Abstract
Objectives:
In platelets, α-synuclein is important in calcium-dependent granule release. Notably, cells release α-synuclein in setting of cell damage or death. Therefore, we investigated α-synuclein levels in plasma of single donor platelet (SDP) units during storage.
Methods:
Aliquots were obtained from same SDP units for 7 days from day of donation. Additionally, randomly sampled SDP units at same storage time points were also assayed by enzyme-linked immunosorbent assay.
Results:
α-synuclein in SDP plasma increased continuously over time at each assayed time point. Significant increases were measured on day 3 (11.7 ± 9.6 ng/mL, p=0.025), day 5 (15.3 ± 5.9 ng/mL, p=0.002), and highest on day 7 (23.7 ± 5.6 ng/mL, p<0.0001) compared to day 0 (1.1 ± 0.8 ng/mL). Similar significant results were obtained in randomly sampled SDP units at same corresponding time points. Flow cytometry showed that platelets had strong expression of α-synuclein and lacked expression of other synucleins.
Conclusions:
Increases of α-synuclein during SDP storage is a steady and continuous process that increases with time. Our findings indicate that α-synuclein may represent a biomarker of platelet biological state during storage. Further research will be needed to determine how α-synuclein increases correlate with platelets’ function.
Keywords: α-synuclein, platelets, storage, senescence, biomarker
INTRODUCTION
α-synuclein is part of a family of small proteins (14–17 kDa) that were first described in normal and malignant brain tissue.(1) In the central nervous system, though its function is not fully understood, it is expressed in both neuronal cytoplasm and in presynaptic terminals where it regulates neurotransmitter release, synaptic function, and neuronal plasticity.(2) Of interest, α-synuclein has been described as being in close proximity to all membranous structures in neurons. However, under certain conditions structural changes leading to both α-synuclein oligomerization and fibrillary growth lead to one of the central pathologic findings of Parkinson’s disease and other synucleinopathies.(2)
α-synuclein is also found in the hematopoietic system where it has been shown to be expressed in erythroid precursors and megakaryocytes in bone marrow, as well as erythrocytes and platelets in blood.(3, 4) Importantly, recent reports have shown that α-synuclein is essential to immunity since its absence leads to impairment in the late maturation stages of hematopoiesis, marked B cell and T cell lymphopoiesis, functional lymphocyte dysregulation, T-helper (h)1 bias in setting of Th2 differentiation, impairment in immunoglobulin isotype-switching, and impaired adaptive immune responses.(5–7)
In the megakaryocytic lineage, α-synuclein is expressed once megakaryocytes are fully mature and cease to express to β-synuclein which is only expressed early in megakaryoblastic development.(3) Along the same lines, α-synuclein has been shown to be a highly specific biomarker of neoplasms of the megakaryocytic cell lineage.(8) In platelets, α-synuclein is highly expressed and loosely associated with the plasma membrane, and it is in close association with membranous organelles and with secretory α-granules.(3) The function of α-synuclein in platelets is not known, but it appears to function as a negative regulator of calcium-dependent α-granule release.(9) This inhibitory function to keep platelets from degranulating seems to be closer to its biological function since exogenous α-synuclein inhibits von-Willebrand factor release from endothelial cells upon stimulation.(10) Furthermore, α-synuclein deficiency leads to morphologically abnormal platelets in α-synuclein −/− mice.(5)
In the United States platelets are stored at room temperature (22–24oC) with continuous gentle agitation for a period of 5 days. However, data from US centers which have extended units’ shelf life to 7 days have not reported increases in adverse events secondary to transfusion.(11) Therefore, based on the aforementioned studies we set up to determine if α-synuclein could be measured in platelet components during prolonged storage (7 days) and if changes in its concentration occur over time.
MATERIALS AND METHODS
Platelet Units
Single donor platelet (SDP) samples were obtained from our local blood suppliers: American Red Cross (Cleveland, OH, USA) and LifeShare Community Blood Services (Lorain, OH, USA). All units were O+ from healthy volunteers, leukoreduced, collected by apheresis, suspended in plasma, stored in PVC bags (Fenwal Inc., Lake Zurich, IL, USA) with ACD-A (10–15%) as anticoagulation, and with similar platelet counts as established by the Food and Drug Administration’s Code of Federal Regulations Title 21. Units were kept at room temperature (22–24oC) with continuous gentle agitation. One mL aliquots were obtained from five randomly chosen SDPs units obtained on day of donation (day 0), and the same units were sampled on days 3, 5, and 7 (post-collection). Separately, random SDP units were sampled at the same corresponding storage time points of the first experiment that followed the same units over time. All samples at time of aliquot were centrifuged at 834(g) and at room temperature conditions to minimize platelet activation as previously described;(12) plasma supernatant was removed and kept frozen at −80oC until assayed.
α-synuclein enzyme-linked immunosorbent assay (ELISA)
An ELISA kit containing a monoclonal antibody to the full length human α-synuclein protein was performed according to manufacturer’s protocols (Invitrogen Co. Carlsbad, CA). Briefly, to quantify total α-synuclein concentration, purified recombinant α-synuclein at known concentrations was assayed in parallel to develop a standard concentration curve using serial protein dilutions to permit conversion of absorbance (optical density) into protein concentration. Samples were assayed at either 1:5–1:10 dilutions. Recombinant α-synuclein and SDP samples were first added to plates in triplicate with capture antibody (immobilized) and to solution phase rabbit polyclonal antibody specific for α-synuclein and incubated for 3 hours at room temperature. Wells were washed four times using a model 1575 ImmunoWash microplate washer (BioRad Laboratories, Inc., Hercules, CA) with 250 µL/well of phosphate buffer saline (PBS) buffer with 0.05% Tween. Horseradish peroxidase-labeled anti-rabbit IgG was added and incubated for 30 min. Wells were washed, substrate solution was added, plates were incubated for 30 min in the dark, stop solution was added, and plates were read at an absorbance of 450 nm using a model 680 microplate reader (Bio-Rad). Recombinant α-synuclein standard was plotted with the absorbance (y-axis) against protein concentration (x-axis) to generate protein standard curve. α-synuclein concentration was determined using the concentration curve times the dilution factor.
Flow cytometry of SDP
α-synuclein expression was determined as previously described.(8) Briefly, SDP aliquots were spun, pelleted, resuspended, and washed in PBS. Cells were then fixed and permeabilized using Fix and Perm Cell Fixation and Permeabilization kit according to manufacturer’s protocols (Life Technologies, Carlsbad, CA). Rabbit anti-human α-synuclein and rabbit anti-human β-synuclein monoclonal antibodies (Novus Biologicals, Littleton, CO) were used (the latter was used as negative control due to lack of expression of β-synuclein in platelets (3, 8). Rabbit anti-human γ-synuclein (Abcam, Cambridge, MA) was used as an additional negative control. Mouse anti-human β-spectrin was used as positive control (BD Biosciences San Jose, CA). Goat anti-rabbit IgG (FITC-conjugated) and donkey anti-mouse IgG (PE-conjugated) were used as secondary antibodies (Abcam). Additionally, rabbit and mouse isotype controls were used during analyses (Abcam). Immunostaining was analyzed using FACSCanto II flow cytometer (BD Biosciences).
Statistical analysis:
Statistics were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA) Results are presented as mean ± standard deviation (SD). All results were analyzed for statistical power,(13) and intergroup data comparisons were performed using one-way ANOVA and the Dunnett’s multiple comparison tests. A p value of <0.05 was set for significance.
RESULTS
α-synuclein concentration in same SDP units followed for 7 days
In each experiment a set of five SDP units were tested (3 independent experiments using different SDP units, N=15). Units on day of donation (day 0) had very low concentration of α-synuclein in the supernatant plasma concentration of 1.1 ± 0.8 ng/mL (Fig. 1A). This is important since it is a concentration similar to that reported in plasma from normal subjects,(14) and it is within the limits of detection of the ELISA (1–100 ng/mL) as described by the manufacturer. By day 3, α-synuclein concentration began to increase when it reached a concentration of 11.7 ± 9.6 ng/mL (p=0.025 [95% confidence interval 4.2–19.1] compared to day 0). Further sampling of the same units on day 5 showed that mean α-synuclein concentration continued to increase to 15.3 ± 5.9 ng/mL (p=0.002 [95% CI 10.8–19.9] compared to day 0). Finally, by day 7 α-synuclein reached its highest concentration of 23.7 ± 5.6 ng/mL (p<0.0001 [95% CI 19.4–28.1] compared to day 0).
Figure.1:
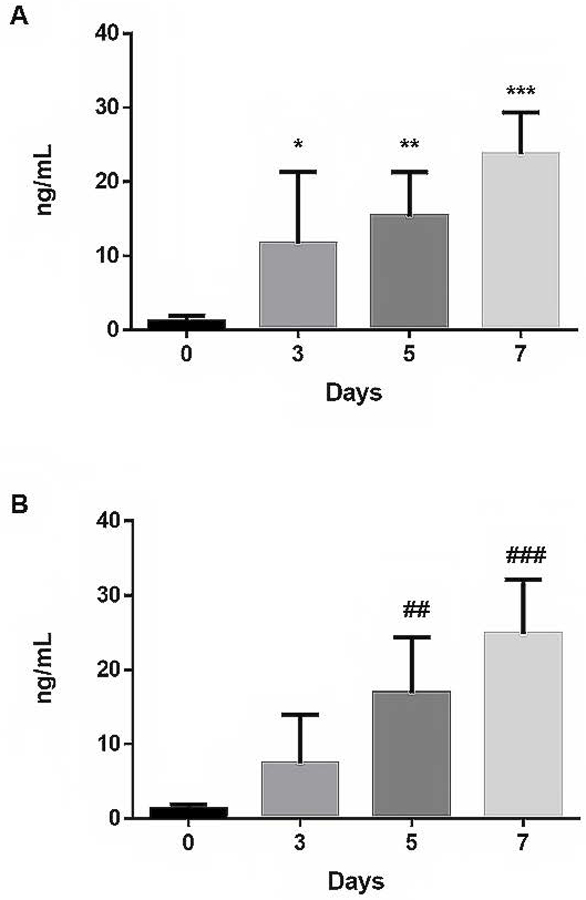
A. Plasma α-synuclein concentration measured by ELISA in the same SDP units sampled on days 0, 3, 5, and 7. Significant increases in α-synuclein were seen at day 3 (*p=0.025), day 5 (**p=0.002) which reached highest levels on day 7 (***p<0.0001) in comparison to day 0. Data are representative of 3 independent experiments on different set of five SDP units followed for the indicated time points. B. Plasma α-synuclein levels measured by ELISA in random SDP units sampled on days 0, 3, 5, and 7. Steady and continuous increase in α-synuclein was noted on day 5 (#p=0.0047), which reaches highest levels on day 7 of storage (##p=0.0002) in comparison to day 0. Data are representative of 3 independent experiments sampling five different random units per experiment (N=15).
Separately, randomly chosen SDP units were sampled on the same storage time points as before (Fig. 1B). Similar to results of same SDP units followed for 7 days, randomly sampled units had increases in α-synuclein concentration by day 3 of 7.4 ± 6.5 ng/mL [95% CI 2.9–17.9]. By day 5 α-synuclein concentration increased to 16.9 ± 7.4 ng/mL (p=0.0047 [95% CI 5.0–28.8] compared to day 0). On day 7, α-synuclein reached its highest concentration of 24.8 ± 7.3 ng/mL (p=0.0002 [95% CI 13.2–36.4] compared to day 0).
Flow cytometric analysis of α-synuclein
Flow cytometry analysis indicated that the cells expressing α-synuclein and therefore source of this protein increases in SDP units’ plasma supernatant were platelets. Both β- and γ-synuclein showed flow staining similar to that of the isotype control (Figure 2A). The histograms of these three antibodies did not differ from samples stained with just the secondary antibody alone (data not shown). On the other hand, α-synuclein staining indicated a strong expression of this protein in platelets as shown by the signal shift to the right (Figure 2B). Each of the runs measured at least 100,000 events and results are representative of three independent experiments.
Figure 2.
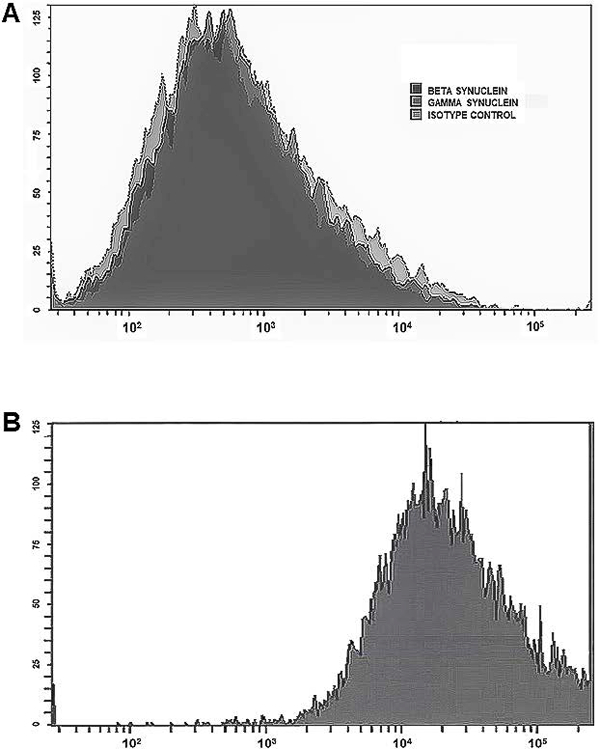
Flow analysis of α-synuclein expression in platelets (y axis represents counts). A. Isotype control, β-synuclein and γ-synuclein staining shows that platelets lack expression of these two synucleins since they did not differ from isotype control. B. Strong right shift in protein expression characterized staining with α-synuclein. Experiments analyzed a minimum of 100,000 events per sample and were similar in 3 independent experiments (N=15).
DISCUSSION
We have previously reported the importance of α-synuclein in hematopoiesis and lymphopoiesis as shown by the lack of B cell maturation, isotype-switching in response to antigenic challenge, effacement of lymphoid organ architecture, T cell maturation deficiency, lymphocyte dysregulation of cytokine profile, anemia, and abnormal platelets.(5, 6) In relationship to the current findings, considering that platelets appear to be dependent on α-synuclein to regulate granular release, (9) increases in α-synuclein concentration in platelet units while in storage may indicate that this molecule can correspond to changes platelets undergo in a time-dependent manner during storage. These results were apparent regardless if the same units were sampled over time or when units were randomly tested. This is the first report indicating that α-synuclein can potentially be used to follow platelet units while in storage and may represent a biomarker of time-dependent cellular changes.
Looking closer at our results, we found at least one SDP unit in each experiment that on day of donation had no measurable α-synuclein. Interestingly, these same units by day 3 had levels of α-synuclein comparable to the other units from the same time point. Furthermore, at least one unit on day 3 had larger increases of α-synuclein compared to the other units on that day but these units continued to increase α-synuclein on days 5 and 7 at comparable rates to the rest of the units of that time point so that by day 7 all units all units had similar α-synuclein concentration. It could be suggested that RBC contamination may be behind these increases based on a report that indicated RBCs contained most of the α-synuclein in blood.(15) However, this same report in its results and discussion revealed that their conclusions were based on the fact that there are more RBCs than other cell types in blood and in fact that by far the highest concentration of α-synuclein per milligram of cellular proteins is actually in platelets.(15) Since all units used experimentally had hematocrits <2%. RBC contamination of a unit does not explain the outliers. Potentially, other factors during storage may have influenced this increase which was not seen during the rest of storage time measurements.
Despite over two decades of intense investigation in the central nervous system the function of α-synuclein is still not well understood. However, current data indicate that its close interaction with neurotransmitter vesicles and with the transport system mediating their release imply that, at least under normal conditions, it is essential to axonal release of neurotransmitters.(1) While more research is needed to understand how α-synuclein mediates its function in hematopoiesis and lymphopoiesis, the close relationship of α-synuclein with membranes, and its ability to closely associate with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes and promote their assembly(16) may provide clues to its function in the hematopoietic system and in lymphopoiesis. Platelets contain both plasma/target membrane t-SNARE and vesicular/granular v-SNARE protein complexes which are essential to granule release.(17) Platelets’ α-synuclein has been shown by electron microscopy to be closely associated with the plasma membrane, membranous organelles, and secretory granules.(3, 9) Since this protein regulates α-granule release from platelets in a calcium-dependent manner,(9) these findings indicate that α-synuclein through its ability to mediate SNARE assembly may be essential to normal platelet responses.
Platelets are anucleated cellular structures which lack the ability to transcribe “de novo” RNA yet undergo an intrinsic apoptotic program that controls their survival and dictates their lifespan.(18) This pre-programing does not stop during their storage post-collection since they can also undergo apoptosis in-vitro as they age.(19) Additionally, during processes of senescence and apoptosis, platelets release proteins and vesicles into plasma that reflect their biological decay.(20) These processes together with morphological changes, signs of activation/ degranulation (CD62P, CD63 expression, release of β-thromboglobulin and platelet factor-4), proteolysis, and changes to surface receptor expression such as GPIb and GPIIb/IIIa are known as the platelet storage lesion.(21–23) Since as mentioned earlier α-synuclein mediates α-granule release, its concentration increases may be indicative of granular release and changes that platelets have undergone during storage.
α-synuclein is also known to be immunogenic since in patients with Parkinson’s both T helper and T cytotoxic cells can readily recognize α-synuclein epitopes and mount strong reactions.(24) It is unclear, however, in light of our results whether these immune responses to α-synuclein would lead to immunogenicity in patients receiving platelet units closer to the end of their shelf life; nevertheless, this should be a matter of investigation to determine whether increasing amounts of α-synuclein in older platelet units lead to equivalent responses in their recipients.
In regard to pathogen reduction technology implementation which seeks to make the platelet inventory safer for recipients, it should be of interest that use of psoralen and ultraviolet A light therapy on platelets inhibits signal transduction and normal docking of liposomes onto α-synuclein.(25) These findings suggest that looking at α-synuclein concentration increases as a surrogate biomarker of changes to platelet biology in response to such technology may prove essential to determine if use of pathogen reduction leads to changes in platelets’ health. Similarly, in light of the current increased interest in using cold-stored platelets,(26–28) it would also be of interest to establish if α-synuclein concentration changes can also be indicative of platelet health in this setting as well. These areas should be investigated in future studies.
In summary, α-synuclein is a protein that earned its reputation as an important biological mediator in the central nervous system, and structural dysregulation in its expression or assembly may be responsible for the pathology seen in synucleinopathies. α-synuclein is also important in hematopoiesis and changes to its concentration occur while platelets are in storage. Therefore, α-synuclein is not only an important mediator of hematopoiesis and lymphopoiesis but may also represent a biomarker for storage changes in platelets that will require further studies to gauge if concentration increases correlate with biochemical changes that occur during storage conditions. Since the highest plasma concentrations of α-synuclein were observed on day 7, it remains to be elucidated the significance of these findings in platelets stored for this extended period.
ACKNOWLEDGEMENTS
This study was fully supported by grant funding from the Office of Diversity and Inclusion of the University Hospitals Cleveland Medical Center and by the Dr. Harry Taylor Scholar Award also from UHCMC both awarded to R.W.M. Data included was presented by C.M.S. and received Top Poster Award at AABB’s annual meeting in Anaheim, CA. The authors would like to acknowledge the technological and logistical assistance provided by Mr. Jamal Nofal at UHCMC Blood Bank, Ms. Nancy Nagy at CWRU who was supported by NIH R01 AI034343 grant (awarded to C.V.H). Additionally, we thank Mrs. Annette Graves for assistance in preparation of the manuscript, and Dr. Hollie M. Reeves for critical review of the manuscript.
Footnotes
Disclosure of Conflicts of Interest: The authors report no conflicts of interest with the submission of this manuscript
REFERENCES
- 1.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 2013; 14(1): 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol 2001; 58(2): 186–90. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A, Saitoh T, Miller A, Masliah E. NACP, a synaptic protein involved in Alzheimer’s disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun 1997; 237(3): 611–6. [DOI] [PubMed] [Google Scholar]
- 4.Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, Ohtaka-Maruyama C, Okado H, Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun 2007; 358(1): 104–10. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Shameli A, Harding CV, Meyerson HJ, Maitta RW. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology 2014; 219(11): 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shameli A, Xiao W, Zheng Y, Shyu S, Sumodi J, Meyerson HJ, Harding CV, Maitta RW. A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology 2016; 221(2): 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettle B, Kuhbandner K, Jorg S, Hoffmann A, Winkler J, Linker RA. alpha-Synuclein deficiency promotes neuroinflammation by increasing Th1 cell-mediated immune responses. J Neuroinflammation 2016; 13(1): 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maitta RW, Wolgast LR, Wang Q, Zhang H, Bhattacharyya P, Gong JZ, Sunkara J, Albanese JM, Pizzolo JG, Cannizzaro LA, Ramesh KH, Ratech H. Alpha- and beta-synucleins are new diagnostic tools for acute erythroid leukemia and acute megakaryoblastic leukemia. Am J Hematol 2011; 86(2): 230–4. [DOI] [PubMed] [Google Scholar]
- 9.Park SM, Jung HY, Kim HO, Rhim H, Paik SR, Chung KC, Park JH, Kim J. Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood 2002; 100(7): 2506–14. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, Park JY, Jou I, Park SM. Regulation of Weibel-Palade body exocytosis by alpha-synuclein in endothelial cells. J Biol Chem 2010; 285(28): 21416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay SN, Immel CC, McClannan LS, Brecher ME. The introduction of 7-day platelets: a university hospital experience. J Clin Apher 2007; 22(5): 283–6. [DOI] [PubMed] [Google Scholar]
- 12.Soderstrom AC, Nybo M, Nielsen C, Vinholt PJ. The effect of centrifugation speed and time on pre-analytical platelet activation. Clin Chem Lab Med 2016; 54(12): 1913–20. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li J, Reeves HM, Reyes R, Maitta RW. Comparison of two apheresis systems during hematopoietic progenitor stem cell collections at a tertiary medical center. Transfusion 2016; 56(11): 2833–8. [DOI] [PubMed] [Google Scholar]
- 14.El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 2003; 17(13): 1945–7. [DOI] [PubMed] [Google Scholar]
- 15.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 2008; 5(2): 55–9. [DOI] [PubMed] [Google Scholar]
- 16.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010; 329(5999): 1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol 2008; 15(5): 537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell 2007; 128(6): 1173–86. [DOI] [PubMed] [Google Scholar]
- 19.Leytin V Apoptosis in the anucleate platelet. Blood Rev 2012; 26(2): 51–63. [DOI] [PubMed] [Google Scholar]
- 20.Pienimaeki-Roemer A, Kuhlmann K, Bottcher A, Konovalova T, Black A, Orso E, Liebisch G, Ahrens M, Eisenacher M, Meyer HE, Schmitz G. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion 2015; 55(3): 507–21. [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava M The platelet storage lesion. Transfus Apher Sci 2009; 41(2): 105–13. [DOI] [PubMed] [Google Scholar]
- 22.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med 2010; 30(2): 475–87. [DOI] [PubMed] [Google Scholar]
- 23.Ng MSY, Tung JP, Fraser JF. Platelet Storage Lesions: What More Do We Know Now? Transfus Med Rev 2018. [DOI] [PubMed] [Google Scholar]
- 24.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 2017; 546(7660): 656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Aelst B, Devloo R, Zachee P, t’Kindt R, Sandra K, Vandekerckhove P, Compernolle V, Feys HB. Psoralen and Ultraviolet A Light Treatment Directly Affects Phosphatidylinositol 3-Kinase Signal Transduction by Altering Plasma Membrane Packing. J Biol Chem 2016; 291(47): 24364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddoch-Cardenas KM, Montgomery RK, Lafleur CB, Peltier GC, Bynum JA, Cap AP. Cold storage of platelets in platelet additive solution: an in vitro comparison of two Food and Drug Administration-approved collection and storage systems. Transfusion 2018. [DOI] [PubMed] [Google Scholar]
- 27.Johnson L, Tan S, Jenkins E, Wood B, Marks DC. Characterization of biologic response modifiers in the supernatant of conventional, refrigerated, and cryopreserved platelets. Transfusion 2018; 58(4): 927–37. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Yin W, Zhang Y, Sun Y, Ma T, Gu S, Gao Y, Zhang X, Yuan J, Wang W. Evaluation of the advantages of platelet concentrates stored at 4 degrees C versus 22 degrees C. Transfusion 2018; 58(3): 736–47. [DOI] [PubMed] [Google Scholar]


