Abstract
Purpose
We characterized both physician- and patient-reported rates of gastrointestinal (GI) toxicity in patients treated with proton beam therapy (PBT) at our institution for prostate adenocarcinoma and identified factors associated with toxicity.
Methods and materials
We treated 192 patients with PBT between July 2013 and July 2016. Included patients had ≥1 year of follow-up. Potential preexisting clinical and treatment-related risk factors for GI toxicity were recorded. Common Terminology Criteria for Adverse Events version 4.0 was used to score toxicity. Expanded Prostate Cancer Index Composite (EPIC) bowel domain questionnaires assessed patient-reported quality of life. Associations between grade (GR) 2+ toxicity and clinical, treatment, and dosimetric factors were assessed using Cox models and corresponding hazard ratios.
Results
The median follow-up was 1.7 years. Most of the observed GI toxicity (>90%) was in the form of rectal bleeding (RB). GR2+ GI toxicity and RB actuarial rates specifically at 2 years were 21.3% and 20.4%, respectively. GR3 toxicity was rare, with only 1 observed RB event. No GR4/5 toxicity was seen. The EPIC bowel domain median score was 96 (range, 61-100) pretreatment, 93 (range, 41-100) at 1 year, 89 (range, 57-100) at 1.5 years, and 89 (range, 50-100) at 2 years. Anticoagulation use was the only factor selected during multivariate analysis for predicting GR2+ RB, with a resulting concordance index of 0.59 (95% confidence interval, 0.48-0.68; P = .088). Type of proton technology (pencil beam scanning vs uniform scanning) and number of fields treated per day (1 vs 2) showed no significant difference in toxicity rate.
Conclusions
PBT was associated with acceptable rates of GR2+ transient GI toxicity, mostly in the form of RB, which correlated with anticoagulation use. High EPIC bowel domain quality of life was maintained in the 2 years after treatment.
Introduction
Gastrointestinal (GI) toxicity is a key dose-limiting side effect of definitive external beam radiation therapy (EBRT) for localized prostate cancer and significantly affects patient quality of life (QoL).1 With the increasing availability of proton therapy around the world, proton beam therapy (PBT) is becoming a more common modality for EBRT. Dosimetry planning studies in prostate cancer have shown that, compared with intensity modulated radiation therapy (IMRT), PBT-based plans can offer excellent target volume coverage with a significantly reduced low-to-moderate dose profile to organs at risk (OARs), including the rectum and bladder, especially with newer proton technology such as pencil beam scanning (PBS).2
Thus, an open question remains regarding whether PBT offers an additional QoL benefit to patients who elect to undergo definitive radiation therapy for prostate cancer. A small number of centers have reported their observed rates of GI-related toxicities in patients treated with PBT, with rates of grade (GR) 2+ toxicities (those requiring intervention) ranging from 1% to 15.4%.3, 4, 5 There is a need to better characterize the incidence of GI toxicity in patients receiving PBT for prostate cancer.
Although randomized evidence comparing IMRT and PBT in patients with low-intermediate risk prostate cancer is currently being collected (NCT01617161) with the primary endpoint being difference in patient-reported GI toxicity at 2 years, the results will not be available for years. To date, there is conflicting evidence with regard to comparative toxicities. Although PBT produces lower low-dose bath to the rectum than does IMRT, several retrospective studies using the Surveillance Epidemiology and End Results database have suggested that PBT may actually be associated with higher rates of rectal toxicity than IMRT.6, 7, 8 A more recent study using private insurance claim codes suggests a higher rate of rectal toxicity at nearly twice the cost of IMRT.9
However, these studies have been criticized for their dependence on correlative data (eg, Medicare claims codes) as surrogates for patient- and physician-reported toxicities10 and are in contrast with institutional studies that report exceedingly low rates of GR2+ toxicities.4 Also, since the initiation of PBT usage in patients with prostate cancer in the United States more than 2 decades ago, PBT delivery techniques have improved with the advent of PBS, which can produce a more conformal dose distribution than uniform scanning (US)/passive scatter techniques, the use of lateral fields rather than a single perineal beam (which may have more unfavorable rectal dosimetry), and image guided radiation therapy with intraprostatic fiducials, which allow smaller planning target volume (PTV) margins.11
The goals of the present study were to characterize both physician- and patient-reported GI-related adverse effects of PBT in patients with prostate cancer treated at our institution and to identify factors associated with GI toxicity.
Methods and materials
Patients
Records of 231 consecutive patients with prostate cancer treated with PBT at our institution between 2013 and 2016 were reviewed in a prospective institutional review board–approved study. Patients were excluded if they received previous radical prostatectomies, received prior radiation, received pelvic IMRT followed by a proton boost, or had follow-up <1 year. A total of 192 patients were included in this analysis, and the baseline characteristics are shown in Table 1. All patients had their pathology test results reviewed at our institution to ensure consistency of diagnosis. All patients had pretreatment serum prostate-specific antigen tests. Intermediate-risk patients underwent staging pelvic computed tomography and/or magnetic resonance imaging. Patients with high-risk disease also had staging technectium bone scans.
Table 1.
Patient characteristics and dosimetry (N = 192)
| Variable | n (%) or median (range) |
|---|---|
| Age, y | 68 (50-85) |
| Race and ethnicity | |
| African American | 3 (1.6) |
| Asian | 4 (2.1) |
| Hispanic | 2 (1.0) |
| White | 176 (91.7) |
| Unknown | 7 (3.6) |
| T stage | |
| T1 | 104 (54.2) |
| T2a | 49 (25.5) |
| T2b | 25 (13.0) |
| T2c | 4 (2.1) |
| T3-T4 | 10 (5.2) |
| Prostate-specific antigen, ng/mL | 7.2 (1.6-69.6) |
| Gleason score | |
| ≤6 | 42 (21.9) |
| 7 = 3 + 4 | 80 (41.7) |
| 7 = 4 + 3 | 36 (18.8) |
| 8 | 14 (7.3) |
| 9-10 | 20 (10.4) |
| Risk category | |
| Low | 38 (19.8) |
| Intermediate | 104 (54.2) |
| High | 50 (26.0) |
| Baseline EPIC score∗ | 96 (60-100) |
| Comorbidities | |
| Diabetes | 19 (9.9) |
| Hypertension | 96 (50.0) |
| Inflammatory bowel disease | 2 (1.0) |
| Hemorrhoids | 26 (13.5) |
| Irritable bowel syndrome | 5 (2.6) |
| Smoking status∗ | |
| Never | 103 (57.5) |
| Former | 65 (36.3) |
| Current | 11 (6.1) |
| Medication use | |
| Aspirin | 74 (38.5) |
| Anticoagulation | 22 (11.5) |
| Alpha blocker | 37 (19.3) |
| Androgen deprivation therapy | 71 (37.0) |
| Low risk† | 1 of 71 (1.4) |
| Intermediate risk† | 27 of 71 (38.0) |
| High risk† | 43 of 71 (60.6) |
| Pencil beam scanning (vs uniform scanning) | 143 (74.9) |
| Number of fields treated/day | |
| 1 | 92 (47.9) |
| 2 | 100 (52.1) |
| Seminal vesicle radiation | 154 (80.2) |
| Whole pelvis radiation | 19 (9.9) |
| Ultrasound-based prostate volume∗, cm3 | 40.0 (12.0-100.4) |
| Dose | |
| <79.2 Gy (RBE) | 27 (14.1) |
| ≥79.2 Gy (RBE) | 165 (85.9) |
| DVH parameters | |
| Rectal wall V50, % | 30.7 (8.0-56.6) |
| Rectal wall V75, % | 16.2 (0.0-30.6) |
| Rectum V50, % | 18.6 (2.0-39.4) |
| Rectum V70, % | 9.2 (0.0-34.7) |
| Bowel maximum dose Gy (RBE)‡ | 51.4 (46.0-54.0) |
Abbreviations: DVH = dose-volume histogram; EPIC = Expanded Prostate Cancer Index Composite; RBE = relative biological effectiveness.
Patients with missing values were excluded from the corresponding summary: EPIC score (n = 28), smoking status (n = 13), uniform scanning–based prostate volume (n = 12).
Based on patients receiving androgen deprivation therapy (n = 71).
In patients receiving whole pelvis proton beam therapy.
Proton therapy treatment
All patients underwent intraprostatic placement of 3 Visicoil fiducial markers (IBA Dosimetry GmbH, Schwarzenbruck, Germany) under transrectal ultrasound guidance. Patients underwent a computed tomography simulation at 1.25 mm slice thickness with a full bladder and prior bowel preparation for an empty rectum. Supine positioning was secured with a vacuum-locked body mold. Rectal balloons were inserted in all patients and filled with 90 mL of saline. Rectal spacer (SpaceOAR, Augmenix) use at our center began in mid-2016; at the time of this analysis, most did not have adequate follow-up for inclusion. Urethrograms and magnetic resonance imaging prostate scans were performed per the physician's preference.
OARs including the bladder, bladder wall, rectum, rectal wall, femoral heads, penile bulb, and bowel were manually contoured per the Radiation Therapy Oncology Group (RTOG) contouring atlas guidelines. The rectum was defined and contoured as the segment between the ischial tuberosities inferiorly to the sigmoid flexure superiorly. The rectal wall and bladder wall OAR ring structures were automatically created using a 3-mm-thick contraction of the rectal and bladder structures, with the rectal wall only extending 1 cm superior-inferior to the PTV.
The primary clinical target volume (CTV) included the prostate ± seminal vesicles depending on the risk group. PTV expansion from the CTV was 5 mm uniformly, except for the posterior margin, which was 4 mm. Patients with high-risk cancer were treated with whole pelvis radiation, which included a nodal CTV of 7 mm around vessels and a 5-mm PTV margin, at the discretion of the treating physician.
Proton therapy was delivered using the Proteus Plus system (IBA, Louvain-la-Neuve, Belgium) with either US or PBS. Our center transitioned from US to PBS in 2015. In US, patient-specific brass apertures were created based on PTV structures with a 0.8 to 1.2 cm margin to account for penumbra. Wax range compensators were designed with an additional 2.5% + 2-mm range uncertainty added to the distal and proximal ranges, as well as 1- to 2-cm smearing margins.
Commercially available Xio treatment planning software (Impac Medical Systems, Maryland Heights, MO) was used. The dose was verified with an ion chamber measurement performed in water, and field shape was verified by comparing the physical shape of the apertures and compensators with the treatment planning system. For PBS delivery, treatment plans were created using RayStation (RaySearch Laboratories AB, Stockholm, Sweden) with single-field uniform dose optimization. Dose and fluence were measured before treatment using an ion chamber array device. Delivered and predicted dose maps were compared by calculating a gamma index. The standard for verification was a gamma pass rate of >90% using acceptance criteria of 3% per 3 mm. Two laterally opposed beams were used in the majority of treatment plans, with approximately half of patients treated with 2 fields per day (52%) versus 1 field per day (48%).
After positioning and rectal balloon placement, daily orthogonal kilovoltage films were obtained for fiducial localization for all patients. A digital imaging positioning system was used to determine optimal table shifts along 3 axes to reproduce fiducial localization within 2 mm of the simulation images (digitally reconstructed radiography). Treatment positioning was re-evaluated if more than 5 minutes passed before beam availability.
Outcome measurement and follow-up
Toxicity grading was based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, with assessments of toxicity performed weekly during treatment, at 3-month intervals for the first year of follow-up, and then at 6-month intervals afterward.12 Expanded Prostate Cancer Index Composite (EPIC) QoL questionnaires were collected prospectively at the above intervals.
GR1 rectal bleeding (RB) involved self-limited bleeding, including patients who received colonoscopies if no intervention was performed. To isolate instances of RB related to treatment alone, patients were excluded from the GR1 RB category if they had a history of hemorrhoids (n = 29) or inflammatory bowel disease, including Crohn disease or ulcerative colitis (n = 2). GR2 RB was subdivided by intervention into medical (GR2A; eg, rectal suppositories or enemas) or procedural (GR2B, including laser photocoagulation, topical formalin application, or electrocautery). No patients received hyperbaric oxygen.
Preexisting clinical conditions that might contribute to toxicity were recorded. Acute toxicity was defined as occurring either during PBT or up to 90 days after completion of PBT. Late toxicity was defined as toxicity occurring at any point after 90 days. Patient-reported QoL surveys were administered at each follow-up visit using EPIC bowel domain, with a minimum score of 0 (worst) to 100 (best). Scores were analyzed at pretreatment, 1 year, 1.5 years, and 2+ years (for patients with >2-year follow-up, the last follow-up score was used in this analysis).
Statistical analysis
Statistical computations were performed with OriginLab software and R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Cumulative incidence of post-PBT toxicity was estimated using the Kaplan-Meier product limit estimator. Univariate analysis of associations between GR2+ RB and potential prognostic factors was performed using Cox proportional hazards regression. The univariate analysis was exploratory and hypothesis-generating, so P-values were not adjusted to account for the number of comparisons.
Because of the low number of GR2+ RB events, the multivariate analysis was conducted on a smaller, prespecified set of factors that were thought to be potentially predictive of GR2+ RB on the basis of existing literature (history of hypertension, aspirin use, anticoagulant use, and dose-volume histogram [DVH] parameters) or unique to our analysis (PBS vs uniform scanning, number of treatment fields per day). The least absolute shrinkage and selection operator technique was used to perform simultaneous variable selection and model fitting. The overall prediction performance was summarized using the concordance index (c-index). The .632 bootstrap method was used for internal validation of the multivariate model performance to limit any upward bias in c-index estimates resulting from training and testing a model using the same data set.13
Changes in EPIC bowel QoL scores were analyzed using generalized estimating equation–based linear regression to account for the repeated measurements per patient. Throughout the data analysis, 2-sided P values < .05 were considered statistically significant.
Results
Patients
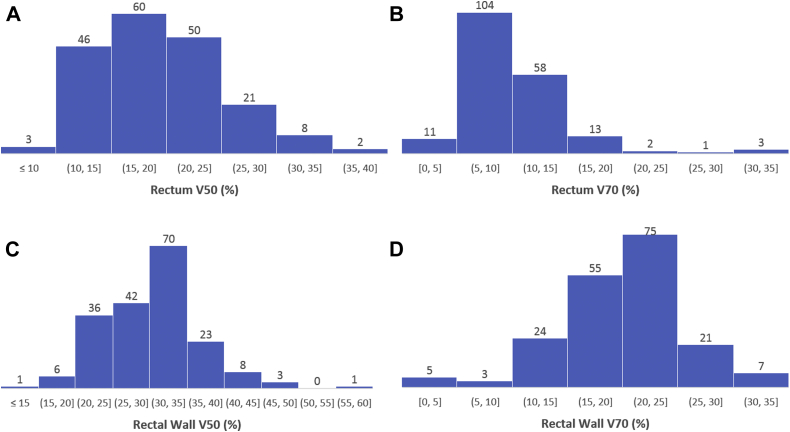
The median follow-up across all 192 patients was 1.7 years (range, 1-3.7 years), and 24% of patients had >2.5 years of follow-up. The rectal DVH parameters are shown in histogram form in Figure 1. Most patients (165 of 192; 86%) were treated to 79.2 Cobalt gray equivalents (CGE) in 44 fractions. Most patients (143 of 192; 75%) were treated using PBS, and the other 49 patients (25%) were treated with the US technique. A total of 100 patients (52%) were treated using 2 lateral fields per day, and 92 (48%) were treated using 1 field per day (Table 1).
Figure 1.
Rectal dose-volume histogram parameter distributions for 192 patients. (A) Rectum V50 Cobalt gray equivalents (CGE); (B) rectum V70 CGE; (C) rectal wall V50 CGE; and (D) rectal wall V70 CGE.
Overall GI toxicities
GR2+ GI toxicity in the acute and late windows combined was observed in 35 of 192 patients (18%) with an actuarial 2-year rate of 21.3%. The majority of GR2+ GI toxicity (>90%) was in the form of RB, with the remaining events being due to transient GR2 proctitis (isolated rectal discomfort) or diarrhea. No GR3+ diarrhea or proctitis was observed.
RB
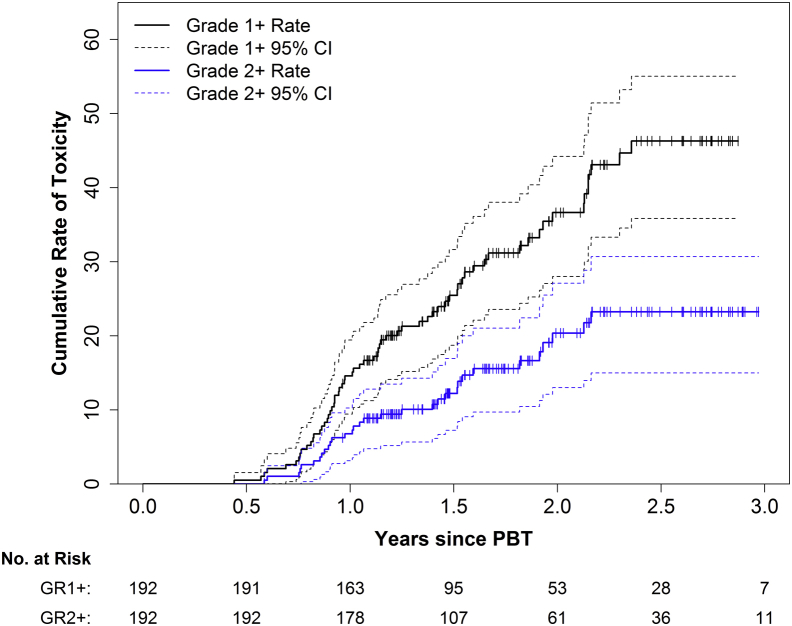
Most RB (GR1+) was transient and resolved without intervention. The actuarial rates of GR1+ and GR2+ RB at 2 years were 36.6% (95% confidence interval [CI], 28.0%-44.2%) and 20.4% (95% CI, 13.0%-27.1%), respectively (Figure 2). Thirty-five patients experienced a maximum of GR1 RB, and 8 of these patients received diagnostic colonoscopies that noted radiation-related changes without intervention. Thirty-one patients experienced a maximum of GR2 RB, of whom 17 received medical management only (GR2A) and 14 received argon photocoagulation or electrocautery (GR2B). Only 1 patient experienced GR3 RB, resulting in admission and transfusion. There were no GR4 or GR5 events. All RB events were transient and eventually resolved with treatment.
Figure 2.
Cumulative toxicity rates for grades 1+ and 2+ rectal bleeding. Solid lines indicate the Kaplan-Meier estimate, and dashed lines indicate the 95% confidence intervals.
The most common time for developing rectal toxicity was between 1 and 2 years posttreatment. The actuarial risk of GR2 or higher toxicity was 6.8% from 90 days posttreatment to 1 year of follow-up, 13.6% from 1 to 2 years, and 2.8% from 2 to 3 years.
Univariate and multivariate analyses of factors associated with RB
Table 2 shows the results of a univariate analysis of factors potentially associated with GR2+ RB. Anticoagulant use (hazard ratio [HR], 3.82; P = .001) and alpha blocker use (HR, 2.21; P = .033) were significantly associated with GR2+ RB. The 2-year incidence of RB was 46.5% for patients on anticoagulation versus 17.4% for patients not on anticoagulation. DVH parameters and treatment technique differences such as PBS and 1 field per day versus 2 fields per day and pelvic nodal irradiation were not significantly associated with GR2+ RB (P > .19 for all). Table 3 compares the characteristics of patients who developed GR2+ RB versus patients who did not.
Table 2.
Univariate analysis of clinical and treatment factors potentially associated with late grade 2+ rectal bleeding
| Variable | HR∗ | (95% CI) | P-value† |
|---|---|---|---|
| Age | 1.20 | (0.85-1.70) | .29 |
| Prostate-specific antigen‡ | 0.79 | (0.53-1.16) | .22 |
| Baseline EPIC score | 1.21 | (0.75-1.96) | .43 |
| History of diabetes | 0.27 | (0.04-1.96) | .19 |
| History of hypertension | 1.51 | (0.75-3.05) | .25 |
| History of hemorrhoids | 0.42 | (0.10-1.75) | .23 |
| Current smoker | 1.53 | (0.47-5.04) | .48 |
| Aspirin use | 1.54 | (0.77-3.09) | .22 |
| Anticoagulant use | 3.82 | (1.71-8.55) | .001 |
| Alpha blocker use | 2.21 | (1.07-4.59) | .033 |
| Androgen deprivation therapy | 0.76 | (0.36-1.61) | .47 |
| Pencil beam (vs uniform scanning) | 0.97 | (0.45-2.07) | .93 |
| Two fields treated/day (vs 1 field/day) | 0.70 | (0.34-1.43) | .33 |
| Elective seminal vesicle radiation | 0.80 | (0.36-1.80) | .60 |
| Whole pelvis radiation | 0.27 | (0.04-1.96) | .19 |
| Ultrasound-based prostate volume, cm3 | 0.90 | (0.63-1.29) | .58 |
| Dosimetry | |||
| Rectal wall V50 | 0.97 | (0.68-1.40) | .88 |
| Rectal wall V75 | 1.13 | (0.79-1.61) | .51 |
| Rectum V50 | 0.87 | (0.60-1.25) | .44 |
| Rectum V70 | 1.12 | (0.84-1.50) | .42 |
| Rectal Eval V75 | 1.07 | (0.76-1.52) | .70 |
Abbreviations: CI = confidence interval; EPIC = Expanded Prostate Cancer Index Composite; HR = hazard ratio.
HRs are presented as the difference per 1 standard deviation increase for continuous variables.
Wald test of the HR = 1 without adjustment for multiple comparisons.
Variable was log-transformed before including in the model to reduce right-skewness.
Table 3.
Characteristics of patients who experienced late grade 2+ rectal bleeding versus those who did not
| Variable | GR2+ RB (n = 32) |
No RB (n = 160) |
|---|---|---|
| n (%) or median (range) | n (%) or median (range) | |
| Age, y | 69 (55-86) | 69 (51-83) |
| Ultrasound-based prostate volume, cm3 | 42.0 (12.0-77.6) | 40.0 (12.0-100.4) |
| Medication use | ||
| Aspirin | 15 (46.9) | 59 (36.9) |
| Anticoagulation | 8 (25.0) | 14 (8.8) |
| Alpha blocker | 11 (34.4) | 26 (16.3) |
| Risk category | ||
| Low | 8 (25.0) | 30 (18.8) |
| Intermediate | 16 (50) | 88 (55.0) |
| High | 8 (25) | 42 (26.2) |
| Baseline EPIC score | 98.2 (62.5-100) | 96.4 (60.7-100) |
| Comorbidities | ||
| Diabetes | 1 (3.1) | 18 (11.3) |
| Hypertension | 18 (56.3) | 78 (48.8) |
| Hemorrhoids | 2 (6.3) | 24 (15.0) |
| Androgen deprivation therapy | 10 (31.3) | 61 (38.1) |
| Pencil beam scanning (vs uniform scanning) | 22 (68.8) | 122 (76.3) |
| Number of fields treated/day | ||
| 1 | 15 (46.9) | 77 (48.1) |
| 2 | 17 (53.1) | 83 (51.9) |
| Seminal vesicle radiation | 24 (75.0) | 130 (81.3) |
| Whole pelvis radiation | 1 (3.1) | 18 (11.3) |
Abbreviations: EPIC = Expanded Prostate Cancer Index Composite; GR = grade; RB = rectal bleeding.
For multivariate analysis of pretreatment clinical factors and DVH parameters, the least absolute shrinkage and selection operator selected anticoagulant use only (HR, 2.52) for predicting GR2+ RB, which ultimately had only modest predictive performance per the bootstrap .632 c-index (c-index, .59; 95% CI, 0.48-0.68; P = .088 for a test of c-index = .5).
Patient-reported QoL
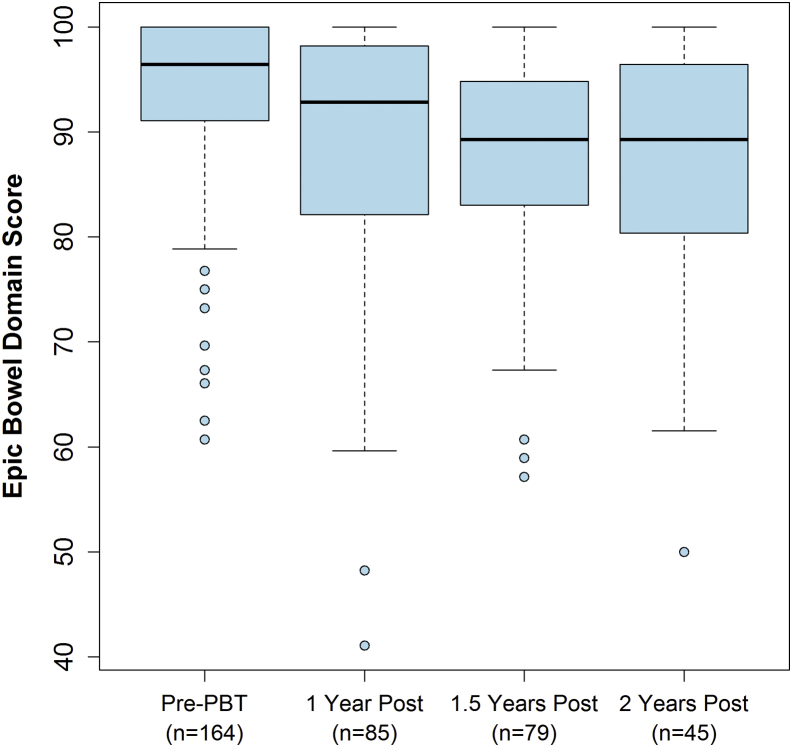
The number of patients who completed EPIC bowel QoL questionnaires before treatment and at 1, 1.5, and 2 years was 164 (85%), 85 (44%), 79 (41%), and 45 (23%), respectively. Median scores at pretreatment and 1, 1.5, and 2 years of follow-up are shown in Figure 3. The average decrease in score at 1 year from the pretreatment value was 5.4 points (95% CI, 2.5-8.2; P < .001). Previous studies evaluating the minimal important difference (MID) for the EPIC bowel questionnaire have shown that a 5-point change should be considered clinically relevant.14 There was an estimated average decrease in score of 1.2 points per year afterward, but this trend was not statistically significant (P = .57). GR2+ RB did not appear to be associated with an additional decrease in the EPIC bowel score because there was no significant difference in posttreatment EPIC score trends before and after RB (P = .86). Table 4 shows the changes in EPIC scores for patients divided into subgroups on the basis of whether they were on anticoagulation or alpha-blockers. Differences between subgroups were not statistically significant, possibly because of the small patient numbers.
Figure 3.
Box-whisker representation of the Expanded Prostate Cancer Index Composite bowel domain survey scores at pretreatment and 1, 1.5, and 2 years posttreatment. Score decrease from pretreatment to 1 year was statistically significantly different (P < .001). There was no significant further decrease in score. Solid bolded black lines indicate median scores. Upper and lower box edges indicate 75th and 25th percent quartile score, respectively. Whiskers indicate 1.5 times the interquartile range of the box. Circular data points indicate individual recorded scores falling outside the boxes and whiskers.
Table 4.
Median EPIC bowel summary scores separated by anticoagulation and alpha blocker use
| Pretreatment | 1 year | 1.5 year | 2 year | |
|---|---|---|---|---|
| Anticoagulation+ (n = 22) | 96 | 93 | 88 | 92 |
| Anticoagulation– (n = 170) | 96 | 93 | 89 | 89 |
| Alpha blocker+ (n = 37) | 95 | 91 | 89 | 94 |
| Alpha blocker– (n = 155) | 96 | 93 | 89 | 89 |
Abbreviation: EPIC = Expanded Prostate Cancer Index Composite.
Discussion
We report the largest series of patients with prostate cancer treated with mostly PBS proton therapy. There are few detailed institutional reports of GI toxicity after proton radiation for prostate cancer, and most studies used older passive scatter proton technology. Loma Linda published their experience in 2004 with patients treated with passive scatter technology, but 75% of our patients were treated with PBS.3 The University of Florida has the largest prospective modern series, reported in 2015, but their patients were also treated with passive scatter.5 In addition, Massachusetts General Hospital published in 2010 on their mixed proton/photon patient experience using scattering technology.15
In accordance with previous studies, we found that by far the most common physician-reported GI toxicity in patients with prostate cancer treated with PBT is transient RB. Other toxicities such as diarrhea, proctitis, and fecal incontinence were rare. Only a few other published PBT series exist.3, 16, 17, 18 Loma Linda University Medical Center reported a 3-year RTOG GR2+ toxicity rate of 21%,3 and Zietman et al reported a CTCAE version 4.0 GR2+ GI toxicity rate of 17% in patients treated with combined photons and protons.16 Conversely, others have reported exceedingly low rates of GI-related toxicities, such as Nihei et al. in Japan, who reported a 2-year CTCAE version 2.0 GR2+ RB rate of 2%.4, 19
Gastrointestinal toxicity rates in IMRT series also vary widely, ranging from 2% to 29% late GR2+ toxicity, with many studies averaging around 13%.20, 21, 22, 23, 24 The major question surrounding PBT in prostate cancer treatment is whether its toxicity profile compares favorably with other EBRT methods, especially IMRT. However, variability among studies using different versions of the RTOG and CTCAE scales makes direct comparisons difficult. For example, CTCAE GR2 toxicity as defined by the University of Florida and the present study includes any interventions, whether medical or procedural, if admission to a hospital is not required. However, the RTOG scale describes GR2 bleeding as “excessive rectal mucus or intermittent bleeding” and GR3 bleeding as “obstruction or bleeding requiring surgery.” There is room for interpretation regarding where minor interventions such as steroid suppositories or enemas may fall.
Similar to many published series using dose-escalated IMRT,25, 26 specific interventions that were counted in our study as GR2 toxicity (minor procedures such as cortisone suppositories) are not specifically reported in the Japanese series, which could be a driver of such a low toxicity rate. Although previous analyses using procedural codes and correlative endpoints (Medicare claims) have made conclusions comparing IMRT and proton therapy based on different rectal toxicity scoring criteria, using the same scoring criteria may lead to greater reproducibility of results.
Our study shows that patient-reported QoL and physician-reported toxicity are not always in concordance. Interestingly, patients with GR2+ RB did not have QoL score changes that were statistically different from those of patients who did not experience GR2+ RB. Previous studies evaluating the MID for the EPIC bowel questionnaire have shown that a 5-point change should be considered clinically relevant.14 Using this range as the definition for MID, we note that at 1 year, the average decrease in score for the overall cohort was clinically relevant (5.4 points).
IMRT experiences have suggested an increase in bowel toxicity incidence over time.27 All 3 major proton experiences reported to date indicate that all or nearly all severe GI-related toxicities are experienced within 2 to 2.5 years of treatment.3, 4, 5 The present EPIC analysis indicates that in our cohort, patients do not have a statistically significant or clinically relevant decrease in score at 2 years compared with the 1-year time point. We acknowledge that our ability to detect significant changes in score past 1 year may be affected by the fact that the number of patients who completed questionnaires decreased by almost one half from 1-year to 2-year follow-up. As more patients in our cohort reach ≥2-year follow-up over time, we will continue to investigate these endpoints.
Our results contrast with those of other published studies involving protons, which show increased risk of GR2+ RB with higher doses delivered to the rectum/rectal wall (selected rectal and rectal wall DVH parameters).5, 16 We found no significant correlation between selected DVH parameters and incidence of GI toxicity. This may be because of the tight dosimetric constraints we employ in treatment planning.
Anticoagulant use was significantly associated with GR2+ RB in this analysis. Previous groups have also demonstrated an increased risk of RB with anticoagulant use.28, 29 On the basis of the results of our study, we would recommend that patients receiving anticoagulation be counseled on the risk of RB. However, we also find that RB incidence in the majority of cases is a transient toxicity and that GR3+ events are exceedingly rare.
The present study was limited mainly by the number of patients, many of whom had a relatively short follow-up. As our follow-up data expand and mature, factors such as aspirin use (P = .22 in our study) that have been found to be significant predictors of RB in prior studies may emerge as significant predictors in future analyses.6 The loss of 29 patients to follow-up also introduced a selection bias to our findings, as well as the completion rate for patient-reported outcomes.
Conclusions
We report a rate of GR2+ GI toxicity after PBT for localized prostate cancer that is comparable with that of other published literature. The majority of GI treatment-related adverse effects involved transient RB. Anticoagulant use was correlated with GR2+ RB, and patients with this risk factor should be counseled on their risk for toxicity.
Acknowledgments
The authors acknowledge Jordan Dickson, BA, and Prachie Banthia, BA, for their assistance with the formatting of the blinded datasets containing results from our patient database.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Gami B., Harrington K., Blake P. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther. 2003;18:987–994. doi: 10.1046/j.1365-2036.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 2.Vargas C., Fryer A., Mahajan C. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–751. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 3.Slater J.D., Rossi C.J., Yonemoto L.T. Proton therapy for prostate cancer: The initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–352. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Nihei K., Ogino T., Onozawa M. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys. 2011;81:390–396. doi: 10.1016/j.ijrobp.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Colaco R.J., Hoppe B.S., Flampouri S. Rectal toxicity after proton therapy for prostate cancer: An analysis of outcomes of prospective studies conducted at the University of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91:172–181. doi: 10.1016/j.ijrobp.2014.08.353. [DOI] [PubMed] [Google Scholar]
- 6.Yu J.B., Soulos P.R., Herrin J. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S., Shen S., Moore D.F. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheets N.C., Goldin G.H., Meyer A. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H.Y., Jiang J., Hoffman K.E. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J Clin Oncol. 2018;36:1823–1830. doi: 10.1200/JCO.2017.75.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendenhall N.P., Schild S., Slater J. Radiation therapy modalities for prostate cancer. JAMA. 2012;308:450. doi: 10.1001/jama.2012.8112. [DOI] [PubMed] [Google Scholar]
- 11.DeLaney T.F. Proton therapy in the clinic. Front Radiat Ther Oncol. 2011;43:465–485. doi: 10.1159/000322511. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute . 2010. Common Terminology Criteria for Adverse Events v4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed September 12, 2018. [Google Scholar]
- 13.Steyerberg E.W., Harrell F.E., Borsboom G.J.J.M., Eijkemans M.J.C., Vergouwe Y., Habbema J.D.F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 14.Skolarus T.A., Dunn R.L., Sanda M.G. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015;85:101–105. doi: 10.1016/j.urology.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talcott J.A., Rossi C., Shipley W.U. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA. 2010;303:1046–1053. doi: 10.1001/jama.2010.287. [DOI] [PubMed] [Google Scholar]
- 16.Zietman A.L., Bae K., Slater J.D. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology Group/American College Of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater J.D., Yonemoto L.T., Rossi C.J. Conformal proton therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1998;42:299–304. doi: 10.1016/s0360-3016(98)00225-9. [DOI] [PubMed] [Google Scholar]
- 18.Coen J.J., Bae K., Zietman A.L. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: Initial report of American College of Radiology Phase II study 03-12. Int J Radiat Oncol Biol Phys. 2011;81:1005–1009. doi: 10.1016/j.ijrobp.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute . 1999. Common Toxicity Criteria, v2.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf. Accessed September 12, 2018. [Google Scholar]
- 20.Yu T., Zhang Q., Zheng T. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: A meta-analysis of the literatures. PloS One. 2016;11:e0154499. doi: 10.1371/journal.pone.0154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thor M., Jackson A., Zelefsky M.J. Inter-institutional analysis demonstrates the importance of lower than previously anticipated dose regions to prevent late rectal bleeding following prostate radiotherapy. Radiother Oncol. 2018;127:88–95. doi: 10.1016/j.radonc.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharfo A.W.M., Dirkx M.L.P., Bijman R.G. Late toxicity in the randomized multicenter HYPRO trial for prostate cancer analyzed with automated treatment planning. Radiother Oncol. 2018;128:349–356. doi: 10.1016/j.radonc.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Budaus L., Bolla M., Bossi A. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur Urol. 2012;61:112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Di Franco R., Borzillo V., Ravo V. Rectal/urinary toxicity after hypofractionated vs conventional radiotherapy in low/intermediate risk localized prostate cancer: Systematic review and meta analysis. Oncotarget. 2017;8:17383–17395. doi: 10.18632/oncotarget.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolnerovski M., Salleron J., Beckendorf V. Intensity-modulated radiation therapy from 70Gy to 80Gy in prostate cancer: Six-year outcomes and predictors of late toxicity. Radiat Oncol. 2017;12:1–10. doi: 10.1186/s13014-017-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delobel J.B., Gnep K., Ospina J.D. Nomogram to predict rectal toxicity following prostate cancer radiotherapy. PLoS One. 2017;12:1–16. doi: 10.1371/journal.pone.0179845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spratt D.E., Pei X., Yamada J., Kollmeier M.A., Cox B., Zelefsky M.J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda K., Ogawa Y., Ariga H., Koto M. Clinical correlations between treatment with anticoagulants/antiaggregants and late rectal toxicity after radiotherapy for prostate cancer. Anticancer Res. 2009;1834:1831–1834. [PubMed] [Google Scholar]
- 29.Hamstra D.A., Stenmark M.H., Ritter T. Age and comorbid illness are associated with late rectal toxicity following dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:1246–1253. doi: 10.1016/j.ijrobp.2012.10.042. [DOI] [PubMed] [Google Scholar]