Abstract
Delayed graft function (DGF) complicates 20–40% of deceased-donor kidney transplants and is associated with increased length of stay and subsequent allograft failure. Accurate prediction of DGF risk for a particular allograft could influence organ allocation, patient counseling, and post-operative planning. Mitochondrial dysfunction, a reported surrogate of tissue health in ischemia-perfusion injury, might also be a surrogate for tissue health after organ transplantation. To understand the potential of MMP in clinical decision-making, we analyzed whether lower mitochondrial membrane potential (MMP), a measure of mitochondrial dysfunction, was associated with DGF. In a prospective, single-center proof-of-concept study, we measured pre-transplant MMP in 28 deceased donor kidneys and analyzed the association between MMP and DGF. We used hybrid registry-augmented regression to adjust for donor and recipient characteristics, minimizing overfitting by leveraging SRTR data. The range of MMP levels was 964–28,333 units. Low-MMP kidneys (MMP<4000) were more likely from female donors (75% vs. 10%, p=0.002) and DCD donors (75% vs. 12%, p=0.004). For every 10% decrease in MMP levels, there were 38% higher odds of DGF (adjusted-OR=1.081.381.78, p=0.01). In summary, MMP might be a promising pre-transplant surrogate for tissue health in kidney transplantation and, after further validation, could improve clinical decision-making through its independent association with DGF.
INTRODUCTION
Delayed graft function (DGF) following kidney transplantation, defined as a need for dialysis in the first week post-transplant, occurs in 20–40% of deceased donor kidney transplant recipients and increases the risk of short- and long-term morbidity.1 Patients who experience DGF are at an increased risk of acute rejection, graft failure, and post-transplant mortality.2,3 In fact, DGF is associated with a 6.6-fold higher risk of interstitial fibrosis and tubular atrophy, making it a stronger risk factor than pre-transplant diabetes or hypertension for long-term graft dysfunction.4 Therefore, accurate prediction of DGF could influence organ allocation, improve patient counseling about organ offers and anticipated post-transplant course, and allow for more informed post-operative planning. While donor, recipient, and transplant factors associated with DGF (e.g. cold ischemia time) have helped to improve outcome prediction, understanding of the biological mechanisms driving these differences in outcomes and the ability to translate such mechanisms into readily available clinical tests remains limited.5–7 A better understanding of these mechanisms could further improve risk classification of organs and resulting treatment decisions.
In models of cardiac and cerebral ischemia-reperfusion injury, mitochondrial dysfunction has proven to be a surrogate of tissue health.8–11 Cellular injury leads to loss of mitochondrial membrane potential (MMP), which leads to compromised ATP production and cell viability.12 Despite increasing acceptance of MMP as an indicator of general cell health and viability and the clear need for a similar metric in the field of organ transplantation, its utility in assessing the tissue health of deceased donor organs has not been explored.
We hypothesized that MMP would be a surrogate for tissue health in donor organs and would correlate with an independent domain of DGF risk not captured by conventional clinical characteristics. We developed an assay for MMP measurement in kidney allograft biopsies that could be performed rapidly using minimal allograft tissue and analyzed whether loss of MMP was independently associated with DGF.
METHODS
Study population
Core biopsy samples were obtained from a convenience sample of 28 deceased donor kidneys transplanted at our center between September 27, 2007 to December 12, 2008. Biopsy material was taken before reperfusion and placed immediately into cold University of Wisconsin (UW) solution for short-term storage. This study was approved by the Institutional Review Board of Johns Hopkins Hospital.
Donor and recipient data
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.13 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Machine perfusion was used at the discretion of the procuring organ procurement organization (OPO) and/or accepting surgeon for the preservation.
Mitochondrial preparation and MMP measurement
Biopsy tissue was homogenized in cold extraction buffer using a hand-held tissue homogenizer. Cell debris was pelleted by centrifugation at 800 × g at 4°C. Supernatants containing mitochondria were centrifuged at 11,000 × g at 4°C. The resultant mitochondrial pellets were resuspended in cold storage buffer. Total protein content of the mitochondrial fraction was measured by Bradford Assay using commercially available reagents (Bio-Rad Laboratories, Hercules, CA). Mitochondria (2–4 μg) were incubated with 1μg/ml JC-1 probe (Invitrogen, Carlsbad, CA) in 96-well plates for 10 min at 37°C. In healthy cells, JC-1 localizes to mitochondria and forms aggregates that emit red fluorescence under illumination. Decreased MMP is quantitatively correlated with decreased formation of red fluorescent JC-1 in mitochondria. Relative fluorescence units (RFU) of the red emission by JC-1 were detected and quantified using a FlexStation II plate reader (Molecular Devices, San Jose, CA). RFU per microgram of protein was calculated to provide a measurement of MMP.
MMP assay validation
A number of cell membrane permeable fluorescent dyes including JC-1 have widely been used in determining the MMP of cells. We applied the MMP assay to mitochondria extracted from human kidney biopsy samples based on our findings in two animal studies.
To determine if JC-1 could also be used in determining MMP of extracted mitochondria and whether this assay provided accurate measurements of MMP, a rat tubular epithelial cell line (NRK-52E, ATCC) was co-cultured with mesoxalonitrile 4-trifluoromethoxyphenylhydrazone (FCCP) to depolarize MMP of cells, mitochondria were then isolated from cultured cells at 1 hour after FCCP treatment, and the MMP levels were subsequently measured by using the JC-1 fluorescent probe. The MMP levels decreased in a FCCP-dose dependent fashion. These results show that the levels of red fluorescent aggregates (emission at 590nm) of JC-1 are correlated with depolarization of MMP, indicating that this provides an accurate read out of MMP and that the JC-1 assay can be used in determining the MMP of extracted mitochondria.
To determine if MMP levels correlated with the ischemia time in cold preserved organs, we extracted mitochondria from cold UW preserved rat livers or kidneys at sequential time points and measured the MMP levels using a JC-1 fluorescent probe. The MMP levels of cold preserved livers were significantly decreased at 48 hours and further declined in a time-dependent manner. The MMP levels of cold UW preserved kidneys remained the same at 72 hours, but significantly decreased at 96 hours after cold preservation.
Post-transplant outcomes
Our primary outcome of interest was DGF, defined as a need for dialysis within one week post-transplant. Both DGF status and serum creatinine at post-transplant day 5 were manually abstracted from recipients’ electronic medical records.
Statistical analysis
We analyzed the association between pre-transplant MMP level and risk of DGF. We also compared MMP levels by post-operative renal function (DGF vs. no DGF) using Kruskal-Wallis equality-of-populations rank test.
Post-transplant delayed graft function
We explored the independent association between MMP level and DGF using logistic regression, adjusting for donor characteristics (age, race, cause of death, diabetes, hypertension, and creatinine), recipient characteristics (age, gender, race, previous kidney transplant, BMI, primary diagnosis, hepatitis C status, insurance, renal replacement therapy time), and organ/transplant characteristics (cold ischemia time, donation following cardiac death status, pump use, zero mismatch, donor/recipient weight ratio, organ share type).
Hybrid registry-augmented regression
We performed hybrid registry-augmented regression, a method developed and previously described by our group, to leverage the large sample of kidney donors and recipients in SRTR to calculate precise coefficients adjust for potential confounders.14 This technique allowed us to calculate precise coefficients for covariates present in the SRTR database. Using data on 19,087 kidney transplants between 2007 and 2008, we calculated coefficients for the following SRTR-measured confounders: recipient (age, gender, race, BMI, primary diagnosis, HCV, PRA, previous kidney transplant, insurance, years on renal replacement therapy), donor (age, race, cause of death, diabetes, hypertension, creatinine), and transplant characteristics (cold ischemia time, DCD, machine perfusion, recipient/donor weight ratio, HLA mismatches, organ share type) age, sex, and race. These coefficients were then introduced into the single-center model, allowing us to adjust for potential confounders of the association between MMP and DGF without sacrificing degrees of freedom.
Stepwise association of MMP and delayed graft function
In order to explore the stepwise association between MMP and graft function, we subdivided patients who did not experience DGF (non-DGF patients) by their serum creatinine level 5 days post-transplant. Patients who had a post-transplant day 5 serum creatinine greater than 3.0 mg/dL but did not require dialysis were classified as having slow graft function (SGF), while those who had a post-transplant day 5 serum creatinine of 3.0 mg/dL or less were classified as having immediate graft function (IGF). Multinomial logistic regression was used to model the association of graft function (IGF, SGF, DGF) and every 10% increase in MMP. IGF is the base outcome in the regression. Relative risk ratio (RRR) from the regression indicates relative risk to have SGF or DGF (vs IGF) associated with increase in MMP.
Confidence intervals are reported as per the method of Louis and Zeger.15 All analyses were performed using Stata 14.1/MP for Windows (College Station, Texas).
RESULTS
Study population
Of the 28 patients included in final analysis, 8 (29%) had MMP below 4000 units (“low MMP”) and 20 (71%) had MMP of at least 4000 (“high MMP”). Compared to kidneys with high MMP, kidneys with low MMP were more likely to be from female donors (75% vs. 10%, p=0.002), from donors after cardiac death (DCD; 88% vs. 25%, p=0.004), to receive ex-vivo machine perfusion (50% vs. 5%, p=0.01), and to be transplanted into female recipients (0% vs. 50%, p=0.02) (Table 1). Kidneys with low MMP and kidneys with high MMP had similar cold ischemia time, HLA mismatching, PRA, and frequency of national or regional sharing (Table 1). At post-operative day 5, four patients had DGF, 11 had SGF, and 13 had IGF.
Table 1.
Patient characteristics by pre-transplant mitochondrial membrane potential (MMP) levels
| Characteristic | MMP≤4000 RFU* (N=8) |
MMP>4000 RFU (N=20) |
P |
|---|---|---|---|
| Donor characteristics, N (%) | |||
| Age (years), median (IQR) | 25.5 (22.5–33) | 36 (22.5–44) | 0.4 |
| Female | 6 (75%) | 2 (10%) | 0.002 |
| African American | 0 (0%) | 0 (0%) | - |
| Cause of death | 0.2 | ||
| Head trauma | 3 (38%) | 8 (40%) | |
| Anoxia | 5 (68%) | 5 (25%) | |
| Stroke | 0 (0%) | 5 (25%) | |
| Other | 0 (0%) | 2 (10%) | |
| Diabetes | 0 (0%) | 1 (5%) | 1 |
| Hypertension | 2 (25%) | 3 (15%) | 0.6 |
| Creatinine (mg/dL), median (IQR) | 2 (1.1–3.3) | 1.1 (0.9–1.5) | 0.1 |
| Recipient characteristics, N (%) | |||
| Age (years), median (IQR) | 45 (37–62) | 52 (43–64) | 0.3 |
| Female | 0 (0%) | 10 (50%) | 0.02 |
| African American | 4 (50%) | 6 (30%) | 0.4 |
| Previous kidney transplant | 2 (25%) | 3 (15%) | 0.6 |
| BMI, median (IQR) | 25.3 (21.9–31.0) | 25.6 (23.1–29.1) | 0.7 |
| Primary diagnosis | 0.6 | ||
| Glomerular disease | 1 (13%) | 6 (30%) | |
| Diabetes | 2 (25%) | 5 (25%) | |
| Hypertension | 4 (50%) | 4 (20%) | |
| Cystic disease | 0 (0%) | 2 (10%) | |
| Other | 1 (13%) | 3 (15%) | |
| Hepatitis C status | 1 (13%) | 2 (10%) | 1 |
| Private insurance | 3 (38%) | 12 (60%) | 0.4 |
| Time on renal replacement therapy (years), median (IQR) | 4.4 (1.2–10.4) | 5 (2.5–5.3) | 0.7 |
| Transplant characteristics, N (%) | |||
| Cold ischemia time (hours), median (IQR) | 23.5 (16.4–39.8) | 19.0 (10.6–24.0) | 0.2 |
| Donation following cardiac death | 7 (88%) | 5 (25%) | 0.004 |
| Machine perfusion | 4 (50%) | 1 (5%) | 0.01 |
| HLA mismatches, zero | 0 (0%) | 2 (10%) | 1 |
| Donor/recipient weight ratio, median (IQR) | 0.9 (0.7–1.3) | 1.1 (0.8–1.4) | 0.2 |
| National/regional share | 3 (38%) | 5 (25%) | 0.4 |
| PRA, median (IQR) | 0 (0–45) | 0 (0–0) | 0.9 |
RFU = relative fluorescence units
MMP levels and DGF
Patients who experienced DGF had significantly lower MMP levels than patients who did not experience DGF (median (IQR): 2,225 (1,357–3,350) vs. 7,773 (5,375–9,697), p=0.005) (Figure 1). Donors with lower MMP levels (≤4,000 RFU) were more likely to be female (75% vs. 10%, p=0.002) than donors with higher MMP levels, but did not differ significantly in age, creatinine, cause of death, or frequency of diabetes or hypertension. Recipients of kidneys with lower MMP levels were less likely to be female (0% vs. 50%, p=0.02) than recipients of kidneys with higher MMP levels, but were otherwise similar (Table 1). Transplants of kidneys with lower MMP levels were more likely to be donations after cardiac death (88% vs. 25%, p=0.004) and were more likely to have received machine perfusion (50% vs. 5%, p=0.01) but had similar cold ischemia time, number of HLA mismatches, donor/recipient weight ratio, frequency of national or regional sharing, and recipient sensitization (Table 1).
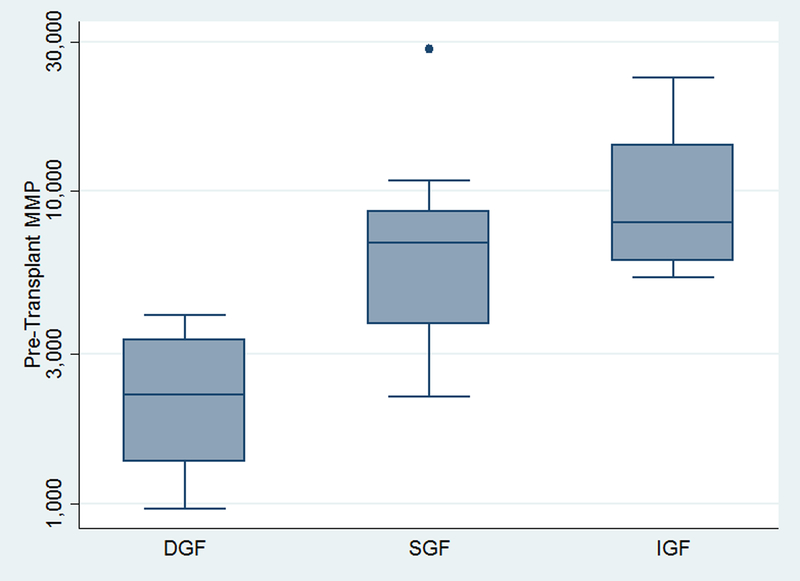
Figure 1. Pre-transplant MMP levels by post-transplant day 5 graft function groups.
Patients were classified as having delayed graft function (DGF; required post-transplant dialysis during hospitalization), slow graft function (SGF; serum creatinine >3.0 mg/dL), or immediate graft function (serum creatinine ≤3.0 mg/dL). Patients with DGF had significantly lower MMP levels than patients with SGF or IGF (overall Kruskal-Wallis test p=0.003).
Higher MMP levels were associated with lower odds of developing DGF (OR of DGF per 10% increase in MMP: 0.510.700.97, p=0.03); this association was unchanged after adjusting for donor, recipient, and transplant characteristics (aOR of DGF per 10% increase in MMP: 0.560.720.93, p=0.01). When we analyzed patients by graft function at 5 days, we found that a 10% increase in pre-transplant MMP levels was associated with a significantly lower risk of developing SGF or DGF than IGF (RRR DGF vs. IGF: 0.390.580.86, p=0.007; RRR SGF vs. IGF: 0.630.790.98 vs. IGF, p=0.03).
DISCUSSION
In this single-center proof-of-concept study of mitochondrial membrane potential analysis in 28 pre-transplant donor kidney biopsies, we found that MMP levels were an independent predictor of DGF risk and that an MMP threshold of 4,000 differentiated patients with DGF from those with immediate graft function. For every 10% decrease in pre-transplant MMP, recipients had a 38% higher risk of DGF (p=0.01), even after adjusting for donor, recipient, and organ/transplant characteristics. Since MMP levels can be rapidly determined and are available pre-transplant, our findings suggest that MMP levels might be a promising surrogate of tissue health of donor kidneys and, pending further validation, potentially serve as an independent predictor of DGF risk following transplantation.
Our findings that MMP was associated with risk of delayed graft function are consistent with previous literature linking MMP levels with tissue function in models of cardiac and cerebral ischemia and reperfusion, as well as septic acute kidney injury16,17 and drug-related toxicity.18,19 MMP has also been explored as a method of testing the effects of cooling and rewarming on renal cells in animal models.20 These findings further support the use of MMP in renal transplantation, an as-yet unexplored application of this marker of tissue function that warrants further study. Notably, mitochondrial function has been noted to differ by sex, including in particular tissues,21–24 and the proportion of female and male donor kidneys in the high and low MMP groups differed in this study. However, differences in MMP by sex have not been specifically studied and should be investigated.
Limitations of this study include a pilot, proof-of-concept sample size which will require further validation in larger populations. However, we were able to leverage risk adjustment using the SRTR dataset, allowing us to test whether MMP risk prediction was independent of 21 other donor, recipient, and transplant characteristics. Our findings using registry-augmented regression analysis suggest that MMP measures risk of DGF beyond that predicted by other variables currently available in the national transplant dataset. However, additional validation in larger sample sizes is indicated to confirm these findings. Another limitation of the study is potential imprecision in the quantity of mitochondria in each sample. Since the assay is standardized per microgram of protein, variability in the results could theoretically arise from variation in the precise degree to which a fraction of protein from stroma lacking mitochondria was included in each biopsy. However, such variation would bias our findings toward the null, suggesting that differences in the MMP levels between groups might be even larger than those found in this study. Additionally, we recognize that this MMP assay will need to be standardized for each organ type, as it is unknown whether there are organ-specific differences in the density of mitochondria per microgram of protein.
In conclusion, we found that mitochondrial membrane potential independently predicted delayed graft function among our sample of kidney transplant recipients. The findings of this pilot study suggest future work to be done to investigate the potential of MMP as a marker of tissue function in renal transplantation. If validated in larger studies, a marker of tissue function such as MMP could influence organ allocation, pre-transplant management of donor organs, patient counseling, and post-transplant monitoring and management of patients identified to be at higher risk for DGF due to donor organ MMP levels. Additionally, as a marker of tissue function used in multiple medical fields, we believe that MMP has the potential to further our understanding of the mechanisms of tissue damage during organ recovery and could aid in the discovery of interventions to improve transplant outcomes.
ACKNOWLEDGMENTS
This work was supported by grant numbers K23DK115908 and K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The third author (JR) is supported by a Doris Duke Clinical Research Foundation grant.
Abbreviations:
- DGF
delayed graft function
- HR
hazard ratio
- HRSA
Health Resources and Services Administration
- IGF
immediate graft function
- IQR
interquartile range
- MMP
mitochondrial membrane potential
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- RFU
relative fluorescence units
- SGF
slow graft function
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Daly PJ, Power RE, Healy DA, Hickey DP, Fitzpatrick JM, Watson RW. Delayed graft function: a dilemma in renal transplantation. BJU Int. 2005;96(4):498–501. [DOI] [PubMed] [Google Scholar]
- 2.Patel SJ, Duhart BT Jr., Krauss AG, et al. Risk factors and consequences of delayed graft function in deceased donor renal transplant patients receiving antithymocyte globulin induction. Transplantation. 2008;86(2):313–320. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff WW, Howard RJ, Patton PR, Adams VR, Rosen CB, Reed AI. Delayed graft function after renal transplantation. Transplantation. 1998;65(2):219–223. [DOI] [PubMed] [Google Scholar]
- 4.Khalkhali HR, Ghafari A, Hajizadeh E, Kazemnejad A. Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction. Exp Clin Transplant. 2010;8(4):277–282. [PubMed] [Google Scholar]
- 5.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. [DOI] [PubMed] [Google Scholar]
- 6.Sharif A, Borrows R. Delayed graft function after kidney transplantation: the clinical perspective. Am J Kidney Dis. 2013;62(1):150–158. [DOI] [PubMed] [Google Scholar]
- 7.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadenbach B, Ramzan R, Moosdorf R, Vogt S. The role of mitochondrial membrane potential in ischemic heart failure. Mitochondrion. 2011;11(5):700–706. [DOI] [PubMed] [Google Scholar]
- 9.Quarrie R, Lee DS, Steinbaugh G, et al. Ischemic preconditioning preserves mitochondrial membrane potential and limits reactive oxygen species production. Journal of Surgical Research. 2012;178(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Molecular Neurobiology. 2013;47(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solhjoo S, O’Rourke B. Mitochondrial instability during regional ischemia–reperfusion underlies arrhythmias in monolayers of cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2015;78:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 2004;66(2):514–517. [DOI] [PubMed] [Google Scholar]
- 13.Massie AB, Kuricka LM, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. American Journal of Transplantation. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G-D, Zhang J-L, Chen Y-T, Zhang J-X, Wang T, Zeng Q-Y. Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Exp Ther Med. 2018;15(4):3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arulkumaran N, Pollen S, Greco E, et al. Renal Tubular Cell Mitochondrial Dysfunction Occurs Despite Preserved Renal Oxygen Delivery in Experimental Septic Acute Kidney Injury. Critical Care Medicine. 2018;46(4):e318–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva JP, Carmo H, Carvalho F. The synthetic cannabinoid XLR-11 induces in vitro nephrotoxicity by impairment of endocannabinoid-mediated regulation of mitochondrial function homeostasis and triggering of apoptosis. Toxicology Letters. 2018;287:59–69. [DOI] [PubMed] [Google Scholar]
- 19.Wongmekiat O, Peerapanyasut W, Kobroob A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2018;391(4):385–394. [DOI] [PubMed] [Google Scholar]
- 20.Hendriks KDW, Lupi E, Hardenberg MC, Hoogstra-Berends F, Deelman LE, Henning RH. Differences in mitochondrial function and morphology during cooling and rewarming between hibernator and non-hibernator derived kidney epithelial cells. Scientific Reports. 2017;7(1):15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardinale DA, Larsen FJ, Schiffer TA, et al. Superior Intrinsic Mitochondrial Respiration in Women Than in Men. Frontiers in Physiology. 2018;9(1133). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. Journal of Bioenergetics and Biomembranes. 2015;47(1):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vona R, Ascione B, Malorni W, Straface E. Mitochondria and Sex-Specific Cardiac Function In: Kerkhof PLM, Miller VM, eds. Sex-Specific Analysis of Cardiovascular Function. Cham: Springer International Publishing; 2018:241–256. [DOI] [PubMed] [Google Scholar]
- 24.Silaidos C, Pilatus U, Grewal R, et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol Sex Differ. 2018;9(1):34 http://europepmc.org/abstract/MED/30045765 http://europepmc.org/articles/PMC6060503?pdf=render http://europepmc.org/articles/PMC6060503http://europepmc.org/abstract/MED/30045765http://europepmc.org/articles/PMC6060503?pdf=renderhttp://europepmc.org/articles/PMC6060503 10.1186/s13293-018-0193-7http://europepmc.org/articles/PMC6060503?pdf=render http://europepmc.org/articles/PMC6060503 https://doi.org/10.1186/s13293-018-0193-7 . Accessed 2018/07//. [DOI] [PMC free article] [PubMed] [Google Scholar]