Abstract
Pyrethroids are a class of neurotoxic insecticides, and some studies have used single-time wiping of hard surface flooring to estimate indoor pyrethroid concentrations. Considering that human activities may affect concentrations, knowledge of temporal variability is needed to reduce the uncertainty of exposure estimates that are calculated using wipe sampling of pyrethroids in occupied housing. During weeks one, two, and six of a 6-week study, two wipe samples of hard surface kitchen flooring were collected in each of 50 occupied residences and used to estimate the temporal variability of eight pyrethroids and six pyrethroid degradation products. Beginning 1 month prior to sample collection, the participants kept pesticide use diaries. All pyrethroids were widely distributed among the houses, and co-occurrence of multiple pyrethroids was common structured. Application diaries and detection frequencies appeared unconnected, but the applications were correlated with measurable changes in pyrethroid concentrations. In general, degradation products were detected less frequently and at lower concentrations than their parent pyrethroids. Estimates of the intraclass correlation coefficient (ICC) for individual pyrethroids ranged from 0.55 (bifenthrin) to 0.80 (deltamethrin), and two sampling events at each residence would have been sufficient to estimate the mean concentration of most pyrethroids with an ICC of 0.80.
Keywords: co-occurrence, indoor, pyrethroid degradation products, pyrethroids, temporal measurements, wipe sampling
1. INTRODUCTION
Pyrethroid insecticides are a class of relatively non-volatile, synthetic neurotoxicants that are considered to act collectively under the current United States (U.S.) Environmental Protection Agency’s (EPA) human risk assessment paradigm.1 In the United States, pyrethroids have many approved applications and are commonly used in the residential market. National surveys have demonstrated that pyrethroid residues are commonly found within the living spaces of the U.S. housing stock2 and child care centers.3 Because they are commonly detected where children spend much of their time, it is important to accurately estimate pyrethroid exposures within these environments.
To estimate indoor human pyrethroid exposure, a variety of sampling techniques has been used, for example, wiping of toys, hands, and hard surfaces; collection of house dust from vacuum cleaners; air sampling; and extraction of clothing.4–11 A subset of these studies also included measurement of urinary pyrethroid metabolites concentrations5, 6, 8, 11 and apportionment models.6, 11
A common limitation of these investigations was their reliance on single-time-point sampling to estimate exposure. Although one-time sampling minimizes costs and subject burden, common human activities could increase (pesticide application), reduce (cleaning), or redistribute (human transport of adhering residues) indoor pyrethroid concentrations and one-time sampling may inadequately represent residue concentrations even over short periods of time.
Because indoor pyrethroid concentrations can change over time, longitudinal measurements of residues are needed to reduce uncertainty in models estimating indoor exposure. Our review of the literature found only three pyrethroid studies where this assessment was made. Of these, two were conducted in unoccupied housing12, 13 and therefore did not include a human activity component. In the sole longitudinal study of occupied housing, Deziel et al.14 found that concentrations of pyrethroids in carpet dust samples collected in 21 homes in California (2003-2005) were relatively stable over a period of 2 years and suggested that a one-time measurement would sufficiently represent concentrations over that time frame. However, relative to hard surface flooring, carpeting may shield chemicals from degradation due to indoor lighting, reduce translocation from direct contact, and be cleaned less frequently with chemicals that degrade pyrethroids. For example, Snyder et al.15 showed that incandescent lighting and commercially available cleaning and disinfection agents such as bleach, ammonia, and hydrogen peroxide would degrade the pyrethroid permethrin on a variety of hard surfaces. Berger-Preieß et al.12 used a commercially available cleaner to degrade permethrin and deltamethrin on settled and suspended house dust and on various indoor hard surfaces. Stout and Leidy16 showed that occupant activities could physically translocate a pyrethroid from the outdoors to indoor hard surfaces.
The goals of this study were to (i) evaluate the temporal variability and co-occurrence of pyrethroids and pyrethroid degradation products as measured by wipe sampling of hard surface flooring in inhabited residences and (ii) determine whether records of current-use pesticide applications informed the measured pyrethroid concentrations. To do this, the occupants collected three wipe samples from the hard-surfaced flooring (eg, vinyl and tile) of their kitchen over a 6-week period and, beginning 1 month prior to the onset of sampling, maintained pesticide use diaries. The kitchen was selected as the sampling location because it is a heavily trafficked living space and, due to the presence of food, more likely to be both cleaned and treated for pests. Measurements of pyrethroid degradate concentrations were included because they have been postulated to contribute to the pyrethroid metabolite levels found in samples of human urine.17, 18 The results of this study may assist in decisions for longitudinal sampling approaches when measuring pyrethroid concentration in inhabited dwellings.
2. MATERIALS AND METHODS
2.1. Study population
The study cohort and recruitment methods have been described previously.19 In summary, the 50 participants were required to be healthy, not pregnant, between the ages of 19 and 50 with no occupational exposure to pyrethroids, and residing within 40 miles of the U.S. EPA’s Human Studies Facility (HSF) located in Chapel Hill, North Carolina (NC).
2.2. Pesticide use diaries
The method used to populate the pesticide use diaries was reported earlier.19 In brief, the participants were individually trained by a technician at the HSF on how to properly fill out the diary, and each participant completed a diary on day 5 of each sampling week (1, 2, and 6). Week 1 diaries also included information on pesticide use in the month immediately prior to onset of sample collection, while week 6 diaries included information on weeks 3 through 6. The diaries recorded each application at the residence of all products containing a pyrethroid(s). Diary information included the EPA registration number, day, location, and number of times applied.
2.3. Chemicals and solvents
The structures and degradation pathways of all pyrethroids and pyrethroid degradates included in this study are presented in Figure 1. The purity of all chemicals used to make standards, recovery validation spikes, and to determine method detection and quantitation limits (MDL/MQL) were ≥97% pure and acquired in solution. Cis- and trans-permethrin, cyhalothrin, esfenvalerate, cypermethrin, cyfluthrin, bifenthrin, and deltamethrin were purchased from Absolute Standards Inc. (Hamden, CT). Pyrethroid degradation products including 3-phenoxybenzoic acid (3-PBA), 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA), cis/trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis/trans-DCCA), cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (DBCA), and isotopically labeled compounds including 13C6 cis-permethrin, 13C6 trans-permethrin, 13C6 cypermethrin, 13C6 cyfluthrin, 13C6 3-PBA, 13C6 4-F-3-PBA, 13C6 cis-DCCA, 13C6 trans-DCCA, and 13C12bisphenol A were purchased from Cambridge Isotopes (Andover, MA). Trans-permethrin D6and fipronil des F3 were obtained from EQ Laboratories, Inc. (Atlanta, GA), and MPA was provided by FMC Agricultural Products Group (Philadelphia, PA).
Figure 1:
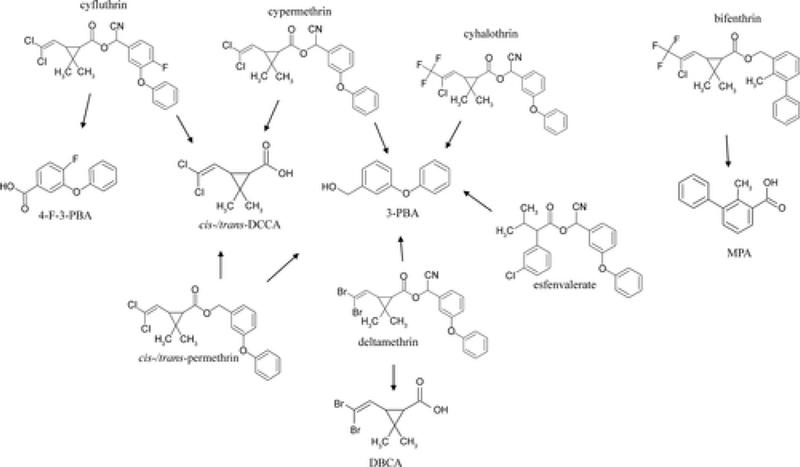
Structures of the pyrethroids and pyrethroid degradation products measured in this study
Silica and C18 were purchased from Supelco (Bellefonte, PA). All organic solvents were pesticide grade or equivalent and screened prior to use to ensure they did not contain measurable concentrations of pyrethroids or pyrethroid degradation products. Methanol, 2-propanol, and acetonitrile were obtained from Honeywell Burdick & Jackson (Muskegon, MI), while n-hexanes and ethyl acetate were acquired from EMD Chemicals (Gibbstown, NJ). Hydrochloric acid was purchased from Fisher Scientific (Fairlawn, NJ).
2.4. Sample collection
The hard floor surface wipes were collected as part of a larger study that examined the variability of pyrethroid metabolites in urine.19 The study design was approved by the US EPA’s Human Subjects Research Review Official and the University of North Carolina’s Institutional Review Board (study number 09-0741) and has been described previously.19 Briefly, the participants were individually trained by a technician at the HSF on how to properly collect a surface wipe sample from their kitchen flooring, and each participant practiced collecting a surface wipe sample from flooring in a study room at the HSF prior to field sample collection. On the 4th day of weeks 1, 2, and 6 of the study, each participant collected two hard surface wipe samples from “highly trafficked” areas of their kitchen (ie, near a sink, refrigerator, or entryway). “Highly trafficked areas” were self-defined, and the participants were given no instructions as to whether they could or could not resample the same location.
The process used to collect each wipe sample has also been described19 and used a 100 cm2, 1 ply pre-cleaned cotton pad (M.G. Chemicals; Toronto, Canada) that was wetted with 10 mL of isopropanol immediately prior to surface wiping. Each sample area was delineated by an aluminum template (960 cm2), and the pad was wiped on the floor across the entire area encompassed by the template. The pad was then folded in half, and the entire sampling area was rewiped. After collection of the samples from both areas was complete, the two pads were placed (together) into a 60-mL amber jar. The jars were sealed and stored in a provided portable thermoelectric cooler (Vinotemp or Princess International®) and transported to the HSF 2 days later. After arrival at the HSF, the samples were cataloged and then transported in the coolers (on blue ice) to a U.S. EPA laboratory in Research Triangle Park, NC, where they were stored at −20°C until extraction.
2.5. Sample extraction and cleanup
Surrogate standards (Table S1) were added after placing the paired wipe samples in 34-mL extraction cells. The cells were then sealed and extracted three times (5 minutes static, 50% flush) with hexane:acetone, 25:75 (v/v) pressurized to 1500 psi at a temperature of 75°C. This was followed by two additional extractions using methanol (5 minutes static, 50% flush) which were accomplished using the same temperature and pressure as during the hexane:acetone extraction.
Next, the hexane:acetone and methanol fractions were combined in a 250-mL flat bottom flask and reduced to approximately 2 mL using a heated water bath and a rotary evaporator. This volume was transferred to a culture tube, and the flat bottom flask was rinsed with 1 mL acetone (3×), followed by 1 mL methanol (3×), and all rinsates were transferred to the same tube. The combined extract and rinsate were dried completely, without heat, using nitrogen. After drying, the samples were reconstituted in 2 mL of 6:94 ethyl acetate:hexane (v/v), vortexed, and sonicated.
Silica solid-phase extraction cartridges (500 mg) were used to remove interfering compounds from the extracts and to separate the parent pyrethroids from the degradates. After sample loading (2 mL of 6:94, ethyl acetate: hexane v/v), the pyrethroids were eluted with 7 mL of 6:94 of ethyl acetate:hexane (v/v) and collected as fraction 1. Degradates were eluted from the silica with 6 mL methanol and collected in a separate tube as fraction 2. Because our experience showed that a significant proportion of the degradates remained in the original sample tube, it was rinsed with 2 mL methanol, and the rinsate was added to the fraction 2 tube.
Prior to purification of the pyrethroid degradates, the volume of fraction 2 was reduced to approximately 200 μL using nitrogen and a water bath (≤30°C). Then, 800 μL of acetonitrile, followed by 1 mL methanol:acetonitrile, 1:4 (v/v) was added to the sample tube. The samples were then loaded onto a C18 cartridge (500 mg) that had been pre-cleaned and conditioned with 3 mL of methanol, followed by 3 mL of methanol:acetonitrile, 1:4 (v/v), and 3 mL of acetonitrile. The extracts were eluted with 2 mL acetonitrile followed by 6 mL of methanol:acetonitrile, 1:4 (v/v).
After elution, 100 and 300 μL of 0.01N HCl were added to fraction 1 (pyrethroids) and fraction 2 (degradation products), respectively, and the volume of each fraction was reduced under nitrogen so that the organic solvent was removed. Next, the appropriate internal standards (Table S1) were added, followed by an additional amount of methanol and water such that the respective ratios of aqueous: organic for fraction 1 and fraction 2 were 1:9 and 3:7. Both fractions were vortexed, then transferred to autosampler vials, and stored at −20°C until analyzed.
2.6. Sample analysis
An LC-MS/MS with an electrospray interface and a C18 column (3.0 × 150 mm, 3.5 μm) was used for the analysis of all samples, and the retention times and MS voltages are provided in Table S1. The mobile phase for all analyses was 5 mM ammonium acetate: methanol, 2:98 (aqueous:organic) for pyrethroids, and 3:7 (aqueous:organic) for the degradation products, at a flow rate of 0.4 mL/min. All degradates, cyfluthrin, and cyhalothrin were ionized in the negative mode, and positive ionization was used for the remaining pyrethroids. This required three different LC-MS/MS runs (pyrethroid+, pyrethroid−, and degradate−) for each extracted sample.
Calibration standards used to generate six-level concentration-response curves were established using matrix matched mixtures of authentic and internal standards. To do this, six pairs of wipes were first extracted and processed according to the finalized procedure. Each extract was then spiked with a known mass of each analyte and internal standard. The concentration of the analytes in the calibration standards was 1, 10, 25, 50, 75, and 100 ng/mL (respective concentrations of cis- and trans-DCCA ranged from 0.3 and 0.7 ng/mL, through 30 and 70 ng/mL), while the concentration of all internal standards in all calibration standards was fixed at 50 ng/mL.
2.7. Method performance: Determination of percent recovery and limits of detection (MDL) and quantitation (MQL)
Analyte recovery using the finalized method was evaluated with pre-cleaned wipes that were spiked with the pyrethroids and degradates. Spiking levels were 25, 50, and 100 ng/wipe for each analyte at each of the three levels, and there were 12 spiked wipes per level. The spiked wipes were processed, and the measured concentration of each analyte was compared to the mass that had been spiked onto the wipe. MDL and MQL limits were estimated by spiking each analyte at concentrations of 2, 5, 10, and 20 ng per wipe (n = 4 wipes per level). One set of four wipes was processed each day for 4 days, and all purified extracts were analyzed once each day for four consecutive days. The pooled standard deviation of the results at each spiking level was calculated, and linear regression (x = spike level, y = SD) was used to determine the slope and intercept of the best-fit line. The MDL and MQL were defined, respectively, as 3× and 10× the intercept value for each analyte.20
2.8. Data analysis
Pyrethroid and degradate concentration data were analyzed using SAS version 9.4 (SAS, Cary, NC).21 Pyrethroid co-occurrence was evaluated using a chi-square test employing a simple 2 × 2 contingency table. The frequency of observed occurrence for all 120 possible combinations of the seven (cis-/trans-permethrin combined) pyrethroids was calculated from the array of detected pyrethroids above the detection limit using PROC SQL. This provided the number of study samples where a particular combination was observed (O) and the number of study samples containing other combinations (Ooth). The expected probability of each pyrethroid combination and expected number of samples (E) was calculated (considering co-occurrence as a random event) by multiplying all individual pyrethroid detection frequencies of a particular combination together. The expected number of study samples containing other combinations (Eoth) was obtained by subtracting the expected probability of E from 1 and multiplying by the total number of study samples. All expected values were rounded to the nearest integer leading to a few expected values being equal to zero. Where the value was 0, a value of 1 was substituted for 0 and used to calculate the chi-square statistic. The chi-square statistic (χ2) was calculated using a Yates correction22 to adjust for small sample sizes as follows:
and evaluated for statistical significance using the PROBCHI function to calculate the probability the value came from a chi-square distribution and considering degrees of freedom determined by the number of pyrethroids involved in the particular combination minus 1.
The variance between (σ2BH) and within homes (σ2WH) for each pyrethroid was estimated using a mixed-effects regression model (PROX MIXED) that considered the study home as a random effect.14 Using the natural log-transformed pyrethroid concentrations (ln(Yij)), effectively this model distinguishes an overall population mean (μy) along with the contribution from the two variance components of interest as follows:
where bi represents the random effect associated with home i, and εij is the residual error associated with the ith home and jth week of repeated measurement. Both the random effects and residual error were assumed to be normally distributed with means equal to 0, along with their respective variances equal to (σ2BH) and (σ2WH). The observed intraclass correlation coefficient (ICCobs) was calculated to compare the between-home variance with the sum of both variance components:
The derived ICC was used to approximate the number of repeated sampling events (m) required to achieve a particular ICC of acceptable reliability (ρm) for the pyrethroid data was determined using the equation derived by Fleiss et al.23:
The estimation of the variance components (and hence ICCs) was generated first using only data above the detection limit, then again after including substituted data for non-detected values. The substituted data were calculated using the geometric means and standard deviations of the measured concentration distribution for each analyte. A single set of concentrations below the limit of detection was generated by randomly sampling from these distributions and used to fill the missing values for each pyrethroid.
To calculate the ratios of co-occurring pyrethroids and their degradation products in each wipe sample, the concentrations of each analyte were first converted to nmol/m2. The numerator for each ratio was the concentration of the degradation product, while the denominator was the summed concentrations of all potential precursors (Figure 1) present in that sample.
Agreement between the information contained in the pesticide use diaries and the measurement data from the wipe samples was evaluated first by comparing pyrethroid detections with recorded applications. Next, a simple model was developed to determine whether within-home pyrethroid concentration variability (from the wipe sample measurements) could predict the pesticide applications recorded in the diaries. To construct the model, concentration variability was first assessed using (i) the coefficient of variation (COV) of the pyrethroid across the three sampling periods, (ii) week of maximum concentration for each pyrethroid, and (iii) whether the applied pyrethroid had the highest concentration of all pyrethroids (in that sample) during the peak week (week of highest concentration). The values of these three indicators were determined for each pyrethroid and compared to the same indicators for a benchmark pyrethroid in each home. Selection of the benchmark pyrethroids was based on detection frequency for all residences, and permethrin (cis- + trans-permethrin) was used as the benchmark for comparison with all other applied pyrethroids, while cypermethrin was used as the benchmark for permethrin. Finally, a pesticide application was predicted to have occurred when a pyrethroid had either (i) a COV of at least 25% and was 50% greater than the COV of the benchmark during its peak week or (ii) the highest concentration, during its peak week.
2.9. Quality assurance and control
At the start of each of the 3 weeks where field samples were to be collected, six field blanks, (each blank = 2 wipes, spiked with 50 ng surrogates) and six field spikes (each spike = 2 wipes, spiked with 100 ng pyrethroids, 100 ng degradates, and 50 ng surrogates) were prepared then transported to the HSF where they were stored in a portable electric cooler. At the end of each sampling week, these samples were returned to the laboratory and stored at −20°C. During sample processing, one laboratory blank (2 wipes, spiked with 50 ng surrogate) and one laboratory spike (2 wipes, spiked with 25-100 ng pyrethroids and degradates, and 50 ng surrogates) were prepared and processed for every 14 field samples.
Surrogate standards provided method performance information, whereas the internal standards were used to compensate for changes in apparent concentrations due to matrix effects.
3. RESULTS AND DISCUSSION
3.1. Method validation and quality control
The percent recovery and MDL/MQL for each of the pyrethroids and degradates are listed in Table S2. Using analysis of variance followed by Student’s t tests to compare mean recovery of spiking levels showed minor concentrated related recovery differences (P < .05) for cyfluthrin (50 ng vs 25 ng) and MPA (100 ng vs 50 and 25 ng). After pooling the results of the three spiking levels (100, 50, and 25 ng), the percent mean recovery of the analytes ranged from 76 ± 14 (bifenthrin) to 118 ± 16 (deltamethrin). Respectively, the MQL and MDL were <9 and <3 ng/sample for all pyrethroids and degradates.
Analyte concentrations were obtained for 139 of the 150 collected samples, and all houses had samples that were successfully analyzed for at least two of the three collection weeks. One pair of wipes was not collected because the participant dropped out during the last week of the study, and 10 pairs of wipes (week 1: n = 6: week 2: n = 4) were lost due to laboratory equipment malfunction.
No sample results were discarded due to quality control issues. However, among the 139 samples, the following deficiencies were noted. A single sample had recoveries of both parent surrogate (trans-permethrin D6) and metabolite surrogate (fipronil des F3) that were <60%. There were three samples where the recovery of fipronil des F3 was <60% and five samples with fipronil des F3 and trans-permethrin D6 recoveries that were >130%. Both surrogates in all other samples were all within a range of 60%-130%.
Considering the 18 field blanks: the recovery of the metabolite surrogate (fipronil des F3) was <60% in one sample, and >130% in one sample. The recovery of all pyrethroid and degradate surrogates from all other blank samples was within 60%-130%.
For the 18 field spikes, recovery of 3-PBA (metabolite) in three samples was <60% and recovery of cis-DCCA in one other sample was <60%. The recoveries of all other pyrethroids, surrogates, and degradates from all other samples were between 60% and 130%.
3.2. Pyrethroid application diaries
Summary information from the pesticide application diaries is presented in Table 1. During the time period covered by the diaries (1 month prior to sampling through the end of the 6-week study period), 20 of the study participants recorded the use of products containing one or more of the target pyrethroids, and, in total, there were 41 logged applications. Of these 20 participants, the majority (12) used a product that contained a single pyrethroid and that pyrethroid was only applied once. During the month prior to sample collection, there were 17 pyrethroid applications. Of these, eight were single indoor, and five were single outdoor only applications. Of the remaining applications, two were single pyrethroids applied both indoors and outdoors, and one participant applied two pyrethroids (not coformulated) both indoors and outdoors. During the 6 weeks of the measurement portion of the study, the number of pyrethroid applications (in parentheses) was as follows: week 1 (1), week 2 (6), weeks 3-5 (5), and week 6 (12).
Table 1.
Pyrethroid usage from participant diariesa
| Participant usageb | Pyrethroid specific usagec | ||||||
|---|---|---|---|---|---|---|---|
| Applications | Participants | Pyrethroid | Applications | Houses | Indoor | Outdoor | Pet |
| 0 | 30 | Permethrind | 10 | 6 | 3 | 3 | 4 |
| 1 | 12 | Cypermethrin | 4 | 3 | 3 | 1 | 0 |
| 2 | 4 | Esfenvalerate | 1 | 1 | 1 | 0 | 0 |
| 3 | 1 | Deltamethrin | 1 | 1 | 0 | 1 | 0 |
| 4 | 2 | Bifenthrin | 8 | 5 | 3 | 4 | 1 |
| 5 | 0 | Cyfluthrin | 5 | 3 | 4 | 1 | 0 |
| 6 | 0 | Cyhalothrin | 12 | 5 | 6 | 5 | 1 |
| 7 | 0 | ||||||
| 8 | 0 | ||||||
| 9 | 0 | ||||||
| 10 | 1 | ||||||
Data collected from 1 month prior, through week 6 of the study.
n = 50 study participants.
n = The 41 recorded pyrethroid applications were distributed across 24 houses
Permethrin represents both cis- and trans-permethrin which are coformulated pesticides.
When considering all houses in the study, the presence of the majority of the pyrethroids in most of the houses (Table 2) contrasts with the low rate of recorded pyrethroid applications, and therefore, the information in the diaries did not explain pyrethroid detection frequencies. For example, cis/trans-permethrin was detected in 92% of the study houses, but only 11% of these residences documented a permethrin application. Deltamethrin and esfenvalerate were detected in 40% and 28% of the houses, respectively, but each had only a single recorded application. Further, those houses having the highest concentrations of cis/trans-permethrin, deltamethrin and esfenvalerate, had no diary entries indicating application of these pyrethroids.
Table 2.
Detection frequencies of pyrethroids and pyrethroid degradation products
| Percentage of houses (n = 50) where analyte was detected |
Probability of detection in all weeks if in ≥1 week |
|||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 6 | ≥1 week | All weeks | ||
| Pyrethroid | ||||||
| Trans-permethrin | 91 | 91 | 90 | 92 | 90 | 98 |
| Cis-permethrin | 91 | 91 | 88 | 92 | 88 | 96 |
| Cypermethrin | 74 | 74 | 73 | 74 | 72 | 97 |
| Esfenvalerate | 26 | 24 | 20 | 26 | 20 | 77 |
| Deltamethrin | 37 | 39 | 37 | 40 | 34 | 85 |
| Bifenthrin | 63 | 59 | 57 | 60 | 58 | 97 |
| Cyfluthrin | 56 | 54 | 51 | 58 | 48 | 83 |
| Cyhalothrin | 47 | 52 | 45 | 54 | 42 | 78 |
| Degradate | ||||||
| 3-PBA | 57 | 58 | 57 | 58 | 54 | 93 |
| 4-F-3-PBA | 32 | 22 | 20 | 28 | 20 | 71 |
| trans-DCCA | 20 | 22 | 20 | 22 | 20 | 91 |
| cis-DCCA | 18 | 18 | 20 | 20 | 18 | 90 |
| DBCA | 9 | 11 | 10 | 10 | 8 | 80 |
| MPA | 11 | 13 | 10 | 16 | 8 | 50 |
While pyrethroid use during this study was recorded by only 40% of the participants, it is possible that some applications may have occurred prior to the collection of the diary information. For example, previous studies24, 25 with multiyear data have reported pesticide use by >90% of the subjects or households. Alternatively, the presence of pesticides in dust collected inside never use houses was noted by both Simcox et al.26 and Colt et al.,24 suggesting that translocation from an external source, into the home, was also a possibility.
The lack of agreement between the pesticide application diaries and the frequencies with which the pyrethroids were detected was also noted in the analysis of data from the national survey of child care centers in the United States3 where there was only 43% agreement between questionnaire response and the presence or absence of a measured pyrethroid in the companion floor wipe sample. In contrast, Colt et al.24 and Deziel et al.25 did find associations between measured pyrethroid concentrations in carpet dust and self-reported pest treatments.
When using the COV and peak concentration model, there was, however, an indication that recorded applications were reflected by changes in measured concentration. For example, considering only the houses (n = 20) where there were recorded pesticide use events (n = 24), the model correctly predicted 75% of the applications. When considering the concentration variability indicators of the non-applied pyrethroids in these homes (n = 116), the model was correct 83% of the time in predicting that there was not an application. In considering all other homes that did not indicate any pesticide usage during the study period (n = 210), the model correctly predicted 76% of the time that there was not a pyrethroid application.
Approximately 20% of the model application/non-application predictions did not correspond with the pesticide diary information. This could be a function of applications that did not substantially alter within-home week-to-week. Alternatively, measurement/diary discrepancies could be related to common shortcomings of survey data collection such as incorrect identification of active ingredients and over- or under-reporting usage).
3.3. Detection frequencies of pyrethroids and pyrethroid degradates
In two houses, no pyrethroids or pyrethroid degradates were detected in any of the surface wipe samples. Interestingly, the pesticide diary for one of these houses indicated no pesticide applications during the study, while the other diaries noted an indoor application of cyfluthrin during week one. In all other houses, one or more of the pyrethroids or degradates was detected in at least one sample. Table 2 shows the frequency of detection for the study analytes in individual samples and by house. Excepting esfenvalerate and deltamethrin, all pyrethroids were present in the majority of the samples and houses, with cis/trans-permethrin being the most frequently detected. The pyrethroid degradates were generally detected less frequently than their precursors (Table 2), with only 3-PBA present in more than 50% of the samples and residences.
The frequency with which individual analytes were detected across the three sampling times was stable. When comparing the percent detects for each analyte in weeks one, two, and six, the relative standard deviations were less than 10% for all compounds except MPA (12%), esfenvalerate (14%), and 4-F-3-PBA (25%). The within-house analyte detections of the analytes were also consistent (Table 2). When an analyte was detected in one sample, the probability that it was detected in all samples from that house ranged from 68% to 95% for all compounds except MPA (50%).
Distributed among the 90 wipe samples that contained degradation products were 150 instances where a degradate and one or more of its precursor pyrethroids were both detected. There were 14 instances where the degradate was present but the relevant pyrethroid(s) was not. Where a single degradation product was detected in a sample, the relevant pyrethroid was also present most of the time (4-F-3-PBA: cyfluthrin 82%; MPA: bifenthrin 81%; and DBCA: deltamethrin 100%).
The use of hard surface floor wipe sampling to measure indoor pyrethroid2, 3, 11 and pyrethroid degradate11 concentrations has been reported previously. Compared to those studies, the detection rates of all pyrethroids except cis/trans-permethrin and bifenthrin in this study were higher. The detection frequencies of 3-PBA and 4-F-3-PBA in this study were below those found by Trunnelle et al.,11 while cis/trans-DCCA and DBCA were higher (MPA not measured by Trunnelle et al.11). As the methods used to sample the flooring were similar for all studies, and the method detection limits in this study were comparable to those of Tulve et al.3 and higher than those achieved by Stout et al.2 and Trunnelle et al.,11 the increase in detection frequency of the pyrethroids was probably not attributable to methodological differences. There may be, however, regional differences in product availability or other factors that resulted in increased use of pyrethroids in this study population, and this is consistent with the results reported by Tulve et al.3 who found higher detection rates for cis/trans-permethrin, esfenvalerate, cyfluthrin, cypermethrin, and cyhalothrin in the Southern United States when compared to other regions of the country.
3.4. Pyrethroid co-occurrence
Based on the frequencies with which individual pyrethroids were detected, chi-square analysis showed that none of the houses would be expected to contain all seven (cis/trans-permethrin combined) pyrethroids. However, in eight of the houses, all of the pyrethroids were present at detectable levels in at least one of the samples, and in five of these houses, all pyrethroids were detected in all samples. Considering the remaining three houses, esfenvalerate was not detected in one wipe sample in two homes, and cyfluthrin was not detected in one sample. In addition to the homes where all pyrethroids were detected, there were several 5- and 6-way pyrethroid combinations (Table S3) that were also observed at significantly (P ≤ .05) greater than expected frequencies. While no specific combination of pyrethroids was markedly more common than other groupings, only two of the houses where any pyrethroid was detected had just one pyrethroid (cis/trans-permethrin).
These groupings indicate a non-random pyrethroid distribution among the houses which is consistent with the multipyrethroid co-occurrences structures found by Tornero-Velez et al.27 when analyzing hard surface wipe data collected from U.S. child care centers. According to Tornero-Velez et al.,27 these co-occurrences reflect structuring processes such as availability of manufacturer formulations. However, because pyrethroids are frequently sold with only one or two active ingredients, it also suggests multiproduct usage by many of the households applying these insecticides.
3.5. Concentrations and variability
The mean, maximum, and distribution of each analyte concentration are presented in Table 3. Whether considering all data, or only data where individual pyrethroids were detected, the rank order of mean pyrethroid concentration approximated the ranking of their respective detection frequency (P ≤ .05). This relationship remained valid when median concentrations (non-detects excluded) were used instead of arithmetic averages. For example, cis-/trans-permethrin was present in all samples in 86% of the houses, and among those houses, the median concentration was 1130 ng/m2. In contrast, esfenvalerate was present in all samples in only 20% of the houses, and the median concentration in those houses was 238 ng/m2. The low frequency with which most of the pyrethroid degradates were detected precluded this comparison using all data. When only results with measurable degradate concentrations were included, there was no statistical relationship (P > .05) between rank order of detection frequency and concentration.
Table 3.
Concentrations of pyrethroids and pyrethroid degradation products in surface wipes collected on kitchen flooring
| n detected |
Concentration (ng/m2) | ||||||
|---|---|---|---|---|---|---|---|
| Mean ± std | 50th pct |
75th pct |
90th pct |
95th pct |
Maximum | ||
| Pyrethroid | |||||||
| trans-permethrin | 125 | 11,00 ± 59,000 | 890 | 3100 | 7100 | 39 000 | 620 000 |
| cis-permethrin | 124 | 8,000 ± 41,000 | 580 | 2300 | 5000 | 29 000 | 430 000 |
| Cypermethrin | 102 | 4,200 ± 12,000 | 240 | 690 | 4300 | 24 000 | 80 000 |
| Esfenvalerate | 32 | 520 ± 690 | <MDL | <MDL | 340 | 600 | 3100 |
| Deltamethrin | 52 | 2,590 ± 5,200 | <MDL | 260 | 1400 | 7000 | 22 000 |
| Bifenthrin | 82 | 1,500 ± 2,300 | 180 | 910 | 3000 | 4200 | 17 000 |
| Cyfluthrin | 74 | 6,500 ± 28,000 | 42 | 290 | 2000 | 12 000 | 210 000 |
| Cyhalothrin | 66 | 5,900 ± 27,000 | <MDL | 250 | 570 | 1400 | 160 000 |
| Degradate | |||||||
| 3-PBA | 79 | 180 ± 270 | 22 | 82 | 290 | 560 | 1500 |
| 4-F-3-PBA | 34 | 50 ± 53 | <MDL | <MDL | 37 | 64 | 280 |
| trans-DCCA | 29 | 250 ± 420 | <MDL | <MDL | 130 | 210 | 2200 |
| cis-DCCA | 14 | 70 ± 54 | <MDL | <MDL | 48 | 84 | 1200 |
| DBCA | 26 | 130 ± 250 | <MDL | <MDL | 7 | 53 | 180 |
| MPA | 16 | 220 ± 240 | <MDL | <MDL | 45 | 150 | 960 |
MDL, Method detection limit.
The detection frequency and concentration both provided order to the potential pyrethroid exposure of the study participants. Therefore, detection frequency and concentration can be combined to rank the relative exposure to the individual pyrethroids measured in this study, for example, after multiplying the median concentration of a pyrethroid by its detection frequency (houses where pyrethroid was detected in all weekly samples), results in; cis-/trans-permethrin > cypermethrin > bifenthrin > deltamethrin > cyfluthrin cyhalothrin > esfenvalerate. However, it is important to note that pyrethroids have differing toxicological potencies. Wolansky et al.28 showed that esfenvalerate is approximately 35 times as potent as permethrin in reducing the motor function of rats and if the relative potency is included as a factor (detection frequency × median concentration × relative potency), the order of importance becomes: bifenthrin > cyhalothrin > deltamethrin > cyfluthrin > esfenvalerate > cypermethrin > cis-/trans-permethrin.
Where co-occurring with pyrethroids, the degradates were usually present at lower concentrations than their precursors and the geometric means of the percent of degradate to relative to their precursor(s) were as follows: 3-PBA 1.6%, 4-F-3-PBA 3.2%, cis/trans-DCCA 1.8%, DBCA 3.3%, and MPA 32.1%. Assuming the measured pyrethroids were the source of the measured degradation products, these ratios suggest that indoor degradation rates for most of the pyrethroids are low, or alternatively, that most of the degradates are unstable intermediate products that further degrade relatively rapidly. As the degradation products are generally considered to be less toxic than the parent pyrethroid, their main importance lies in the potential contribution to urinary pyrethroid metabolite concentrations following ingestion. The ratios found in this study indicate that, excepting MPA, the degradates would not be significant contributors and indicates they do not need to be included in the suite of analytes in studies comparing indoor residential pyrethroid exposures and excreted pyrethroid metabolites.
Table 4 shows the intraclass correlations (ICC) of pyrethroid concentrations measured in this study, and number of sampling events that would be required to achieve an ICC of 0.80 (m). ICCs and m values were not calculated for the degradation products because they were infrequently detected and, when detected. were usually present at relatively low concentrations. In Table 4, ICC and m values are provided with and without using substitution for non-detected pyrethroids and further divided into present estimates using only results from houses where data were obtained for all 3 weeks (39 homes), and homes where there were data for at least 2 weeks (ie, the remaining 11 homes). Regardless of which data set was used, the ICCs for all pyrethroids were ≥0.55, and only the models using both 2- and 3-week data for bifenthrin and cyhalothrin (no substitutions) and cis-/trans-permethrin (substitutions) had ICCs that were below 0.71. Regardless of whether substitution was used, or 2-week data were included, the ICCs of the individual pyrethroids remained relatively constant, changing by <20% for all pyrethroids except cyhalothrin (25%) and bifenthrin (39%).
Table 4.
Intraclass correlations (ICC) and sample size (m) required to estimate temporal variability of pyrethroids over a 6-week period with a reliability of 0.8
| Homes with 3 weeks of data |
Homes with at least 2 weeks of data |
Homes with 3 weeks of data |
Homes with at least 2 weeks of data |
|||||
|---|---|---|---|---|---|---|---|---|
| ICC | m | ICC | m | ICC | m | ICC | m | |
| trans-permethrin | 0.75 | 1.4 | 0.71 | 1.7 | 0.77 | 1.2 | 0.73 | 1.5 |
| cis-permethrin | 0.74 | 1.4 | 0.74 | 1.4 | 0.76 | 1.3 | 0.7 | 1.7 |
| Cypermethrin | 0.72 | 1.6 | 0.71 | 1.7 | 0.94 | 0.3 | 0.92 | 0.3 |
| Esfenvalerate | 0.71 | 1.6 | 0.8 | 1 | 0.87 | 0.6 | 0.86 | 0.6 |
| Deltamethrin | 0.82 | 0.9 | 0.74 | 1.4 | 0.92 | 0.4 | 0.89 | 0.5 |
| Bifenthrin | 0.75 | 1.4 | 0.55 | 3.3 | 0.95 | 0.2 | 0.94 | 0.3 |
| Cyfluthrin | 0.84 | 0.8 | 0.78 | 1.2 | 0.96 | 0.2 | 0.88 | 0.5 |
| Cyhalothrin | 0.91 | 0.4 | 0.67 | 2 | 0.91 | 0.4 | 0.86 | 0.6 |
| All pyrethroids | 0.82 | 0.9 | 0.74 | 1.4 | 0.82 | 0.9 | 0.77 | 1.2 |
n = 50 houses when 2 and 3 sample collection weeks are included. Thirty-nine houses when data for houses having only 3 sample collection weeks, n for non-substituted data is further reduced according to frequency of detection (Table 2).
Starr et al.13 demonstrated that pyrethroid concentrations in an unoccupied residential setting were stable over a 5-week period. In contrast, this study included a human element and measured pyrethroid concentrations on hard surface flooring in kitchens, an area likely to be highly used. Therefore, the stability of the pyrethroid concentrations in the participants’ kitchens over the 6-week study was somewhat unexpected. It is possible that the study itself changed the participants’ behavior, and this change resulted in more stability than would normally occur. For example, the relatively low apparent pyrethroid use evidenced by the application diaries did not reflect the rather ubiquitous presence of the pyrethroids.
Considering the largest data set (2- and 3-week homes with substitution), the m value indicates that one sample would be sufficient to describe the variability (assuming 0.80 is sufficient) of deltamethrin, while two samples would be required for cis-/trans-permethrin, cypermethrin, esfenvalerate, cyfluthrin, and cyhalothrin, and four samples would be needed for bifenthrin. If all pyrethroids in this data set are considered together, two temporal samples would have been adequate to achieve an ICC of 0.8.
The ICC’s of the pyrethroids in this study are comparable with those estimated by Deziel et al.14 for pyrethroids in indoor residential carpet dust. However, it would be incorrect to extrapolate the within-home stability of pyrethroid concentrations measured over 6 weeks using hard surface floor wipes, to those measured by vacuuming carpets over a 2-year period.
4. CONCLUSIONS
The pyrethroid insecticides measured in this research were widely distributed across the study homes and their distributions were well structured, indicating multiple pyrethroids were used in individual residences. While the pesticide use diaries were not correlated with the presence or absence of a pyrethroid, they were related to changes in concentration. Overall, the results did not support the use of pesticide use diaries as a substitute for concentration measurement data, but did indicate that diaries could successfully predict whether or not an application had occurred.
Relative to the pyrethroids, the pyrethroid degradation products were detected less frequently and at much lower concentrations. This suggests that exposure to the degradates present on hard flooring would not contribute significantly to excreted pyrethroid metabolite concentrations, rendering their measurement unnecessary in studies using urinary metabolites to reconstruct dose.
Considering all residences in the study, within-home concentrations of the pyrethroids were relatively stable, and, for this study (assuming a required ICC of 0.8), two wipe samples collected from the hard surface kitchen flooring over the 6-week study period would have been sufficient to differentiate within- and between-home concentration variability.
Supplementary Material
Acknowledgments
DISCLAIMER
The US EPA through its Office of Research and Development funded and managed the research described here. It has been subjected to Agency review and approved for publication. Although this work was reviewed by US EPA and approved for publication, it may not necessarily reflect official Agency policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
REFERENCES
- 1.EPA US Science Policy Paper: Common Mechanism Grouping for the Pyrethrins and Synthetic Pyrethroids. August 28, 2013. [Google Scholar]
- 2.Stout DM II, Bradham KD, Egeghy PP, et al. American healthy homes survey: a national study of residential pesticides measured from floor wipes. Environ Sci Technol. 2009;43:4294–4300. [DOI] [PubMed] [Google Scholar]
- 3.Tulve NS, Jones PA, Nishioka MG, et al. Pesticide measurements from the first national environmental health survey of child care centers using a multi-residue GC/MS analysis method. Environ Sci Technol. 2006;40:6269–6274. [DOI] [PubMed] [Google Scholar]
- 4.Quandt SA, Arcury TA, Rao P, et al. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ Health Perspect. 2004;112:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradman A, Whitaker D, Quiro′s L, et al. Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J Expo Sci Env Epid. 2007;17:331–349. [DOI] [PubMed] [Google Scholar]
- 6.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK. An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ Res. 2007;104:266–274. [DOI] [PubMed] [Google Scholar]
- 7.Julien R, Adamkiewicz G, Levy JI, Bennett D, Nishioka M, Spengler JD. Pesticide loadings of select organophosphate and pyrethroid pesticides in urban public housing. J Expo Sci Env Epid. 2008;18:167–174. [DOI] [PubMed] [Google Scholar]
- 8.Tulve NS, Egeghy PP, Fortmann RC, et al. Multimedia measurements and activity patterns in an observational pilot study of nine young children. J Expo Sci Env Epid. 2008;18:31–44. [DOI] [PubMed] [Google Scholar]
- 9.Quirós-Alcalá L, Bradman A, Nishioka M, et al. Pesticides in house dust from urban and farmworker households in California: an observational measurement study. Environ Health. 2011;10:19 Retrieved from http://www.ehjournal.net/content/10/1/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C, Adamkiewicz G, Attfield KR, et al. Household pesticide contamination from indoor pest control applications in urban low-income public housing dwellings: a community-based participatory research. Environ Sci Technol. 2013;47:2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trunnelle KJ, Bennett DH, Tulve NS, et al. Urinary pyrethroid and chlorpyrifos metabolite concentrations in Northern California families and their relationship to indoor residential insecticide levels, part of the Study of Use of Products and Exposure Related Behavior (SUPERB). Environ Sci Technol. 2014;48:1931–1939. [DOI] [PubMed] [Google Scholar]
- 12.Berger-Preieß E, Preieß A, Sielaff K, Raabe M, Ilgen B, Levsen K. The behaviour of pyrethroids indoors: a model study. Indoor Air. 1997;7:248–262. [Google Scholar]
- 13.Starr JM, Gemma AA, Graham SE, Stout DM II. A test house study of pesticides and pesticide degradation products following an indoor application. Indoor Air. 2014;24:390–402. [DOI] [PubMed] [Google Scholar]
- 14.Deziel NC, Ward MH, Bell EM, et al. Temporal variability of pesticide concentrations in homes and implications for attenuation bias in epidemiologic studies. Environ Health Perspect. 2013;121:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder E, Tabor D, Starr J, et al. Developing decontamination methods to address indoor pesticide contamination from improper bed bug treatments. US EPA International Decontamination and Development Conference. RTP, NC May 2015. [Google Scholar]
- 16.Stout DM, Leidy RB. A preliminary examination of the translocation of microencapsulated cyfluthrin following applications to the perimeter of residential dwellings. J Environ Sci Health, Part B 2000;35:477–489. [DOI] [PubMed] [Google Scholar]
- 17.Starr JM, Graham SE, Stout DM 2nd, Andrews K, Nishioka M. Pyrethroid pesticides and their metabolites in vacuum cleaner dust collected from homes and day-care centers. Environ Res. 2008;108:271–279. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Zhao T, Pan C, Ross JH, Krieger RI. Preformed biomarkers including dialkylphosphates (DAPs) in produce may confound biomonitoring in pesticide exposure and risk assessment. J Agric Food Chem 2012;60:9342–9351. [DOI] [PubMed] [Google Scholar]
- 19.Morgan MK, Sobus JR, Barr DB, et al. Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ Res. 2016;144: 81–91. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JK. Quality Assurance of Chemical Measurements New York: Lewis Publishers; 1987. [Google Scholar]
- 21.SAS. Base SAS® 9.4 Procedures Guide, Seventh Edition. 2017. Available at: http://documentation.sas.com/api/collections/pgm-mvacdc/9.4/docsets/proc/content/proc.pdf?locale=en#nameddest=bookinfo.
- 22.Yates F Contingency tables involving small numbers and the X2 test. J R Stat Soc. 1934;1:217–235. [Google Scholar]
- 23.Fleiss JL. The Design and Analysis of Clinical Experiments New York, NY: John Wiley & Sons; 1985. [Google Scholar]
- 24.Colt JS, Lubin J, Camann D, et al. Comparison of pesticide levels in carpet dust and self reported pest treatment practices in four US sites. J Expo Anal Environ Epidemiol. 2004;14: 74–83. [DOI] [PubMed] [Google Scholar]
- 25.Deziel NC, Colt JS, Kent EE, et al. Associations between self reported pest treatments and pesticide concentrations in carpet dust. Environ Health. 2015;14:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simcox NJ, Fensky RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ Health Perspect. 1995;103:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tornero-Velez R, Egeghy PP, Cohen-Hubel EA. Biogeographical analysis of chemical co-occurrence data to identify priorities for mixtures research. Risk Anal. 2012;32:224–236. [DOI] [PubMed] [Google Scholar]
- 28.Wolansky MJ, Gennings C, Crofton KM. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol Sci. 2006;89:271–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


