Abstract
Objectives
Vitamin D has been believed to have a bearing in the pathogenesis of knee osteoarthritis (OA). This study was done to search the literature and review the correlation between vitamin D levels in knee OA in the adult population and the outcome of vitamin D supplementation in knee OA.
Methods
An exhaustive search of Pub Med and Cochrane library database was done with keywords vitamin D, knee and osteoarthritis for a period from Jan 2005 to December 2015. All Randomized Control Trials (RCT), Cohort, Case-control, cross-sectional studies were included in the present systematic review.
Results
The search resulted in a total of 86 studies; out of which 11 studies were included in the current review. There were two Randomized Control Trial (RCT), one case-control, four cross-sectional and four cohort studies. These studies comprised of a total of 5137 participants (ranging from 46 to 1248) Our results suggested there was a moderate evidence of positive association in vitamin D deficiency (VDD) and progression of radiographic OA (ROA), as assessed by Kellgren and Lawrence (KL) grading. However, VDD was not associated with the incidence of ROA and MRI-detected change in focal cartilage defect. However, this study has a limited evidence for a positive correlation in VDD and the cartilage volume loss. There was also limited evidence showing no role of vitamin D therapy in reducing cartilage volume loss and knee pain in Knee OA.
Conclusion
The VDD is common and has been associated with knee OA, in an adult population. However, there is still inconsistent evidence regarding the prevention of incidence and progression of ROA after vitamin D therapy. There is a need for multicentric and well-conducted randomized studies with larger samples to conclude the positive effect of Vitamin D therapy.
Keywords: Knee, Osteoarthritis, Vitamin D deficiency, Systematic review
1. Introduction
Osteoarthritis (OA) is one of the major contributing factors for joint pain and impaired mobility, worldwide.1 Obesity and aging population have raised the number of patients with symptomatic OA.2 Knee OA was graded as the 11th highest cause of global disability. The global age- standardized prevalence of knee OA was 3.8% with no change from 1990 to 2010.3 In the USA, the prevalence of OA is 13.5% of adults aged 25 yrs and 33.6% for 65yrs of age and older in 2005.4 Vitamin D deficiency (VDD) is highly prevalent, and the over a billion people worldwide are vitamin D deficient or insufficient.5 Vitamin D reserve in the body decrease with the aging process and hence elderly women are more susceptible to subclinical VDD.6 The incidence of VDD in developing country is rising dramatically and therefore, VDD has gained increasing interest from the physicians.7
Receptors of Vitamin D have been demonstrated in the human chondrocytes. Through these receptors, vitamin D may regulate matrix metalloproteinase and prostaglandin E2 production.8 Vitamin D has been shown to have a direct impact on cartilage via vitamin D receptors by metabolic transformation, which stimulates proteoglycan synthesis in mature chondrocytes.9 Vitamin D deficiency inhibits bone remodeling, which may affect the bone changes, which eventually may evolve into OA by various pathophysiological processes. Alternately, Vitamin D could help in the prevention of progression of OA by enhancing bone remodeling and by reducing the abnormal pathophysiological phenomenon.10,11
There are several published systematic reviews which have shown the association of vitamin D and OA, physical activity, muscle strength, quality of life, obesity, bone density and common health outcomes.12, 13, 14, 15, 16, 17 However, no reviews have been performed specifically to investigate between vitamin D level and knee OA in the adult population. The aim of this systematic review is to review the association of vitamin D and knee OA in the adult population and the effect of vitamin D therapy on progression of knee OA.
2. Methods
We performed a search of the entire database of Pubmed and Cochrane Library from Jan 2005 to December 2015. Search terms included were the “Knee”, “Osteoarthritis” and “Vitamin D”. We included only studies published in English literature. Studies were included if they were randomized controlled trials, case-control, cohort and cross-sectional studies showing the association of vitamin D and Knee OA. The study was excluded if it showed an association between osteoporosis and any other joint OA, other than the knee. The level of evidence was further defined using the criteria employed by Lievense et al and Cao et al18,19 (Table 1).
Table 1.
Table showing level of evidence and their explanation.
| Level of evidence | Explanation |
|---|---|
| Strong evidence (High) | Consistent findings in multiple high-quality cohort studies. |
| Moderate evidence (Moderate) | Consistent results in two high-quality cohort studies, or in one high-quality cohort study and two or more high-quality case control/cross-sectional studies, or in three or more high-quality case-control/ cross-sectional studies. |
| Limited evidence (Low) | Consistent findings in a single cohort study, or in two or more case-control/cross-sectional studies. |
| Insufficient evidence (Very low) | Finding in single case control or cross-sectional study; conflicting evidence—conflicting findings (i.e., <75% of the studies reported consistent results). |
| No evidence | No studies can be found. |
The studies were further filtered using the keywords – “human studies”, “case-control studies”, “randomized control studies”, and “cohort studies”. All the studies were further assessed, and data was extracted including the name of the author, years of publication, country of origin, the number of participants, gender ratio, the mean age of participants, baseline Vitamin D levels, study design, results and conclusion. The results were extracted by two investigators (PL and VV). The results were cross-checked by the senior author (RV) (Table 1). The studies were included if they met all the inclusion criteria and were not blinded regarding their source, affiliation and funding. The review articles, expert opinion, and abstracts from scientific meetings were excluded from this study. The systematic review was done using the PRISMA guidelines. The primary research question in this present systematic review was to find out the association of vitamin D with knee OA.
3. Results
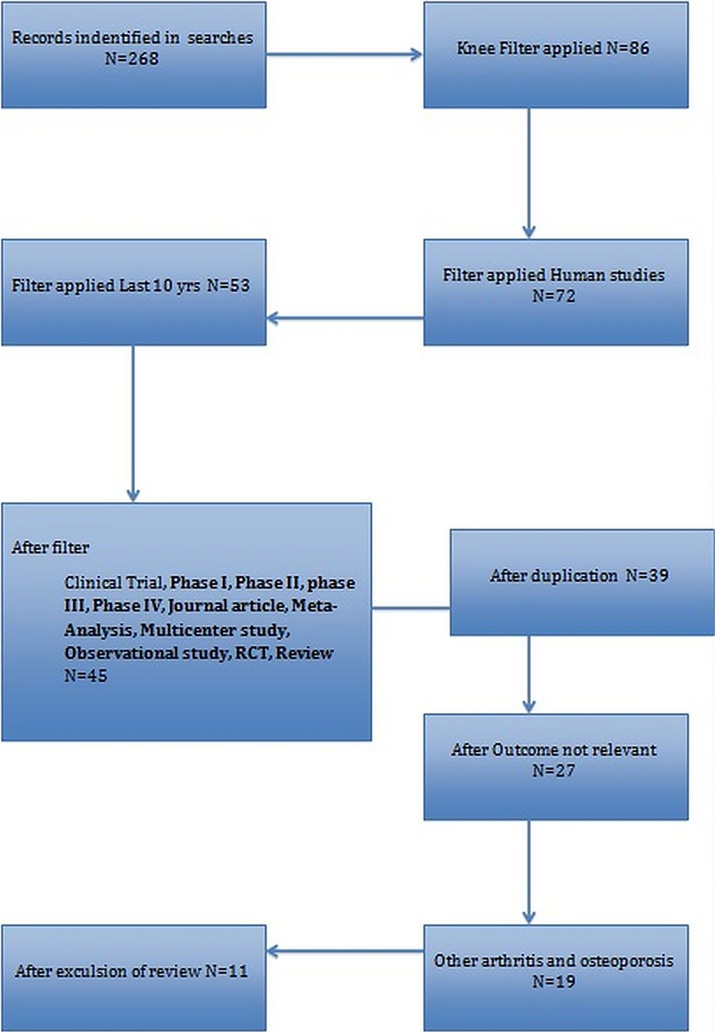
3.1. Prisma chart
Out of 286 search results for OA and knee, 86 articles were selected. After further application of filters and removal of duplicates, a total of eleven articles were found to be relevant and were finally included in the systematic review (Fig. 1).
Fig. 1.
PRISMA chart.
3.2. Characteristics of studies
There were 11 articles, which included two RCTs and nine observational studies regarding the vitamin D status and Knee OA. One article citing an observational study included the combination of 2 cohort studies.20 Out of a total number of 12 studies, four were done in the USA, 2 in the UK, one each in Iran, Egypt, Australia, India, Netherland, and Japan. The total numbers of the participants were 5137 (ranged from 46 to 1248), with the age ranging from 20 to 73.1 years and the gender ratio, male to female ranging from 0 to 100%. Two studies had all females, and one study has not given any details about the sex ratio of male to female.
3.3. Diagnosis of knee osteoarthritis
For the diagnosis of knee OA, a radiograph of the knee (Antero-Posterior view in standing position) was used. Kellgren and Lawrence (KL) grading, which combines assessments of joint space narrowing (JSN), osteophytes, sclerosis and deformity with a score of 0–4, was used for classification of the knee OA.20, 21, 22, 23, 24, 25, 26
MRI was used to assess bone marrow lesion and cartilage defect, cartilage loss.20,21 Musculoskeletal ultrasonography was used to determine the distal femur cartilage thickness of both knees in supine position from the midpoints of the medial condyle (MC), intercondylar area (IA) and lateral condyle (LC) in one study.27
3.4. Vitamin D assay
Serum vitamin D level was measured by the iodine-125 radioimmunoassay in 6 studies,1,28,29,30,31 chemiluminescence micro particle immunoassay in two studies,22,27 ELISA method in two studies24,26 and one study each used the TQD triple quadruple mass spectrometer23 and high-performance liquid chromatography (HPLC) method.25
3.5. Observational studies for the association between vitamin D and OA
Heidari et al found a significant association (p-value<0.05) between VDD and knee OA in young patients (aged<60 years) and suggested to measure serum vitamin D level in any patient with early knee OA symptoms.26 Fevziye et al reported that distal femoral cartilage is thinner in-patient with low vitamin D level (<10 ng/ml) when compared to patient with vitamin D level (>10 ng/ml).27 Jansen et al reported 24% prevalence of VDD in patients with severe knee OA, who were scheduled for TKA.29 Abu et al concluded that low level of the vitamin was found with newly diagnosed OA of the knee in postmenopausal Egyptian women (50–60 yrs).25 Berginik et al concluded that Vitamin D supplementation would prevent the progression of radiological progression of osteoarthritis (ROA) in an elderly population with low Bone Mineral Density (BMD).28
Ding et al found that there is a significant association between the sunlight exposure and VDD with knee cartilage loss.31 However, there was no significant association with cartilage defects in older adult (51–79 yrs.) people. Felson et al included two longitudinal studies (Framingham and Boston Osteoarthritis of the Knee Study (BOKS) study) and concluded that Vitamin D was not related to the joint space narrowing (JSN) and cartilage volume loss.20 Muraki et al22 also reported that there was no association between vitamin D level and ROA but had shown the gene polymorphism in Vitamin D receptor (VDR) was associated with knee pain (Table 2).
Table 2.
Observational studies and its characteristics.
| Author (Year of publication, country) | Number of Sample | Male :Female (M/F) | Baseline age | Baseline 25OHD level (ng/ml) | Study design | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Heidari et al (2011, Iran)26 | 298 | NA | Knee OA 60.2 ± 12.9 years and controls 60.1 ± 10.2 years | Knee OA : 23.8 ± 18.8, Control :34.5. ± 29.6 ng/ml, | case control | significant association between Vitamin D deficiency and knee OA in young patients (aged<60 years) and suggested to measure serum vitamin D level in any patient with early Knee OA symptoms. | Vitamin D was associated with knee OA in patients aged <60 years. |
| Fang Fang Zhang et al (2015 USA)23 | 418 | 46.6:53.4(223/195) | 61 ± 9.2 | 26.2 ± 10.3 | cohort | Participants with low vitamin D [25(OH)D < 15 mg/L] had >2-fold elevated risk of knee osteoarthritis progression | vitamin D deficiency has a increased risk of knee osteoarthritis |
| Fevziye Onsal Malas et al (2013, UK)27 | 80 | 0:100(0/80) | 20 to 45 | 20 | cross sectional | distal femoral cartilage is thinner in patient with low vitamin D level(<10 ng/ml) when compared to patient with vitamin D level(>10 ng/ml | Vitamin D deficiency is associated with thinning of distal femoral cartilage. |
| JA Jansen et al (2013, UK)29 | 139 | 42:58(58/81) | 71.4 | 40 | cross sectional | 24% prevalence of VDD in patients with severe knee OA who were scheduled for knee arthroplasty | Vitamin D is associated with high prevalence in elderly patients with advanced knee osteoarthritis. |
| Mohamed A.Abu Maaty et al (25)2013, Egypt | 46 | 22:78(10/36) | 54.7 ± 3.2 | 25 ± 1.6 | Cross-sectional | low level of Vitamin were found with newly diagnosed OA of Knee in post menopausal Egyptian women(50-60yrs | Vitamin D deficiency is associated with new OA knee in post menopausal |
| Ding C et al (2009, Australia)31 | 880 | 50:50(440/440) | 61 | 52.8 | Cohort | No significant association between vitamin D and sunlight exposure in Knee ROA but significant association was found between knee cartilage loss and VDD. | Vitamin D and sunlight exposure were both associated with reducing knee cartilage loss and concluded that vitamin D had a protective effect in knee cartilage. |
| Felson DT et al (2007, USA)21 | 992 | Framingham: 46.9:53.1 BOKS radiographic follow-up: 58.6:41.4 BOKS MRI follow-up: 61.6:38.4 |
Framingham study: 53.1 8.7 BOKS with radiographic follow-up: 66.2 (9.3); BOKS with MRI follow-up: 66.5 (9.6) |
Framingham study: 19.7 ± 7.4; BOKS radiographic follow up:20.2 ± 8.3; BOKS with MRI follow up: 20.3 ± 8.3 | 2 prospective cohort studies | No significant association between vitamin D and ROA and Knee cartilage loss | Vitamin D was not related to the joint space narrowing(JSN) and cartilage volume loss in Frangminham(mean age 53.1 yrs) and BOKS(mean age 66.2yrs) |
| Muraki S et al (2011, Japan)22 |
787 | 49:51 (388/399) | 65.6 ± 2.7 | 42.5 | cross sectional | No significant association of polymorphism of VDR with radiological OA but low tertile level vitamin D is associated with knee pain than high tertile of vitamin D level | VDD is not associated with radiographic Knee OA but is associated with knee pain and association between genetic polymorphism of VDR and knee pain were very weak evidence. |
| Bergink AP et al (2009, Netherland)28 | 1248 | 58:42(728/520) | 66.2 (6.7) | 26.4 (10.8) | cohort | Significant association between JSN and Knee ROA progression with low tertile vitamin D. | Low vitamin D intake increases the risk of progression of knee ROA particularly in subjects with low baseline BMD |
3.6. Randomized controlled trial for the relationship between 25-(OH) D and OA
Sanghi et al followed up their patients for 12 months and concluded that vitamin D supplementation had the positive association in reducing knee pain at 12 months.24 VAS (visual analogue score) was decreased by 0.26 unit and WOMAC pain by 0.55 unit in the vitamin D group whereas VAS was increased by 0.13 unit and WOMAC by 1.16 unit in the placebo group.
However, McAlindon et al followed up the patient for 24 months and reported that Vitamin D supplementation had no role in reducing knee pain or cartilage volume loss in symptomatic knee OA at two years.21 In their study, Vitamin D group had slightly higher scores for WOMAC pain and WOMAC function and less femoral cartilage volume, compared to placebo group. Vitamin D supplementation group had an increase in the mean plasma vitamin D level from 22.7 to 38.5 ng/mL in 2 yrs., whereas in the placebo group it had only rose from 21.9 to 24.7. Similarly, knee pain (WOMAC) was similar in both groups and cartilage volume loss was also not significant.
3.7. Evidence level of studies for association of vitamin D with knee OA
The standard of evidence of the association between two parameters was further graded into strong, moderate, mild and limited levels of evidence.18
3.7.1. Vitamin D and prevalence of symptomatic OA
Four studies reported on the association of vitamin D with symptomatic OA. Three studies reported on osteoarthritis using a questionnaire for symptoms of knee OA. One study used the Knee Society score (KSS) for studying the association of vitamin D and symptomatic OA. All these studies showed a positive association between the two factors. There was moderate evidence of association between VDD and prevalence of symptomatic osteoarthritis (1 case control/2 cross-sectional studies).22,25,26
3.7.2. Vitamin D and prevalence of ROA
There were three studies (all cross-sectional studies), which tried to associate vitamin D with the prevalence of radiographic osteoarthritis. Two studies22,31 suggested there was no significant association between OA and prevalence of ROA using KL and presence of osteophytes and one study31 which used joint space narrowing as radiological evidence of OA suggested there was a positive relationship between the VDD and prevalence of ROA. There was limited level of evidence for the prevalence of knee OA.
3.7.3. Vitamin D and incidence of ROA
Two studies21,28 reported on the incidence of ROA using the KL grading (one cohort20 and one RCT28). They reported no association between the two and the level of evidence was moderate. Only a single cohort study reported on the positive association between VDD and incidence of JSN but the level of evidence was limited.
3.7.4. Vitamin D and thickness of cartilage
The level of evidence was limited since there is the only single retrospective study to demonstrate that VDD had a negative correlation with the thickness of distal femoral cartilage using ultrasonography.27
3.7.5. Vitamin D and progression of OA
Two studies used the KL grading for studying the progression of OA (one cohort study19 and one RCT27), and both reported an association between low vitamin D levels and radiographic progression of OA. The level of evidence was moderate (two high-quality studies - 1 cohort and 1 RCT to demonstrate the positive association between VDD and progression of ROA assessed by KL. Three studies reported the progression by the presence of osteophytes (all cohort studies).20,21 One study reported positive association and two negative associations but overall the level of evidence was conflicting. One cohort study reported a positive association of progression of joint space narrowing (JSN), but the level of evidence was limited.23
3.7.6. Vitamin D and focal cartilage defect progression and volume loss
The level of evidence was moderate (two high-quality cohort studies1,30) to suggest that VDD was not associated with focal cartilage defects but limited evidence for cartilage volume loss using MRI.28
4. Discussion
Recently, vitamin D has been related to the possibility of changing disease course in some systemic diseases.7 Although there have been some studies, the results have been ambiguous. Since vitamin D plays a pivotal role in the bone and cartilage metabolism, it seems logical to correlate that it may have an association with incidence and progression of arthritis of all the joints in general and the weight bearing joints like knee and hip, in specific. The purpose of the present systematic review was to review the literature for the association of vitamin D with knee OA.
In the present review, there was moderate evidence of the prevalence of symptomatic OA of the knee in VDD patients in three studies20,23,25 (Table 3). Hedari et al reported that VDD prevalence is higher in younger adults, and there was a significant positive association with knee OA in younger patients with age <60 yrs.26 They did not perform X-rays of asymptomatic patients and included all of them in the control group. This may skew the results as asymptomatic patients may also have OA. There is also a possibility that asymptomatic patients may have a low vitamin D level, and hence, this might decrease the difference in the mean value of the study and the control group.
Table 3.
Randomized control trial studies for the association between 25-(OH) D and OA.
| Author (Year of publication, country) | Number of Sample | Male :Female (M/F | Baseline age | Baseline 25OHD level (ng/ml | Study design | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Timothy McAlindon et al (2013 USA)20 | 146 | 0:100(0/146) | 62.4 | placebo:37.52 ± 7.53 vitamin D group 37.03 ± 7.54 |
RCT | knee pain and function worsened, cartilage volume decreased in both groups | Vitamin D supplementation had no role in reducing knee pain or cartilage volume loss in symptomatic knee OA at 2 years |
| Divya sanghi et al (2013, India)24 | 103 | Placebo Males 21 (40.6%) Females 30 (59.4%) Vitamin D group Male 16 (30.3%) Female 36 (69.7%) |
Placebo 53.00 ± 7.44 (40–74) Vitamin D group 53.24 ± 9.64 (40–70) |
placebo:37.52 ± 7.53 vitamin D group 37.03 ± 7.54 |
RCT | Vitamin supplementation is associated with reducing knee pain and function and not significant in ROA | vitamin D supplementation had positive association in reducing knee pain at 12 months |
Muraki et al reported that vitamin D status had no association with radiographic knee OA but was associated with knee pain.22 There was a weak level of evidence for an association between the genetic polymorphism of vitamin D receptors and knee OA pain. It may be because they did not correlate JSN or osteophyte with Vitamin D or Vitamin D receptor polymorphisms.
Mohamed et al studied the prevalence of vitamin D in newly-diagnosed knee OA in postmenopausal women and reported a sex-specific difference because only female patients were selected.25 Healthy male participant’s vitamin D level is not comparable to that of the female patients as the clothing habits, and outdoor activity levels are different. Jenson et al concluded that there is a high prevalence of VDD with advanced knee OA in elderly patients scheduled for knee arthroplasty however in their total sample size of 139, only 33 had low VDD and remaining patients had normal vitamin D, hence there is only 24% prevalence of VDD.29 One study has reported that VDD was related to quadriceps weakness, which leads to the knee pain.31
Ding et al concluded that VDD was not associated with ROA in a knee.31 The mean vitamin D level (52.8 mol/liter) was higher in their study than other baselines and hence may be a source of potential bias. Moreover, the radiographs of the knee were not obtained at the first follow-up hence; the correlation of vitamin D with the change in osteophytes was not reported. Mala et al had shown a negative association of VDD in distal femoral cartilage thickness using ultrasound.27 Since they included only females so their results cannot be extrapolated to males and the evidence is limited. There is moderate evidence showing the progression of ROA changes with low vitamin D level. Berginik et al28 and Mc Alindon et al21 have shown the positive association with progressing of KL scoring in knee OA with low vitamin D, however conflicting evidence regarding the osteophyte progression in the knee was reported in Felson et al in their study.20
Framingham et al reported that those with VDD had a modest decreased risk of ROA changes than those with sufficient levels of vitamin D, but the result was not significant (Table 3).20 In the BOKS et al, there was no association of VDD with osteophyte progression (Table 3).20 The radiograph of the knee without fluoroscopy guidance is not an acceptable method of defining the ROA progression.33 It may be because the radiographs used were full extension AP view of the knee by BOKS et al and were fluoroscopy guided in Framingham study, that the two studies showed the difference in their results.19,20
In Ding et al’s study no significant association was reported between VDD and increased cartilage defects of the knee or an increase in total knee cartilage volume loss.31 However in BOKS et al, there was a lower risk of cartilage loss with sufficient Vitamin D level (9.9%) when compared to VDD (13.1%) and concluded that vitamin D plays a protective role in cartilage volume loss (Table 3).20 This may be attributed to the fact that Ding et al measured the tibial plateau to gauge the cartilage of the knee joint rather than tibiofemoral cartilage for knee joint cartilage loss.31 The role of vitamin D and its association with quadriceps weakness has also been studied and found to have a positive association.32 It is possible that VDD is associated differently with femoral cartilage and tibial cartilage.33 In BOKS et al, MRI measurement of tibiofemoral cartilage was scored on all five plates (both tibia and femoral cartilage planes) in both the medial and lateral tibiofemoral joints.19 Therefore, MRI results of the two studies cannot be compared significantly to conclude the effect of vitamin D on cartilage defect or volume loss in the knee joint.
There was a moderate evidence showing the prevalence of symptomatic OA in VDD and limited evidence showing a decrease in Knee Society Score with VDD. There was moderate evidence showing that prevalence of radiographic OA (joint space narrowing and osteophytes) was not associated with VDD. However, moderate evidence supported the “progression” of radiographic OA; it is an association with VDD and that vitamin D supplementation can decrease the progression of ROA. There is conflicting evidence showing the association of progression of osteophytes (ROA) with VDD. There is limited evidence regarding the progression of JSN and Vitamin D supplementation (Table 3).
There was moderate evidence showing that progression of MRI detected cartilage defect is not associated with VDD, but there is limited evidence showing the progression of MRI detected cartilage volume loss in low vitamin D levels (Table 3). There is limited evidence which suggests showing the distal femoral cartilage thickness is inversely proportional to the VDD using the musculoskeletal ultrasonography (Table 3). There is conflicting evidence indicating the role of vitamin D in decreasing subjective knee pain (Table 4).
Table 4.
Evidence level for VD Association with Knee OA.
| Association of VDD to OA knee | Study | Effective Evidence level | Types of studies | |
|---|---|---|---|---|
| SOA prevalence | Questionnaire | Muraki S et al + Heidari et al + Mohamed A.Abu + |
Moderate (4+) | 1case control/3cross-sectional) |
| KSS score | Ja jenson et al + | |||
| ROA Prevalence | KL | Muraki S et al(-) | Limited 1- | cross-sectional study |
| Osteophytes | Ding C. et al(-) | limited 1- | cross-sectional study | |
| JSN | Ding C. et al(+) | limited1+ | cross-sectional study | |
| ROA Incidence | KL | Berginik AP. et al(-) Mc Alindon et al(-) |
Moderate 2- / | 1Cohort study 1RCT |
| JSN | Bergnik AP. et al (+) | Limited 1+ | Cohort study | |
| Distal femoral cartilage thickness | Ultrasonography | Fevziye Ünsal Malas et al (+) | Limited 1+ | retrospective study |
| ROA Progression | KL | Bergnik AP. et al (+) Mc Alindon et al (+) |
Moderate (2+ | 1Cohort 1RCT |
| Osteophytes | Mc Alindon et al (+) Felson DT. et al (Framingham)(-) Felson DT et al (BOKS) (-) |
Conflicting (2-and 1+)) | Cohort studies | |
| JSN | Fang fang et al (+) | Limited 1+ | Cohort studies | |
| cartilage defect Progression | Felson DT et al BOKS (-) Ding C. et al(-) |
Moderate 2- | Cohort studies | |
| cartilage volume loss Progression | Ding C. et al(+) | 1+ | Cohort studies |
There were some limitations in our study. Firstly, the study samples were few; only 11 studies were included relevant to Knee OA because of few high-quality article relating particularly to knee OA and vitamin D and secondly meta- analysis was not performed due to the heterogeneity of the studies. However, most studies which were included in this systematic review were high-quality samples >200.
In summary, the VDD is common and has been associated with knee OA, in the adult population. However, there is still inconsistent evidence regarding the association of VDD and knee OA and its effect (Table 5). There is a need for multicentric and well-conducted randomized studies with larger samples to conclude the positive effect of Vitamin D supplementation.
Table 5.
Table summarizing the association of Vitamin D deficiency with osteoarthritis knee.
| Association of VDD to OA knee | Types of studies | Type of association with vitamin D deficiency |
|---|---|---|
| SOA prevalence | 1case control 3cross-sectional |
Positive |
| ROA Prevalence | 2 cross-sectional studies | Negative |
| 1 cross-sectional study | Negative | |
| ROA Incidence | 1Cohort study 1RCT |
Negative |
| Cohort study | Positive | |
| Distal femoral cartilage thickness | Retrospective study | Positive |
| ROA Progression | 2Cohort 1RCT |
Positive |
| 2 Cohort studies | Negative | |
| Cartilage defect Progression | Cohort studies | Negative |
| Cartilage volume loss Progression | Cohort studies | Positive |
Abbreviations – osteoarthritis – OA; SOA – symptomatic osteoarthritis; ROA – radiological osteoarthritis.
Contributor Information
Raju Vaishya, Email: raju_vaishya@apollohospitalsdelhi.com.
Vipul Vijay, Email: v@apollodelhi.com.
Amit Agarwal, Email: dramit_a@apollodelhi.com.
References
- 1.Felson D.T. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Yuguing Z., Hordan Joanne M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damian H., Lyn M., Anthony W. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):949–950. doi: 10.1136/annrheumdis-2013-204763. Epub 2014 Feb 19. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence R.C., Felson D.T., Helmrick C.G. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollick M.F., Chen T.C. Vitamin deficiency a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 6.Tsaei K.S., Wahner H.W., Offord K.P., Melton L.J., Kumar R., Rigs B.L. Effect of aging on vitamin D stores and bone density in women. Calcif Tissue Int. 1987;40(5):241–243. doi: 10.1007/BF02555255. [DOI] [PubMed] [Google Scholar]
- 7.Raju V., Vipul V., Amit K.A., Jabed J. Resurgence of vitamin D: Old wine in new bottle. J Clin Orthop Trauma. 2015;6(3):173–183. doi: 10.1016/j.jcot.2015.02.002. Epub 2015 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetlow L.C., Woolley D.E. Expression of vitamin D receptors and matrix metalloproteinase in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartil. 2001;9(5):423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 9.Corvol M.T., Dumontier M.F., Tsagris L., Lang F., Bourguignon J. Cartilage and vitamin D in vitro (author’s transl) Ann Endocrinol (Paris) 1981;42:482–487. [PubMed] [Google Scholar]
- 10.McAlindon T.E. Nutraceuticals: do they work and when should we use them? Best Pract Res Clin Rheumatol. 2006;20:99–115. doi: 10.1016/j.berh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Holick M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 12.Urquhart D.M., Tobing J.F., Hanna F.S. What is the effect of physical activity on the knee joint? A systematic review. Med Sci Sports Exerc. 2011;43:432–442. doi: 10.1249/MSS.0b013e3181ef5bf8. [DOI] [PubMed] [Google Scholar]
- 13.Stockton K.A., Mengersen K., Paratz J.D. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–871. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 14.Muir S.W., Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011;59:2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 15.Renzaho A.M., Halliday J.A., Nowson C. Vitamin D, obesity, and obesity-related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27:868–879. doi: 10.1016/j.nut.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Winzenberg T., Powell S., Shaw K.A. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi: 10.1136/bmj.c7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung M., Balk E.M., Brendel M. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 18.Lievense A., Bierma-Zeinstra S., Verhagen A. Influence of work on the development of osteoarthritis of the hip: a systematic review. J Rheumatol. 2001;28(11):2520–2528. [PubMed] [Google Scholar]
- 19.Yuelong C., Tania W., Kay N., Jianhao L. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology (Oxford) 2013;52:1323–1334. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 20.Felson D.T., Niu J., Clancy M. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 21.McAlindon Timothy, LaValley Michael, Schneider Erica. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis a randomized controlled trial. JAMA. 2013;309(2):155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraki S., Dennison E., Jameson K. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:1301–1306. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F.F., Driban J.B., Grace H.L. Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr. 2014;144:2002–2008. doi: 10.3945/jn.114.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Divya S., Abhishek M., Amar C.S. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res. 2013;471:3556–3562. doi: 10.1007/s11999-013-3201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed A.A., Hanafi R.S., Badawy S.E. Association of suboptimal 25-hydroxyvitamin D levels with knee osteoarthritis incidence in post-menopausal Egyptian women. Rheumatol Int. 2013;33(11):2903–2907. doi: 10.1007/s00296-012-2551-9. [DOI] [PubMed] [Google Scholar]
- 26.Heidari B., Heidari P., Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. International Orthopaedics (SICOT) 2011;35:1627–1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malas F.U., Kara M., Aktekin L., Ersoz M. Does vitamin D affect femoral cartilage thickness? An ultrasonographic study. Clin Rheumatol. 2014;33(9):1331–1334. doi: 10.1007/s10067-013-2432-y. [DOI] [PubMed] [Google Scholar]
- 28.Bergink A.P., Uitterlinden A.G., Van Leeuwen J.P. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: the Rotterdam study. J Clin Rheumatol. 2009;15:230–237. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 29.Jansen J.A., Haddad F.S. High prevalence of vitamin D deficiency in elderly patients with advanced osteoarthritis scheduled for total knee replacement associated with poorer preoperative functional state. Ann R Coll Surg Engl. 2013;95 doi: 10.1308/003588413X13781990150374. 569–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Jarallah K.F., Shehab D., Al-Awadhi A. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med Princ Pract. 2012;21:74–78. doi: 10.1159/000330025. [DOI] [PubMed] [Google Scholar]
- 31.Ding C., Cicuttini F., Parameswaran V. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009;60:1381–1389. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 32.Annweiler C., Schott-Petelaz A.M., Berrut G. Vitamin D deficiency related quadriceps weakness: results of the Epidemiologie De l’Osteoporose cohort. J Am Geriatr Soc. 2009;57(2):368–369. doi: 10.1111/j.1532-5415.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 33.Mazzuca S.A., Brandt K.D. Plain radiography as an outcome measure in clinical trials involving patients with knee osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):467–480. doi: 10.1016/s0889-857x(05)70079-x. ix. [DOI] [PubMed] [Google Scholar]