Abstract
Purpose
Even when index pain (pain caused by the irradiated tumor) is palliated after radiation therapy (RT), patients may not derive the full benefits of RT in the presence of another, more intense pain. In this case-control study with prospectively collected data, we sought to identify predictors of the predominance of nonindex pain after palliative RT.
Methods and Materials
Brief Pain Inventory data were collected from patients who received RT for painful tumors. The treating radiation oncologists prospectively evaluated the intensity and origin of nonindex pain. Patients were diagnosed with predominance of other pain (POP) if nonindex pain of malignant or unknown origin was present and had a greater worst pain score than the index pain at the 1- or 2-month follow-up. Changes in pain interference from baseline to follow-up were compared between the 2 groups using Mann-Whitney U tests. Using variables that were identified as significant in a multivariable logistic regression analysis, we developed a prediction model for POP.
Results
Of the 170 patients who were evaluable at the 2-month follow-up, 24 (14%) were diagnosed with POP. At the 2-month follow-up examination of the patients with POP, none of the items of the pain interference scores were reduced from baseline; in contrast, patients without POP experienced significant reductions in all items. Multivariable analysis using the backward elimination method indicated that age ≤65 years, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline were significant independent predictors of POP. As the number of the risk factors increased, the proportion of patients with POP increased.
Conclusions
We identified three predictors of POP. For patients likely to have POP, careful follow-up is important, and new palliative RT or analgesics should be used when needed.
Summary.
Predominance of other pain (POP) was diagnosed if nonindex pain of malignant or unknown origin was present and had a greater pain score than the index pain after palliative radiation therapy. Patients with POP did not experience improvement in pain interference with daily life. Younger age, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline were significant independent predictors of POP.
Introduction
Pain response after radiation therapy (RT) is assessed using the intensity of the index pain (pain caused by the irradiated tumor).1 Patients with cancer often have more than 1 pain location.2, 3, 4 Even when the index pain from the irradiated tumors is controlled, new pain from tumors outside of the irradiated area may have a negative effect in terms of the interference of pain in the patient's life and may negatively affect quality of life.5 To our knowledge there are no data regarding the frequency or causes of nonindex pain in patients treated with palliative RT. Predictors of the presence of nonindex pain are also unknown.
In the present study we set an endpoint to detect a situation in which patients cannot derive full benefit from palliative RT. For instance, when an index pain score of 8 is reduced to 3 after RT and other pain with a score of ≤3 is simultaneously present, the patient may derive the full benefit from RT; the 5-point decrease in the patient's pain score may be beneficial. In contrast, when the index pain score of 8 is reduced to 3 after RT and other pain with a pain score of 6 is simultaneously present, the patient may not receive the full benefit from RT; the 2-point (and not 5-point) decrease in the pain score may be beneficial to the patient. Accordingly, we presumed the presence of a malignant pain with greater intensity than the index pain after RT to be the relevant endpoint. In a secondary analysis of our prospective observational study, we sought to identify predictors of the predominance of pain other than the index pain after palliative RT. Another objective was to evaluate the influence of such predominance of nonindex pain on pain interference with daily life.
Methods and Materials
Patients and study design
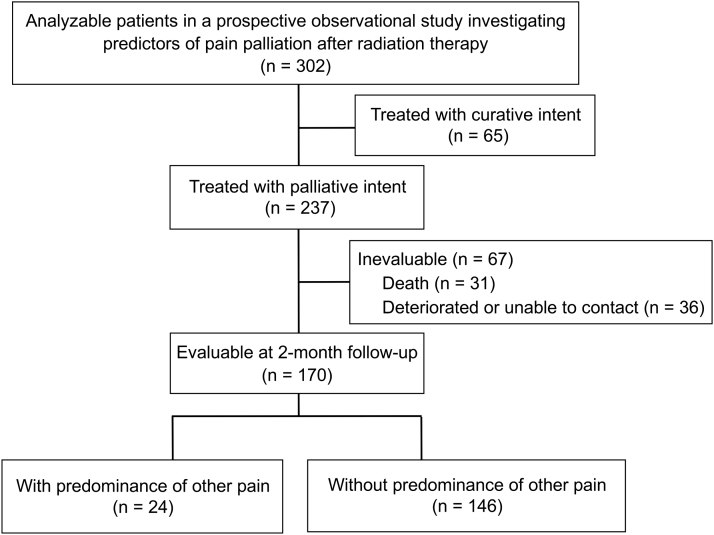
We used the data from our previously published prospective observational study6 in this secondary study. The primary study included 302 analyzable patients for whom RT was planned for painful tumors, with the aim of identifying the predictors of pain response. The treatment was performed with palliative intent for 237 of these 302 patients (Fig 1). RT was defined as palliative if the primary purpose of treatment was pain relief or if the radiation field did not cover all of the tumors identified by diagnostic imaging.6 After the exclusion of 67 patients who were not evaluable, 170 patients who were evaluable at 2 months of follow-up were included in the present study. This secondary study was approved by the participating centers' institutional review boards; we received written informed consent from all of the patients who were enrolled in the primary study. In the present case-control study, cases (patients with predominance of other pain [POP]) and controls (patients without POP) were enrolled at the 2-month follow-up evaluation; baseline characteristics of the participants were assessed to identify factors that could lead to POP.
Figure 1.
Flow diagram of the study cohort. In the present case-control study, cases (patients with predominance of other pain) and controls (patients without it) were enrolled at the 2-month follow-up evaluation.
Follow-up and evaluation
The primary study previously reported how the patients were followed up and evaluated.6 In brief, at baseline, the treating radiation oncologists identified the index pain (pain caused by the irradiated tumor) using a physical examination and diagnostic imaging. The Brief Pain Inventory (BPI) short form was used to evaluate the intensity of pain and the interference of pain in the patient's life using an 11-point scale.7 Patients rated their worst pain in the previous 3 days (in terms of index pain, and nonindex pain if present). Pain interference was evaluated with 7 subscales: general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life. The BPI data and analgesic data were collected at baseline and at 1 month (±7 days), 2 months (±7 days), and 3 months (±7 days) after the start of RT. The pain response in terms of index pain was assessed using the International Consensus Endpoint for clinical trials in bone metastases.1 Patients who received RT for painful tumors were categorized as responders or nonresponders. Responders included patients who experienced complete and partial responses. A complete response was defined as an index pain score of 0 with no increase in the daily oral morphine equivalent dose (OMED).1 A partial response was defined as a reduction of the pain score of ≥2 points without an increase in OMED or a reduction in analgesic use of ≥25% without an increase in the pain score.1
Assessment of pain other than the index pain
At baseline and at follow-up, the treating radiation oncologists prospectively evaluated whether the patients had pain other than the index pain; for the patients with this pain, they recorded its intensity (the worst pain in the previous 3 days) and origin. When more than 1 nonindex pain was present, the pain of the greatest intensity was recorded. Nonindex pain was classified as pain having a malignant (tumor-related), unknown, or benign origin or as treatment related. POP was defined to detect the situation in which there was nonindex pain of malignant origin that was more intense than the index pain. Pain of unknown origin was treated as malignant pain because tumor-related malignant pain is often not identified by, or not evaluated by, diagnostic imaging. Therefore patients were diagnosed with POP if nonindex pain of a malignant or unknown origin was present and had a greater worst pain score than the index pain at the 1- or 2-month follow-up. When the treating radiation oncologists were uncertain whether the reported pain was related to the index tumor in patients evaluated by mail or fax, they telephoned the patients to distinguish between index and nonindex pain.
Statistical analysis
Patient characteristics were analyzed using Mann-Whitney U tests for continuous variables and Fisher exact tests for categorical variables. We treated Eastern Cooperative Oncology Group performance status (≤1 vs >1) and worst pain score at baseline (≤7 vs >7) as binary variables and age and total radiation dose as continuous variables. Changes in pain intensity, pain interference, and opioid analgesic dose from baseline to 2-month follow-up were compared between the patients with POP and those without POP using Mann-Whitney U tests. Pain response rate was compared between the 2 groups using a Fisher exact test. Overall survival, calculated from 2 months of follow-up, was estimated using the Kaplan-Meier method; differences were determined with the log-rank test.
To identify factors associated with POP, we performed multivariable regression after univariable logistic regression analysis using the backward elimination method with a P < .05 criterion for retention. In the logistic regression analysis, age and total radiation dose were dichotomized at their median values. Using the variables significant in multivariable logistic regression analysis, we developed a prediction model for POP; we assigned risk scores based on the odds ratios of the variables. Discrimination of the prediction model was evaluated using the receiver operating characteristic (ROC) curve.
All tests were two-tailed; P < .05 was considered significant. All statistical analyses were performed with SPSS software Version 24 (IBM Corp, Armonk, NY).
Results
Patients
We analyzed 170 patients treated with palliative intent and evaluable at the 2-month follow-up (Fig 1; Table 1). At baseline, of the 29 patients who had nonindex pain, 24 had nonindex pain of malignant or unknown origin (Table 2). In the primary study, patients were excluded if they had another tumor with more severe pain than the one scheduled to receive RT; accordingly, at baseline, all nonindex pain had lower pain scores than the index pain.
Table 1.
Baseline patient characteristics
| Characteristic | Included (n = 170) |
Excluded (n = 67) |
P∗ | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age, y | .14 | ||||
| Median | 65 | 70 | |||
| Range | 21-91 | 35-89 | |||
| Sex | .008 | ||||
| Female | 78 | 46 | 18 | 27 | |
| Male | 92 | 54 | 49 | 73 | |
| ECOG performance status | .001 | ||||
| 0 | 27 | 16 | 6 | 9 | |
| 1 | 75 | 44 | 18 | 27 | |
| 2 | 48 | 28 | 25 | 37 | |
| 3, 4 | 20 | 12 | 18 | 27 | |
| Irradiated tumor | .84 | ||||
| Solid tumor | 146 | 86 | 57 | 85 | |
| Hematologic tumor | 24 | 14 | 10 | 15 | |
| Worst pain score for the index pain† at baseline | .061 | ||||
| 1-2 | 5 | 3 | 2 | 3 | |
| 3-4 | 29 | 17 | 6 | 9 | |
| 5-7 | 61 | 36 | 20 | 30 | |
| 8-10 | 75 | 44 | 39 | 58 | |
| Neuropathic component of the index pain† | .45 | ||||
| No | 110 | 65 | 47 | 70 | |
| Yes | 60 | 35 | 20 | 30 | |
| Nonindex pain of malignant or unknown origin at baseline | .55 | ||||
| No | 146 | 86 | 55 | 82 | |
| Yes | 24 | 14 | 12 | 18 | |
| Opioid analgesic use at baseline | .009 | ||||
| No | 84 | 49 | 20 | 30 | |
| Yes | 86 | 51 | 47 | 70 | |
| Adjuvant analgesic use at baseline | .76 | ||||
| No | 111 | 65 | 42 | 63 | |
| Yes | 59 | 35 | 25 | 37 | |
| Total radiation dose, Gy | .014 | ||||
| Median | 30 | 30 | |||
| Range | 8-50 | 6-60 | |||
| ≤10 | 25 | 15 | 13 | 19 | |
| 10-20 | 25 | 15 | 13 | 19 | |
| 20-30 | 70 | 41 | 32 | 48 | |
| >30 | 50 | 29 | 9 | 13 | |
Abbreviation: ECOG = Eastern Cooperative Oncology Group.
Mann-Whitney U test or Fisher exact test.
Pain caused by the tumor scheduled to receive radiation therapy.
Table 2.
Pain other than the index pain∗
| Cause of pain | Baseline (n = 170) |
1-month follow-up (n = 163) |
2-month follow-up (n = 170) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Malignant (tumor related) | 19 | 11 | 11 | 7 | 17 | 10 |
| Solid tumor | 14 | 8 | 9 | 6 | 14 | 8 |
| Hematogenous metastasis | 10 | 6 | 6 | 4 | 8 | 5 |
| Bone metastases | 9 | 5 | 4 | 2 | 5 | 3 |
| Other | 1 | 1 | 2 | 1 | 3 | 2 |
| Lymph node metastasis | 2 | 1 | 1 | 1 | 1 | 1 |
| Pleural dissemination | 1 | 1 | 2 | 1 | 2 | 1 |
| Other | 1 | 1 | 0 | 0 | 3 | 2 |
| Hematologic tumor | 5 | 3 | 2 | 1 | 3 | 2 |
| Unknown | 5 | 3 | 9 | 6 | 14 | 8 |
| Benign | 5 | 3 | 2 | 1 | 8 | 5 |
| Treatment related | 0 | 0 | 7 | 4 | 3 | 2 |
| Radiation related | 0 | 0 | 6 | 4 | 2 | 1 |
| Other treatment related | 0 | 0 | 1 | 1 | 1 | 1 |
Pain caused by the tumor scheduled to receive radiation therapy.
Pain other than the index pain
The causes of nonindex pain are presented in Table 2. At the 1- or 2-month follow-up, 35 patients had some nonindex pain with a higher pain score than the index pain. After we excluded 11 patients whose nonindex pain was benign or treatment related, 24 (14%) had nonindex pain of malignant or unknown origin that had greater pain intensity than the index pain; they were diagnosed with POP (Table 1). For the patients who experienced POP, the median worst pain score was 2 (interquartile range, 0-3.25) for the index pain and 4 (interquartile range, 2.5-6.5) for the nonindex pain at the 1-month follow-up; at the 2-month follow-up, the median worst pain score was 1.5 (interquartile range, 0-4) for the index pain and 8 (interquartile range, 5-8.75) for the nonindex pain.
Pain response
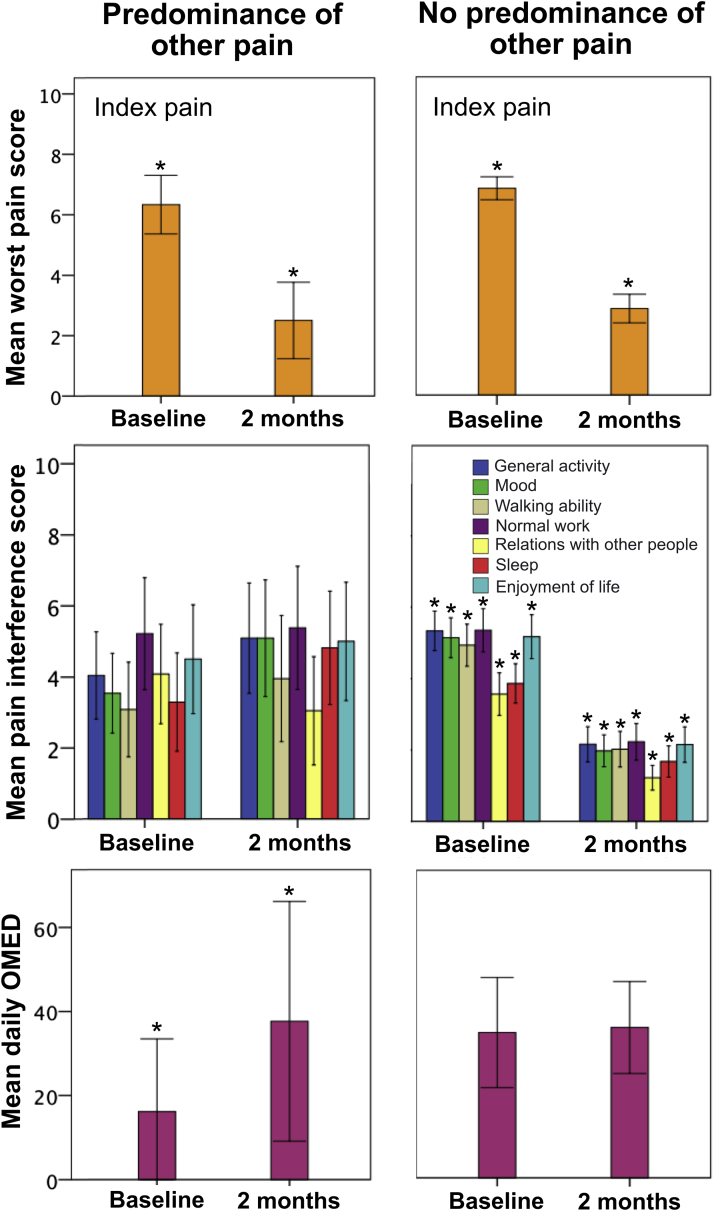
At the 2-month follow-up, for the patients with POP and those without POP, the mean change in worst pain score (in terms of the index pain) from baseline (ie, follow-up minus baseline) was −3.83 (95% confidence interval [CI], −5.28 to −2.38) and −3.98 (95% CI, −4.49 to −3.47), respectively (P = .79; Fig 2). For the patients with POP and those without POP, the per-protocol pain response rate for the index pain was 42% and 57%, respectively, at the 2-month follow-up (P = .19).
Figure 2.
Pain intensity (in terms of the index pain), pain interference, and opioid analgesic dose at baseline and 2-month follow-up. The error bars indicate the 95% confidence intervals. Asterisks indicate a significant difference between baseline values and 2-month follow-up values. Abbreviation: OMED = oral morphine equivalent dose.
Of the 24 patients who experienced POP, 9 (38%) received secondary palliative RT for tumors causing nonindex pain at a median interval of 54 days (range, 19-231 days) after the initiation of the primary RT courses. Of the 9 patients who received secondary RT, 5 were evaluable within 3 months from the start of the secondary RT; 1 patient experienced a pain response, and the other 4 did not.
Opioid analgesic use
At the 2-month follow-up, for the patients with POP and those without POP, the mean change in daily OMED from baseline (ie, follow-up minus baseline) was 21.5 mg (95% CI, 8.2-34.7 mg) and 1.2 mg (95% CI, −5.7 to 8.1 mg), respectively (P = .005; Fig 2).
BPI pain interference scores
At the 2-month follow-up, for the patients with POP and those without POP, the mean change in pain interference scores from baseline (ie, follow-up minus baseline) was 0.86 (95% CI, –1.15 to 2.88) and –3.31 (95% CI, −3.98 to −2.64) for general activity (P < .001); 1.32 (95% CI, −0.47 to 3.11) and −3.21 (95% CI, −3.91 to −2.50) for mood (P < .001); 0.86 (95% CI, −1.15 to 2.88) and −2.94 (95% CI, −3.62 to −2.26) for walking ability (P < .001); −0.25 (95% CI, −2.49 to 1.99) and −3.18 (95% CI, −3.93 to −2.44) for normal work (P = .007); −1.27 (95% CI, −3.56 to 1.02) and −2.50 (95% CI, −3.15 to −1.86) for relations with other people (P = .38); 1.36 (95% CI, −1.04 to 3.77) and −2.15 (95% CI, −2.77 to −1.53) for sleep (P = .001); and 0.14 (95% CI, −2.30 to 2.58) and −3.10 (95% CI, −3.87 to −2.33) for enjoyment of life (P = .005), respectively (Fig 2). At the 2-month follow-up, patients with POP did not have a reduction in any item of the pain interference scores from baseline (Fig 2). In contrast, patients without POP experienced significant reductions in all items of the pain interference scores from baseline (Fig 2). The changes in pain interference scores from baseline to the 2-month follow-up were significantly different between the patients with POP and those without POP for all items except relations with other people.
Survival
Median follow-up of censored patients was 7.4 months. The median overall survival for all patients was 9.4 months (95% CI, 7.5-11.3 months). The median overall survival for the patients with POP was 3.5 months (95% CI, 2.7-4.3 months), and it was 10.2 months (95% CI, 7.9-12.5 months) for those without POP (P < .001).
Predictors of POP
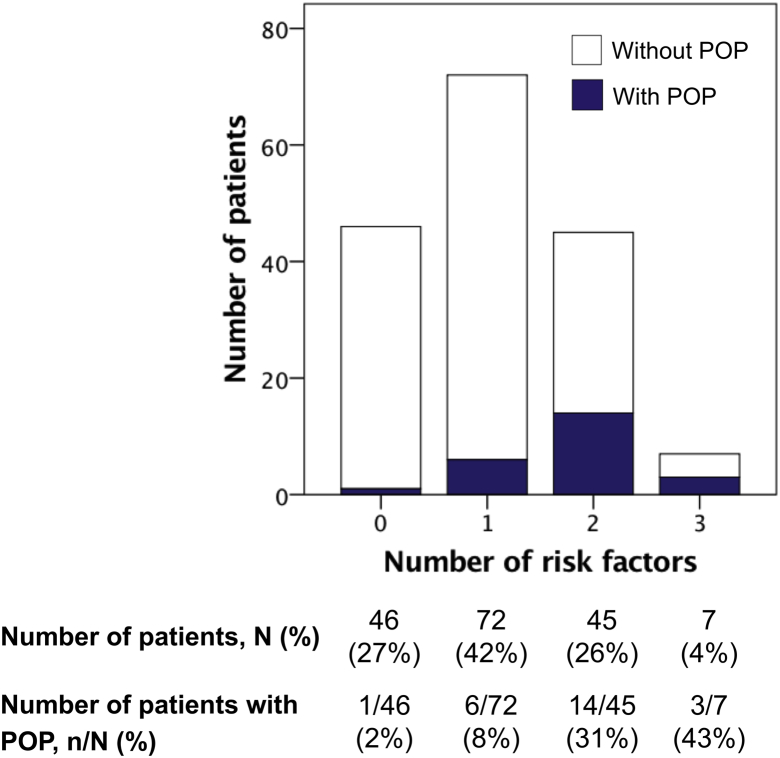
Univariable logistic regression analysis indicated that age ≤65 years, the absence of a neuropathic component of the index pain, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline were significant predictors of POP (Table 3). Multivariable analysis using the backward elimination method indicated that age ≤65 years, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline were significant independent predictors. The odds ratios of these 3 risk factors identified in multivariable logistic regression analysis were 3.00 to 4.29. Thus a prediction model for POP was developed using the simple method of counting the number of risk factors (Fig 3). As the number of the risk factors increased, the proportion of patients with POP increased (Fig 3). The area under the ROC curve for our model was 0.77 (95% CI, 0.67-0.87; P < .001).
Table 3.
An analysis to identify the predictors of predominance of other pain∗ after radiation therapy for painful tumors (n = 170)
| Variable | Univariable analysis† |
Multivariable analysis† |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age ≤65 y | ||||||
| No | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 3.69 | 1.44-9.44 | .007 | 3.00 | 1.12-8.05 | .029 |
| Sex | ||||||
| Female | 1.00 (reference) | |||||
| Male | 1.84 | 0.74-4.57 | .19 | |||
| ECOG performance status | ||||||
| 0, 1 | 1.00 (reference) | |||||
| 2-4 | 0.72 | 0.29-1.78 | .47 | |||
| Irradiated tumor | ||||||
| Solid tumor | 1.00 (reference) | |||||
| Hematologic tumor | 1.76 | 0.59-5.27 | .31 | |||
| Bone involvement by the tumor | ||||||
| No | 1.00 (reference) | |||||
| Yes | 0.74 | 0.27-2.04 | .57 | |||
| Worst pain score for the index pain† at baseline | ||||||
| 0-7 | 1.00 (reference) | |||||
| 8-10 | 0.59 | 0.24-1.46 | .26 | |||
| Neuropathic component of the index pain‡ | ||||||
| No | 1.00 (reference) | |||||
| Yes | 0.22 | 0.06-0.78 | .019 | |||
| Nonindex pain of malignant or unknown origin at baseline | ||||||
| No | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 4.06 | 1.50-10.99 | .006 | 4.24 | 1.40-12.85 | .011 |
| Without opioid analgesic use at baseline | ||||||
| No | 1.00 (Reference) | 1.00 (reference) | ||||
| Yes | 3.64 | 1.37-9.69 | .010 | 4.29 | 1.49-12.35 | .007 |
| Without adjuvant analgesic use at baseline | ||||||
| No | 1.00 (reference) | |||||
| Yes | 2.23 | 0.79-6.32 | .13 | |||
| Total radiation dose > 30Gy | ||||||
| No | 1.00 (reference) | |||||
| Yes | 1.54 | 0.62-3.79 | .35 | |||
Abbreviations: ECOG = Eastern Cooperative Oncology Group; OR = odds ratio; CI = confidence interval.
Multivariable analysis was performed using the backward elimination method with a P < .05 criterion for retention.
When some other pain of malignant (tumor-related) or unknown origin was more intense than the index pain.
Logistic regression analysis.
Pain caused by the tumor scheduled to receive radiation therapy.
Figure 3.
Number of the risk factors for the predominance of other pain. Patients were diagnosed with predominance of other pain if nonindex pain of malignant or unknown origin was present and had greater pain score than the index pain at 1- or 2-month follow-up. Abbreviation: POP = predominance of other pain.
Discussion
We found that patients with nonindex pain more intense than the index pain after RT did not experience improvement in pain interference from baseline. Significant predictors of such a predominance of nonindex pain were younger age, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline. Based on the area under the ROC curve, our prediction model had moderate discriminative ability.
For patients who have more than one pain simultaneously, the most intense pain would determine the pain interference with daily life and quality of life. Therefore, even when the index pain caused by the irradiated tumor is palliated after RT, patients cannot derive full benefits from RT under the presence of another, more intense pain. We found that in patients with POP, the opioid analgesic dose significantly increased after RT; more analgesics would have been needed to control the nonindex pain.8 This increase in analgesic use may lower the pain response rate of POP patients, although we could not test the statistical significance of the difference in response rate between the POP and non-POP patients with sufficient statistical power.
In palliative RT for painful tumors, uncontrolled index pain may be caused by a tumor's low sensitivity to radiation or low RT dose; in contrast, deterioration of pain other than the index pain may be caused by rapid systemic tumor growth or suboptimal target selection. Assessment of both index and nonindex pain would be essential for a more personalized treatment strategy for palliating tumor-related cancer pain.
We found that significant predictors of the predominance of nonindex pain were younger age, the presence of nonindex pain of malignant or unknown origin at baseline, and no opioid analgesic use at baseline. A past study investigating the influence of age on pain characteristics in cancer patients found that there was a trend toward younger patients having more pain locations,9 which is consistent with our results. The difference in age may also reflect the differences in metastatic patterns of cancers that have different incidences based on age. However, we could not draw any specific conclusions regarding this based on the results of the present study. The influence of age on the number of pain locations and on systemic progression of cancer pain should be investigated in further studies. In patients who taking opioid analgesics, the analgesics may sometimes mask some nonindex pain even if it is present. In patients who do not take opioid analgesics at baseline, pain other than the index pain may tend to emerge clinically. For patients more likely to experience predominance of nonindex pain, careful follow-up after RT is important, and new intervention with palliative RT or analgesics should be considered when needed.
We found that patients with nonindex pain more intense than the index pain after RT had worse survival than those without it. Systemic progression of malignant pain may reflect high systemic tumor burden and therefore may be associated with worse prognosis.
As a limitation of the study, the attrition rate was relatively high and not all of the patients who received palliative RT could be analyzed. The second limitation is the small sample size, particularly that of patients with POP. Further, larger studies examining patients with various patient and pain characteristics are warranted. Third, in this observational study in which there was no intervention for research purposes, we could not explain why nonindex pain was not better treated by the treating physician by offering RT for nonindex tumors or changing analgesia regimens. Our findings may reflect underlying clinical practice patterns. Finally, our prediction model should be validated in future studies. A temporal validation with data from our facilities or an external validation with independent data from other facilities would be necessary to confirm our results.
In conclusion, patients with pain more intense than the index pain after RT did not experience improvement in pain interference. We would like to emphasize that even patients with POP may benefit from RT. Without RT, the index lesion might have caused more problems. Evaluation of other pain in addition to the index pain will contribute to a more personalized strategy for palliating tumor-related pain. The present study may be the first to investigate pain other than the index pain for patients who receive palliative RT. To our knowledge, the predominance of pain other than the treated pain has not been investigated for other local pain treatments such as nerve blocks or vertebroplasty. Further studies investigating the frequency, clinical relevance, and predictors of the predominance of nonindex pain are warranted.
Footnotes
Sources of support: This work had no specific funding
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Chow E., Hoskin P., Mitera G. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Grond S., Zech D., Diefenbach C. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64:107–114. doi: 10.1016/0304-3959(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 3.Caraceni A., Portenoy R.K. An international survey of cancer pain characteristics and syndromes. IASP task force on cancer pain. International association for the study of pain. Pain. 1999;82:263–274. doi: 10.1016/S0304-3959(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 4.Moryl N., Dave V., Glare P. Patient-reported outcomes and opioid use by outpatient cancer patients. J Pain. 2018;19:278–290. doi: 10.1016/j.jpain.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westhoff P.G., de Graeff A., Monninkhof E.M. Quality of life in relation to pain response to radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2015;93:694–701. doi: 10.1016/j.ijrobp.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Saito T., Toya R., Tomitaka E., Matsuyama T., Ninomura S., Oya N. Predictors of Pain Palliation After Radiation Therapy for Painful Tumors: A Prospective Observational Study. Int J Radiat Oncol Biol Phys. 2018;101:1061–1068. doi: 10.1016/j.ijrobp.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland C.S., Ryan K.M. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 8.Mercadante S. Scoring the effect of radiotherapy for painful bone metastases. Support Care Cancer. 2006;14:967–969. doi: 10.1007/s00520-006-0036-7. [DOI] [PubMed] [Google Scholar]
- 9.Green C.R., Hart-Johnson T. Cancer pain: An age-based analysis. Pain Med. 2010;11:1525–1536. doi: 10.1111/j.1526-4637.2010.00957.x. [DOI] [PubMed] [Google Scholar]