Abstract
Periodic limb movements in sleep (PLMS) are associated with adverse outcomes in patients with heart failure (HF). The aim of this study was to investigate whether PLMS change in response to adaptive servo-ventilation (ASV) for central sleep apnea (CSA) in patients with HF. We examined polysomnographic studies conducted between 2010 and 2014 at Mayo Clinic, Rochester, MN (N =14,444). Among those, 314 of 579 patients with CSA completed the sleep study with a protocol that began with diagnostic polysomnography, followed by continuous positive airway pressure, and, for persistent CSA, by ASV titration. Patients with HF (n=118) had a significantly higher median PLM index compared to those without HF (n=196): 33.7 vs. 6.1 events/h (p<0.001). HF was associated with a significant PLM arousal index (PLMAI) increase from diagnostic trial to ASV (odds ratio =1.79, p=0.032) after adjusting for demographics, comorbidities and medications. In patients aged >68 years, HF was associated with PLMI and PLMAI increases during ASV (odds ratio =2.16, p=0.016 and odds ratio =2.05, p=0.024), which persisted in multivariable models (odds ratio =2.36, p=0.025 and odds ratio =2.33, p=0.026). In multivariable analysis, patients with ejection fraction ≤45% had higher odds of increased PLMAI during ASV than those with ejection fraction >45% (odds ratio =1.98, p=0.022). In conclusion, PLMS may increase in HF patients after suppression of CSA by ASV. While the clinical significance of increased post-ASV PLMS in HF prognosis needs to be determined, these increases may contribute to worsening outcomes in HF patients with CSA treated with ASV.
Keywords: periodic limb movements in sleep, heart failure, adaptive servo-ventilation, central sleep apnea
Periodic limb movements of sleep (PLMS) are described as episodes of nocturnal repetitive limb movements. They have been independently associated with the development of cardiovascular diseases and predict mortality in patients with heart failure (HF) and other cardiovascular diseases.1–5 The clinical significance of PLMS may be more evident in older patients, in whom PLMS are often more prevalent.2, 6 The etiological mechanisms linking PLMS and cardiovascular risk may relate to autonomic deregulation.7–9 Central sleep apnea (CSA) is highly prevalent in patients with HF, affecting 30–40% 10 of this clinical population, in whom it has been associated with an adverse prognosis.11, 12 By providing breath-by-breath adjustment of respiratory pressure support to normalize breathing patterns, adaptive servo-ventilation (ASV) effectively reduces CSA. Prior data have shown PLMS decreased in patients with mild obstructive sleep apnea (OSA) after treatment of continuous positive airway pressure (CPAP), but increased in subjects with severe OSA.13 To our knowledge, no published data are available on the changes in PLMS after treatment of CSA. Considering the high prevalence of PLMS in patients with HF1 and its close association with long-term mortality,4 it may be relevant to determine whether suppression of respiratory events results in heightened PLMS in this group. In the present study we investigated changes in PLMS during ASV titration for CSA in patients with HF.
METHODS
A sample of 14,444 consecutive adult patients who underwent polysomnographic evaluation at the Center for Sleep Medicine at Mayo Clinic, Rochester from January 1st, 2010 to September 30th, 2014 were retrospectively investigated (Figure 1). A total of 579 patients met the criteria for CSA14, 15 during the diagnostic portion of the study. Of these, 422 had split night titration. Since most patients who accepted ASV titration underwent an unsuccessful trial of CPAP before ASV, and since the aim of study was to investigate PLMS changes after ASV, only those subjects who completed the sleep study in the sequence of diagnosis-CPAP-ASV were included (n=314). Patients were excluded if other interventions (e.g., bi-level positive airway pressure) were adopted during the study. Abnormal overnight oximetry (n=154), observed sleep apnea or snoring (n=102), or excessive daytime sleepiness (n=15) were the main reasons for referral for sleep test.
Figure 1. Flow Chart of Study Progress.
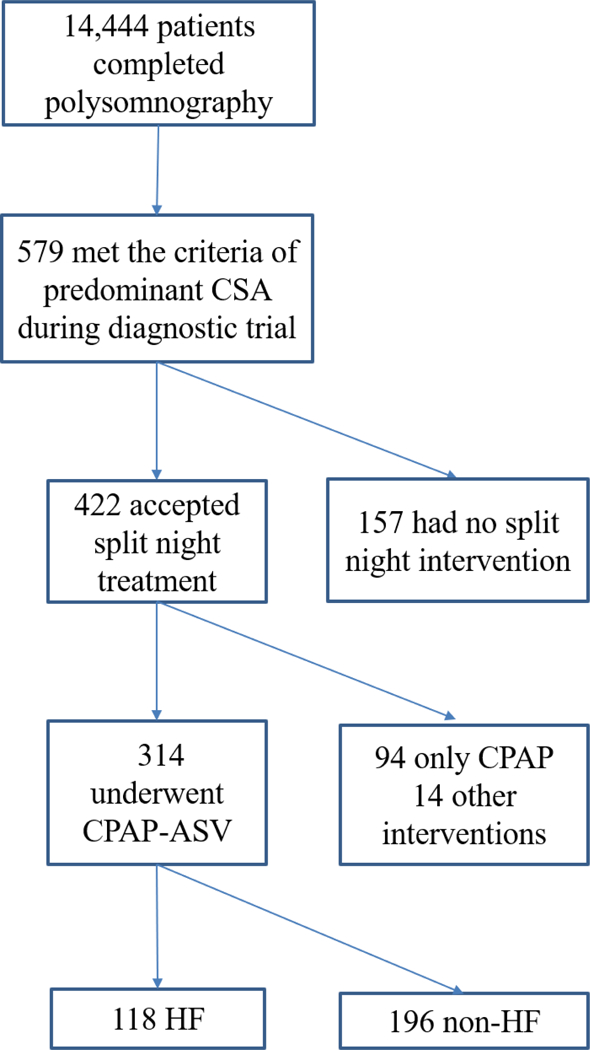
ASV= adaptive servo-ventilation; CPAP = continuous positive airway pressure; CSA = central sleep apnea; HF = heart failure.
All diagnoses made during any clinical visits and inpatient or outpatient procedures in the Mayo Clinic were uniformly recorded and assigned the searchable International Classification of Diseases adaptation codes.2 Clinical information on HF diagnosis (including subjects with no-to-mild symptoms) and comorbidities, demographics and medications were extracted by using an advanced query tool.16 Additionally, a retrospective chart review was conducted manually to validate that the diagnosis of HF was consistent with the guidelines.17 New York Heart Association Functional Classification within 1-year interval before or after polysomnography was noted. This study was approved by the Mayo Clinic Institutional Review Board.
Sleep evaluations were performed at the Mayo Clinic Center for Sleep Medicine, an American Academy of Sleep Medicine-accredited sleep center, using standardized scoring.18, 19 Electroencephalogram, electro-oculogram, electromyogram, electrocardiogram, thoraco-abdominal excursion, pulse oximetry, and oronasal airflow were monitored. Apneas were defined as a ≥90% decrease of airflow for at least 10s, and hypopneas were defined by a ≥30% decline in airflow for at least 10s accompanied by an oxygen desaturation of ≥4%. Apneas without evidence of respiratory effort were scored as central, while those with respiratory effort were categorized as obstructive. Apnea-hypopnea index (AHI) was calculated as the average number of apneas and hypopneas per hour of sleep. Patients with AHI ≥15 events/h were diagnosed with predominant CSA if central apneas accounted for >50% of all breathing events.14,15 Presence of a limb movement was scored as such if its duration was between 0.5 to 10 seconds and there was a >8uV amplitude increase from baseline in a limb electromyogram channel. A limb movement would not be scored if it occurred during a period from 0.5 s preceding an apnea, hypopnea, or respiratory effort-related arousal to 0.5 s following, according to guidelines.21, 22 To be considered periodic, at least 4 limb movements had to occur sequentially, 5 to 90 seconds apart. Periodic limb movement index (PLMI) was the total number of periodic limb movements per hour of sleep. Periodic limb movement arousal index (PLMAI) was the total number of periodic limb movements per hour of sleep in which an arousal was associated with limb movement. Arousals were scored as breathing related arousal index if immediately preceded by any breathing event including respiratory effort-related arousal, while movement-related arousal index referred to the arousals promptly after any limb movement.
A proper nasal or nasal-mouth mask was selected to fit the patients and positive airway pressure titration was completed by sleep technologists. CPAP was started at 4 cm water pressure and increased to control breathing events following recommendations.20 During ASV titration, minimum expiratory positive airway pressure was 4 or 5 cm water pressure; pressure support started at 3 cm of water and was increased to achieve optimal ventilation.
Data are presented as counts (percentage) for categorical variables or median (interquartile range [IQR]: 25th–75th percentile) for continuous variables and compared by groups by Pearson chi-square tests or nonparametric Wilcoxon rank-sum tests, respectively. PLMI and PLMAI changes from the diagnostic portion to the ASV trial were computed, and multivariable logistic regression models were used to explore factors associated with PLMI and PLMAI increases (dependent variables) during ASV titration. Covariables included demographics (age, sex and smoking), comorbidities (hypertension, diabetes, coronary artery disease, cerebrovascular disease and renal disease), and medications (antiplatelets, diuretics, angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers, beta-blockers and statins). Note that the aforementioned medications were included in multivariable model not only for the statistical significance but also for their known beneficial effects on HF syndrome which is closely associated with PLMS prevalence. Comparison of the odds of having increased PLMS during ASV trial was conducted in HF vs. non-HF subjects and in subgroups stratified by median age, since PLMS have been proven to be pathologically associated with adverse events in elderly subjects.2, 6, 21 Univariable and adjusted multivariable odds ratios (OR) are provided with 95% confidence intervals (CI). Analyses were performed using JMP, version 13 (SAS Institute; Cary, North Carolina), and two-way a priori p-values < 0.05 were considered statistically significant.
RESULTS
Of 314 CSA patients included, 118 subjects had a clinical diagnosis of HF. Most HF patients [75 (63.6%)] had New York Heart Association classification I or II at the time of polysomnography, with a median LVEF of 44% (IQR: 35–59 %). Demographic and clinical characteristics are presented in Table 1. HF patients were older, mostly males, with higher prevalence of comorbidities including hypertension, diabetes, coronary artery disease, cerebrovascular disease, and renal disease. Cardioprotective medications were taken more frequently by HF subjects than non-HF subjects.
Table1.
Demographic and Clinical Characteristics of Study Patients with Central Sleep Apnea (n=314)
| Characteristics | Heart Failure |
p-value | |
|---|---|---|---|
| Yes (n=118) | No (n=196) | ||
| Age (years) | 75(64, 80) | 65(53, 73) | <0.001 |
| Men | 109(92.4%) | 155(79.1%) | 0.002 |
| White | 109(92.4%) | 174(88.8%) | 0.301 |
| LVEF (%) | 44(35, 59) | 63(59, 66) | 0.001 |
| Body mass index (kg/m2) | 30(27, 36) | 32(28, 36) | 0.560 |
| Smoking | 82(69.5%) | 109(55.6%) | 0.015 |
| Hypertension | 84(71.2%) | 97(49.5%) | <0.001 |
| Diabetes mellitus | 33(28.0%) | 33(16.8%) | 0.019 |
| CAD | 59(50.0%) | 43(21.9%) | <0.001 |
| Cerebrovascular disease | 22(18.6%) | 16(8.2%) | 0.005 |
| Renal disease | 19(16.1%) | 14(7.1%) | 0.012 |
| Iron deficiency anemia | 14(11.9%) | 17(8.7%) | 0.359 |
| Major depressive disorder | 5(4.2%) | 19(9.7%) | 0.078 |
| Parkinson’s disease | 1(0.9%) | 3(1.5%) | 0.601 |
| Medications | |||
| Antiplatelets | 72(61.0%) | 78(39.8%) | <0.001 |
| Diuretics | 50(42.4%) | 30(15.3%) | <0.001 |
| Calcium channel blockers | 10(8.5%) | 21(10.7%) | 0.519 |
| Angiotensin converting enzyme inhibitors /angiotensin II receptor blockers |
59(50.0%) | 51(26.0%) | <0.001 |
| Beta-blockers | 71(60.2%) | 58(29.6%) | <0.001 |
| Ferritin supplements | 6(5.1%) | 4(2.0%) | 0.137 |
| Dopamine agonists | 1(0.9%) | 9(4.6%) | 0.067 |
| Statins | 67(56.8%) | 74(37.8%) | 0.001 |
Data are median (interquartile range) or counts (percentage). Abbreviations: CAD = Coronary artery diseases of angina and myocardial infarction; HF = heart failure; LVEF = left ventricular ejection fraction.
As shown in Table 2, during the diagnostic portion of the study, HF vs. non-HF patients had significantly higher CSA. Compared with non-HF subjects, HF patients also had higher PLMI and PLMAI. Patients with HF vs. non-HF had higher percentage of subjects with PLMI≥15 and PLMAI≥1. During the ASV trial, both obstructive and central breathing events were reduced significantly in both HF and non-HF patients. Nevertheless, HF patients still exhibited higher median value of PLMI and PLMAI than non-HF subjects. The proportion of patients with PLMI≥15 and PLMAI≥1 remained greater in the HF group compared to the non-HF group. The percentage of movement related arousal index increased in patients with and without HF, and it was substantially higher in HF patients vs. non-HF subjects during the ASV trial. A total of 114(83.2%) patients with severe CSA (AHI ≥30 events/h) had PLMI increase during ASV while only 23(16.8%) patients with moderate CSA (AHI<30 events/h) had increased PLMI(P=0.005).
Table 2.
Sleep Characteristics for Subjects with Central Sleep Apnea during Diagnostic and Adaptive Servo-Ventilation Titration (n=314)
| Heart failure | ASV therapy | |||||
|---|---|---|---|---|---|---|
| Variable | Yes (n=118) |
No (n=196) |
p- value |
With heart failure (n=118) |
Without heart failure (n=196) |
p- value |
| Apnea-hypopnea index (events/h) |
57(42.8, 75.3) |
42.0(25.3, 63.8) |
<0.001 | 5.0(1.0, 14.0) | 5.0(1.0, 15.0) | 0.915 |
| CSA (events/h) | 42.5(25.8, 59.3) |
29.0(18.0, 46.0) |
<0.001 | 0 | 0 | 0.195 |
| Obstructive apnea (events/h) |
1(0, 4) | 1(0, 4) | 0.655 | 0(0, 0) | 0(0, 0) | 0.760 |
| Mixed apnea index (events/h) |
1(0, 4) | 1(0, 3) | 0.196 | 0(0, 0) | 0(0, 0) | 0.007 |
| Hypopnea index (events/h) |
7(3, 12) | 6(3, 10) | 0.399 | 3(0, 9) | 2(0, 8) | 0.407 |
| Respiratory effort-related arousal (events/h) |
2(1, 6) | 5(2, 10) | <0.001 | 2(0, 6) | 2(0, 6) | 0.832 |
| Arousal index (events/h) |
56.0(42.9, 82.8) |
47.5(32.0, 65.4) |
0.001 | 28.8(16.5, 43.0) |
27.4(16.2, 43.8) |
0.823 |
| Percentage of movement related arousal index (%) |
4.0(0, 14.1) |
1.1(0, 13.1) |
0.058 | 24.8(2.3, 53.2) |
2.9(0, 28.4) | <0.001 |
| Percentage of breathing related arousal index (%) |
76.7(58.7, 90.2) |
76.8(62.5, 85.4) |
0.695 | 29.3(5.9, 49.7) |
26.0(9.4, 53.1) |
0.927 |
| Sleep efficiency (%) |
67.0(52.7, 77.0) |
73.9(59.1, 83.6) |
<0.001 | 73.9(54.4, 86.4) |
71.7(53.8, 85.3) |
0.357 |
| Percentage of N1 sleep (%) |
36.3(20.4, 55.5) |
23.8(14.7, 39.9) |
<0.001 | 14.3(6.7, 27.2) |
16.5(8.6, 29.2) |
0.260 |
| Percentage of N2 sleep (%) |
40.6(27.7, 54.7) |
44.6(34.4, 59.4) |
0.036 | 42.6(30.3, 57.0) |
43.0(31.8, 59.5) |
0.434 |
| Percentage of N3 sleep (%) |
4.9(0, 16.1) |
10.3(0, 22.9) |
0.004 | 11.6(0, 25.1) | 6.4(0, 22.8) | 0.048 |
| Percentage of rapid eye movement sleep (%) |
8.7(0, 16.1) |
8.8(0, 14.7) |
0.959 | 19.5(7.9, 30.5) |
17.7(9.0, 25.8) |
0.519 |
| Oxygen saturation during sleep (%) |
93(92, 94) | 93(92, 94) | 0.062 | 94(93, 95) | 94(93, 95) | 0.311 |
| PLMI (events/h) | 33.7(2.2, 80.5) |
6.1(0, 35.9) |
<0.001 | 32.2(6.8, 86.3) |
7.1(0, 36.9) | <0.001 |
| PLMAI (events/h) | 2.1(0, 8.3) | 0.5(0, 5.6) | 0.028 | 5.1(0.6, 13.1) | 0.5(0, 7.7) | <0.001 |
| PLMI ≥15 | 74(62.7%) | 74(37.8%) | <0.001 | 78(66.1%) | 81(41.3%) | <0.001 |
| PLMAI ≥1 | 71(60.2%) | 91(46.4%) | 0.018 | 84(71.2%) | 93(47.5%) | <0.001 |
P-values refer to between-group differences in HF and non-HF patients. Data are median (interquartile range ) or counts (percentage). Abbreviations: ASV = adaptive servo-ventilation; CSA=central sleep apnea; PLMI = periodic limb movement index; PLMAI = periodic limb movement arousal index
Compared with the diagnostic portion, during ASV PLMI increased in 51.7% of HF patients, in contrast to 38.8% of non-HF patients (Chi-Square=4.0, p=0.025). Similarly, a higher proportion of HF patients had PLMAI increases in comparison to non-HF subjects (67[56.8%] vs. 75[38.3%], Chi-Square=10.2, p=0.001). Patients who experienced PLMI increase during ASV had higher baseline AHI in diagnostic test than those who demonstrated PLMI decrease(57[39, 77] events/h vs. 40[26, 54] events/h, p<0.001). In crude logistic analysis (Figure 2), HF was associated with increased PLMI and PLMAI from diagnostic to ASV trial (PLMI: odds ratio =1.69, 95% CI 1.07–2.68, p=0.026; PLMAI: odds ratio =2.12, 95% CI 1.33–3.37, p=0.002). In multivariable logistic analysis, HF remained associated with PLMAI increases (odds ratio =1.79, 95% CI 1.05–3.07, p=0.032) after adjusting for prespecified covariates. Similar results (odds ratio =1.70, 95% CI 1.04–2.79, p=0.035) were obtained after further adjustment for clinical variables that differed between patients with and without increment in PLMAI during ASV titration (i.e., diagnosis of diabetes and use of angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers and beta-blockers). None of the covariates was significantly associated with either PLMI or PLMAI increases.
Figure 2. Odds Ratio (95% CI) of Increased PLMS Indexes during ASV Trial in HF vs. Non-HF patients.
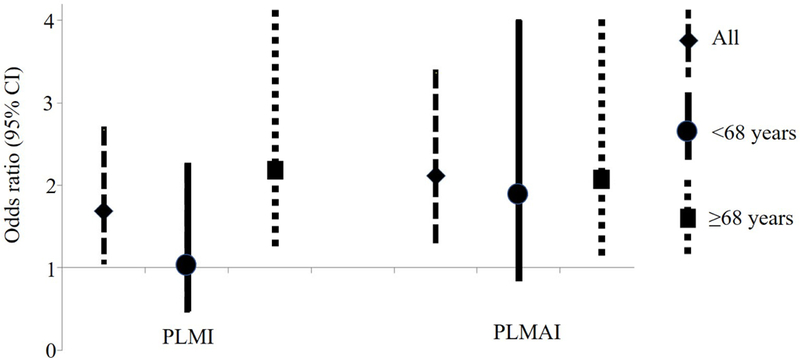
In crude logistic analysis, HF was associated with increased PLMI and PLMAI from diagnostic to ASV trial in all subjects. Stratified analysis by median age showed that the associations were statistically significantly only in those subjects ≥68 years old.
ASV = adaptive servo-ventilation; HF=heart failure; PLMI = periodic limb movement index; PLMAI = periodic limb movement arousal index; PLMS = periodic limb movements in sleep
In a subgroup of patients older than the median age of 68 years-old, HF vs. non-HF subjects had higher odds of increased PLMI during the ASV trial compared with the diagnostic portion of the study in both unadjusted (odds ratio =2.16, 95% CI 1.16–4.05, p=0.016) (Figure 2) and adjusted models (odds ratio =2.36, 95% CI 1.11–4.99, p=0.025). Similarly, in those ≥68 years old, HF was associated with PLMAI increase during the ASV trial in univariate analysis (odds ratio =2.05, 95% CI 1.10–3.83, p=0.024), with the significance persisting after taking into account confounding variables (odds ratio =2.33, 95% CI 1.10–4.93, p=0.026). HF patients younger than 68 did not have higher odds of PLMS increment than their non-HF counterparts.
To investigate the potential role of LVEF, subjects were classified into two groups according to their LVEF (≤45% vs. >45%). Patients with LVEF ≤45% vs. those with LVEF >45% had higher median values of PLMI during both diagnostic (36.7 events/h [IQR: 2.1–88.7 events/h] vs. 12.4 events/h [0–56.7 events/h], p=0.020) and ASV titration (38.0 events/h [IQR: 13.4–77.0 events/h] vs. 14.4 events/h [0–55.8 events/h], p=0.001) phases, while PLMAI was higher in those with LVEF ≤45% than in those with LVEF >45% only during the ASV trial (5.7 events/h [IQR: 0.8–17.1 events/h] vs. 2.2 events/h [0–9.7 events/h], p=0.007). In multivariable logistic analysis, patients with LVEF ≤45% had higher odds of increased PLMAI during the ASV trial than their counterparts with LVEF >45% (odds ratio =1.98, 95% CI 1.10–3.55, p=0.022) (Figure 3). Significance persisted after all covariates were adjusted for (odds ratio =1.91, 95% CI 1.01–3.63, p=0.048).
Figure 3. Odds Ratio (95% CI) of Increased PLMS Indexes during ASV Trial in subjects with LVEF ≤45% vs. LVEF >45%.
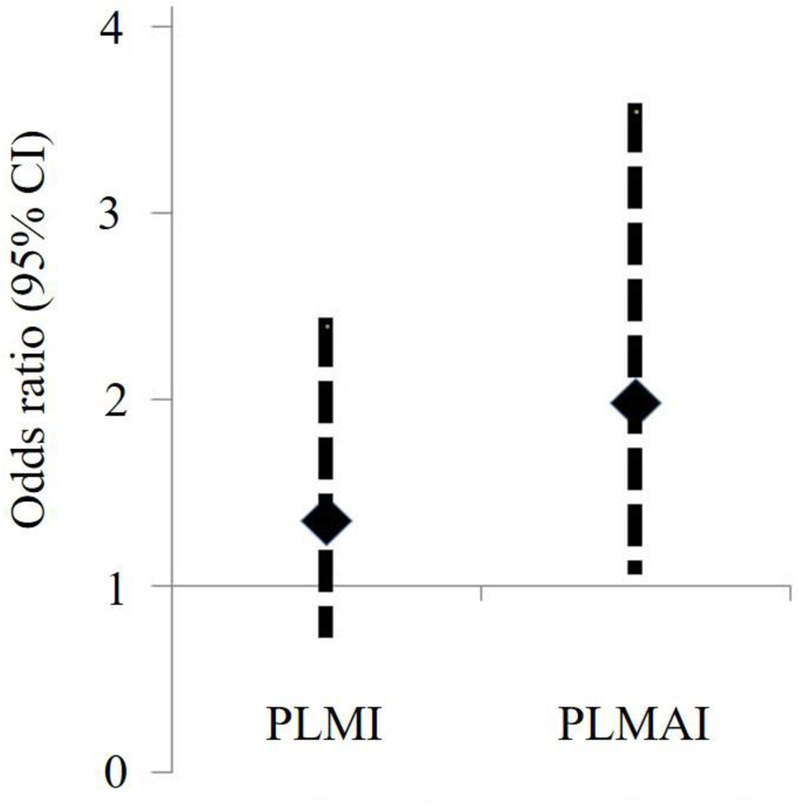
Patients with LVEF ≤45% exhibited higher PLMAI compared to those with LVEF >45%.
ASV = adaptive servo-ventilation; LVEF = left ventricular ejection fraction; PLMI = periodic limb movement index; PLMAI = periodic limb movement arousal index; PLMS = periodic limb movements in sleep
DISCUSSION
Our study has two main novel findings. First, HF patients with CSA manifest more frequent PLMS compared to CSA patients without comorbid HF; and second, PLMS increase significantly during treatment of CSA by ASV. Since PLMS are associated with adverse cardiovascular prognosis,4, 5, 22 the impact of post-ASV PLMS on cardiovascular risk warrants further investigation.
Although only patients with CSA were assessed for the purpose of the study, our results are in line with prior reports showing prevalent PLMS in HF.23 The finding that HF subjects with CSA in our study had worse PLMS than non-HF subjects is supported by the following: (i) HF patients had higher median values of PLMS during both the diagnostic study and the ASV trial; (ii) HF patients presented more PLMS than their non-HF counterparts in spite of higher AHI, which was shown to be inversely associated with PLMI/PLMAI in a previous study;13 (iii) A greater proportion of HF patients had increased post-ASV PLMS than non-HF subjects.
The high prevalence of post-ASV PLMS in CSA patients with HF is consistent with a previous study conducted in non-HF patients titrated on CPAP for moderate-severe OSA.13 Spontaneous PLMS, which may be concealed by frequent breathing events, may be unmasked and become apparent after effective treatment of sleep-disordered breathing, irrespective of its obstructive or central nature, especially among subjects with severe obstructive sleep apnea and CSA. Thus, it is plausible that adequate ASV or CPAP therapy may merely unveil a preexisting sleep phenomenon, rather than increase PLMS in and of itself.
Our study indicates that age may influence the association between HF and post-ASV increase in PLMS. The significant rebound of unmasked PLMS among older HF patients is indeed noteworthy and potentially important, because both we and other investigators have shown that PLMS may be more strongly associated with cardiovascular risk in older patients than in younger ones.2, 6
Another factor likely implicated in the observed associations is LVEF. Patients with LVEF ≤45% exhibited higher PLMS indexes compared to those with LVEF >45%, and low LVEF predicted augmented PLMS following ASV trial independently of conventional covariables. Thus, although speculative it is conceivable that PLMS may be the extraneous variable contributing to the poor outcomes reported by a recent large clinical trial 24 on HF patients with LVEF ≤45% who underwent ASV therapy for comorbid CSA.
PLMS in isolation (i.e., in the absence of symptoms of restless legs) are not typically treated. Despite the relationship of PLMS with impaired cardiovascular function and adverse prognosis, it remains unknown whether treatment of PLMS impacts the cardiovascular risk profile. There is limited evidence that dopaminergic therapy may reduce atrial fibrillation burden,25 normalize the PLMS-related heart rate response,26 and reduce blood pressure,27, 28 though these studies have been conducted in non-HF populations. Finally, it is worth considering that medical optimization of HF, which has been shown to attenuate CSA,29 might also result in a similar improvement in PLMS. Indeed, there is an anecdotal evidence of resolution of PLMS after heart transplant.30 Randomized controlled trials and experimental physiological studies are needed to test such hypotheses.
One of the strengths of our study is the inclusion of consecutive patients who underwent attended polysomnography scored by technicians who were not part of the study team and thus masked to outcomes. Meanwhile, prior to 2007, scoring of PLMS during breathing events was technically at the scorers’ discretion with some practitioners including and others excluding this measure. The standardized approach adopted to score PLMS for our subjects reduces bias and improves generalizability. There are, however, limitations inherent to the retrospective study design. The major shortcoming is the practical split-night titration rather than separate diagnostic and titration studies on different nights. Therefore, any increase in PLMS may be a rebound from PLMS suppression by breathing events in the first part of night, and may not completely reflect a patient’s usual condition. Even so, it does not diminish our finding of a close association of HF with PLMS, since HF subjects always had higher PLMI/PLMAI than their non-HF comparators in any conditions, and more HF patients had increased PLMS during ASV than non-HF patients. Second, given the high percentage of male patients and complex medical conditions of study subjects who were neither simple HF subjects nor healthy controls, but rather with medical concerns requiring referral for sleep study, generalizability of the results may be limited.
In conclusion, PLMS are prevalent in HF patients with CSA. Although treatment with ASV reduces breathing events, a paradoxical increase in PLMS is observed in HF patients, which is particularly more pronounced among elderly subjects and subjects with low LVEF. As PLMS are linked to unfavorable outcomes, these data thus reinforce the current recommendations against the use of ASV therapy in HF patients with low LVEF.
Acknowledgments
Funding
This study was supported by funding from National Institutes of Health grants HL65176 and Beijing Municipal Administration of Hospital Incubating Program PX2018073.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
JX has served as a consultant for ResMed and Philips. He has spoken at meetings sponsored by Philips and ResMed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Gottlieb DJ, Somers VK, Punjabi NM, Winkelman JW. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med 2017;31:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Chahal CAA, Covassin N, Schulte PJ, Singh P, Srivali N, Somers VK, Caples SM. Periodic limb movements of sleep are associated with an increased prevalence of atrial fibrillation in patients with mild sleep-disordered breathing. Int J Cardiol 2017; 241:200–204. [DOI] [PubMed] [Google Scholar]
- 3.Li YP, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation 2012;126:1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yumino D, Wang H, Floras JS, Newton GE, Mak S, Ruttanaumpawan P, Parker JD, Bradley TD. Relation of periodic leg movements during sleep and mortality in patients with systolic heart failure. Am J Cardiol 2011;107:447–451. [DOI] [PubMed] [Google Scholar]
- 5.Yatsu S, Kasai T, Suda S, Matsumoto H, Shiroshita N, Kato M, Kawana F, Murata A, Kato T, Hiki M, Daida H. Impact on clinical outcomes of periodic leg movements during sleep in hospitalized patients following acute decompensated heart failure. Circ J 2017;81:495–500. [DOI] [PubMed] [Google Scholar]
- 6.May AM, Blackwell T, Stone KL, Cawthon PM, Sauer WH, Varosy PD, Redline S, Koo BB, Mehra R; Osteoporotic Fractures in Men (MrOS) Study Group. Longitudinal relationships of periodic limb movements during sleep and incident atrial fibrillation. Sleep Med 2016;25:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophysiol 2005;116:1096–1104. [DOI] [PubMed] [Google Scholar]
- 8.Walter LM, Foster AM, Patterson RR, Anderson V, Davey MJ, Nixon GM, Trinder J, Walker AM, Horne RS. Cardiovascular variability during periodic leg movements in sleep in children. Sleep 2009;32:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep 2007;30:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mookadam F, Calvin AD, Somers VK. Prevalence and management of central sleep apnea in heart failure patients. Curr Heart Fail Rep 2008;5:233–237. [DOI] [PubMed] [Google Scholar]
- 11.Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C, Horstkotte D, Oldenburg O. Cheyne–stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011; 32:61–74. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007;49:2028–2034. [DOI] [PubMed] [Google Scholar]
- 13.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep 2003;26:717–720. [DOI] [PubMed] [Google Scholar]
- 14.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353: 2025–2033. [DOI] [PubMed] [Google Scholar]
- 15.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: A post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial. Circulation 2007;115:3173–3180. [DOI] [PubMed] [Google Scholar]
- 16.Cartee AK, Owens LA, Lahr BD, Yawn BP, Murray JA, Kudva YC. Incidence of type 1 diabetes is not increasing in a population-based cohort in Olmsted county, Minnesota, USA. Mayo Clin Proc 2016;91:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1–e82. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan S. For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications 1st ed Winchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA; Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 21.Winkelman JW, Blackwell T, Stone K, Ancoli-Israel S, Redline S. Associations of incident cardiovascular events with restless legs syndrome and periodic leg movements of sleep in older men, for the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep Study). Sleep 2017;40(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza M, Shen WK, Sofi A, Jahangir A, Mori N, Tajik AJ, Jahangir A. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J Am Soc Echocardiogr 2013;26:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skomro R, Silva R, Alves R, Figueiredo A, Lorenzi-Filho G. The prevalence and significance of periodic leg movements during sleep in patients with congestive heart failure. Sleep Breath 2009;13:43–47. [DOI] [PubMed] [Google Scholar]
- 24.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirza M, Shen WK, Sofi A, Tran C, Jahangir A, Sultan S, Khan U, Viqar M, Cho C, Jahangir A. Frequent periodic leg movement during sleep is an unrecognized risk factor for progression of atrial fibrillation. PLoS One 2013;8:e78359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, Ferini-Strambi L. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med 2011;12:47–55. [DOI] [PubMed] [Google Scholar]
- 27.Bauer A, Cassel W, Benes H, Kesper K, Rye D, Sica D, Winkelman JW, Bauer L, Grieger F, Joeres L, Moran K, Schollmayer E, Whitesides J, Carney HC, Walters AS, Oertel W, Trenkwalder C; SP0977 study investigators. Rotigotine’s effect onPLM-associated blood pressure elevations in restless legs syndrome: An RCT. Neurology 2016;86:1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocchi C, Albanese M, Placidi F, Romigi A, Lauretti B, Marfia GA, Liguori C, Marciani MG, Mercuri NB, Izzi F. Chronic dopaminergic treatment in restless legs syndrome: Does it affect the autonomic nervous system? Sleep Med 2015;16:1071–1076. [DOI] [PubMed] [Google Scholar]
- 29.Tamura A, Kawano Y, Naono S, Kotoku M, Kadota J. Relationship between beta-blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest 2007;131:130–135. [DOI] [PubMed] [Google Scholar]
- 30.Hanly P, Zuberi N. Periodic leg movements during sleep before and after heart transplantation. Sleep 1992;15:489–492. [PubMed] [Google Scholar]


