Abstract
Feeding and swallowing disorders have been described in children with a variety of neurodevelopmental disabilities, including Down syndrome (DS). Abnormal feeding and swallowing can be associated with serious sequellae such as failure to thrive and respiratory complications, including aspiration pneumonia. Incidence of dysphagia in young infants with DS has not previously been reported. To assess the identification and incidence of feeding and swallowing problems in young infants with DS, a retrospective chart review of 174 infants, ages 0 to 6 months was conducted at a single specialty clinic. Fifty-seven percent (100/174) of infants had clinical concerns for feeding and swallowing disorders that warranted referral for Videofluroscopic Swallow Study (VFSS); 96/174 (55%) had some degree of oral and/or pharyngeal phase dysphagia and 69/174 (39%) had dysphagia severe enough to warrant recommendation for alteration of breast milk/formula consistency or non-oral feeds. Infants with certain comorbidities had significant risk for significant dysphagia, including those with functional airway/respiratory abnormalities (OR=7.2). Infants with desaturation with feeds were at dramatically increased risk (OR=15.8). All young infants with DS should be screened clinically for feeding and swallowing concerns. If concerns are identified, consideration should be given to further evaluation with VFSS for identification of dysphagia and additional feeding modifications.
Keywords: Down syndrome, Trisomy 21, dysphagia, respiratory aspiration, failure to thrive, swallow study
Introduction
The majority of individuals with Down syndrome (DS) have 47 chromosomes including an extra copy of human chromosome 21. DS has an incidence of ~1/800 live births [de Graaf et al., 2017], and the phenotypes associated with DS are variable in manifestation and severity. Cognitive impairment is a hallmark of DS, and 40–50% of those with Ts21 have cardiac abnormalities. Essentially all individuals with DS have distinguishing craniofacial features including brachycephaly, midface hypoplasia, flattened face, short nose with a flat nasal bridge, and a small oral cavity [Allanson et al., 1993; Fischer-Brandies et al., 1986; Hennequin et al., 1999; Richtsmeier et al., 2000; Starbuck et al., 2011; Venail et al., 2004].
The risk of orofacial malformations is over five times greater in infants with DS than those without [Cleves et al., 2007]. Oropharyngeal deficits in Trisomy 21 (Ts21) may begin in utero and alter the suckle-swallow movement after birth [Guihard-Costa et al., 2006; Hennequin et al., 1999]. Common consequences of feeding and swallowing abnormalities in DS after birth include delayed suckling, problems with breast and bottle feeding as well as mastication, tendency for the mouth to be open while resting and drooling, and silent aspiration of liquid or semi-liquid food [Hennequin et al., 1999]. Feeding and breathing may be significantly impaired in infants with DS, possibly impacted by low muscle tone, and may continue through adulthood [Hennequin et al., 2005; Shott, 2006; Spender et al., 1996]. Poor chewing, difficulties swallowing, choking and gagging, as well as aspiration are typical feeding and swallowing problems reported in individuals with Ts21 [Cooper-Brown et al., 2008].
Although approximately 25% of adults with DS exhibited some type of feeding problem in a cross-sectional survey of parents of children with DS in France [Hennequin et al., 2000], relatively few studies have examined feeding and swallowing problems and related sequelae within young infant DS populations. Studies of individuals with DS in clinical populations (convenience samples that may not be generalizable to the entire population) ranging from infants to adults found feeding problems in up to 80% of these individuals including delayed feeding milestones, oral motor difficulties, and oral hypersensitivity [Cullen et al., 1981; Field et al., 2003; Hennequin et al., 2000; Pipes and Holm, 1980; Spahis and Wilson, 1999]. Examination of oral and pharyngeal phase swallowing in 19 children with DS (all under 4 years of age, average age 24 months) using an oral and feeding assessment followed by a videofluoroscopic modified barium swallow (VMBS) test identified pharyngeal phase abnormalities in 16 of 19 children and aspiration in 10 of 19 children [Frazier and Friedman, 1996]. In this group, it was determined that the severity of clinically observed oral phase problems did not predict aspiration, and the authors concluded that there was a need to “broaden the assessment of children with Down syndrome to include a look at the pharyngeal phase of their swallow and their oral sensory status.” When pharyngeal dysphagia (PD) was specifically examined in 201 children with DS, 58% of children (ages one month to 16 years, average age at PD diagnosis was 1.69 years) were identified from a clinical setting with clinical evidence of PD that was confirmed by videofluoroscopic swallow studies (VFSS) [O’Neill and Richter, 2013]. The authors concluded from these results that “PD is common, persistent, and should be routinely explored in children with DS.” An examination of oral and pharyngeal dysphagia on 158 children with DS (ages one month to 18 years, average age was 2.10 years) showed that 66% exhibited oral phase dysphagia and 56% of individuals with DS given a VFSS had PD [Jackson et al., 2016]. Additionally, this study found that in this wide age range of children, oral phase dysphagia did not predict PD and that most individuals with DS who aspirated did so silently. They recommended that the “physician threshold for referring children with Down syndrome for swallow studies should be low.” Taken together, these studies indicate that feeding and swallowing abnormalities are common in children with DS and need more detailed examination.
The occurrence of other phenotypes at the same time as feeding and swallowing difficulties (comorbidities) has not been well defined, likely due to small sample sizes and heterogeneous samples. When feeding difficulties were examined with other phenotypes in studies including infants with DS, delayed feeding milestones were correlated with moderate or severe congenital heart disorders and low muscle tone [Cullen et al., 1981]. Others have shown that feeding milestones were influenced by hypotonia, poor suck/swallow coordination, cardiac problems, prematurity and infant fatigue and lethargy [Spahis and Wilson, 1999]. Additionally, a high correlation was found between food refusal or dysphagia and gastroesophageal reflux [Field et al., 2003]. Comorbidities were found in children with PD, but were not analyzed due to the complexity and heterogeneity of the comorbidities [O’Neill and Richter, 2013]. Conversely, other small studies of infants with DS found no correlation between the severity of oral motor difficulties and aspiration [Frazier and Friedman, 1996], no increased incidence of dysphagia or food refusal in children with cardiopulmonary disease [Lewis and Kritzinger, 2004], and no correlations between feeding difficulties and other anomalies [Lewis and Kritzinger, 2004]. Taken together, these varied studies identified feeding and swallowing issues in individuals with DS at all ages, but did not specifically examine enough age-matched infants to consistently and conclusively identify common significant comorbidities and predictive variables that may help identify feeding and swallowing issues in infants with DS.
The present study examined feeding and swallowing difficulties in infants with DS who presented for care to a Down syndrome specialty clinic and examined potential comorbidities that may help identify infants at greater risk for pharyngeal dysphagia.
Method
Participants:
A retrospective chart review was conducted of 178 infants with Down syndrome that were referred to the Down Syndrome Program at Riley Hospital for Children in Indianapolis, IN, a specialty consultation clinic providing multidisciplinary care for children from birth until adulthood. Individuals are referred from throughout the state of Indiana, as well as neighboring states of Ohio, Michigan, Illinois and Kentucky. The clinic provides comprehensive medical, as well as developmental, consultation pertaining to the care of children with DS ranging from birth to 21 years of age. During the study period, a team of three developmental pediatricians, as well as pediatric nurse practitioners, dietitians, social workers and family support personnel staffed the clinic. A pediatric speech-language pathologist was also present for consultation for a portion of the study period. Physicians and nurse practitioners participated in medical assessments, including identifying clinical signs or symptoms of feeding and swallowing disorders (poor growth/failure to thrive [FTT], poor or slow feeding/feeding refusal; coughing or choking with feeds; chronic respiratory concerns; tachypnea or noisy breathing with feeds; desaturation or cyanosis with feeds; or pneumonia). Infants with signs or symptoms of feeding and swallowing disorders (both inpatient and outpatient) underwent Video Fluoroscopic Swallowing Study (VFSS) by a trained, experienced examiner (speech-language pathologist, neonatal nurse practitioner or developmental pediatrician with expertise in feeding issues), and results were independently reviewed and reported by a staff radiologist. Pulse oximetry was used during the VFSS to document presence or absence of oxygen desaturation with feeds, as per institutional standard care at the time this study data was collected.
Chart review:
The criteria for selection for this study were that the infant was diagnosed with DS, received care between August 2005 to June 2010, and during that time was between birth and 6 months (corrected for prematurity, if indicated). All charts from infants that were seen in the clinic during this time that met this criteria were included in the chart review. Four of the 178 charts were eliminated because of insufficient information, and data from 174 infants were included in this study. Data were collected from the hospital records and clinic charts including a wide range of clinical data and the de-identified data was entered into an Excel spreadsheet. Authors NS, NB, and SBJ who were trained and overseen by CD and MAS obtained data. Each individual overlapped with another individual in collecting data from the same chart on ~10% of the charts to assure that each person collected the same data. The majority of the data collected were qualitative and not subject to interpretation, and any questions were referred to CD or MAS. Data collection included basic demographic information including race and gender, gestational age at birth, and growth status along with information regarding a wide range of medical issues and comorbidities including cardiac, pulmonary, endocrine, gastrointestinal (GI), developmental, and neurologic. Specific information collected on feeding and swallowing included route of feeding (breast, bottle, nasogastric or gastrostomy tube), type of feed (breast milk or formula), and alterations in feeding (thickened liquid or increased caloric intake), oromotor dysfunction, desaturation, tachypnea, feeding refusal, nasopharyngeal regurgitation and pharyngeal pooling. For the feeding evaluation, aspiration (with thin, thick [nectar], thick [honey], or puree feeds), and penetration (with thin, thick [nectar], thick [honey], or puree feeds) were noted. Institutional review board approval was obtained for this study from Indiana University-Purdue University Indianapolis (IUPUI) and Clarian Institutional Review Boards.
Statistical analysis:
Basic descriptive and bivariate statistics with odds ratios and confidence intervals were calculated using IBM SPSS (Armonk, New York) version 20. An alpha of 0.05 was used. Infants with oral and/or pharyngeal phase dysphagia with alterations in feeding strategies compared to those without these parameters were stratified on the comorbidities of interest.
Results
The 174 infants with DS included in this study were 0–6 months of age, with an average gestational age at birth of 37.2 weeks. Age was corrected for prematurity, if indicated. The average birth weight was 2978 g, 52% of the infants were male, and ethnicity was 68% White/non-Hispanic; 9% Black; 7% Hispanic; 5% Asian; and 11% unknown/not documented. Karyotypes showed Down syndrome was caused by trisomy 21 (93%), Robertsonian translocation (4%), mosaic trisomy 21 (1.5%) and unknown (1.5%, i.e. karyotype report not available in medical record due to being performed at outside institution). Patients were referred from both inpatient and outpatient settings. Infants in this study presented with a variety of medical comorbidities including cardiac, neurological, gastrointestinal, feeding, pulmonary, auditory, and endocrine abnormalities.
Based on clinical concerns indicative of feeding and swallowing disorders observed by physicians and nurse practitioners in the Down Syndrome Clinic (slow growth/FTT, slow feeding/feeding refusal, coughing or choking with feeds, chronic respiratory problems including pneumonia, noisy breathing with feeds, or oxygen desaturation or cyanosis with feeds), it was determined that 100/174 infants warranted referral for a Videoflouroscopic Swallowing Study (VFSS). Three individuals that were referred for a VFSS also had a tracheostomy and one was chronically ventilator dependent. VFSS results indicated that 96/100 of the infants referred for the study (55% of the total group of infants) had some degree of oral (weak, inefficient or uncoordinated suck) or pharyngeal (pooling, penetration or aspiration) phase dysphagia. Of those studied, 69/100 or 69% (39% of the total group), had dysphagia severe enough to warrant recommendation for alteration of the consistency of breast milk or formula or non-oral feeds.
Other variables that may have contributed to the oral and/or pharyngeal phase dysphagia with required alterations in feeding strategies were also evaluated. These included respiratory issues such as dynamic upper airway obstruction as revealed by a bronchoscopy and/or abnormal sleep study (NPSG or OPSG); wheezing or reactive airway disease (RAD) requiring treatment with bronchodilators; chronic inhaled steroid use; history of pneumonia; or oxygen requirement. Additionally, desaturation with feedings, weight at birth (below 10% of CDC defined weight percentile was considered underweight for this study), prematurity and severe congenital heart disease were evaluated (Table 1). Severe congenital heart disease for the purpose of this study was defined as structural congenital heart disease requiring medication treatment (diuretics or digoxin) and/or requiring surgical intervention in the first 6 months of life. This analysis revealed that infants with desaturation with feedings (OR=15.8), and infants with functional airway/respiratory anomalies (OR=7.2) had a significantly increased risk for oral and/or pharyngeal phase dysphagia that required alterations in feeding strategies. Infants that were underweight (OR=2.4) and premature infants (OR=1.7) displayed an increased risk for dysphagia. Infants with severe CHD were not at a higher risk for dysphagia (OR=0.5) (Figure 1).
Table 1.
Prevalence of Comorbidities and Number of Videofluoroscopic Swallow Studies (VFSS) Performed in Infants with Down Syndrome
| Total (% of 174) |
Completed VFSS (N=100) |
Abnormal VFSS (N=96) |
Abnormal VFSS and required alteration of feeding (N=69) |
Infants without VFSS (N=74) |
|
|---|---|---|---|---|---|
| Respiratory Abnormalities | n=63 (36%) |
55 | 51 | 41 | 8 |
| Desaturation with feeds | n=41 (24%) |
41 | 39 | 33 | 0 |
| Severe CHD | n=40 (23%) |
29 | 27 | 18 | 11 |
| Prematurity | n=37 (21%) |
25 | 25 | 20 | 12 |
| Underweight | n=25 (14%) |
14 | 14 | 9 | 11 |
Figure 1.
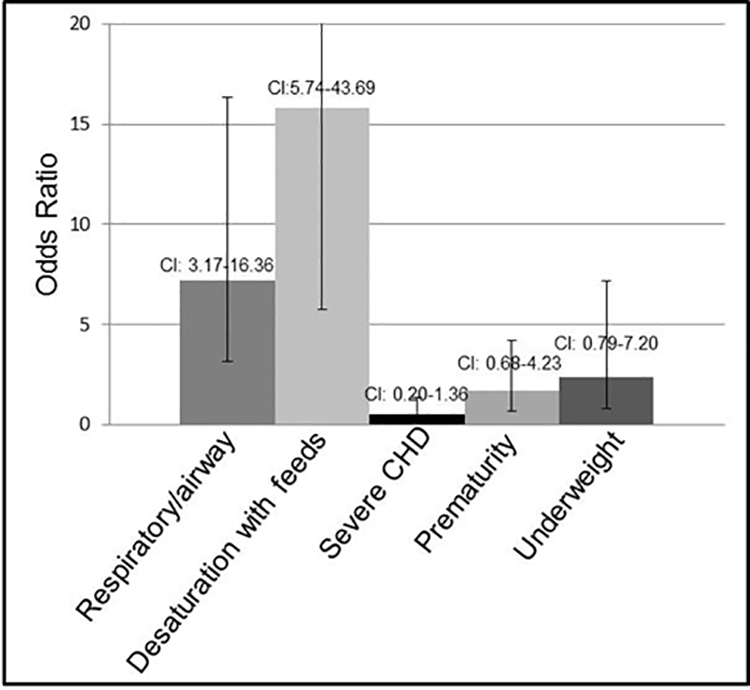
Odds ratios of traits comorbid with oral and/or pharyngeal phase dysphagia with alterations in feeding strategies in infants with DS. A significant risk for oral and/or pharyngeal phase dysphagia requiring alterations in feeding strategies was seen with respiratory/airway abnormalities and desaturation with feeds (p<0.0001). An increased risk of dysphagia was found in infants who were premature or underweight. No significant increased risk was seen with severe CHD. Confidence intervals (CI) are listed above each category.
Discussion
Over 50% of infants with DS in this study from a DS specialty clinic had a clinical concern that indicated a possible problem with feeding and swallowing. VFSS indicated that 96% percent of these infants with a clinical feeding and/or swallowing concern had some manifestation of oral and/or pharyngeal phase dysphagia. VFSS findings suggesting increased safety with altered feeding consistency or route in 69% of those examined with VFSS. It is noted that for this study, alterations of breast milk or formula consistency or recommendation for non-oral feeds documentation was collected, but recommendations related to changes in nipple type or flow or recommendations for other feeding strategies, such as change in positioning or pacing of feeds, were not included in the information gathered. This is important, given variations in institutional practice and evolving practices related to interventions that have occurred over time. Given that this study was focused on identification of dysphagia, rather than specific interventions, the actual interventions are only relevant in that some of the 31/100 infants with oropharyngeal dysphagia that did not require alteration of feeding consistency or non-oral feeds may still have required interventions to support safe oral feeding.
Because the data was taken from a large tertiary center that is partially comprised of a referral population, there was the potential for higher clinical severity in the population studied, leading to a possible overestimate of the incidence of feeding or swallowing disorders and therefore dysphagia. Interestingly, however, comparable to the 40–50% incidence of CHD found in other studies, CHD was observed in 41% of the infants in this study, suggesting a representative population that was not skewed toward higher severity. Our finding of 55% of infants with dysphagia agrees with three large studies in clinical settings that found 57% (≤ 2 years), 58% (0–17 years) and 56% (0–18 years) incidence of feeding problems and dysphagia, in children with DS [Jackson et al., 2016; O’Neill and Richter, 2013; Spahis and Wilson, 1999].
This study may, however, underestimate the incidence of dysphagia in infants with DS. Patients identified by this study as having feeding and swallowing disorders include only those identified by physicians or nurse practitioners with clinical concerns for feeding or swallowing disorders who additionally underwent VFSS. Not included are patients for whom clinical concern for feeding and/or swallowing was identified, but VFSS was not completed due to parent preference, or other reasons (number unknown). Additionally, the decision for VFSS was based entirely on clinical judgment. Although poor growth, feeding, and respiratory issues were used clinically as indication for study, not all patients with these risk factors were studied; nor did all patients recommended for VFSS complete the study. Also, an additional 7% of the infants in this study received non-oral feeding without having a VFSS, based presumably on clinical judgment of the child’s medical care team. Thus, the reported incidence of feeding and swallowing disorders in infants with DS in this study may underestimate the actual incidence.
Two previous studies of feeding and swallowing difficulties in children with DS found no correlation between oral phase dysphagia and aspiration [Frazier and Friedman, 1996], or oral and pharyngeal phase dysphagia [Jackson et al., 2016]. While oral and pharyngeal phase dysphagia were not specifically divided in the current study, we found that 96% of infants with clinical signs and symptoms of feeding and swallowing disorders (poor growth/failure to thrive [FTT], poor or slow feeding/feeding refusal; coughing or choking with feeds; chronic respiratory concerns; tachypnea or noisy breathing with feeds; desaturation or cyanosis with feeds; or pneumonia) had an abnormal VFSS that was characterized as oral and/or pharyngeal dysphasia. Differences between this and previous studies include analysis of a large population of infants ages 0–6 months, and a possible different clinically defined threshold for identification of potential feeding and swallowing difficulties in infants with DS.
We examined the prevalence of respiratory abnormalities, desaturation with feeds, severe CHD, prematurity and weight at birth as possible comorbid conditions of a large number of 0–6 month old infants with DS with dysphagia. Previous reports indicated a correlation of delays in feeding milestones in children with DS with moderate to severe heart disease (n = 114, 6–36 months) [Cullen et al., 1981], and feeding difficulties in children with DS (n = 20, 12–48 months) were correlated with exhaustion (associated with heart defects and low muscle tone) and aspiration [Lewis and Kritzinger, 2004]. Aspiration was also listed as a concern for children with DS (n = 19, 3–36 months) with feeding and swallowing problems [Frazier and Friedman, 1996]. Cardiac abnormalities were implicated in feeding difficulties of infants with DS (n=216, less than 24 months) [Spahis and Wilson, 1999]. In contrast, cardiac problems were not correlated with feeding difficulties in other studies of 204 (1–39 years) [Hennequin et al., 2000], 22 (1 month-12 years) [Field et al., 2003], or 403 (infant) individuals with DS [Ergaz-Shaltiel et al., 2017]. Our retrospective study of 174 infants with DS 0–6 month of age found that those infants with desaturation with feedings and functional airway/respiratory anomalies were at a significantly higher risk for oral and/or pharyngeal phase dysphagia that required alterations in feeding strategies. Infants with DS who were underweight or premature were at increased risk for dysphagia. We found that severe CHD did not provide a significant increased risk for dysphagia. However, there were eight patients with severe CHD and one additional individual with (non-severe) CHD who had non-oral feedings without documentation of VFSS, possibly underestimating risk for this group. Our large number of infants with DS at a similar time point likely allowed us to identify significant comorbidities related to dysphagia at an early developmental stage, whereas other studies with more scattered ages of individuals with DS were not able to identify specific comorbidities.
The current findings provide additional support for the 2011 (reaffirmed in 2018; see: http://pediatrics.aappublications.org/content/141/5/e20180518) AAP Health Supervision for Children with Down Syndrome recommendation that “all infants with clinical signs for feeding abnormalities be given a radiographic swallowing assessment” [Bull and Committee on, 2011]. The current results indicate that infants with DS who have functional airway/respiratory anomalies, or had desaturation with feedings were at a significant risk for dysphagia. Other abnormalities including slow feeding, choking with feeds, chronic aspiration, marked hypotonia or an unexpected failure to thrive may also be indicative of the need for a radiographic swallowing assessment [Bull and Committee on, 2011; McDowell and Craven, 2011]. A study of speech language pathologists found that feeding was examined in ~35% of 0–3 year old individuals with DS [Meyer et al., 2017]. Both feeding and swallowing management were among the top five areas that speech language pathologists wished they had more time to address in 0–3 year old individuals with DS. Many have advocated for a low threshold of clinical signs to examine feeding and swallowing in children with DS [Bassett and Musso, 2017; Jackson et al., 2016]. Taken together, prior studies and current data from this retrospective study suggest the need for a prospective controlled longitudinal study of dysphagia in infants and children with DS to identify measures that can be taken in infants with DS that will improve feeding and swallowing abnormalities with increased age. Overall, the current results indicate a need for a heightened awareness and examination for feeding and swallowing abnormalities in infants with DS and support clinical screening of all young infants with DS.
Conclusions
Infants with DS presented with a high incidence of clinical concerns for feeding or swallowing abnormalities exhibited a high incidence of dysphagia that was confirmed by VFSS. Of those displaying oral and/or pharyngeal phase dysphagia, the majority merited recommendations for alterations in feeding protocols. Infants with desaturation with feedings and respiratory/airway anomalies had a significant risk for feeding and swallowing disorders. Infants with DS who were premature or underweight had an increased risk for dysphagia. These data support the clinical screening of all infants with DS for feeding and swallowing abnormalities and evaluation with appropriate studies as indicated.
Acknowledgements
This study received financial support from NIH Grant DE021034, NSF Grant DGE0742475 and IUPUI Undergraduate Research Opportunity Grants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the National Institutes of Health. The funders had no involvement of the in study design, data collection, data analysis, manuscript preparation and /or publication decisions. The authors declare no conflict of interest.
Grant numbers: NIH Grant DE021034, NSF Grant DGE0742475 and IUPUI Undergraduate Research Opportunity Grants.
Abbreviations:
- CHD:
congenital heart disease
- DS:
Down syndrome
- OR:
odds ratio
- PD:
Pharyngeal Dysphagia
- Ts21:
Trisomy 21
- VFSS:
Videofluroscopic Swallow Study
References
- Allanson JE, O’Hara P, Farkas LG, Nair RC. 1993. Anthropometric craniofacial pattern profiles in Down syndrome. Am J Med Genet 47:748–752. [DOI] [PubMed] [Google Scholar]
- Bassett EC, Musso MF. 2017. Otolaryngologic management of Down syndrome patients: what is new? Curr Opin Otolaryngol Head Neck Surg 25:493–497. [DOI] [PubMed] [Google Scholar]
- Bull MJ, Committee on G. 2011. Health supervision for children with Down syndrome. Pediatrics 128:393–406. [DOI] [PubMed] [Google Scholar]
- Cleves MA, Hobbs CA, Cleves PA, Tilford JM, Bird TM, Robbins JM. 2007. Congenital defects among liveborn infants with Down syndrome. Birth Defects Res A Clin Mol Teratol 79:657–663. [DOI] [PubMed] [Google Scholar]
- Cooper-Brown L, Copeland S, Dailey S, Downey D, Petersen MC, Stimson C, Van Dyke DC. 2008. Feeding and swallowing dysfunction in genetic syndromes. Dev Disabil Res Rev 14:147–157. [DOI] [PubMed] [Google Scholar]
- Cullen SM, Cronk CE, Pueschel SM, Schnell RR, Reed RB. 1981. Social development and feeding milestones of young Down syndrome children. Am J Ment Defic 85:410–415. [PubMed] [Google Scholar]
- de Graaf G, Buckley F, Skotko BG. 2017. Estimation of the number of people with Down syndrome in the United States. Genetics in medicine : official journal of the American College of Medical Genetics 19:439–447. [DOI] [PubMed] [Google Scholar]
- Ergaz-Shaltiel Z, Engel O, Erlichman I, Naveh Y, Schimmel MS, Tenenbaum A. 2017. Neonatal characteristics and perinatal complications in neonates with Down syndrome. Am J Med Genet A 173:1279–1286. [DOI] [PubMed] [Google Scholar]
- Field D, Garland M, Williams K. 2003. Correlates of specific childhood feeding problems. J Paediatr Child Health 39:299–304. [DOI] [PubMed] [Google Scholar]
- Fischer-Brandies H, Schmid RG, Fischer-Brandies E. 1986. Craniofacial development in patients with Down’s syndrome from birth to 14 years of age. Eur J Orthod 8:35–42. [DOI] [PubMed] [Google Scholar]
- Frazier JB, Friedman B. 1996. Swallow function in children with Down syndrome: a retrospective study. Dev Med Child Neurol 38:695–703. [DOI] [PubMed] [Google Scholar]
- Guihard-Costa AM, Khung S, Delbecque K, Menez F, Delezoide AL. 2006. Biometry of face and brain in fetuses with trisomy 21. Pediatr Res 59:33–38. [DOI] [PubMed] [Google Scholar]
- Hennequin M, Allison PJ, Faulks D, Orliaguet T, Feine J. 2005. Chewing indicators between adults with Down syndrome and controls. J Dent Res 84:1057–1061. [DOI] [PubMed] [Google Scholar]
- Hennequin M, Allison PJ, Veyrune JL. 2000. Prevalence of oral health problems in a group of individuals with Down syndrome in France. Dev Med Child Neurol 42:691–698. [DOI] [PubMed] [Google Scholar]
- Hennequin M, Faulks D, Veyrune JL, Bourdiol P. 1999. Significance of oral health in persons with Down syndrome: a literature review. Dev Med Child Neurol 41:275–283. [DOI] [PubMed] [Google Scholar]
- Jackson A, Maybee J, Moran MK, Wolter-Warmerdam K, Hickey F. 2016. Clinical Characteristics of Dysphagia in Children with Down Syndrome. Dysphagia 31:663–671. [DOI] [PubMed] [Google Scholar]
- Lewis E, Kritzinger A. 2004. Parental experiences of feeding problems in their infants with Down syndrome. Downs Syndr Res Pract 9:45–52. [DOI] [PubMed] [Google Scholar]
- McDowell KM, Craven DI. 2011. Pulmonary complications of Down syndrome during childhood. J Pediatr 158:319–325. [DOI] [PubMed] [Google Scholar]
- Meyer C, Theodoros D, Hickson L. 2017. Management of swallowing and communication difficulties in Down syndrome: A survey of speech-language pathologists. Int J Speech Lang Pathol 19:87–98. [DOI] [PubMed] [Google Scholar]
- O’Neill AC, Richter GT. 2013. Pharyngeal dysphagia in children with Down syndrome. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 149:146–150. [DOI] [PubMed] [Google Scholar]
- Pipes PL, Holm VA. 1980. Feeding children with Down’s syndrome. Journal of the American Dietetic Association 77:277–282. [PubMed] [Google Scholar]
- Richtsmeier JT, Baxter LL, Reeves RH. 2000. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn 217:137–145. [DOI] [PubMed] [Google Scholar]
- Shott SR. 2006. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet 142:131–140. [DOI] [PubMed] [Google Scholar]
- Spahis JK, Wilson GN. 1999. Down syndrome: perinatal complications and counseling experiences in 216 patients. Am J Med Genet 89:96–99. [PubMed] [Google Scholar]
- Spender Q, Stein A, Dennis J, Reilly S, Percy E, Cave D. 1996. An exploration of feeding difficulties in children with Down syndrome. Dev Med Child Neurol 38:681–694. [DOI] [PubMed] [Google Scholar]
- Starbuck J, Reeves RH, Richtsmeier J. 2011. Morphological integration of soft-tissue facial morphology in Down Syndrome and siblings. Am J Phys Anthropol 146:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venail F, Gardiner Q, Mondain M. 2004. ENT and speech disorders in children with Down’s syndrome: an overview of pathophysiology, clinical features, treatments, and current management. Clin Pediatr (Phila) 43:783–791. [DOI] [PubMed] [Google Scholar]


