Abstract
Objective:
The Intensive Diet and Exercise for Arthritis (IDEA) trial showed that an intensive diet and exercise (D+E) program led to a mean 10.6 kg weight reduction and 51% pain reduction in knee osteoarthritis (OA) patients. We investigated the cost-effectiveness of adding this D+E program to treatment for overweight and obese (BMI>27 kg/m2) knee OA patients.
Methods:
We used the Osteoarthritis Policy Model to estimate quality-adjusted life-years (QALYs) and lifetime costs for overweight and obese knee OA patients with and without the D+E program. We evaluated cost-effectiveness with the incremental cost-effectiveness ratio (ICER), a ratio of the differences in lifetime cost and QALYs between treatment strategies. We considered three cost-effectiveness thresholds: $50,000/QALY, $100,000/QALY, and $200,000/QALY. Analyses were conducted from healthcare sector and societal perspectives and used a lifetime horizon. Costs and QALYs were discounted at 3% per year. D+E characteristics were derived from the IDEA trial. Deterministic and probabilistic sensitivity analyses (PSA) evaluated parameter uncertainty and the effect of extending the D+E program duration.
Results:
In the base case, D+E led to 0.054 QALYs gained per person and cost $1,845 from the healthcare sector perspective and $1,624 from the societal perspective. This resulted in ICERs of $34,100/QALY and $30,000/QALY. In the healthcare sector perspective PSA, D+E had a 58% and 100% likelihood of being cost-effective with thresholds of $50,000/QALY and $100,000/QALY, respectively.
Conclusion:
Adding D+E to usual care for overweight and obese knee OA patients is cost-effective and should be implemented in clinical practice.
Knee osteoarthritis (OA) is highly prevalent, affecting 14 million Americans, approximately 50% of whom are obese (1, 2). Obesity and knee OA together result in 3.5 quality-adjusted life-years (QALYs) lost per person (one QALY measures the equivalent of one year of perfect health) (2). Weight management and exercise are recommended by OA treatment guidelines, including those of the Osteoarthritis Research Society International and the American Academy of Orthopaedic Surgeons (3, 4).
Other treatment options for knee OA have inherent limitations. Pharmacologic treatments, including non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, are moderately efficacious, but their long-term utilization is frequently limited by side effects, such as cardiovascular and gastrointestinal events and opioid addiction (5, 6). While total knee arthroplasty (TKA) is both effective and cost-effective, it is generally reserved for later stages of OA progression (7, 8).
Several randomized controlled trials (RCTs) have shown that weight loss is associated with reduced knee OA pain (9). A meta-analysis of RCTs found that a weight loss of 10% is expected to have a clinically relevant effect on disability (10). Participants randomized to a diet and exercise (D+E) regimen in the Arthritis, Diet, and Activity Promotion Trial (ADAPT) experienced a 30% pain reduction over 18 months compared to 17% in the healthy lifestyle control group (11). The intention-to-treat analysis of the Intensive Diet and Exercise (IDEA) trial found that D+E participants experienced a 51% reduction in pain severity over 18 months compared to a 28% reduction in exercise alone participants (the attention control). After 18 months, 38% of D+E participants reported little or no pain, compared to 22% of exercise participants and 20% of diet participants (12).
Economic evaluations of D+E regimens are scarce. A recent systematic review identified only one economic evaluation of an OA intervention targeting obesity: a cost-effectiveness analysis of the ADAPT trial (13, 14). This analysis did not investigate the cost-effectiveness of D+E regimens in the context of other OA treatments. It was also limited to the 18-month trial timeframe; this short-term time horizon is problematic because OA is a chronic disease. The review concluded that there is a pressing need for long-term evaluations that report cost per QALY outcomes to facilitate comparisons of D+E cost-effectiveness across healthcare sectors (13). We address this gap in the literature with a formal cost-effectiveness analysis of adding a D+E regimen to usual care for overweight and obese knee OA patients.
Materials and Methods
Analytic Overview
We used the Osteoarthritis Policy (OAPol) Model, a validated, published computer simulation model of knee OA (2, 7, 15, 16), to assess the cost-effectiveness of adding a D+E program to usual care for knee OA. OAPol is a state-transition, Monte Carlo simulation model that estimates quality-adjusted life expectancy (QALE) and lifetime medical costs of knee OA patients. State transition refers to the fact that the model characterizes each knee OA patient’s clinical progress as a sequence of annual transitions between health states. Monte Carlo refers to the process of simulating one hypothetical knee OA patient at a time and determining that person’s health state transitions with a set of transition probabilities.
Our primary outcome was the incremental cost-effectiveness ratio (ICER), a ratio of the differences in costs and QALYs gained between treatments. We considered a treatment cost-effective if its ICER was below a given willingness-to-pay (WTP) threshold. No single WTP threshold is used to make decisions in the United States, and discussion over what would be an appropriate threshold remains unsettled (17). In selecting a threshold, we aim to present how the preferred strategy depends on society’s willingness to pay for an additional QALY and to provide comparative guidance on what ICER might be considered an acceptable value. To this end, we have included three thresholds. $50,000/QALY is a commonly used threshold in the field, and as some evidence suggests that $50,000/QALY is too low for United States healthcare, we also included thresholds of $100,000/QALY and $200,000/QALY (18).
We conducted analyses from both the healthcare sector and societal perspectives as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine (19). The latter includes costs of care-giving and lost productivity due to OA pain and surgery. Appendix Table 1 outlines each perspective’s cost and quality-of-life components. All costs are in 2016 USD, and costs and QALYs were discounted by 3% annually.
Osteoarthritis Policy Model
OAPol generates knee OA patients based on demographic and clinical characteristics including age, BMI, comorbidities, and knee OA pain and structural severity. Model subjects transition annually between health states, defined by obesity, comorbidities, and knee OA severity. Time in each health state, including all associated costs and health-related quality-of-life effects, is accounted for from model entry to death.
QALE was estimated using data from the Osteoarthritis Initiative (OAI), a large longitudinal cohort study of knee OA (20). The OAI measured health-related quality-of-life using the SF-12. We transformed SF-12 responses into preference-based measures (i.e., utilities) using a previously published conversion algorithm (21). These utilities, stratified by age, comorbidities, obesity, and knee OA pain, were the weights employed by the OAPol model to estimate quality-adjusted survival.
Model subjects with a BMI ≥30 kg/m2 are considered obese. Obesity lowers quality-of-life utility, increases the incidence of cardiovascular disease, diabetes, and cancer, and increases mortality (2, 22, 23). The model assumes that subjects with BMI 18.5 – 24.99 kg/m2 and BMI 25 – 29.99 kg/m2 carry similar risks of comorbidities.
Knee OA treatments in OAPol include NSAIDs, TKA, and D+E. All treatments can affect quality-of-life utility by reducing knee OA pain. TKA also alters the presence of structural knee OA, and D+E reduces BMI. Each treatment has an associated cost and likelihood of toxicities. Toxicities carry their own costs and quality-of-life decrements.
OAPol includes all direct medical costs of knee OA treatment as well as non-OA medical costs stratified by age and comorbidities (Appendix Table 2). Costs were adjusted for inflation using the method recommended by the Second Panel on Cost-Effectiveness in Medicine (Appendix Section 1a) (19).
Cohort Characteristics
Cohort characteristics were from the IDEA trial (Table 1). Mean age was 66 (SD 6) years, 72% were female, and average BMI was 33.6 kg/m2. OA pain severity was assigned using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Subscale (24). The mean baseline WOMAC pain was 32.5 (SD 15.5; 0-100, 100 = worst). At initiation, 13% of the cohort had diabetes and 10% had cardiovascular disease. Other comorbidity incidence was derived from NHANES 2011-2014 data and relative risks of mortality were derived using data from NHANES and CDC 2011 life tables (25, 26).
Table 1.
Cohort Characteristics
| Parameter | Estimate | Data Source | ||||
|---|---|---|---|---|---|---|
| Age: Mean (SD) | 66 (6) | Messier et al., 2013(12) | ||||
| Percentage Female | 72% | |||||
| Race | ||||||
| White Non-Hispanic | 81% | |||||
| African American Non-Hispanic | 6.3% | |||||
| African American Hispanic | 6.3% | |||||
| White Hispanic | 6.3% | |||||
| BMI: Mean (SD) | 33.6 (3.7) | |||||
| BMI First Year Minimum, Maximum | 27, 41 | |||||
| KL2 | 50% | |||||
| KL3 | 50% | |||||
| WOMAC Pain: Mean (SD) | ||||||
| Year 1 | 32.5 (15.5) | |||||
| Increase in Years 2+a | 2 (10) | |||||
| Comorbidity Prevalence | ||||||
| Cardiovascular Disease | 10% | |||||
| Diabetes | 13% | |||||
| Indirect Costs | Derived from Losina et al., 2015 (15) Gupta et al., 2005 (43) Inflated to 2016 USD with CPI-U (50) |
|||||
| Annual OA pain productivity costb | $1,037 | |||||
| Annual OA care-giving costb | $1,128 | |||||
| Productivity costs for TKA (year 1) | $3,311 | |||||
| Productivity costs for revision TKA (year 1) | $3,592 | |||||
| Quality-of-Life Utilities (Nonobese/Obese) | Osteoarthritis Initiative(20) Brazier et al., 2004(21) | |||||
| Age | WOMAC Pain (0-100) | |||||
| Group | 0 | 1-15 | 16-40 | 41-70 | 71-100 | |
| 0 Comorbidities | ||||||
| 45-54 | 0.841/0.830 | 0.816/0.806 | 0.780/0.769 | 0.714/0.703 | 0.656/0.645 | |
| 55-64 | 0.847/0.836 | 0.822/0.812 | 0.786/0.775 | 0.720/0.709 | 0.662/0.651 | |
| 65-74 | 0.871/0.860 | 0.846/0.835 | 0.810/0.799 | 0.744/0.733 | 0.685/0.675 | |
| 75+ | 0.854/0.843 | 0.829/0.818 | 0.793/0.782 | 0.727/0.716 | 0.669/0.658 | |
| 1 Comorbidity | ||||||
| 45-54 | 0.818/0.807 | 0.791/0.780 | 0.755/0.744 | 0.679/0.668 | 0.645/0.634 | |
| 55-64 | 0.824/0.813 | 0.797/0.786 | 0.761/0.750 | 0.685/0.674 | 0.651/0.640 | |
| 65-74 | 0.848/0.837 | 0.821/0.810 | 0.785/0.774 | 0.708/0.698 | 0.674/0.664 | |
| 75+ | 0.831/0.820 | 0.804/0.793 | 0.768/0.757 | 0.692/0.681 | 0.658/0.647 | |
| 2+ Comorbidities | ||||||
| 45-54 | 0.806/0.795 | 0.794/0.783 | 0.732/0.721 | 0.635/0.624 | 0.500/0.489 | |
| 55-64 | 0.812/0.801 | 0.800/0.789 | 0.738/0.727 | 0.641/0.630 | 0.506/0.495 | |
| 65-74 | 0.836/0.825 | 0.824/0.813 | 0.762/0.751 | 0.665/0.654 | 0.530/0.519 | |
| 75+ | 0.819/0.808 | 0.807/0.796 | 0.745/0.734 | 0.648/0.637 | 0.513/0.502 | |
Abbreviations: OA, osteoarthritis; SD, standard deviation; BMI, body mass index; KL, Kellegren-Lawrence grade (measure of osteoarthritis severity, 0-4, 4 = most severe); WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Assumption;
Costs incurred by knee OA patients with WOMAC > 40 in base case
Treatment Strategies
For the two treatment strategies, usual care and D+E, we define below regimen duration, efficacy, discontinuation rate, and cost (Table 2). Usual care subjects began their treatment sequence in year 1. D+E subjects began both the D+E regimen and the usual care treatment in parallel in year 1.
Table 2.
Diet and Exercise Characteristics
| Parameters | Data Source | |||
|---|---|---|---|---|
| Percent reduction in BMI | Probability of BMI reduction | Percent reduction in WOMAC Pain: Mean (SD) | Probability of weight loss failure (subsequent years) | |
| 20-25% | 0.08 | 52.1 (40.3) | 0.04 | Derived from IDEA trial datasets |
| 15-20% | 0.11 | 42.6 (53.1) | 0.04 | |
| 10-15% | 0.23 | 27.5 (51.6) | 0.04 | |
| 5-10% | 0.29 | 27.5 (51.6) | 0.34 | |
| 0% | 0.30 | 11.9 (44.6) | NA | |
| Probability of pain reduction failure (subsequent years)) | ||||
| Given weight loss success | 0.21 | |||
| Given weight loss failure | 0.57 | |||
| Discontinuation | ||||
| Overall Discontinuationa, b | 8% [12%, 16%] | |||
| Discontinuation due to Treatment Failurea | 0% [50%, 100%] | |||
| Duration of D+Ea | 2 years [3, 5, 8 years, no limit] | |||
| Cost | First Year | Subsequent Years | Derived from IDEA trial datasets, GNC Inc. (40) | |
| Personnel | $328 | $281 | ||
| Meal Replacementsa | $455 [0] | $0 | ||
| Gym Membershipa | $600 [$0] | $600 [$0] | Assumption | |
Abbreviations: SD, standard deviation; BMI, body mass index; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Values in brackets were assessed in one-way sensitivity analyses
In the base case, we assumed that the D+E program would run for two years; thus, overall discontinuation only occurred in the first year. In sensitivity analyses, we tested longer durations of the D+E program and in those instances, the probability of discontinuation is annual.
Usual Care
Usual care treatment consisted of a pharmacologic NSAIDs regimen, followed by TKA among those eligible and willing to undergo surgery, and revision TKA among those who failed primary TKA. Based on NSAID utilization in the IDEA cohort at baseline (unpublished data), half of the participants were assumed to begin with the NSAID regimen. The other half used analgesics intermittently, without any long-term efficacy, until eligible for TKA (Appendix Section 2c). Usual care does not include steroid injections because injections, though efficacious in the short-term, have not been shown to have long-term efficacy in reducing knee OA pain (27).
Intervention duration:
Subjects could remain on the NSAID regimen for up to 20 years. As we describe below, minor toxicity discontinuation or treatment failure caused most subjects to end treatment before this maximum was reached.
Efficacy:
NSAID efficacy was derived from the GAIT study (28). TKA structural efficacy rates were derived from data published by Paxton and colleagues (29). TKA pain decrements were derived from two longitudinal studies of knee OA patients undergoing TKA: AViKA and STARs (30, 31). (Appendix Section 2a and Appendix Table 3).
Discontinuation:
11.28% of model subjects on NSAIDs discontinue treatment in the first year due to minor toxicities. This probability was derived from multiple, large randomized controlled trials of NSAIDs in arthritis (32–36). We assume that all minor toxicity discontinuation occurs in the first year. In all years of treatment, subjects can discontinue NSAIDs due to treatment failure. Treatment failure discontinuation occurs if subjects return to within 9 points of their starting pain (half of the average initial decrease in pain).
Subjects only discontinue from primary TKA if they require a revision surgery. Therefore, we assume that minor toxicity discontinuation is 0%. Treatment failure discontinuation can only occur if a subject experiences a structural failure and if the subject’s pain returns to within 21 points of their starting pain (half of the average initial decrease in pain) (Appendix Section 2b).
Cost:
NSAID cost was calculated using an average of the cost of treatment (from Red Book Online (37)) weighted by the utilization of treatments by OA patients (MCBS 2009 (38)). The cost also included an annual office visit and labs. NSAIDs cost $841 in the first year and $810 in subsequent years. TKA cost, derived from Medicare Fee Schedules, was $17,976 (primary) and $24,985 (revision) and $109 each year after the year of surgery (39). Cost derivation methods for NSAIDs and TKA are described in a prior publication (15).
Diet and Exercise
Intervention duration:
Consistent with the IDEA trial duration (18 months), in the base case, subjects could remain on the D+E program for up to two years. Sensitivity analyses evaluated implementing D+E for longer durations. As data on long-term weight loss are limited, we conservatively assumed that benefits from D+E were not maintained after the program ended (Appendix Section 3a).
Efficacy:
Subjects on the D+E regimen were assigned a probability of percentage BMI reduction, derived from IDEA trial data. The BMI reduction was associated with a percentage reduction from baseline WOMAC pain (Table 2).
Each subsequent year, a subject had a probability of either losing the BMI reduction (termed weight loss failure) or losing the pain reduction (pain failure). Pain and weight loss failures did not necessarily occur together, but weight loss failure increased the probability of pain failure. Probability of weight loss failure was stratified by the amount of BMI reduction. If a subject experienced weight loss or pain failure, they reverted to the weight or pain level that they would have experienced had they not been on the D+E regimen. Likewise, in the year following termination of D+E, all subjects revert to the weight or pain levels that they would have experienced had they not received the D+E intervention (Appendix Section 3b).
We validated OAPol-predicted weight loss from the D+E regimen by comparing mean BMI in the D+E arm of the IDEA trial at the five-year follow-up (18% of subjects reporting data) to OAPol-predicted BMI at five years. The BMI in the IDEA trial D+E cohort was 31.65kg/m2 and OAPol-predicted BMI for D+E participants was 32.94 kg/m2.
Discontinuation:
Subjects had an overall probability of discontinuing the D+E regimen each year. This overall discontinuation was divided into general discontinuation (discontinuation unrelated to treatment efficacy) and treatment failure discontinuation (discontinuation due to failure to maintain weight loss). As we do not have data on why subjects discontinued from the D+E program in the IDEA trial, in the base case, we assumed that the 8% discontinuation was entirely general discontinuation; subjects who had lost weight had an equal probability of discontinuing as those who did not. In sensitivity analyses, we varied the percentage of discontinuation due to treatment failure (Appendix Section 3c).
Cost:
D+E cost was estimated using three components: personnel ($328 in the first year, $281 in subsequent years), meal replacements ($455 in the first year), and gym membership ($600 each year). Personnel costs were derived using 2015-2016 interventionist salaries at Wake Forest University. Meal replacement costs were derived using the current retail cost of the meal replacements (40) and the average number of meal replacement containers used by study participants (unpublished trial data). Gym costs were an assumption. Fixed costs were not included (Appendix Section 3d).
Process Benefits:
Exercise may confer additional increases in quality-of-life utility beyond those due to reduction in pain and weight. We refer to these increases as “process benefits” because they occur due to the process of exercising, rather than the outcomes (41). Based on published data, we estimated the process benefits from the exercise intervention by increasing a subject’s annual utility by 0.026 QALYs (41). The base case analysis did not include process benefits. In a one-way sensitivity analysis, they were added to the first year or all years of exercise (Appendix Section 3e).
Base Case Analysis
Health Sector Perspective
In the base case, we used D+E characteristics derived from IDEA trial data with added costs for a gym membership. We assumed that D+E regimen costs included personnel costs, meal replacements, and a hypothetical $600/year gym membership. Discontinuation was estimated at 8% annually (the IDEA trial had a 12% discontinuation over 18 months). Process benefits were not included.
Societal Perspective
The societal perspective included all health and quality-of-life components from the healthcare sector with added costs accounting for lost productivity and care-giving. Productivity costs reflect work absenteeism among individuals with OA in the US labor force (42). Knee OA pain costs $1,037 in lost productivity annually. Primary and revision TKA cost $3,311 and $3,592 respectively in lost productivity during the year of the surgery (15).
Based on a study by Gupta and colleagues that reported that 52.1% of OA indirect costs were related to care-giving (43), we assumed that productivity costs due to knee OA pain represented 47.9% of annual indirect costs. This resulted in a total annual indirect cost of $2,166. In the base case, only subjects with a WOMAC pain score greater than 40 incurred care-giving and OA pain productivity costs. We varied this threshold in sensitivity analyses.
Sensitivity Analyses
Deterministic Sensitivity Analyses
One-way sensitivity analyses varied key D+E parameters. D+E duration was assessed at 2 (base case), 3, 5, and 8 years as well as without limit. D+E cost was varied from only personnel costs to 50% more than the base case cost. Overall discontinuation was assessed at 8% (base case), 12%, and 16% annually; the percentage of that discontinuation due to treatment failure was assessed at 0% (base case), 50%, and 100%. Process benefits were not included (base case), or received by subjects during the first year or all years of D+E. The societal perspective included an additional analysis varying the WOMAC pain threshold for productivity and care-giving costs from 1 point (any pain), to 15, 40, or 70 points.
Probabilistic Sensitivity Analyses
We used probabilistic sensitivity analyses (PSA) to determine the effect of the uncertainty around the D+E regimen parameters (Appendix Table 4). We varied cost, overall discontinuation, the relative reduction in pain severity, and the probability of pain and BMI failure. The societal perspective analysis also varied costs from OA-related care-giving. In all iterations, the duration of D+E was two years, discontinuation due to treatment failure was 0%, and process benefits were not included.
Results are shown with acceptability curves, which report the percentage of PSA simulations out of 1000 for which an intervention was cost-effective at different values of willingness to pay for additional QALYs.
Results
Base Case Analysis
The D+E regimen led to a gain of 5.4 QALYs for every 100 participants (increasing per-person QALE to 8.963 QALYs from 8.909 QALYs). From the healthcare sector and societal perspectives, the D+E regimen raised per person cost by $1,845 and $1,624, respectively. D+E was cost-effective at all thresholds. D+E had an ICER of $34,100/QALY from the healthcare sector perspective and an ICER of $30,000/QALY from the societal perspective (Table 3).
Table 3.
Cost-Effectiveness of Diet and Exercise
| QALEa | Lifetime Costa | ICER | ||
|---|---|---|---|---|
| Healthcare Perspective | Usual Care | 8.909 | $116,200 | $34,100/QALY |
| Diet and Exercise | 8.963 | $118,100 | ||
| Societal Perspective | Usual Care | 8.909 | $130,700 | $30,000/QALY |
| Diet and Exercise | 8.963 | $132,400 |
Abbreviations: QALE, quality-adjusted life expectancy; ICER, incremental cost-effectiveness ratio
QALE and cost reported as per-person values and discounted at 3% per year
The improvement in quality of life from D+E is due to decreases in BMI and WOMAC Pain. During the first year of the D+E program, the D+E cohort’s average WOMAC Pain was 6.8 (out of 100) points lower than the usual care cohort, and their average BMI was 2.8kg/m2 lower than the usual care cohort. During the second year, the D+E cohort’s average WOMAC Pain was 4.9 points lower and their BMI was 2.3kg/m2 lower than the usual care cohort. Due to the assumption that D+E benefits would not extend beyond two years, D+E delayed, but did not avert, total knee replacement.
One-way Sensitivity Analyses – Healthcare Sector Perspective
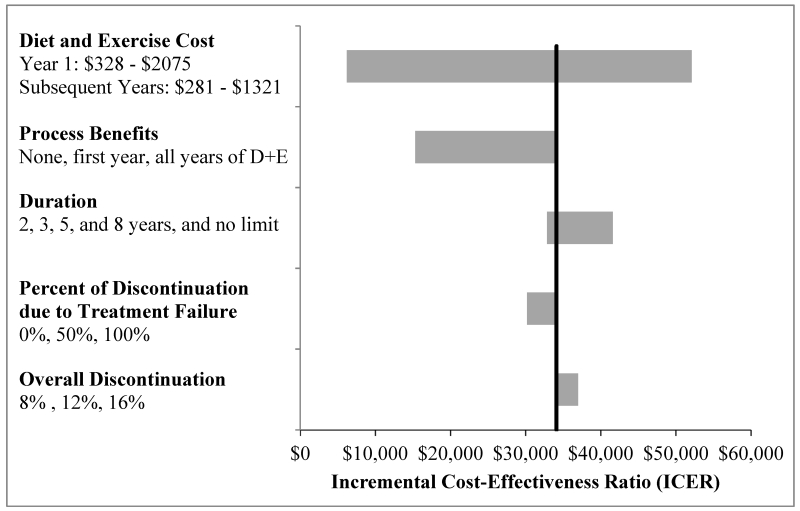
D+E was cost-effective at a WTP threshold of $100,000/QALY in all variations (Figure 1). As D+E was always cost-effective at a threshold of $100,000/QALY, we do not report specific results for the $200,000/QALY threshold. The ICER for D+E was only above $50,000/QALY when cost was increased to 150% of the base case (ICER = $52,100/QALY). When only personnel costs were included, the ICER was $6,200/QALY and when personnel and meal replacement costs were included, the ICER was $12,900. The inclusion of process benefits in the first year of D+E lowered the ICER to $22,600/QALY and including process benefits in all years lowered the ICER further to $15,300/QALY. Varying discontinuation changed the ICER minimally.
Figure 1. One-way Sensitivity Analysis of Diet and Exercise Parameters (Healthcare Sector Perspective).
This figure illustrates the incremental cost-effectiveness ratio (ICER) estimated for the diet and exercise regimen under a variety of conditions. In each analysis, all parameters were held at base case values except for the parameter listed on the vertical axis which was varied according to the values listed. The leftmost end of each bar reports the ICER when the parameter of interest is set to its most favorable value; the rightmost end of each bar reports what happens when the parameter assumes its least favorable value. The vertical black bar shows the base case ICER. Process benefits are the increase in quality-of-life utility that occurs from the process of exercising (in addition to increases from weight loss and pain reduction). Overall discontinuation refers to the cohort’s overall discontinuation rate and the percentage of discontinuation due to treatment failure is the percentage of that discontinuation that occurs specifically in subjects who did not maintain their weight loss.
When compared to usual care, all D+E durations were cost-effective at a WTP threshold of $50,000/QALY. We also compared D+E programs of different durations incrementally. The two-year program was dominated by the three-year program. The three-year, five-year, eight-year, and indefinite D+E programs had ICERs of $32,800/QALY, $33,400/QALY, $42,100/QALY, and $79,200/QALY respectively.
One-way Sensitivity Analyses – Societal Perspective
D+E was somewhat more cost-effective when analyzed from the societal perspective than from the healthcare sector perspective. The highest ICER, $48,700/QALY, occurred when cost was 150% of the base case. Varying the WOMAC pain threshold for productivity and care-giving costs had a small effect on cost-effectiveness. ICERs ranged from $26,800/QALY (WOMAC > 15) to $32,700/QALY (WOMAC > 1) (Appendix Figure 1).
Probabilistic Sensitivity Analyses
From the healthcare sector perspective, at WTP thresholds of $50,000/QALY and $100,000/QALY, D+E had a 58% and 100% likelihood of being cost-effective (Figure 2). From the societal perspective, at WTP thresholds of $50,000/QALY and $100,000/QALY, D+E had a 68% and 100% likelihood of being cost-effective (Appendix Figure 2).
Figure 2. Cost-Effectiveness Acceptability Curve (Healthcare Sector Perspective).
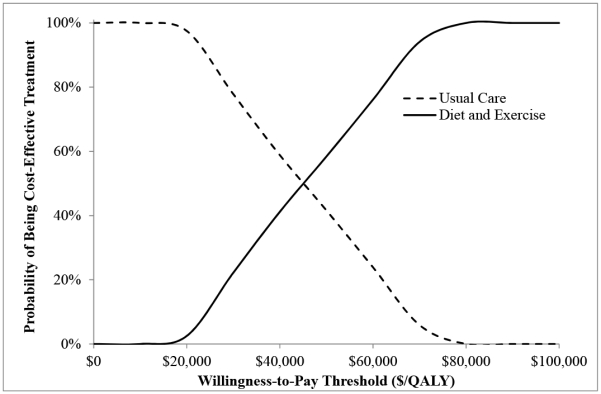
The curves show the percentage of simulations (out of 1000) for which an intervention was cost-effective at a given willingness-to-pay threshold. Each of the 1000 analyses independently sampled model input parameters from the specified distribution (Appendix Table 4).
Discussion
Our analysis suggests that incorporating a D+E regimen into usual care treatment for knee OA would be highly cost-effective from both societal and healthcare sector perspectives. In our base case evaluation and the majority of sensitivity analyses, D+E had an ICER below $50,000/QALY, the more conservative cost-effectiveness threshold. D+E was always cost-effective with a threshold of $100,000/QALY, which is increasingly used in United States cost-effectiveness analyses (18).
Both weight loss and D+E are effective in reducing knee OA pain and improving function (10–12, 44). Cost-effectiveness analyses of exercise as treatment for knee OA (without weight loss as an explicit goal) have also generally indicated that exercise programs are cost-effective (45, 46). A previous analysis of D+E in the ADAPT trial found that D+E was cost-effective for self-reported function, pain, and stiffness (14). Our findings corroborate this and suggest that D+E would be cost-effective as a program with limited duration or as a program in which patients can continue to participate indefinitely. Our comparison of D+E durations suggests that with a WTP threshold of $50,000/QALY, the eight-year program provides the best value. If the WTP threshold is raised to $100,000/QALY, the program without a limit on duration offers the best value.
Of note, we showed that D+E is cost-effective when the effectiveness measure includes an adjustment for quality of life, which permits comparisons with other treatments. While the 0.054 difference in QALE between the two-year D+E program and usual care is small, this is because OA treatments primarily improve quality, rather than quantity, of life. The improvements in QALE from the two-year D+E program are similar to those from over-the-counter (OTC) Naproxen (0.081 QALYs) (16). The base case D+E ICER is also comparable to other OA treatments. OTC Naproxen and TKA, for example, have ICERs of $57,100/QALY and $22,500/QALY respectively (updated to 2016 USD) (7, 16, 47, 48).
Given the number of Americans with OA, implementing a D+E program into usual care may lead to substantial improvement in quality of life on a population level, but funding the program may have a non-trivial effect on payers’ budgets. The major components of the cost of D+E are meal replacements and a gym membership. Payers considering coverage for D+E programs could develop strategic partnerships with gyms and meal replacement manufacturers to minimize budget impact.
We note several limitations. First, the D+E regimen was based on results from a clinical trial D+E regimen, which was led by professional interventionists and required significant participant investment. The outcomes of D+E regimens should also be established in community settings, where participants may not be as strongly motivated. In addition, the trial and model cohort had an average starting KL grade of 2.5, so the results may not apply to a cohort with more severe OA.
We made several assumptions to project the results of an 18-month clinical trial over a longer duration. As data on long-term weight loss maintenance is limited, we assumed that subjects lost all weight and pain reduction benefits once the D+E program ended and that the base case D+E program would only last for two years. Long-term data on D+E adherence and the sustainability of weight loss and pain reduction are needed to more accurately model D+E treatments. We also did not consider potential correlations between baseline characteristics (e.g., age, pain) and D+E outcomes, which may have biased our point estimates.
Model inputs were derived from a variety of national data sources and published literature (Appendix Table 5). QALE was derived from the OAI using the SF-12, rather than directly measured in the IDEA cohort. The utility increase from the process of exercising (process benefit) was derived from the Health Survey for England (HSE) using the EQ-5D. As the populations and measures in the OAI and HSE differ, the process benefit quality-of-life values were only included in a one-way sensitivity analysis. As our costs were trial-based, they may not entirely reflect the cost of implementing D+E outside of a clinical trial, though we added the gym membership cost to more accurately reflect what participants may have to contribute. In conformity with widely accepted guidelines for the conduct of economic evaluation (19, 49), we employed extensive sensitivity analyses to address uncertainty surrounding our findings. Our estimates were robust to uncertainty from the trial data.
Our findings strongly suggest that implementing D+E in knee OA treatment provides good value and should be a priority for clinicians and policy-makers. Further studies should consider how best to implement these programs and make them accessible to knee OA patients.
Supplementary Material
Significance and Innovations.
While previous work has established the clinical efficacy (pain reduction) of diet and exercise programs for knee osteoarthritis (OA) treatment, this study is the first to confirm that such programs also provide excellent economic value when compared to alternative uses of scarce OA treatment resources.
We considered multiple willingness-to-pay thresholds ($50,000, $100,000, and $200,000 per quality-adjusted life-year (QALY)). In the base case, the diet and exercise program was cost-effective at all thresholds considered.
If payers are willing to spend $50,000 per QALY gained, allowing participants to participate in the diet and exercise program for up to eight years provides the best value. If they are willing to spend $100,000 per QALY gained, allowing participants to participate in the diet and exercise program indefinitely provides the best value.
Programs to provide knee OA patients with access to diet and exercise treatment should be implemented into clinical care.
Acknowledgments
Support
NIH/NIAMS R01AR064320, K24AR057827
Footnotes
Disclosures:
EL is on the Samumed Payers Advisory Board and has provided statistical consulting to Tissuegene (both <$10,000); DJH provides consulting advice for Flexion, Merck Serono, Tissuegene, and TLCBio (all <$10,000). All other authors have no disclosures.
References
- 1.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. The number of persons with symptomatic knee osteoarthritis in the United States: impact of race/ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken). 2016;68(12):1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med 2011;154(4):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. [DOI] [PubMed] [Google Scholar]
- 4.Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition J Bone Joint Surg Am. 2013;95(20):1885–6. [DOI] [PubMed] [Google Scholar]
- 5.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016;24(6):962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarberg B, Tenzer P. Complexities in the pharmacologic management of osteoarthritis pain. Curr Med Res Opin. 2013;29(5):539–48. [DOI] [PubMed] [Google Scholar]
- 7.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–21; discussion 21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597–606. [DOI] [PubMed] [Google Scholar]
- 9.Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9(4):225–35. [DOI] [PubMed] [Google Scholar]
- 10.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. [DOI] [PubMed] [Google Scholar]
- 12.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flego A, Dowsey MM, Choong PF, Moodie M. Addressing obesity in the management of knee and hip osteoarthritis - weighing in from an economic perspective. BMC Musculoskelet Disord. 2016;17(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevick MA, Miller GD, Loeser RF, Williamson JD, Messier SP. Cost-effectiveness of exercise and diet in overweight and obese adults with knee osteoarthritis. Med Sci Sports Exerc. 2009;41(6):1167–74. [DOI] [PubMed] [Google Scholar]
- 15.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(2):203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2016;24(3):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 19.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, eds. Cost-effectiveness in health and medicine. 2 ed: Oxford University Press; 2017. [Google Scholar]
- 20.Osteoarthritis Initiative (OAI). San Francisco: University of California; 2013. [Google Scholar]
- 21.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–9. [DOI] [PubMed] [Google Scholar]
- 22.Lean ME, Han TS, Seidell JC. Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Arch Intern Med. 1999;159(8):837–43. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Nordhorn J, Muckelbauer R, Englert H, Grittner U, Berger H, Sonntag F, et al. Longitudinal association between body mass index and health-related quality of life. PLoS One. 2014;9(3):e93071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention U.S. Department of Health and Human Services. National Center for Health Statistics. National Health and Nutrition Examination Survey; 2011-2014. [Google Scholar]
- 26.DeLemos BP, Xiang J, Benson C, Gana TJ, Pascual ML, Rosanna R, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2011;18(3):216–26. [DOI] [PubMed] [Google Scholar]
- 27.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. [DOI] [PubMed] [Google Scholar]
- 29.Paxton EW, Namba RS, Maletis GB, Khatod M, Yue EJ, Davies M, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92 Suppl 2:117–32. [DOI] [PubMed] [Google Scholar]
- 30.Losina E, Collins JE, Wright J, Daigle ME, Donnell-Fink LA, Strnad D, et al. Postoperative care navigation for total knee arthroplasty patients: a randomized controlled trial. Arthritis Care Res (Hoboken). 2016;68(9):1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dave AJ, Selzer F, Losina E, Usiskin I, Collins JE, Lee YC, et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis Cartilage. 2017;25(5):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376(9736):173–9. [DOI] [PubMed] [Google Scholar]
- 33.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–55. [DOI] [PubMed] [Google Scholar]
- 34.Lisse JR, Perlman M, Johansson G, Shoemaker JR, Schechtman J, Skalky CS, et al. Gastrointestinal tolerability and effectiveness of rofecoxib versus naproxen in the treatment of osteoarthritis: a randomized, controlled trial. Ann Intern Med. 2003;139(7):539–46. [DOI] [PubMed] [Google Scholar]
- 35.Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368(9549):1771–81. [DOI] [PubMed] [Google Scholar]
- 36.Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364(9435):665–74. [DOI] [PubMed] [Google Scholar]
- 37.Red Book Online. Truven Health Analytics Inc. Data Accessed 2/5/2016. [Google Scholar]
- 38.Centers for Medicare and Medicaid Services. Medicare Current Beneficiary Survey (MCBS) 2009. Baltimore, MD. [Google Scholar]
- 39.Centers for Medicare and Medicaid Services. Medicare Fee Schedules 2015. Baltimore, MD. [Google Scholar]
- 40.GNC Total Lean™ Lean Shake™. General Nutrition Centers. Accessed: March 12, 2018 Available from: http://www.gnc.com/weight-management/meal-replacements/?landing=1.
- 41.Pavey TG, Anokye N, Taylor AH, Trueman P, Moxham T, Fox KR, et al. The clinical effectiveness and cost-effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess. 2011;15(44):i–xii, 1–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger A, Hartrick C, Edelsberg J, Sadosky A, Oster G. Direct and indirect economic costs among private-sector employees with osteoarthritis. J Occup Environ Med 2011;53(11):1228–35. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531–7. [DOI] [PubMed] [Google Scholar]
- 44.Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis. 2011;70(10):1798–803. [DOI] [PubMed] [Google Scholar]
- 45.Richardson G, Hawkins N, McCarthy CJ, Mills PM, Pullen R, Roberts C, et al. Cost-effectiveness of a supplementary class-based exercise program in the treatment of knee osteoarthritis. Int J Technol Assess Health Care. 2006;22(1):84–9. [DOI] [PubMed] [Google Scholar]
- 46.Pinto D, Robertson MC, Hansen P, Abbott JH. Cost-effectiveness of nonpharmacologic, nonsurgical interventions for hip and/or knee osteoarthritis: systematic review. Value Health. 2012;15(1):1–12. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Medicare and Medicaid Services. National Health Expenditure Accounts: Personal Health Care. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html, January 5, 2017.
- 48.U.S. Bureau of Economic Analysis. Personal Consumption Expenditures: Services: Health Care [DHLCRC1Q027SBEA], retrieved from FRED, Federal Reserve Bank of St. Louis; https://fred.stlouisfed.org/series/DHLCRC1Q027SBEA, January 5, 2017. [Google Scholar]
- 49.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–32. [DOI] [PubMed] [Google Scholar]
- 50.Consumer Price Index - All Urban Consumers (CPI-U). National Bureau of Labor Statistics; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.