine had a cross-sectional association with higher systolic blood pressure, whereas the inverse was true with potassium. A higher sodium-to-potassium ratio was, as expected, associated with a higher systolic blood pressure (Table 3).
Table 3.
Cross-sectional association between sodium, potassium, sodium-to-potassium ratio, and systolic blood pressure, per standard deviation (SD) at MESA Baseline 2000 to 2002, adjusted for age, gender, race/ethnicity, and hypertension medication usage
| Systolic blood pressure (mmHg) | ||
|---|---|---|
| β-coefficient (95% confidence interval) | p-value | |
| Sodium excretion (per SD) | 1.20 (0.68 to 1.71) | <0.0001 |
| Potassium excretion (per SD) | −2.89 (−3.39 to −2.39) | <0.0001 |
| Sodium-to-potassium ratio (per SD) | 2.74 (2.27 to 3.22) | <0.0001 |
Our primary model (Model 2) showed an increase in risk for all CVD events (Myocardial infarction, definite angina, stroke, transient ischemic attack, coronary heart disease death) of hazard ratio (HR) 1.25 (95% CI:1.08 to 1.45), and stroke, HR 1.54 (95% CI:1.17 to 2.05) after adjustment (Table 4). Associations with HF, all CHD, and PVD did not achieve significance. Increasing the statistical adjustment to increase variables that are potential mediators of the association, like systolic and diastolic blood pressure, left only stroke as statistically significant at HR 1.47 (95% CI:1.07 to 2.00). The stroke association was not influenced by further adjustment for total energy intake, IL-6 levels, and carotid intima-media thickness (HR 1.40, 95% CI: 1.02 to 1.94), done as a sensitivity analysis. Further, we did not exclude individuals on diuretics in the primary analysis as shown in Table 4. However, in follow up test using our primary model (Model 2) for stroke, excluding all participants taking diuretics (approximately 15% of the sample) resulted in a minimal shift in the primary estimate of the association, with an HR of 1.65 (1.16 TO 2.33, P=0.0048).
Table 4.
Hazard ratio of cardiovascular events comparing participants meeting the Sodium-to-Potassium ratio ≤ 1.00, as reference, with those participants who exceed this ratio for all cardiovascular events (CVD), coronary heart disease events (CHD), heart failure, peripheral vascular disease (PVD), and Stroke.
| CVD (n=781) | CHD (n=530) | Heart Failure (n=274) | PVD (n=104) | Stroke (n=236) | |
|---|---|---|---|---|---|
| Model 1 | 1.21 (1.04, 1.40) | 1.13 (0.94, 1.35) | 1.24 (0.96, 1.60) | 1.13 (0.75, 1.69) | 1.44 (1.09, 1.90) |
| Model 2 | 1.25 (1.08, 1.45) | 1.12 (0.97, 1.38) | 1.28 (0.99, 1.65) | 1.22 (0.82, 1.83) | 1.54 (1.17, 2.05) |
| Model 3 | 1.13 (0.96, 1.33) | 1.11 (0.92, 1.34) | 1.07 (0.81, 1.41) | 1.11 (0.70, 1.74) | 1.47 (1.07, 2.00) |
Model 1: Age, sex, race
Model 2: Age, sex, race, diabetes, smoking (current and former), total cholesterol, HDL cholesterol, and treated hypertension
Model 3: Age, sex, race, diabetes, smoking (current and former), total cholesterol, HDL cholesterol, treated hypertension, education, systolic blood pressure, diastolic blood pressure, hip circumference, body mass index, aspirin use, intentional exercise, and GFR.
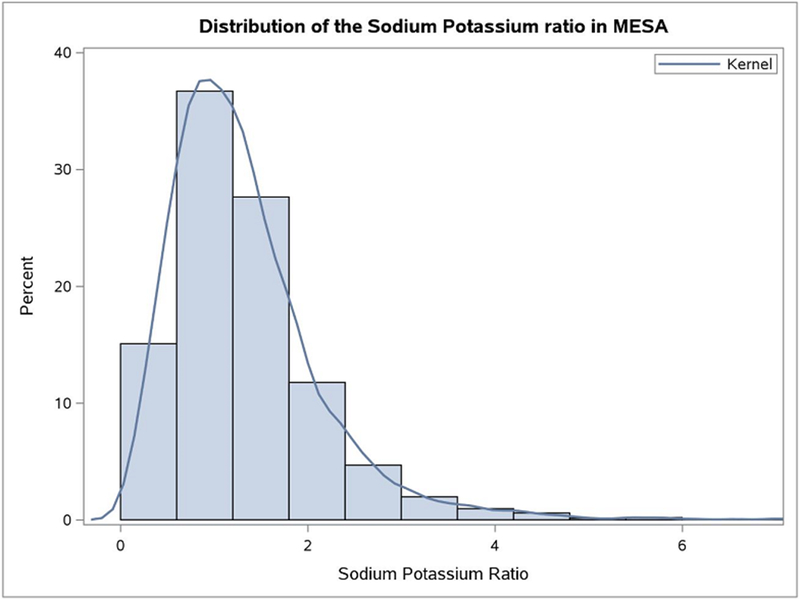
We also considered sodium-to-potassium ratio as a continuous variable, to determine how the risk changes with lower ratios. The data for the sodium-to-potassium ratio is quite skewed (Figure 1). Generalized additive models were used to assess the linearity of the sodium-to-potassium ratio and outcomes. We were not able to reject the null hypothesis of a linear relation between the sodium-to-potassium ratio and stroke (p=0.19), heart failure (p=0.58), PVD (p=0.23), and all CVD (p=0.63) in this data. We found an association with incident stroke events (HR 1.17, 95% CI: 1.03 to 1.32), after full adjustment (Table 5) and when comparing lowest to the highest tertiles of the sodium-to-potassium ratio (HR 1.50, 95% CI: 1.09-2.07; Supplemental Table II). Associations with HF, all CVD, all CHD, and PVD did not achieve statistical significance.
Figure 1.
Distribution of the sodium-to-potassium ratio as measured by baseline spot urine in the Multi-Ethnic Study of Atherosclerosis
Table 5.
Hazard ratio of cardiovascular events (CVD), coronary heart disease events (CHD), heart failure, peripheral vascular disease (PVD), and stroke, by continuous sodium-to-potassium ratio.
| CVD (n=781) | CHD (n=530) | Heart Failure (n=274) | PVD (n=104) | Stroke (n=236) | |
|---|---|---|---|---|---|
| Model 1 | 1.09 (1.02, 1.18) | 1.05 (0.96, 1.16) | 1.01 (0.88, 1.16) | 1.14 (0.96, 1.35) | 1.17 (1.05, 1.31) |
| Model 2 | 1.08 (1.01, 1.16) | 1.04 (.094, 1.14) | 1.01 (0.84, 1.21) | 1.12 (0.95, 1.33) | 1.17 (1.05, 1.31) |
| Model 3 | 1.06 (0.98, 1.15) | 1.01 (0.91, 1.12) | 0.99 (0.87, 1.13) | 1.17 (0.98, 1.41) | 1.17 (1.03, 1.32) |
Model 1: Age, sex, race
Model 2: Age, sex, race, diabetes, smoking (current and former), total cholesterol, HDL cholesterol, and treated hypertension
Model 3: Age, sex, race, diabetes, smoking (current and former), total cholesterol, HDL cholesterol, treated hypertension, education, systolic blood pressure, diastolic blood pressure, urine creatinine, hip circumference, body mass index, aspirin use, intentional exercise, and GFR.
A log transformation of the sodium-to-potassium ratio yielded consistent measures of association. We found an association between log transformed sodium-to-potassium ratio with all CVD events (HR 1.13, 95% CI: 1.01 to 1.26) and stroke events (HR 1.26, 95% CI: 1.03 to 1.55), after adjusting for our primary variables of interest (Model 2). As before, associations with HF, CHD, and PVD did not achieve statistical significance.
The sodium-to-potassium ratio of 1 was selected because it was both easy to interpret in a clinical setting and was significantly associated with stroke. However, we also looked at other ratios based on American Heart Association guidelines, which included a ratio of 0.49, representing the recommendations for the general public, and a ratio of 0.32, which corresponds to recommendations made for individuals at high risk of hypertension2. We assessed these ratios using the Model 2 adjustment covariates. Notably, few MESA participants met either of the AHA ratios (supplemental table I). Only 8 of the 236 strokes occurred among the 256 participants meeting the 0.32 sodium-to-potassium ratio threshold, thus the HR estimate of 1.24 (95% CI:0.61 to 2.52) is imprecise due to the small number of participants who managed to achieve this relatively extreme ratio. Similarly, there were only 25 strokes among the 664 participants meeting the threshold of 0.49, which yield an estimate of HR 0.97 (95% CI:0.64 to 1.48). Last, using values from the Lowess plot (supplemental figure I) where the risk crosses the null yielded quite similar estimates to the ratio of 1, HR 1.41 (95% CI:1.08 to 1.84), suggesting that the proposed ratio of 1 can’t be greatly improved on by fitting the cut-point to the observed data.
DISCUSSION
Urinary sodium-to-potassium ratios of 1 or lower, as measured by spot urines in the Multi-Ethnic Study of Atherosclerosis, were associated with a 40-50% lower risk of strokes. Should this association be causal, this is a clinically meaningful decrease in a common and crippling disease. There was also the expected association between low sodium-to-potassium ratios and lower baseline blood pressure. This evidence is consistent with other studies showing that the sodium-to-potassium ratio is associated with cardiovascular events22, 23. Furthermore, these associations were obtained in a multiethnic cohort of older adults, showing generalizability of these findings across populations with a concern for cardiovascular disease, and the spot urine biomarker is easy to measure in modern routine clinical care.
Studies have only recently begun to explore the feasibility of meeting the longstanding guidelines to lower sodium and increase potassium intake by populations at risk of CVD events14, 16. Our data showing that 2.8% of at risk individuals had urinary ratios of <0.32 (as entailed by the American Heart Association recommendations), suggests that some current guidelines are not really feasible for middle aged and older American adults (Supplemental Table I). Since the sodium and potassium guidelines for at-risk populations appear to be aspirational, we will benefit by identifying a ratio that is both feasible to achieve and clinically meaningful. We propose a ratio where ≤1.0 (on the mg/dL scale) serves as an attainable initial goal for achieving a balance of sodium and potassium in the diet and is close to the median for the population. It is important to note that we do not have data from this study suggesting that lower sodium-to-potassium ratio necessarily needs to be obtained through improved diet quality. However, studies suggest that diets high in fruits, vegetables, whole grains, fish, nuts, and dairy are most likely to improve cardiometabolic health and is the suggested strategy to achieve a lower sodium-to-potassium ratio24. Further, a ratio of 1 is both easy to calculate and explain in a clinical setting. Our study found a ratio meets both the criteria of being achievable (39% of participants met this ratio at baseline) and clinically meaningful, due to the association with stroke, in a multiethnic older American adult cohort. The goal of a sodium-to-potassium ratio of less than 1.0 (on the mg/dL scale) appears to segment risk based on statistical models and the shape of the association (supplemental figure I), making it a possible target for public and individual health efforts, as in a recent clinical trial25.
Spot urines for sodium-to-potassium ratio have large utility in the clinical setting given to their ease of administration, low cost, and quick interpretation. Spot urine may have some errors due to recent intake, although we expect this type of non-differential misclassification to dilute the strength of the association. To guard against such an issue, providers may discuss typical dietary patterns and perform follow-up measures to ensure that clinical care is not dictated by a single error-prone measure, as well as to measure progress in meeting dietary goals.
Since the American Heart Association (AHA) guidelines are for much lower than a ratio of one, a patient meeting a sodium-to-potassium ratio of ≤1 is moving towards the optimal level of intake2. Our goal is not to dispute the high-quality and evidence-based AHA guidelines, so much as to suggest a feasible and clinically relevant intermediate goal on the pathway to improving public health.
Limitations
We used spot urine measures, which despite previous successful use26 are not as ideal as 24-hour measures for determining the absolute levels of sodium and potassium27. We avoided inference on the association of spot urine measures of sodium and potassium on events (Supplemental Table III) due to worries about the raw measures (taking the ratio should at least partially correct for concentration as both measures are simultaneously diluted). It is unclear to what degree potassium is directly causal and could be supplemented in the diet, or if it is a marker of dietary patterns that may lower stroke risk. Participants in MESA are not routinely supplementing with potassium and any use of potassium supplementation to increase the sodium-to-potassium ratio should await high-quality clinical trial evidence, and not be based on these associations. Like all observational studies, there is the possibility of residual confounding. We adjusted for a broad range of covariates, including possible intermediate variables, like systolic blood pressure (Table 3) which was independently associated with the sodium-to-potassium ratio. However, possible additional unknown factors may exist. We did not correct for multiple testing between endpoints, but our stroke finding would be robust to adjustment using the false discovery rate28.
The lack of attenuation for stroke risk across the models was surprising, along with the finding that the association was stronger for stroke than the with CVD, CHD, Heart Failure, and PVD (table 4). It is interesting to speculate that the sodium-to-potassium ratio may be a marker of other risk factors of physiological processes not provided in the model. Further, it is intriguing to consider how causal pathways differ across the cardiovascular disease assessed in this paper, and how that may impact the different associations seen between the sodium-to-potassium ratio and cardiovascular outcomes. Similarly, there is limited ability to be certain about the causal role of sodium, despite decades of high-quality research, as some of the association attributed to sodium levels may still be acting as marker for diet quality. Future studies could explore whether the sodium-potassium ratio is a mediator of disease or a marker of other biological or behavioral factors.
Conclusion
We demonstrate that a sodium-to-potassium ratio over one, collected from spot urines, is associated with a greater risk of stroke over eleven years of follow-up in adult Americans of diverse race/ethnicity. It may also, potentially, be associated with a broader range of cardiovascular events. The collection of spot urines is part of routine clinical care, and the measurement of these biomarkers is inexpensive, suggesting strong potential to use spot urines ratios in clinical care settings. Further, an intermediate target (sodium-to-potassium ratio’s <1) is attractive for future public health interventions and our data continues to provide evidence that sodium and potassium are linked to cardiovascular health.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources
This research was supported by R01 DK 076608 and contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from National Center for Advancing Translational Sciences. This work is also a publication of the US Department of Agriculture, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX) funded in part by the USDA/ARS (Cooperative Agreement 58-3092-5-001). The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement from the US government.
DISCLOSURES
AD Adam Drewnowski has received grants, contracts, and honoraria, and consulting fees from private industry, foundations, numerous food and public beverage companies and other commercial and nonprofit entities with interests in the links between nutrient profiling of foods, diet quality, and health outcomes. The University of Washington has received grants, donations, and contracts from both the public and the private sector.
Footnotes
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Subcommittee AHASCaSS, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 4.United States. Department of Health and Human S, United States. Department of A and United States. Dietary Guidelines Advisory C. Dietary guidelines for Americans, 2010. 2010.
- 5.WHO. Guideline: Sodium intake for adults and children. WHO; 2012. [PubMed] [Google Scholar]
- 6.Jackson SL, Cogswell ME, Zhao L, Terry AL, Wang CY, Wright J, et al. Association Between Urinary Sodium and Potassium Excretion and Blood Pressure Among Adults in the United States: National Health and Nutrition Examination Survey, 2014. Circulation. 2018;137:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J, Cogswell ME, Park S, Jackson SL and Odom EC. Sodium Intake Among U.S. Adults - 26 States, the District of Columbia, and Puerto Rico, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:695–8. [PMC free article] [PubMed] [Google Scholar]
- 8.NHANES 2011 - 2012: Dietary Interview - Individual Foods, First Day Data Documentation, Codebook, and Frequencies. 2014;2015.
- 9.Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 10.Institute of Medicine (U.S.). Panel on Dietary Reference Intakes for Electrolytes and Water Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press; 2005. [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 12.Mercado CI, Cogswell ME, Valderrama AL, Wang CY, Loria CM, Moshfegh AJ, et al. Difference between 24-h diet recall and urine excretion for assessing population sodium and potassium intake in adults aged 18–39 y. Am J Clin Nutr. 2015;101:376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 14.Drewnowski A, Maillot M and Rehm C. Reducing the sodium-potassium ratio in the US diet: a challenge for public health. Am J Clin Nutr. 2012;96:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey RL, Parker EA, Rhodes DG, Goldman JD, Clemens JC, Moshfegh AJ, et al. Estimating Sodium and Potassium Intakes and Their Ratio in the American Diet: Data from the 2011–2012 NHANES. J Nutr. 2016; 146:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drewnowski A, Rehm CD, Maillot M, Mendoza A and Monsivais P. The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open. 2015;5:e006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maillot M, Monsivais P and Drewnowski A. Food pattern modeling shows that the 2010 Dietary Guidelines for sodium and potassium cannot be met simultaneously. Nutr Res. 2013;33:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayedi A, Ghomashi F, Zargar MS and Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. [published online June 1, 2018]. Clin Nutr 2018. https://www.sciencedirect.com/science/article/pii/S0261561418302024?via%3Dihub Accessed December, 20, 2018. [DOI] [PubMed]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 20.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart. 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidula H, Liu K, Criqui MH, Szklo M, Allison M, Sibley C, et al. Metabolic syndrome and incident peripheral artery disease - the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;243:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–38. [DOI] [PubMed] [Google Scholar]
- 23.Prentice RL, Huang Y, Neuhouser ML, Manson JE, Mossavar-Rahmani Y, Thomas F, et al. Associations of Biomarker-Calibrated Sodium and Potassium Intakes With Cardiovascular Disease Risk Among Postmenopausal Women. Am J Epidemiol. 2017;186:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Appel LJ and Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwahori T, Ueshima H, Ohgami N, Yamashita H, Miyagawa N, Kondo K, et al. Effectiveness of a Self-monitoring Device for Urinary Sodium-to-Potassium Ratio on Dietary Improvement in Free-Living Adults: a Randomized Controlled Trial. J Epidemiol. 2018;28:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saulnier PJ, Gand E, Ragot S, Bankir L, Piguel X, Fumeron F, et al. Urinary Sodium Concentration Is an Independent Predictor of All-Cause and Cardiovascular Mortality in a Type 2 Diabetes Cohort Population. J Diabetes Res. 2017;2017:5327352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer IH and Kestenbaum B. Invited commentary: Quantifying salt in urine--a complex solution. Am J Epidemiol. 2013;177:1193–5; discussion 1196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y and Y H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.