Abstract
Background
Adenine phosphoribosyltransferase (APRT) deficiency is a hereditary purine metabolism disorder causing kidney stones and chronic kidney disease (CKD). The purpose of the study was to examine the disease course in patients who presented in childhood.
Methods
The disease course of 21 (35%) patients in the APRT Deficiency Registry of the Rare Kidney Stone Consortium, who presented with manifestations and/or were diagnosed with APRT deficiency before age 18 years, was studied. The effect of pharmacotherapy on renal manifestations and outcomes was thoroughly assessed.
Results
Fourteen children were placed on allopurinol, 100 (25–200) mg/day, at the age of 2.6 (0.6–16.5) years. Six of these patients had experienced kidney stone events and 3 patients had developed acute kidney injury (AKI) prior to allopurinol treatment. During 18.9 (1.7–31.5) years of pharmacotherapy, stones occurred in 2 patients and AKI in 3. Six adult patients started allopurinol treatment, 200 (100–300) mg/day, at age 29.8 (20.5–42.4) years. Five of these patients had experienced 28 stone episodes and AKI had occurred in 2. Stone recurrence occurred in 4 patients and AKI in 2 during 11.2 (4.2–19.6) years on allopurinol therapy. Lack of adherence and insufficient dosing contributed to stone recurrence and AKI during pharmacotherapy. At latest follow-up, estimated glomerular filtration rate (eGFR) was 114 (70–163) and 62 (10–103) mL/min/1.73 m2 in those who initiated treatment as children and adults, respectively. All 3 patients with CKD stages 3–5 at last follow-up were adults when pharmacotherapy was initiated.
Conclusion
Timely diagnosis and treatment of APRT deficiency decreases renal complications and preserves kidney function.
Keywords: Kidney stones, nephrolithiasis, chronic kidney disease, kidney failure, crystal nephropathy, kidney transplantation, allopurinol treatment, children
Introduction
Adenine phosphoribosyltransferase (APRT) deficiency (OMIM 102600) is a rare autosomal recessive disorder of adenine metabolism that leads to kidney stones and chronic kidney disease (CKD) [1–3]. In the absence of APRT activity, adenine is converted by xanthine oxidoreductase (XOR; xanthine dehydrogenase/oxidase) to the poorly soluble 2,8-dihydroxyadenine (DHA) which is excreted in the urine in excessive amounts. Affected individuals develop kidney stones and/or progressive chronic kidney disease (CKD) due to DHA crystal nephropathy [2, 3]. At least 20% of reported adult patients develop end-stage renal disease (ESRD), most commonly in the fifth decade of life [3].
Radiolucent kidney stones are by far the most commonly reported childhood manifestation of APRT deficiency [1, 4]. Other well-known clinical features in children include reddish-brown diaper stains in young children, acute kidney injury (AKI) due to bilateral obstructive DHA calculi, recurrent urinary tract infections and hematuria [1, 2, 5–7]. Many individuals remain asymptomatic for years or even decades and, not uncommonly, the disease is recognized by family screening or incidentally when DHA crystals (Figure 1) are detected on routine urine microscopy [1, 4]. The diagnosis is confirmed by absent APRT enzyme activity in red cell lysates and/or the identification of biallelic pathogenic variants in the APRT gene [8].
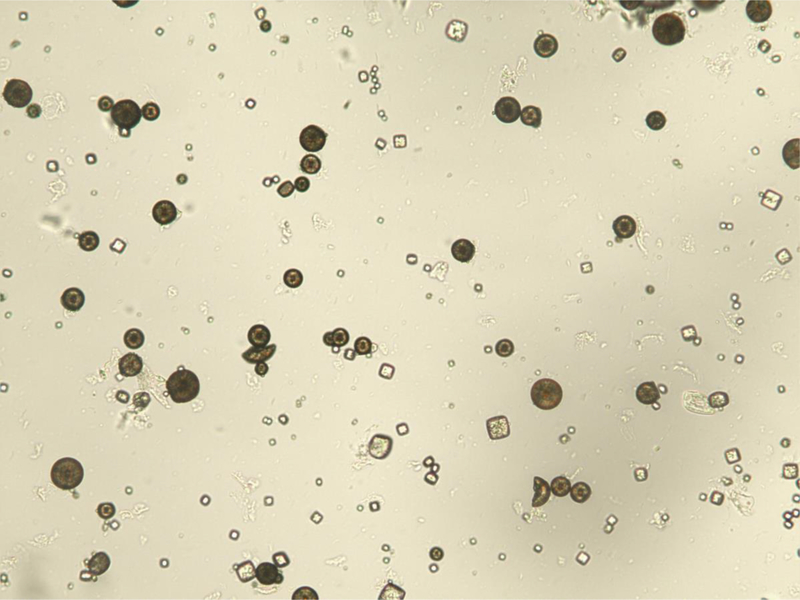
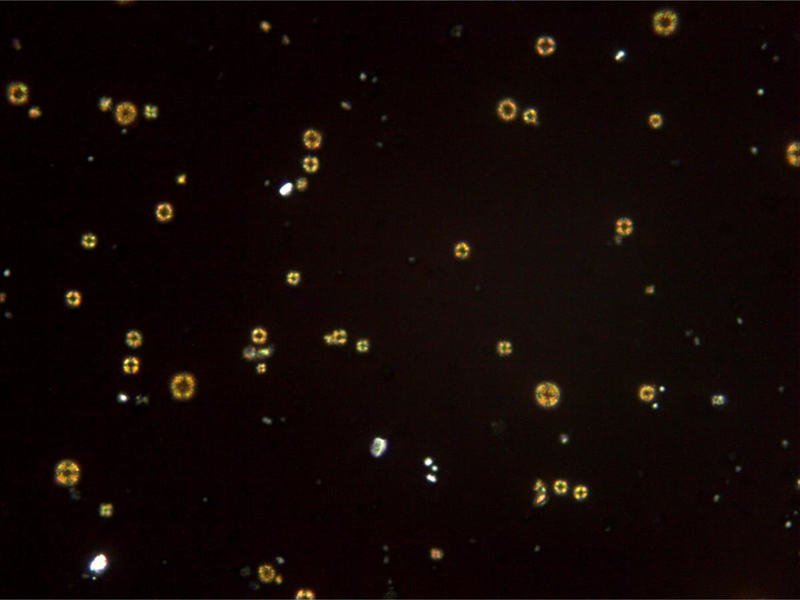
Figure 1.
Urinary 2,8-dihydroxyadenine crystals. (A) The characteristic medium-sized crystals are brown with a dark outline and central spicules. (Original magnification x 400). (B) The same field viewed with polarized light microscopy shows that the small- and medium-sized crystals appear yellow in color and produce a central Maltese cross pattern. (Original magnification x 400)
The outcome of kidney disease in APRT deficient patients who from early childhood have received treatment with an XOR inhibitor, mostly allopurinol and more recently febuxostat, appears to be favorable [2, 3]. To date, only one study has been published focusing on the disease presentation in children [4] and limited data exist on the long-term evolution of kidney function in treated and untreated individuals [3]. The aim of the present study was to compare the disease course in patients with APRT deficiency who initiated XOR inhibitor treatment prior to the age of 18 years with those who did not receive pharmacologic therapy until adulthood.
Methods
Ethical approval
The study was approved by the National Bioethics Committee of Iceland (NBC 09–072) and the Icelandic Data Protection Authority. All living patients or their legal guardians gave a written informed consent for participation in the study. The clinical and research activities reported herein are consistent with the Principles of the Declaration of Helsinki.
Study design
Description of registry data and definitions, which have previously been described by our group [3], are briefly outlined below. The present study is based on extensive observational data from patients in the APRT Deficiency Registry of the Rare Kidney Stone Consortium (RKSC, http://www.rarekidneystones.org/). Included in the current study were 21 (35%) of the 60 enrolled patients, who all presented with clinical manifestations of the disorder or were diagnosed before the age of 18 years. The latest follow-up was on December 31, 2017. Limited data on 20 of the 21 patients have previously been reported [3].
Clinical data
The registry data include age at initial disease presentation and at diagnosis; clinical manifestations; laboratory tests including serum creatinine (SCr) values, results of urine microscopy, including assessment of DHA crystalluria, APRT enzyme activity, APRT genotype, imaging studies, kidney biopsies and kidney stone analyses; XOR inhibitor treatment, surgical management of kidney stones and renal replacement therapy (RRT); and causes of death. The availability of multiple SCr measurements for most patients allowed accurate ascertainment of AKI episodes, CKD staging and characterization of changes in kidney function over time.
Definitions
Symptomatic kidney stones were defined as either a patient-reported stone passage or abdominal pain associated with hematuria and/or a urinary tract stone confirmed with an imaging study. Kidney stones identified by imaging only were considered asymptomatic. Stone recurrence was defined as the detection of a stone in a patient with a history of kidney stones who has previously been shown to be stone-free by medical imaging. Glomerular filtration rate estimates (eGFR) were derived from SCr using the modified Schwartz or Chronic Kidney Disease in Children (CKiD) equation [9] and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in adults [10]. For calculation of eGFR in children, height measurements were either obtained from the registry or extrapolated from data points on individual patient’s growth charts when recent measurements were not available. Non-standardized SCr values were reduced by 5% before eGFR was calculated, as previously described [11]. The KDIGO classification system was used to stage CKD [12]. Serial SCr values were used to identify episodes of AKI, defined according to the KDIGO criteria as an increase in SCr of ≥26.5 μmol/L (≥0.3 mg/dL) within 48 hours or ≥1.5 times baseline, presumed to have occurred over 7 days [13].
Statistical analysis
Statistical analyses were performed using SPSS (IBM SPSS Statistics version 21.0, 2012, Armonk, NY, USA). Data are presented as number, percentage and median (range). For the purpose of calculations, individuals receiving RRT were assigned an eGFR of 10 mL/min/1.73 m2. Slopes of eGFR and staging of CKD were based on all eGFR calculations available for each patient, excluding all values obtained during episodes of AKI. Slopes and eGFR trajectory lines were calculated by fitting a linear regression line through available eGFR values for the 18 patients that had more than one SCr value available in our registry. The long-term renal outcome (kidney stones, AKI, CKD, ESRD and eGFR slope) of patients who were placed on XOR inhibitor treatment prior to 18 years of age was compared with those not receiving pharmacotherapy until they had reached adulthood (≥18 years of age).
Results
Patient characteristics and presenting features
Of the 21 patients in the registry who either presented with clinical manifestations of APRT deficiency or were diagnosed with the disorder at <18 years of age, 16 were from Iceland, 1 from Austria, 1 from Italy, 1 Norwegian child of Turkish descent, 1 from the United States and 1 from India. A total of 12 (57%) were females and the age at first presentation was 1.6 (0.2–16.5) years. Clinical characteristics of the patients at presentation are described in Table 1. Reddish-brown diaper spots and kidney stones were the most common presenting features, occurring in 13 (62%) and 11 (52%) and patients, respectively. The median (range) age at the first symptomatic stone event among the 11 patients was 3.4 (0.3–6.9) years. Three patients presented with AKI due to obstructive stone disease, one of whom required transient hemodialysis. No patient had reached CKD stages 3–5 at the time of presentation, while at diagnosis 4 had progressed to CKD stage 3 or above, including one who had initiated RRT for ESRD.
Table 1.
Clinical characteristics of children (N = 21) with adenine phosphoribosyltransferase deficiency at the time of first presentation, classified according to whether the diagnosis of the disorder was made before or after age 18 years.
| Females, n (%) | 12 (57) | |
|---|---|---|
| Diagnosis at age <18 years | Diagnosis at age ≥18 years | |
| Number of patients | 15 (71) | 6 (29) |
| Age at first presentation, years | 1.5 (0.2–16.5) | 4.4 (0.5–7.1) |
| Age at diagnosis, years | 2.5 (0.6–16.5) | 35.5 (20.5–42.4) |
| Diagnostic delay, years | 1.2 (0.6–10.4) | 29.2 (20.1–39.2) |
| Reddish-brown diaper stain in infancy | 12 (80.0) | 1 (16.7) |
| Kidney stones | 7 (46.7) | 4 (66.7) |
| Acute kidney injury | 2 (13.3) | 1 (16.7) |
| Chronic kidney disease stage 3–5 | 0 | 0 |
| Asymptomatic crystalluria | 2 (13.3) | 1 (16.7) |
Data are presented as number (percentage) and median (range).
Diagnosis of APRT deficiency
The diagnosis of APRT deficiency was initially suggested by detection of urinary DHA crystals in 18 patients and by stone analysis in two. The diagnosis was confirmed by genetic testing (n=20) and/or absent APRT activity (n=4) in all cases. All of the 16 Icelandic patients shared the same biallelic variant c. 194A>T (p.Asp65Val) in the APRT gene, whereas the Austrian and the US patients were found to be homozygous for c.400+2dup (IVS4+2insT), resulting in aberrant splicing and deletion of exon 4. The patient from India was homozygous for the c.2T>C (p.Met1?) variant in exon 1, affecting the translation initiation codon, and the Italian patient was heterozygous for a variant in intron 1, c.81–3C>G.
The age at diagnosis was 4.8 (0.6–42.4) years, while a diagnostic delay of 10.4 (0.6–39.2) years occurred in 13 patients following their first stone event (n=10) and/or detection of diaper stains (n=3). The diagnostic delay was caused by misidentification of urine DHA crystals in 8 patients, erroneous kidney stone analysis (presumed uric acid calculi) in 2, absence of stone analysis in 2 cases and failure to recognize diaper stains in 1 case.
Treatment and outcome
The timeline of diagnosis, XOR inhibitor treatment and ensuing clinical events for each patient with APRT deficiency is shown in Figure 2. In 14 patients, the diagnosis was made and allopurinol treatment commenced in childhood, in the daily dose of 100 (25–200) mg or 6.0 (3.020.8) mg/kg. Six of these 14 patients had already experienced 8 kidney stone events when XOR inhibitor treatment was initiated at the age of 2.6 (0.6–16.5) years. After 18.9 (1.7–31.5) years of drug treatment, one additional patient, who was known to be poorly adherent to drug treatment, had experienced an incident kidney stone, and one receiving insufficient allopurinol dose, 200 mg per day, had suffered stone recurrence. Both these patients had DHA crystalluria confirmed by urine microscopy. Three patients required stone removal procedures, 1 at the time of presentation and 2 patients underwent two therapeutic interventions each shortly following diagnosis. One patient underwent unilateral nephrectomy due to irreversible damage caused by stone obstruction. Four of the 14 patients had experienced AKI episodes, 1 prior to diagnosis and treatment initiation and three following initiation of pharmacologic therapy. Two of these patients had been prescribed insufficient doses (75–200 mg/day) of allopurinol and 1 had been placed on allopurinol 400 mg/day but was non-adherent to the treatment as evidenced by DHA crystalluria. None of the 14 patients who were started on pharmacotherapy in childhood had developed CKD stage 3–5 at the time of last follow-up. Five of these patients had not developed any clinical events at last follow-up.
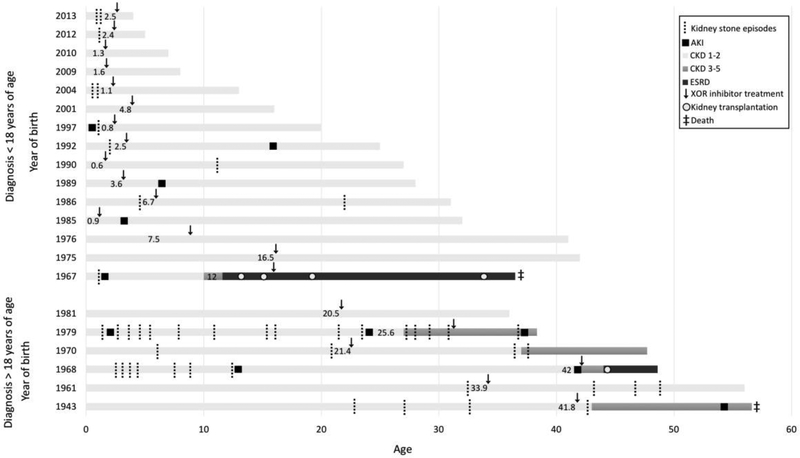
Figure 2.
Timeline of major renal manifestations and treatment in patients with adenine phosphoribosyltransferase deficiency according to age below or above 18 years at diagnosis. Year of birth is shown on the Y-axis. Age at initiation of xanthine oxidoreductase inhibitor (allopurinol or febuxostat) treatment is indicated within each patientߣs timeline. The patient born in the year 1967 did not receive drug treatment prior to progressing to kidney failure.
Six patients first received allopurinol therapy as adults, in a daily dose of 200 (100–300) mg. Five had experienced a total of 28 kidney stone events at the age of 29.8 (20.5–42.4) years when they were diagnosed with the disorder and allopurinol treatment was started (Figure 2). After 11.2 (4.2–19.6) years of pharmacotherapy, 7 stone recurrences had occurred in 4 of these patients; 3 were non-adherent with drug treatment and one was receiving an insufficient allopurinol dose of 200 mg per day. All these patients had DHA crystals detected on urine microscopy. One patient who initiated treatment at the age of 20 years remained free of clinical events. Stone removal procedures were carried out in 2 patients, one of whom underwent 18 procedures before diagnosis while the other had 3 surgical interventions performed following diagnosis of the disorder. One patient underwent a partial nephrectomy in adulthood due to severe kidney damage caused by stone obstruction. Three of the 6 patients suffered 6 episodes of AKI, 5 before pharmacologic therapy was initiated and 1 following prescription of allopurinol treatment. The patient who developed AKI during pharmacotherapy was poorly adherent to drug treatment and the episode occurred during a symptomatic kidney stone event. Three of these 6 patients had progressed to CKD stages 3–5 at latest follow-up, one had stage 3A, one stage 3B, and one patient had reached stage 5 CKD requiring RRT. One patient had improved from stage 3A to stage 2. At last follow-up, 5 of the 6 patients who initiated treatment with an XOR inhibitor as adults were receiving allopurinol in a daily dose of 300 (200–400) mg. Febuxostat 80 mg/day had been prescribed in 1 patient in whom allopurinol therapy was discontinued due to an adverse reaction (pruritus). One patient in this group died at the age of 56 years from breast cancer.
At disease presentation, the eGFR in the 21 patients was 83 (35–165) mL/min/1.73 m2, despite the inclusion of several patients with AKI. Eighteen patients who had two or more SCr values available were included in the assessment of the evolution of kidney function, excluding 2 patients who only had a single SCr value and the patient who developed ESRD at age 11 years. At last follow-up the eGFR was 114 (70–163) mL/min/1.73 m2 and the median eGFR slope (n=12) was 0.04 (range, −5.28 to 2.79) mL/min/1.73 m2 per year in the group of patients who started allopurinol treatment in childhood. As these 14 patients only had SCr values available after they had started pharmacotherapy, the potential effect of XOR inhibitor treatment on kidney function could not be studied. For the 6 patients who initiated drug treatment as adults, the eGFR at last follow-up was 62 (10–103) mL/min/1.73 m2 and the median eGFR slope (n=6) was 0.47 (range, −1.32 to 0.38) mL/min/1.73 m2 per year. Four of these six patients had SCr values available prior to and after initiation of pharmacotherapy, all of whom displayed improvement of kidney function following commencement of treatment. The median eGFR slope was −0.47 (−1.23 to −0.26) before starting therapy and 0.85 (0.29 to 5.13) mL/min/1.73 m2 per year after treatment initiation. The results for both patient groups are depicted in Figure 3.
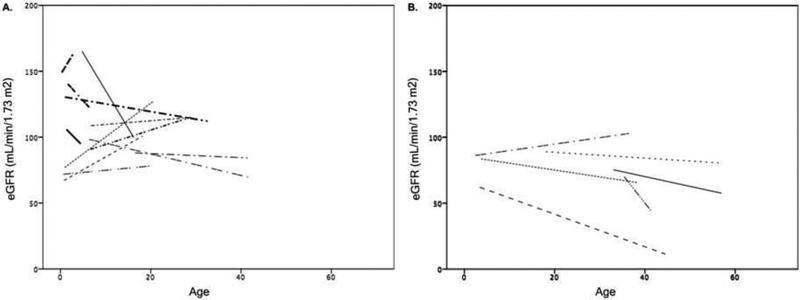
Figure 3.
Changes in estimated glomerular filtration rate (eGFR) over time in patients (Panel A, n=12) who received xanthine oxidoreductase inhibitor treatment before the age of 18 years and in those (Panel B, n=6) who initiated drug therapy in adult life. eGFR trajectory lines were created by fitting a linear regression line through all available eGFR values for each patient, excluding episodes of acute kidney injury (AKI).
The case of the patient who was diagnosed with APRT deficiency at the age of 11 years but did not receive pharmacotherapy until after his second kidney transplant, warrants more in-depth description. The boy first presented with AKI at the age of 18 months due to bilateral obstructive stone disease requiring 3 percutaneous stone removal procedures and transient hemodialysis. By the time of diagnosis at 11 years of age, the boy had already progressed to ESRD. Due to unknown reasons, allopurinol treatment was not initiated at that time. He later received four kidney transplants, at age 14, 16, 19 and 34 years. Treatment with allopurinol, 300 mg/day, was started at 16 years of age when a biopsy disclosed a recurrence of DHA nephropathy in his second renal allograft, resulting in graft loss 3 years later. The third kidney transplant functioned for 8 years with allopurinol therapy. Following a fourth deceased donor kidney transplant, the patient died from sepsis and liver failure associated with intestinal perforation at the age of 36 years.
Discussion
This study of patients with APRT deficiency presenting in childhood demonstrates an improved long-term renal outcome in those initiating treatment with an XOR inhibitor before 18 years of age, even when started late in childhood. A substantial proportion of those who experienced significant diagnostic delay and did not receive pharmacotherapy until after the age of 18 years had progressive CKD, which was not seen in the much larger group of patients who started treatment early. Furthermore, almost all patients who initiated pharmacologic treatment after 18 years of age experienced multiple recurrent kidney stone episodes which were much less frequent in those commencing treatment prior to age 18 years. The most common presenting features of APRT deficiency were reddish-brown diaper spots and kidney stones, while AKI and asymptomatic DHA crystalluria were less commonly observed.
Nephrolithiasis was the presenting feature in half of our patient population and almost 60% had developed kidney stones at the time of diagnosis. This is lower than previously described in a study from France [4], where 80% of patients presented with a symptomatic kidney stone event, subsequently leading to a diagnosis of APRT deficiency. The higher proportion of patients with kidney stones as the presenting feature in the French cohort may have resulted from failure to identify other less apparent manifestations of the disorder in that study, such as diaper stains, lower urinary tract symptoms and asymptomatic crystalluria. As expected, a much higher stone burden was seen in the group of patients in our study who first initiated drug treatment in adult life, and most of these stones were formed before the diagnosis of APRT deficiency was made and pharmacotherapy initiated. Approximately one-third of the patients experienced stone recurrence after the diagnosis of APRT deficiency, despite treatment with allopurinol or febuxostat, which is similar to that observed in the French study [4]. Lack of adherence to treatment or insufficient allopurinol dosing appears to have contributed to new stone formation in all the patients in our study.
AKI episodes caused by bilateral obstructive stone disease was the presenting feature in 3 patients, which is similar to the findings in the aforementioned French study [4]. However, episodes of AKI were more commonly observed in our patient cohort during the follow-up period than previously described, likely due to the advantage of the comprehensive SCr dataset in our registry which allowed for a more detailed analysis of changes in kidney function than has been possible in other published studies. The proportion of patients with AKI at diagnosis in the French study may have been higher than recognized in view of the large number of their patients who experienced a marked improvement in kidney function during the first few months of allopurinol therapy [4]. Diagnostic delay or inadequate pharmacologic therapy, due to either poor adherence or insufficient XOR inhibitor dosing appears to have contributed to all the AKI episodes observed in the current study. Furthermore, episodes of AKI may have contributed to progression of CKD among patients who experienced significant delay in diagnosis and treatment.
Chronic kidney disease stage 3 or above did not develop in any of the patients in our study who initiated treatment at an early age, in contrast to two-thirds of those who did not start pharmacotherapy until in adult life. These findings are similar to those in the French cohort, in which none of the patients diagnosed in childhood developed advanced CKD [4]. Although their study period and follow-up time was considerably shorter than in the present study, this finding emphasizes the importance of early institution of pharmacotherapy.
Our extensive set of SCr values allowed a detailed analysis of the evolution of kidney function over time. In the group that initiated allopurinol treatment in childhood, an improvement in eGFR was observed followed by preservation of kidney function, which was within normal limits at the end of the study period. These findings are in concert with the results of the aforementioned French study, in which all patients started pharmacotherapy at a relatively young age [4]. Interestingly, the patients who experienced a prolonged diagnostic delay experienced improvement in eGFR after initiation of treatment with an XOR inhibitor, but remained much lower than in the group receiving pharmacotherapy at an early age. The initial eGFR rise on treatment is possibly explained by clearance of intratubular DHA crystals, while the subsequent decline in kidney function is likely caused by chronic DHA crystal nephropathy which is characterized by chronic tubulointerstitial inflammation, fibrosis and progressive nephron loss.
The transplanted kidney appears to be particularly susceptible to DHA nephropathy which tends to recur early in the post-transplant period and is frequently severe, leading to shortened allograft survival, even despite XOR inhibitor therapy [14]. This phenomenon is well illustrated by the case of the unfortunate patient who had already progressed to ESRD at the time of diagnosis of APRT deficiency when he was 11 years of age, and who later underwent a total of 4 kidney transplantation procedures. This case also underscores the phenotypic variability of APRT deficiency as the disease was unusually aggressive, resulting in ESRD already in childhood.
Diagnostic delay is an important determinant of adverse renal outcomes and, in contrast to many other rare causes of CKD, effective treatment of APRT deficiency is available. The most frequent reasons for missed diagnosis are lack of awareness of the disease resulting in failure to recognize urinary DHA crystals, misidentification of radiolucent kidney stones as uric acid calculi and confusion of renal histopathological findings with other forms of crystal nephropathy [3]. The observed variability in disease expression between individuals, as is illustrated by only one-third of our overall study population having symptomatic disease during the first two decades of life, makes the diagnosis even more difficult. Reddish-brown diaper stains in infancy, reported in approximately 60% of patients in the current study, is an important presenting feature that should be taken seriously [3]. Indeed, APRT deficiency must be considered in all such patients and in the differential diagnosis of kidney stones and CKD, in children and young adults. The lack of awareness of rare diseases frequently results in unacceptable delay in diagnosis and treatment, frequently with grave consequences.
Timely institution of treatment with an XOR inhibitor is extremely important as both allopurinol and febuxostat have been shown to effectively reduce urine DHA excretion [15]. The currently recommended daily dose of allopurinol in children is 10 mg/kg and at least 400–600 mg in adults, and if well tolerated, this dose should maintained, even in individuals with advanced CKD [3, 15]. The currently recommended starting dose of febuxostat is 80 mg/day in adults as a single daily dose [15], but no dosing recommendations are currently available for children. Both drugs are usually well tolerated. Monitoring of pharmacotherapy, which is important to ensure adequate dosing and adherence, has traditionally been carried out using urine microscopy where the disappearance urine DHA crystals has been considered indicative of adequate treatment. However, detection of crystalluria may not be reliable enough for monitoring of drug treatment. Our group has recently developed a urinary DHA assay using ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), which has the potential to greatly facilitate both clinical diagnosis and therapeutic monitoring of pharmacotherapy of patients with APRT deficiency [16]. As the current study is based on observational registry data only, we did not have the opportunity to include the novel urinary DHA assay in this work.
Most of the cases in our study come from Iceland where all patients are homozygous for the same missense mutation (p.D65V), presumably due to a founder effect. It is noteworthy that no genotype-phenotype correlation has been identified in APRT deficiency, a finding which would be expected as all known pathogenic APRT variants completely obliterate the enzyme activity. Thus, the phenotypic differences observed must be due to other factors.
Our study has several strengths, including the comprehensive dataset. At the time of analysis, the registry contained more than 600 patient-years of clinical information on individuals included in the present study, making this the largest pediatric clinical dataset available and the longest observation time reported to date. The abundance of available SCr values allowed for the identification of AKI episodes and detailed characterization of long-term evolution of kidney function. The main limitation is the retrospective nature of part of the dataset, thus lacking standardization.
In conclusion, our data clearly demonstrate a much more favorable renal outcome in patients with APRT deficiency who are diagnosed and treated early. The diagnostic evaluation of young patients with stone disease or CKD of unknown etiology at any age, should include screening for rare disorders such as APRT deficiency. Timely pharmacologic therapy appears to reduce stone burden and slow or possibly prevent the progression of CKD, even in severely affected individuals. Our results further underscore the significant variability in the clinical characteristics of APRT deficiency, which may include a long asymptomatic period making diagnosis extremely difficult. The relatively frequent occurrence of advanced CKD and even kidney failure at the time of diagnosis is concerning and suggests a lack of familiarity with this treatable condition. Finally, our findings emphasize the importance of kidney biopsy in younger patients with unexplained CKD.
Acknowledgements
Part of this work was presented in an abstract form at the American Society of Nephrology Kidney Week, November 3–8, 2015, San Diego, CA. The authors want to sincerely thank the following physicians for their invaluable assistance in clinical data and biosample collection: Dawn Milliner (Mayo Clinic, Rochester, MN, USA), John Lieske (Mayo Clinic, Rochester, MN, USA), David Goldfarb (New York University, New York, NY, USA), Philipp Eller (Medical University of Graz, Graz, Austria), Hans-Jacob Bangstad (Oslo University Hospital, Oslo, Norway), Amrik Sahota (Rutgers University, Piscataway, NJ, USA), and Lynette Fairbanks (Guy’s and St. Thomas’ Hospital NHS Foundation Trust, London, UK).
Support
This study was supported by the Rare Kidney Stone Consortium (2U54KD083908), a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR). The Rare Kidney Stone Consortium is funded through collaboration between NCATS and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
Disclosure
None of the authors declared financial or other conflicting interests. No honorarium, grant, or any other form of payment was given to anyone to produce the manuscript.
Compliance with ethical standards
The study was approved by the National Bioethics Committee of Iceland (NBC 09–072) and the Icelandic Data Protection Authority. All living patients or their legal guardians gave a written informed consent for participation in the study. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
References
- 1.Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T (2001) Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis 38:473–480. [DOI] [PubMed] [Google Scholar]
- 2.Bollee G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, Deteix P, Daudon M, Knebelmann B, Ceballos-Picot I (2010) Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol 21:679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO (2016) Kidney Disease in Adenine Phosphoribosyltransferase Deficiency. Am J Kidney Dis 67:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harambat J, Bollee G, Daudon M, Ceballos-Picot I, Bensman A (2012) Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol 27:571–579. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood MC, Dillon MJ, Simmonds HA, Barratt TM, Pincott JR, Metreweli C (1982) Renal failure due to 2,8-dihydroxyadenine urolithiasis. Eur J Pediatr 138:346–349. [DOI] [PubMed] [Google Scholar]
- 6.Debray H, Cartier P, Temstet A, Cendron J (1976) Child’s urinary lithiasis revealing a complete deficit in adenine phosphoribosyl transferase. Pediatr Res 10:762–766. [DOI] [PubMed] [Google Scholar]
- 7.Chiba P, Zwiauer K, Muller MM (1988) Characterization of an adenine phosphoribosyltransferase deficiency. Clin Chim Acta 172:141–147. [DOI] [PubMed] [Google Scholar]
- 8.Edvardsson VO, Palsson R, Sahota A (2012) Adenine Phosphoribosyltransferase Deficiency. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K (eds) GeneReviews, Seattle WA. [Google Scholar]
- 9.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, F Van Lente, Greene T, Coresh J; CKD-EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD (2011) Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J 162:548–554. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Raikwar N, Deng L, Yang M, Liang L, Shao C, Evan AP, Stambrook PJ, Sahota A, Tischfield JA (2000) Altered gene expression in kidneys of mice with 2,8-dihydroxyadenine nephrolithiasis. Kidney Int 58:528–536. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) AKI Work Group (2012). KDIGO clinical practice guideline for acute kidney injury. AKI Definition. Kidney Int Suppl 2:19–36. [Google Scholar]
- 14.Zaidan M, Palsson R, Merieau E, Cornec-Le Gall E, Garstka A, Maggiore U, Deteix P, Battista M, Gagne ER, Ceballos-Picot I, Duong Van Huyen JP, Legendre C, Daudon M, Edvardsson VO, Knebelmann B (2014) Recurrent 2,8-dihydroxyadenine nephropathy: a rare but preventable cause of renal allograft failure. Am J Transplant 14:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edvardsson VO, Runolfsdottir HL, Thorsteinsdottir UA, Sch Agustsdottir IM, Oddsdottir GS, Eiriksson F, Goldfarb DS, Thorsteinsdottir M, Palsson R (2018) Comparison of the effect of allopurinol and febuxostat on urinary 2,8-dihydroxyadenine excretion in patients with Adenine phosphoribosyltransferase deficiency (APRTd): A clinical trial. Eur J Intern Med 48:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorsteinsdottir M, Thorsteinsdottir UA, Eiriksson FF, Runolfsdottir HL, Agustsdottir IM, Oddsdottir S, Sigurdsson BB, Hardarson HK, Kamble NR, Sigurdsson ST, Edvardsson VO, Palsson R (2016) Quantitative UPLC-MS/MS assay of urinary 2,8-dihydroxyadenine for diagnosis and management of adenine phosphoribosyltransferase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci 1036–1037:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]