Abstract
By documenting potent anti-oxidative and anti-inflammatory functions, preclinical studies encourage heme oxygenase-1 (HO-1)-inducing regimens in clinical orthotopic liver transplantation (OLT). We aimed to determine the importance of recipient-derived HO-1 in murine and human OLTs. Hepatic biopsies from fifty-one OLT patients were screened for HO-1 expression (Western blots) prior to put-in (basal) and post-reperfusion (stressed) and correlated with the hepatocellular function. In parallel, livers from HO-1 proficient mice (WT; C57/BL6), subjected to ex-vivo cold storage (18hr), were transplanted to syngeneic myeloid HO-1 deficient (mHO-1 KO) or FLOX (control) hosts, and sampled post-reperfusion (6hr). In human OLT, post-transplant but not pre-transplant HO-1 expression correlated negatively with ALT levels (p=0.0178). High post- but not pre-transplant HO-1 expression trended with improved OLT survival. Compared with controls, livers transplanted into mHO-1 KO recipient mice had decreased HO-1 levels; exacerbated hepatic damage/frequency of TUNEL+ cells; increased mRNA levels coding for TNFα/CXCL1/CXCL2/CXCL10; higher frequency of Ly6G+/4HN+ neutrophils; and enhanced MPO activity. Peritoneal neutrophils from mHO-1 KO mice exhibited higher CellRox+ ratio and increased TNFα/CXCL1/CXCL2/CXCL10 expression. By demonstrating the importance of post-transplant recipient HO-1 phenotype in hepatic macrophage/neutrophil regulation and function, this translational study identifies recipient HO-1 inducibility as a novel biomarker of ischemic stress resistance in OLT.
INTRODUCTION
Orthotopic liver transplantation (OLT) has become the standard care for patients with end-stage liver disease and those with hepatic malignancies (1). Ischemia-reperfusion injury (IRI), a leading cause of early graft dysfunction, represents a major risk factor in the development of acute/chronic rejection and contributes to the shortage of donor organs (2). Thus, novel IR-targeted strategies are needed to improve clinical outcomes and expand donor organ pool. Despite its clinical importance, however, the mechanisms that account for liver IRI are not fully appreciated (3).
Liver IR-damage, an innate immune-driven inflammation, is followed by the hepatocellular death. The cellular IR-stress primes secretion of damage-associated molecular patterns, which trigger inflammatory cytokines to further hepatocyte death. In addition to macrophages, neutrophils also serve as early effectors in hepatic IRI by generating/diffusing reactive oxygen species (ROS) and disturbing homeostasis to trigger mitochondrial dysfunction and cell death (4),(5). Indeed, systemic neutrophil depletion attenuated liver IRI by suppressing hepatic neutrophil accumulation (6).
Heme oxygenase-1 (HO-1; HMOX1; hsp32), the inducible isoform of heme oxygenase catalyzing the degradation of heme into biliverdin, iron and carbon monoxide, exerts anti-oxidative and anti-inflammatory functions (7). We reported on benefits of HO-1 induction in hepatic IRI rodent models, using gene transfer (8),(9) and macrophage-based therapies (10),(11). With macrophage recognized as a key mediator of innate inflammation, HO-1 cytoprotection is attributed to its regulation in IR-stressed liver (12),(13). Others have shown that cobalt protoporphyrin (chemical HO-1 inducer) decreased neutrophil superoxide production and suppressed neutrophil migration (14),(15), implying neutrophil regulation as an integral part of the HO-1 cytoprotective phenotype. However, by decreasing BACH1 and increasing NRF2 protein levels, cobalt protoporphyrin acts indirectly on HO-1 (16), while gene specific HO-1 function in neutrophil regulation remains to be defined.
We have reported that post-transplant HO-1 expression negatively correlated with the severity of IRI in liver transplant patients (11). Since macrophages are the main source of HO-1 in IR-stressed livers (12), post-transplant HO-1 phenotype in OLT may include liver-resident (Kupffer cell, donor-origin) and liver-infiltrating (recipient-origin) macrophages. Indeed, Devey et al. reported that Kupffer cells may dictate the hepatic HO-1 levels in a mouse warm liver IRI model (17). By contrast, in a mouse cold IRI-OLT model, adjunctive infusion of HO-1-overexpressing bone marrow-derived macrophages (BMDM) increased graft HO-1 levels; whereas HO-1-silenced (siRNA) BMDM decreased graft HO-1 expression, as compared with unmodified BMDM infusion (10),(18). Although in murine models, liver-infiltrating host BMDM may affect HO-1 expression/function at the graft site, it remains unknown whether recipient-derived HO-1 may influence hepatic HO-1 levels and IRI severity in OLT. In humans, genetic HO-1 induction seems to govern its expression profile, with studies focusing on donor basal HO-1 levels, and no insights into recipients’ HO-1 inducibility (19),(20).
In this study, we analyzed whether recipient-derived HO-1 may affect liver graft HO-1 levels and function. In the experimental arm, we used myeloid-specific HO-1 deficient mice as recipients of HO-1 proficient (WT) livers to highlight how HO-1 macrophage/neutrophil regulation may affect IRI in OLT. Our clinical arm reinforces the importance of recipient HO-1 phenotype by documenting the need for peri-transplant HO-1 enhancement in OLT protection. In the context of preclinical studies paving the way for HO-1 cytoprotective regimens and the need of future inclusion criteria for clinical responders, the current study provides important insights to the role of recipient HO-1 inducibility as a novel biomarker of hepatocellular resistance against IR-stress in liver transplantation.
METHODS
Clinical liver transplant study
Fifty-one adult primary OLT recipients were recruited under an IRB protocol (13-000143; May 2013–August 2015) (11),(21). Routine standard of care and immunosuppressive therapy was administered, as specified by UCLA liver transplant protocols. Study data were collected and managed using REDCap Electronic Data Management System. Livers were perfused with and stored in University of Wisconsin (UW) solution (Niaspan; Bristol-Meyers Squibb Pharma). Cold ischemia time was defined as the time from the perfusion of the donor liver with UW solution to its removal from the cold storage for implantation. Recipient venous blood was collected within the hour prior to the transplant and on post-operative day 1 (POD1). The hepatocellular injury was evaluated by serum alanine aminotransferase (sALT). Protocol Tru-Cut needle biopsies (Bx) from the left hepatic lobe were obtained during back-table preparation (prior to the put-in); and 2h after portal reperfusion (prior to the abdominal closure).
Animals
Myeloid-specific HO-1 deficient (mHO-1 KO; C57BL/6) mice were generated (22). In brief, floxed HO-1 KO mice were crossed with lysM (lysozyme M) Cre transgenic mice (23). Homozygous mice for floxed and Cre transgenic alleles (HO-1fl/fl, lysM Cre+/+) were used as mHO-1 KO, while HO-1fl/fl, lysM Cre−/− served as controls (12). We confirmed depressed HO-1 expression in liver-resident and bone marrow-derived macrophages from mHO-1 KO mice. Wild-type (WT; C57BL/6) and GFP transgenic (GFP-Tg) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were housed at UCLA under pathogen-free conditions, received humane care according to criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
Mouse orthotopic liver transplantation
We used a mouse model of ex-vivo hepatic cold storage and transplantation (21),(24). To mimic “marginal” human OLT, donor livers were stored in UW solution at 4°C for 18h prior to transplant into syngeneic recipients. Liver graft and serum were collected at 6h of reperfusion, the peak of hepatocellular damage in this model. The sham group underwent the same procedures except for OLT.
Hepatocellular function assay
Mouse serum alanine transaminase (sALT) and aspartate transaminase (sAST), an indicator of hepatocellular injury, were measured by IDEXX Laboratories (Westbrook, ME).
OLT histology and IRI grading
Formalin-fixed paraffin-embedded liver sections (5μm) were stained with hematoxylin and eosin (H&E). The severity of IRI was graded using Suzuki’s criteria (25).
TdT-mediated dUTP nick end labeling (TUNEL) assay
Cell death in formalin-fixed paraffin-embedded liver sections (5μm) was detected by Apop Tag Plus Peroxidase in Situ Apoptosis Kit (Millipore, Temecula, CA). Results were scored semi-quantitatively by blindly counting the number of positive cells in 10 HPF/section.
Immunofluorescence
Mouse livers were stained with rabbit anti-CD11b Ab (Abcam, Cambridge, MA), rat anti-Ly6G Ab (BD Biosciences, San Jose, CA), rabbit anti-4-hydroxynonenal Ab (Abcam), rabbit anti-HO-1 Ab (Enzo Life Sciences, Farmingdale, NY) and rat anti-CD68 Ab (Bio Rad, Hercules, CA). Human livers were stained with rabbit anti-HO-1 Ab (Enzo Life Sciences) and mouse anti-CD68 Ab (BD Biosciences). Signals were visualized with secondary Alexa Fluor Abs. Liver-infiltrating CD11b+ and Ly6G+ cells were scored semi-quantitatively by blindly counting positive cells in 10 HPF/section (x400).
Bone marrow-derived macrophage culture
Bone marrow-derived macrophages (BMDM) were generated, as described (10). In brief, bone marrow cells were obtained from the femurs and tibias, cultured in 15% L929-conditioned medium for 7 days, and used for in vitro study.
Neutrophil isolation and flow cytometry
Casein-elicited peritoneal neutrophils were purified by Percoll density gradient centrifugation (Neutrophil Isolation Kit, Cayman Chemical, Ann Arbor, MI) (26). Neutrophils were incubated in a 5μM concentration of the CellROX Green Reagent (Invitrogen, Carlsbad, CA) and stained with the surface marker of PerCP/Cy5.5 conjugated Ly6G (BioLegend, San Diego, CA). Multi parameter FACS was performed using a SORP BD LSRII analytic flow cytometer (BD Bioscience) and results were analyzed using BD FACSDiva software (BD Bioscience).
Western blot assay
Proteins were extracted from tissue/cell samples, and their concentration measured using BCA Protein Assay Kit (Thermo Scientific). Equal amount of protein was electrophoresed, blotted, incubated with primary Ab, secondary HRP-conjugated Ab, and developed. Primary Ab detecting HO-1 (Enzo Life Sciences, Farmingdale, NY), cleaved caspase 3, β-actin (Cell Signaling Technology, Danvers, MA) were used. To compare protein expression in multiple human OLT samples, densitometry quantification was conducted as reported (12, 27). Briefly, in a preliminary study, one of the Bx samples expressing all target proteins was chosen and assigned as a “control” sample. Equal amount of protein lysate from each sample was applied to each well/gel, and the target band intensity was expressed as relative band intensity to that of the positive control in the same gel. The target relative protein value was normalized according to β-actin intensity.
Quantitative RT-PCR analysis
RNA extracted with RNAse Mini Kit (Qiagen, Germantown, MD) was reverse-transcribed into cDNA. PCR was performed using QuantStudio 3 (Applied Biosystems, Foster City, CA). The primer sequences are listed (Table S1). The target gene expression was normalized to housekeeping HPRT or β-actin.
ELISA
Serum MCP1/TNFα concentration was measured by ELISA kits (Thermo Scientific), according to the manufacturer’s protocol.
Myeloperoxidase (MPO) activity assay
The presence of MPO was used as an index of neutrophil accumulation in the liver. The change in absorbance was measured spectrophotometrically at 655nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1μmol peroxide per min at 25°C per gram of tissue (28).
Statistical analysis
Group comparisons were performed using a Student t-test for mouse experiments, while Mann-Whitney U test was used for human data. Spearman’s correlation coefficient (r) was used to evaluate the strength of linear relationship between variables. The cumulative survival rate was analyzed by Kaplan-Meier method, and differences were compared using a log-rank test. JMP for Windows 8.0 (SAS Institute, Cary, NC) was used for statistical analyses. A p-value of <0.05 was considered statistically significant.
RESULTS
Post- but not pre-transplant HO-1 expression negatively correlates with hepatocellular function in human OLT
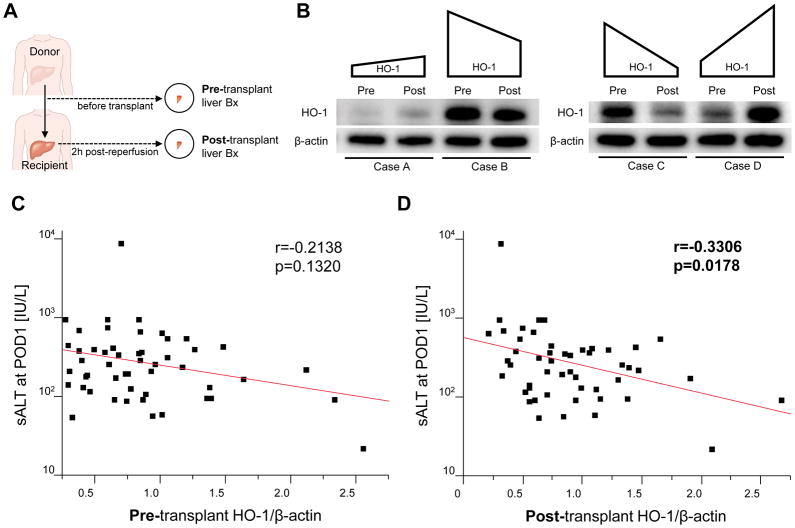
Although Buis et al reported that pre-transplant hepatic high HO-1 expression associated with improved human OLT survival (20), others failed to identify increased pre-transplant HO-1 level as a biomarker of preserved OLT function (19). Having documenting negative correlation between post-transplant HO-1 levels and IRI severity in clinical OLT (11),(12), we now aimed to compare the relationship between pre- / post-transplant HO-1 expression and IR-triggered hepatocellular damage in fifty-one OLT patients. Pre-transplant liver Bx were collected after cold storage (prior to put-in) and post-transplant Bx were obtained 2h after portal reperfusion (prior to the abdominal closure) (Fig. 1A). Representative pre-transplant (basal) and post-transplant (IR-induced) Western blot-assisted HO-1 expression profiles are shown in Fig. 1B. There was no significant correlation between basal hepatic HO-1 expression and donor demographics, pre-procurement blood tests, duration of brain ischemia, or cold ischemia times (Table S2). We also found no significant correlation between post-transplant HO-1 levels and donor/recipient demographics, pre-operative blood tests, duration of brain or cold ischemia time, race, disease etiology, presence of HCC, ABO-compatibility, MELD score, pre-transplant dialysis, or intra-operative blood loss (Table S3). Unlike basal HO-1 levels, which failed to significantly correlate with sALT at POD1 (r=−0.2138, p=0.1320, Fig. 1C), post-transplant HO-1 correlated negatively with sALT at POD1 (r=−0.3306, p=0.0178, Fig. 1D). This indicates that post-transplant rather than basal HO-1 levels were essential for improved hepatocellular function in OLT recipients.
Figure 1. Post-transplant but not pre-transplant graft HO-1 expression correlates negatively with the hepatocellular damage in liver transplant patients.
(A) Pre-transplant (prior to put-in) and post-transplant (2h after reperfusion) protocol liver biopsies (Bx) were collected from fifty-one liver transplant patients. HO-1 expressions in Bx samples was analyzed by Western blots with β-actin normalization, as described in Methods. (B) Representative peri-operative HO-1 profiles (Pre: pre-transplant, Post: post-transplant, case A vs case B: pre-transplant HO-1 level rather than peri-operative HO-1 increase determined post-transplant HO-1 expression; case C vs case D: peri-operative HO-1 enhancement rather than pre-transplant HO-1 was crucial for post-transplant HO-1 level). (C) Relationship between pre-transplant HO-1 and sALT level at postoperative day 1 (POD1). (D) Relationship between post-transplant HO-1 level and sALT level at POD1. r: Spearman’s correlation coefficient.
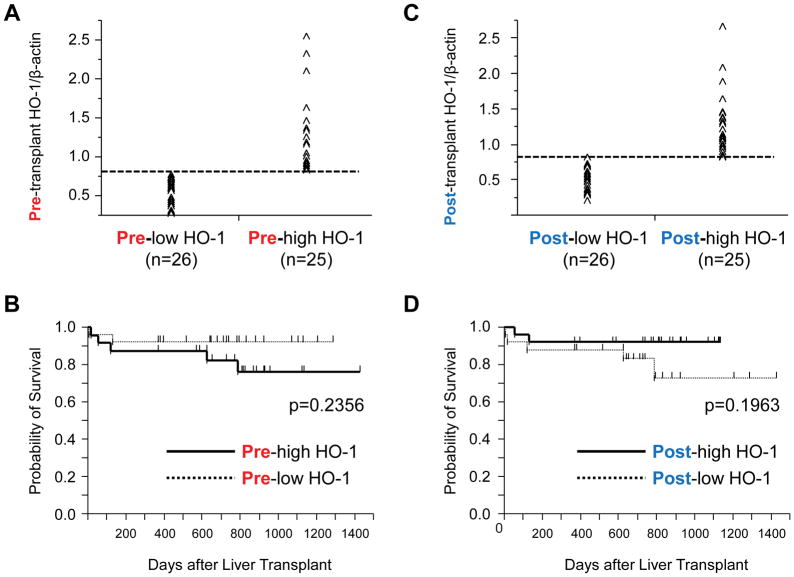
We next evaluated the influence of pre- vs. post-transplant hepatic HO-1 levels on recipient OLT survival, with a median follow-up of 740 days (range, 4–1432 days). None of the patients underwent secondary liver transplantation. Based on Western blot-assisted basal HO-1 quantification, patients were classified into pre-low (n=26) and pre-high (n=25) HO-1 expression groups (Fig. 2A). There was a tendency for pre-high HO-1 having inferior survival as compared with pre-low HO-1 group (2-year: pre-high=81.1% vs. pre-low=92.4%; p=0.2356; Fig. 2B). However, when OLT patients were divided into post-low (n=26) and post-high (n=25) HO-1 expression groups (Fig. 2C), the latter showed a trend toward improved survival, compared with post-low HO-1 cohort (2-year: post-high=92.6% vs. pre-low=80.7%; p=0.1963; Fig. 2D). Despite lacking statistical significance (Fig. 2B/D), these findings are consistent with the notion that post-transplant HO-1 expression profile is likely important for OLT protection against IR-stress, whereas pre-transplant basal HO-1 levels seem to be a less reliable predictor of the clinical outcome (Fig. 1C/D).
Fig. 2. Relationship between basal or post-transplant HO-1 expression and liver transplant patient survival.
Pre-transplant (prior to put-in) and post-transplant (2h after reperfusion) liver biopsies (Bx) were collected from fifty-one liver transplant patients. HO-1 expression in Bx samples was analyzed by Western blots with β-actin normalization. (A) Based on Western blot-assisted HO-1 expression in pre-transplant liver Bx, human OLT recipients were classified into “pre-low HO-1” (n=26) and “pre-high HO-1” (n=25) groups. (B) The cumulative probability of post-transplant survival (Kaplan-Meier method). Solid line indicates pre-high HO-1, while the dotted line pre-low HO-1 groups (log-rank test). (C) Based on Western blot-assisted HO-1 expression in post-transplant liver Bx, recipients were classified into “post-low HO-1 (n=26)” and “post-high HO-1 (n=25)” groups. (D) The cumulative probability of post-transplant survival (Kaplan-Meier method). Solid line indicates post-high HO-1, while the dotted line post-low HO-1 groups (log-rank test).
Both, pre-transplant HO-1 levels and peri-transplant HO-1 enhancement are essential for post-transplant HO-1 levels
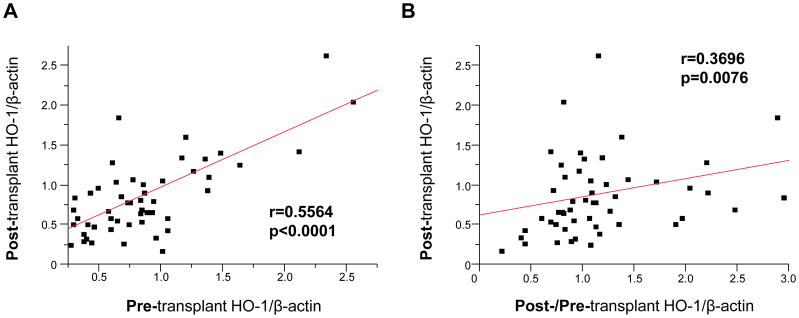
Having demonstrating the importance of post-transplant HO-1 levels in graft cytoprotection, we envisioned two putative scenarios. While comparing clinical case A and B (Fig. 1B left panel), despite IR-stress enhanced graft HO-1 in case A but not in case B, the HO-1 level was lower in case A after reperfusion, indicating basal HO-1 but not peri-operative HO-1 increase determined post-transplant HO-1 phenotype. In contrast, while comparing case C and D (Fig. 1B right panel), although basal HO-1 expression was higher in case C, marked HO-1 enhancement after reperfusion was noted in case D but not in case C, suggesting peri-operative HO-1 increase but not basal HO-1 was crucial for post-transplant HO-1 levels. To verify the importance of these two factors, we tested the relationship between pre- and post-transplant HO-1 expression (Fig. 3A) as well as the correlation between post-/pre-transplant HO-1 ratio (an indicator of peri-transplant HO-1 enhancement) and post-transplant HO-1 expression (Fig. 3B). Post-transplant HO-1 expression significantly correlated with basal HO-1 levels (r=0.5564, p<0.0001), implying pre-transplant HO-1 expression influenced post-transplant HO-1 levels. On the other hand, despite post-/pre-transplant HO-1 ratio was calculated with division by basal HO-1 (which showed strong correlation with post-transplant HO-1 [Fig. 3A]), the post-transplant HO-1 levels nonetheless showed significant correlation with post-/pre-transplant HO-1 ratio (r=0.3696, p=0.0076, Fig. 3B). Thus, post-transplant HO-1 expression was dictated not only by basal HO-1 steady-state but also by IR-stress triggered HO-1 increase.
Figure 3. Both basal HO-1 level and peri-transplant HO-1 enhancement are important for post-reperfusion hepatic HO-1 expression in liver transplant patients.
Pre-transplant (prior to put-in) and post-transplant (2h after reperfusion) liver biopsies (Bx) were collected from fifty-one liver transplant patients. HO-1 expression in Bx samples was analyzed by Western blots with β-actin normalization. (A) Relationship between pre-transplant HO-1 and post-transplant HO-1 levels. (B) Relationship between post-/pre-transplant HO-1 ratio and post-transplant HO-1 level. r: Spearman’s correlation coefficient.
Recipient myeloid HO-1 deficiency decreases HO-1 expression in mouse OLT
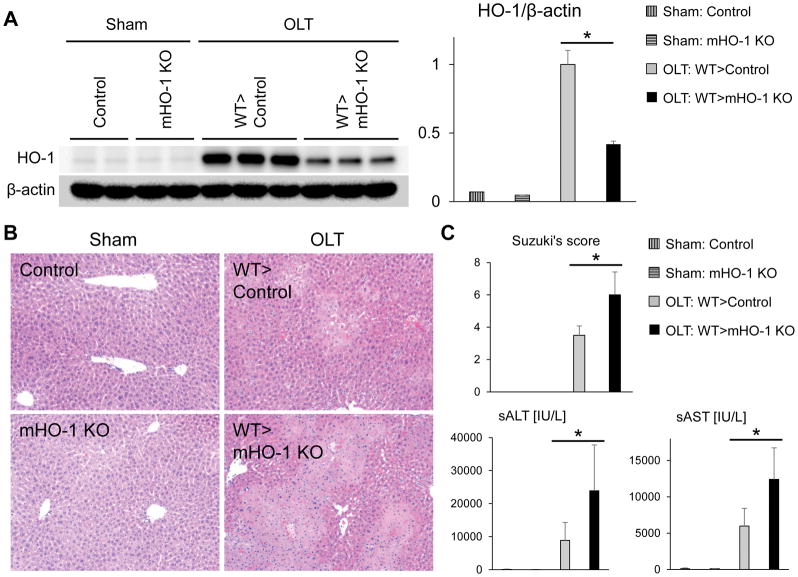
Consistent with published data (12),(19), we found CD68 macrophages are the primary source of HO-1 in IR-stressed human and mouse OLT (Fig. S1). Since post-reperfusion liver grafts contain both donor-origin resident (Kupffer cells) and recipient-origin infiltrating macrophages, theoretically both cell types can produce HO-1 and contribute to IR-triggered peri-transplant HO-1 enhancement, which is one of post-transplant HO-1 determinant factors (Fig. 3B). However, with previous studies focused on HO-1 expression in donor-derived resident macrophages (17),(19),(20), the importance of recipient-derived infiltrating macrophages on graft HO-1 expression remains to be elucidated. To determine HO-1 origin (donor vs. recipient) in OLT, we first transplanted WT livers, subjected to 18h cold storage, into GFP-Tg recipient mice. By 6h of reperfusion, we observed HO-1 expression in donor-derived GFP-negative cells (Fig. S2a/S2b) as well as in recipient-derived GFP-positive cells (Fig. S2c/S2d), indicating both donor- and recipient-origin macrophages were the source of HO-1 in IR-stressed OLT. Next, to determine the impact of recipient HO-1 on graft HO-1 expression, we transplanted HO-1 proficient WT livers (cold-stored for 18h) into groups of myeloid-specific HO-1 knockout (mHO-1 KO) vs. HO-1 proficient (control) mice. We confirmed that compared with controls, HO-1 protein was almost undetectable in BMDM cultures from mHO-1 KO mice (Fig. S3). As shown in Fig. 4A, myeloid-specific HO-1 recipient deficiency decreased graft HO-1 levels by almost half at 6h of reperfusion as compared with controls, indicating IR-stressed recipient macrophage HO-1 was a critical determinant for post-transplant graft HO-1 levels. On the other hand, HO-1 levels in WT livers transplanted into mHO-1 KO mice were enhanced by five-fold as compared with sham-livers, indicating liver-resident macrophages achieved five-fold HO-1 enhancement in IR-stressed OLT without recipient-derived macrophage HO-1. Hence, liver-resident macrophage HO-1 as well as recipient macrophage HO-1 are both essential for post-transplant hepatic HO-1 phenotype.
Figure 4. Recipient myeloid HO-1 deficiency decreases graft HO-1 expression and aggravates hepatocellular damage in mouse OLT.
WT mouse (C57/Bl6) livers subjected to 18h of cold storage were transplanted orthotopically to HO-1 proficient control and myeloid-specific HO-1 knockout (mHO-1 KO) recipient mice. Liver grafts and serum samples were analyzed at 6h post-OLT. The sham group underwent the same procedures except for OLT. (A) Western blot-assisted detection and relative intensity ratio of HO-1. β-actin expression served as an internal control and used for normalization. (B) Representative H&E staining (original magnification, x100) (C) Serum ALT/AST levels (IU/L) and Suzuki’s histological grading of liver IRI (n=3–5/group). Data shown as mean±SD (*p<0.05, Student t-test).
Recipient myeloid HO-1 deficiency accelerates hepatocellular damage in mouse OLT
We next asked whether suppression of HO-1 at the graft site, resulting from recipient myeloid HO-1 deficiency, may influence IR-damage in OLT. At 6h post-reperfusion, WT livers transplanted to mHO-1 KO mice displayed enhanced sinusoidal congestion, edema/vacuolization and hepatocellular necrosis, as compared with OLT in HO-1 proficient controls (Fig. 4B). This correlated with increased Suzuki’s histological grading of liver IRI (control=3.5±0.6 vs. mHO-1 KO=6.0±1.4, n=4–5, p=0.0066, Fig. 4C); elevated sALT/sAST levels (sALT: control=8,895±5,411 vs. mHO-1 KO recipient=23,880±13,891 IU/L, p=0.0370; sAST: control=5,984±2,433 vs. mHO-1 KO=12,405±4,323 IU/L, p=0.0142; n=4–5, Fig. 4C); enhanced cleaved caspase-3 (p=0.0077, Fig. S4A); and increased frequency of TUNEL+ cells (p=0.0011, Fig. S4B). Thus, suppression of graft HO-1 levels due to recipient myeloid HO-1 deficiency exacerbated IR-damage in OLT.
OLTs in mHO-1 KO recipients exhibit increased pro-inflammatory IR-signature
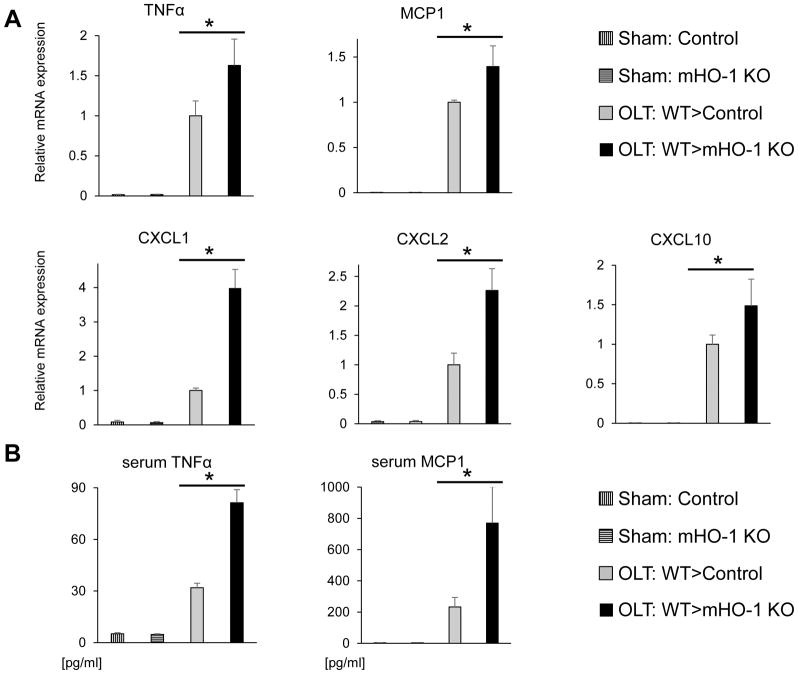
As the release of pro-inflammatory cytokines is critical for the continuum of immune cascade culminating in the hepatocellular death, we next focused on cytokine profile in our IRI-OLT model. At 6h post-reperfusion, WT livers transplanted into mHO-1 KO recipients showed higher levels of TNFα, MCP1, CXCL1, CXCL2 and CXCL10 (p<0.05, Fig. 5A/B), and increased frequency of infiltrating macrophages (CD11b; p=0.0002, Fig. S5A/B). These in vivo findings were corroborated by BMDM cultures (data not shown), consistent with the notion that HO-1 inhibits macrophage pro-inflammatory phenotype (12),(13), a dominant HO-1 producer (Fig. S1) and the key regulator of innate immune response in IR-stressed livers. Thus, recipient myeloid HO-1 deficiency enhanced inflammation in IR-stressed OLT.
Figure 5. Recipient myeloid HO-1 deficiency enhances inflammatory response in IR-stressed mouse OLT.
WT mouse livers subjected to 18h of cold storage were transplanted into HO-1 proficient control and myeloid-specific HO-1 knockout (mHO-1 KO) recipient mice, followed by serum/graft sampling at 6h after reperfusion. (A) qRT-PCR-assisted detection of mRNA coding for TNFα, MCP1, CXCL1, CXCL2 and CXCL10 in OLTs. Data were normalized to HPRT gene expression (n=3–4/group). (B) ELISA-assisted examination of serum TNFα and MCP1 levels (pg/ml, n=3–5/group). Data shown as mean±SD (*p<0.05, Student t-test).
Recipient myeloid HO-1 deficiency enhances neutrophil activation in mouse OLT
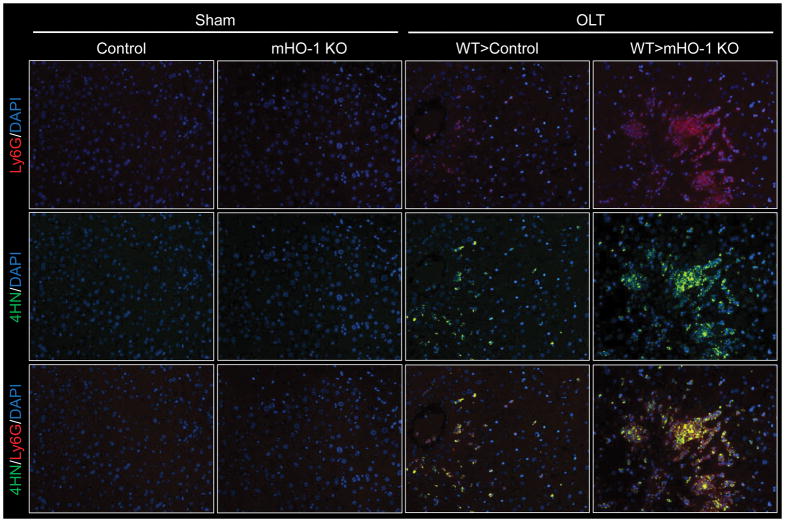
As macrophages play a critical role in IRI, exacerbated inflammation in WT livers transplanted to mHO-1 KO recipients (Fig. 5A/B) could be most likely attributed to disrupted macrophage regulation by HO-1 (12, 13). However, by producing cytotoxic ROS, neutrophils may also be essential in IRI-OLT pathogenesis. With their role largely understudied, we then focused on the impact of HO-1 deficiency upon the function of OLT-infiltrating neutrophils. By 6h of reperfusion, OLTs in mHO-1 KO mice exhibited increased neutrophil (Ly6G) sequestration (Fig. S5A/B, p<0.0001) and higher MPO activity (Fig. S5C, p=0.0013), as compared with controls. Enhanced neutrophil OLT sequestration in mHO-1 KO recipients was accompanied by increased 4-hydroxynonenal (4HN) expression, one of the key oxidative metabolites (Fig. 6).
Figure 6. Recipient myeloid HO-1 deficiency increases neutrophil 4-Hydroxynonenal (4HN) expression in mouse OLT.
WT mouse livers subjected to 18h of cold storage were transplanted into HO-1 proficient control and myeloid-specific HO-1 knockout (mHO-1 KO) recipient mice, followed by hepatic sampling at 6h after reperfusion. Immunohistochemical detection of Ly6G (red), 4HN (green) and DAPI (blue) in OLTs. The sham group underwent the same procedures except for OLT. Representative of three.
HO-1 deficient neutrophils exhibit increased ROS and pro-inflammatory phenotype
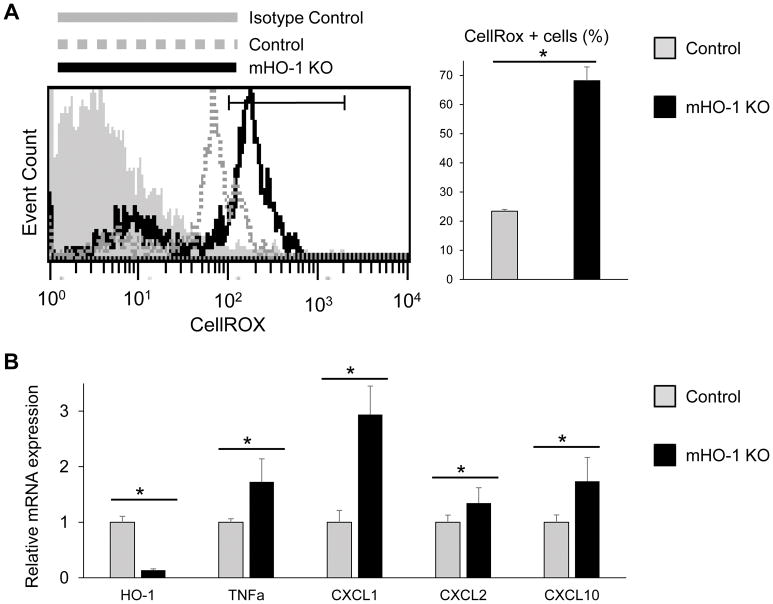
We next focused on the neutrophil function in our model by analyzing the influence of HO-1 disruption in casein-elicited peritoneal neutrophil population. Indeed, Ly6G+ sorted neutrophils from mHO-1 KO mice were characterized by increased levels of ROS as compared with those from HO-1 proficient (control) mice, evidenced by CellRox staining by FACS (Fig. 7A, p=0.0021). In addition, neutrophils from mHO-1 KO mice displayed enhanced mRNA levels coding for TNFα, CXCL1, CXCL2, CXCL10 and concomitantly depressed HO-1 (Fig. 7B, p<0.05). These findings indicate neutrophil HO-1 is essential to regulate ROS and pro-inflammatory gene programs.
Figure 7. Neutrophil HO-1 deficiency enhances reactive oxygen species (ROS) production and inflammatory gene phenotype.
Casein-elicited peritoneal neutrophils were obtained from HO-1 proficient control and myeloid-specific HO-1 knockout (mHO-1 KO) mice. (A) ROS level in neutrophil was analyzed by flow cytometry using fluorescent CellROX probe (n=4/group). (B) qRT-PCR-assisted detection of mRNA coding for HO-1, TNFα, CXCL1, CXCL2 and CXCL10. Data were normalized to β-actin gene (n=4/group). Data shown as mean±SD (*p<0.05, Student t-test).
DISCUSSION
This is the first study, to the best of our knowledge, which demonstrates the hepatoprotective function of recipient myeloid cell-specific HO-1. In the clinical arm, we found that post-transplant low HO-1 level was a reliable predictor of exacerbated hepatic IR-damage (Fig. 1D). Post-transplant HO-1 reflected pre-transplant HO-1 expression (Fig. 3A), while the relationship between sALT (POD1) and basal HO-1 was weaker as compared with post-transplant HO-1 levels (Fig. 1C/D), implying that steady-state HO-1 level was not a singular decisive factor for post-transplant HO-1 phenotype. To bridge the gap, we have identified peri-operative HO-1 increase as one of the critical factors for post-reperfusion HO-1 expression in human OLT (Fig. 3B). In the experimental arm, recipient myeloid HO-1 deficiency increased hepatic tissue injury histology scores, release of liver enzymes (Fig. 4C), cell death (Fig. S4), pro-inflammatory cytokine phenotype (Fig. 5), and leukocyte infiltration in IR-stressed mouse OLT (Fig. S5). To establish macrophage HO-1 regulatory axis (12),(13), we now show the regulatory function of HO-1 in neutrophil ROS and pro-inflammatory gene expression programs (Fig. 7). Taken together, our study documents the importance of recipient HO-1 inducibility (i.e., the ability to trigger HO-1 under IR-stress) for peri-operative HO-1 enhancement and OLT cytoprotection.
Prolonged cold ischemia time (CIT) represents an independent risk factor for OLT loss (29). In our current fifty-one human OLT cohort, livers with CIT<8h showed significantly better post-OLT survival as compared with those subjected to CIT≥8h (Fig. S6A, p<0.05). Although HO-1 is a stress-inducible gene, we found no correlations between pre-/post-transplant HO-1 levels and CIT (Fig. S6B–S6E), suggesting CIT is unlikely determinant factor for graft HO-1 expression. Moreover, we found no significant correlations between graft HO-1 levels and other clinical parameters, including liver function (AST/ALT/T-Bil/INR), duration of brain ischemia, MELD score, intra-operative blood loss, all of which possibly reflect stress severity (Table S2/S3). In contrast, others have reported that cerebral cortex/hippocampus HO-1 levels in normal human brains showed significant positive correlation with age (30); whereas unstressed livers from aged mice had significantly more HO-1 compared with young counterparts (31). Likewise, despite lacking statistical correlation, pre-transplant HO-1 levels in our clinical study trended towards positive correlation with the donor age (r=0.2771, p=0.0514, Table S2); whereas the relationship between donor age and HO-1 weakened in post-reperfusion biopsies (r=0.1422, p=0.3245, Table S3). Noteworthy, human liver grafts with peri-operative HO-1 increase (at 2hr post-reperfusion) had significantly lower pre-operative HO-1 levels (Fig. S7). Similarly, Geuken et al. reported that human liver grafts with abundant pre-transplant HO-1 showed decreased HO-1 expression after reperfusion (by 23%); whereas those with low pre-transplant HO-1 levels were able to induce HO-1 at reperfusion (19). As unlike in “young” mice, oxidative stress failed to increase hepatic HO-1 levels in “old” mice (31), donor age might be one of the factors contributing to discrepant HO-1 dynamics and aforementioned gap between pre- and post-transplant HO-1 levels.
Human HO-1 gene expression is modulated by two functional polymorphisms in the gene promoter. First, a short (GT)n repeat polymorphism has been associated with enhanced transcriptional HO-1 activity (32). Indeed, a short (GT)n repeat in the kidney graft was accompanied by a favorable post-transplant renal function and survival (33, 34). Second, A(-413)T single nucleotide polymorphism (SNP) has also been identified as a functionally relevant variation of the HO-1 gene, while A-allele rather than T-allele of this SNP correlated with a higher promotor activity (35). Interestingly, two studies (19),(20) failed to find correlation between donor (GT)n repeat polymorphism and pre-transplant HO-1 mRNA level in OLT, whereas donor livers with at least one A-allele of A(-413)T SNP could be characterized by higher pre-transplant mRNA levels coding for HO-1 (20). This suggests that A(-413)T SNP may be dominant over (GT)n repeat polymorphism in the hepatic HO-1 promoter activity. Our study suggests the importance of recipient HO-1 inducibility in the mechanism of liver graft protection. A single clinical study reported to date, examining the impact of recipient (GT)n repeat polymorphism but without looking into HO-1 levels, failed to show significant differences on OLT outcomes (36). Further studies on the impact of two functional polymorphisms in organ donor and recipient on post-transplant HO-1 expression levels in OLT patients, are warranted. With previous studies focusing on donor HO-1 polymorphisms, a possibility that recipient polymorphisms may influence HO-1 function in the graft itself is a novel and attractive idea. Indeed, determining the recipient HO-1 polymorphism could be useful to identify prospective transplant patients with poor peri-operative HO-1 inducibility and then utilize, if needed, HO-1 induction regimens beforehand to minimize the risk of a subsequent allograft failure.
We acknowledge the limitations of our study. First, despite recipient HO-1 inducibility affecting post-reperfusion graft HO-1 levels and IRI severity in mouse OLT, we were unable to examine donor/recipient gene polymorphisms in the current patient cohort, or analyze whether putative varieties of HO-1 inducibility in human OLT are indeed decisive to graft HO-1 levels/clinical outcomes. In addition, we found no significant clinical factors related to graft HO-1 levels (Table S2/S4). Second, although post-transplant HO-1 levels negatively correlated with sALT at POD1 (Fig. 1D, r=−0.3306, p=0.0178), the survival differences between post-high and post-low HO-1 groups failed to reach statistical significance (Fig. 2D, p=0.1963). Moreover, although OLTs experiencing biopsy-proven rejection (n=5) had lower HO-1 expression as compared with rejection-free counterparts (n=46), the differences failed to reach statistical significance (data not shown). As many factors influence post-OLT patient/graft survival, encompassing analyses of possible confounders in large patient cohort are required to conclusively determine the importance of HO-1 signaling for OLT clinical outcomes.
We have reported on macrophage HO-1 regulatory functions in the mechanism of liver IRI (12) (13),(37). Consistently, IR-stressed mHO-1 KO livers as well as BMDM cultures generated from mHO-1-deficient mice exhibit increased M1 and decreased M2 gene expression programs as compared with controls (Ming and Nakamura, unpublished data), confirming that HO-1 signaling can drive the phenotypic shift to anti-inflammatory M2 phenotype (37),(38). Of note, HO-1 was highly expressed in hemorrhage-specific macrophages (Mhem), a newly identified anti-oxidative and anti-inflammatory subset in human atherosclerotic plaques (39),(40). Since activating transcription factor 1 drives macrophage adaptation to intraplaque hemorrhage while inducing HO-1, HO-1 may well be crucial for atheroprotective Mhem function (40). Thus, mHO-1 KO mouse used in the current study may be useful to further investigate distinct macrophage states while searching for new immunomodulatory approaches in widely diverse human diseases
Although Kupffer cells are principal ROS producers early post-reperfusion, neutrophil oxidative burst becomes the main source of ROS in the later IRI phase (41),(42). Indeed, by 6 hours of reperfusion (the peak of hepatocellular damage in our murine model), OLT-infiltrating Ly6G-positive neutrophils elaborated large amounts of ROS metabolite, 4HN (Fig. 6), the levels of which along the frequency of CellROX in casein-elicited peritoneal neutrophils, increased further in myeloid-deficient HO-1 hosts (Fig. 7A). These findings, consistent with our data on neutrophil regulation (43),(44), reinforce the role of neutrophil-targeted therapy against liver IRI. Although neutrophil depletion (mAb clone: 1F12) prior to the ischemia insult did not affect hepatocellular damage, despite reducing their infiltration by 60%, repeated mAb treatment alleviated IRI, implying early neutrophil regulation was insufficient for hepatoprotection (45). Paradoxically, disruption of neutrophil signaling (mAb clone: 1F12) was accompanied by activation of Kupffer cells to release toxic oxygen-derived metabolites in vivo and in vitro (46), whereas neutrophil depletion (mAb clone: 1A8) (47) decreased sALT levels at 4 hours post-reperfusion (48). Moreover, adoptive transfer of WT but not TLR4/TRL9 deficient neutrophils, recreated neutrophil extracellular traps (NETs) and cell damage indicating the importance of TLR-dependent netosis in the pathogenesis of liver IRI (49). By shedding light on unappreciated role of neutrophils in the early IRI phase and novel neutrophil-NETs axis in hepatic inflammatory enhancement, these findings further advance the therapeutic potential of neutrophil management in OLT.
In conclusion, this translational study documents the importance of recipient myeloid HO-1 in post-reperfusion HO-1 function, neutrophil regulation and graft protection against IR stress. In the context of encouraging preclinical data on HO-1 inducing regimens in organ transplantation, and the need of inclusion criteria for prospective clinical responders, our study highlights the recipient HO-1 inducibility as one of potentially important biomarkers of hepatic resistance against IR-stress in OLT.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants PO1 AI120944; RO1 DK062357, DK107533, DK102110 (to JWKW); NIH RO1 ES016959, R56 ES016959-06 (to JAA); and The Dumont-UCLA Research Foundation. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility. We thank Ko Takanashi (UCLA-TPCL) for immunohistochemical assistance.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- BMDM
bone marrow-derived macrophages
- Bx
biopsy
- CIT
cold ischemia time
- HE
hematoxylin and eosin
- HO-1
heme oxygenase-1
- IRI
ischemia-reperfusion injury
- mHO-1
myeloid-specific HO-1
- MPO
myeloperoxidase
- OLT
orthotopic liver transplantation
- POD
post-operative day
- ROS
reactive oxygen species
- TUNEL
TdT-mediated dUTP nick end labeling
- UW solution
University of Wisconsin solution
- WT
wild type
- 4HN
4-hydroxynonenal
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Brand DA, Viola D, Rampersaud P, Patrick PA, Rosenthal WS, Wolf DC. Waiting for a liver--hidden costs of the organ shortage. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10(8):1001–1010. doi: 10.1002/lt.20212. [DOI] [PubMed] [Google Scholar]
- 2.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature reviews Gastroenterology & hepatology. 2013;10(2):79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Zhou H, Ni M, Wang X, Busuttil R, Kupiec-Weglinski J, et al. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation. 2016;100(12):2601–2610. doi: 10.1097/TP.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. The American journal of physiology. 1997;272(4 Pt 1):C1286–1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, et al. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology (Baltimore, Md) 2008;48(5):1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1990;4(15):3355–3359. [PubMed] [Google Scholar]
- 7.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annual review of pharmacology and toxicology. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. The Journal of clinical investigation. 1999;104(11):1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke B, Buelow R, Shen XD, Melinek J, Amersi F, Gao F, et al. Heme oxygenase 1 gene transfer prevents CD95/Fas ligand-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Human gene therapy. 2002;13(10):1189–1199. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 10.Ke B, Shen XD, Gao F, Ji H, Qiao B, Zhai Y, et al. Adoptive transfer of ex vivo HO-1 modified bone marrow-derived macrophages prevents liver ischemia and reperfusion injury. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(5):1019–1025. doi: 10.1038/mt.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Kageyama S, Yue S, Huang J, Fujii T, Ke B, et al. Heme Oxygenase-1 Regulates Sirtuin-1 - Autophagy Pathway in Liver Transplantation: From Mouse-to-Human. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017 doi: 10.1111/ajt.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa R, et al. Macrophage HO-1–SIRT1–p53 Axis Regulates Sterile Inflammation in Liver Ischemia–Reperfusion Injury. Journal of hepatology. 2017 doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. Journal of hepatology. 2012;56(2):359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YT, Chen YH, Yang YH, Jao HC, Abiko Y, Yokoyama K, et al. Heme oxygenase-1 suppresses the infiltration of neutrophils in rat liver during sepsis through inactivation of p38 MAPK. Shock (Augusta, Ga) 2010;34(6):615–621. doi: 10.1097/SHK.0b013e3181e46ee0. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2- production in a two-hit model of alcohol intoxication and burn injury. Journal of immunology (Baltimore, Md : 1950) 2008;180(10):6933–6940. doi: 10.4049/jimmunol.180.10.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(14):2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 17.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, et al. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(1):65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen XD, Ke B, Uchida Y, Ji H, Gao F, Zhai Y, et al. Native macrophages genetically modified to express heme oxygenase 1 protect rat liver transplants from ischemia/reperfusion injury. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(2):201–210. doi: 10.1002/lt.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, et al. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 20.Buis CI, van der Steege G, Visser DS, Nolte IM, Hepkema BG, Nijsten M, et al. Heme oxygenase-1 genotype of the donor is associated with graft survival after liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(2):377–385. doi: 10.1111/j.1600-6143.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- 21.Kageyama S, Nakamura K, Fujii T, Ke B, Sosa RA, Reed EF, et al. Recombinant Relaxin Protects Liver Transplants from Ischemia Damage via Hepatocyte Glucocorticoid Receptor: From Bench-to-Bedside. Hepatology (Baltimore, Md) 2018 doi: 10.1002/hep.29787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamiya T, Katsuoka F, Hirayama A, Nakajima O, Kobayashi A, Maher JM, et al. Hepatocyte-specific deletion of heme oxygenase-1 disrupts redox homeostasis in basal and oxidative environments. The Tohoku journal of experimental medicine. 2008;216(4):331–339. doi: 10.1620/tjem.216.331. [DOI] [PubMed] [Google Scholar]
- 23.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 24.Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(10):1273–1281. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine Transplantation. 1993;55(6):1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of Mouse Neutrophils. Current protocols in immunology. 2015;110:3.20.21–23.20.15. doi: 10.1002/0471142735.im0320s110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura K, Kageyama S, Ke B, Fujii T, Sosa RA, Reed EF, et al. Sirtuin 1 Attenuates Inflammation and Hepatocellular Damage in Liver Transplant Ischemia-Reperfusion: From Mouse-to-Human. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2017 doi: 10.1002/lt.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology (Baltimore, Md) 2010;51(4):1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Annals of surgery. 2005;241(6):905–916. doi: 10.1097/01.sla.0000164077.77912.98. discussion 916–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirose W, Ikematsu K, Tsuda R. Age-associated increases in heme oxygenase-1 and ferritin immunoreactivity in the autopsied brain. Legal medicine (Tokyo, Japan) 2003;5(Suppl 1):S360–366. doi: 10.1016/s1344-6223(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 31.Barnes CJ, Cameron IL, Puleo-Scheppke B, Lee M. Age alters expression and inducibility of heme oxygenase isozymes in mice. Age. 1998;21(3):123–128. doi: 10.1007/s11357-998-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. American journal of human genetics. 2000;66(1):187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baan C, Peeters A, Lemos F, Uitterlinden A, Doxiadis I, Claas F, et al. Fundamental role for HO-1 in the self-protection of renal allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(5):811–818. doi: 10.1111/j.1600-6143.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 34.Exner M, Bohmig GA, Schillinger M, Regele H, Watschinger B, Horl WH, et al. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation. 2004;77(4):538–542. doi: 10.1097/01.tp.0000113467.36269.f8. [DOI] [PubMed] [Google Scholar]
- 35.Ono K, Goto Y, Takagi S, Baba S, Tago N, Nonogi H, et al. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173(2):315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Zhang ZY, Guan J, Li H, Zhou ZQ, Zhou GW. Heme Oxygenase-1 Promoter Polymorphism Protects Liver Allograft. The Indian journal of surgery. 2016;78(1):14–19. doi: 10.1007/s12262-015-1309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Shen XD, Yue S, Zhu J, Gao F, Zhai Y, et al. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Molecular medicine (Cambridge, Mass) 2014;20:448–455. doi: 10.2119/molmed.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Archives of biochemistry and biophysics. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. The American journal of pathology. 2009;174(3):1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circulation research. 2012;110(1):20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. American journal of physiology Gastrointestinal and liver physiology. 2005;289(4):G760–767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- 42.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicologic pathology. 2007;35(6):757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 43.Palumbo T, Nakamura K, Lassman C, Kidani Y, Bensinger SJ, Busuttil R, et al. Bruton Tyrosine Kinase Inhibition Attenuates Liver Damage in a Mouse Warm Ischemia and Reperfusion Model. Transplantation. 2017;101(2):322–331. doi: 10.1097/TP.0000000000001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2009;15(8):939–947. doi: 10.1002/lt.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. Journal of hepato-biliary-pancreatic surgery. 1998;5(4):402–408. doi: 10.1007/s005340050064. [DOI] [PubMed] [Google Scholar]
- 46.Bautista AP, Spolarics Z, Jaeschke H, Smith CW, Spitzer JJ. Antineutrophil monoclonal antibody (1F12) alters superoxide anion release by neutrophils and Kupffer cells. Journal of leukocyte biology. 1994;55(3):328–335. doi: 10.1002/jlb.55.3.328. [DOI] [PubMed] [Google Scholar]
- 47.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of leukocyte biology. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 48.Loi P, Yuan Q, Torres D, Delbauve S, Laute MA, Lalmand MC, et al. Interferon regulatory factor 3 deficiency leads to interleukin-17-mediated liver ischemia-reperfusion injury. Hepatology (Baltimore, Md) 2013;57(1):351–361. doi: 10.1002/hep.26022. [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology (Baltimore, Md) 2015;62(2):600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.