Abstract
Purpose
Grade 4 lymphopenia (G4L) during radiation therapy (RT) is associated with higher rates of distant metastasis and decreased overall survival in a number of malignancies, including esophageal cancer (EC). Through a reduction in integral radiation dose, proton RT (PRT) may reduce G4L relative to photon RT (XRT). The purpose of this study was to compare G4L in patients with EC undergoing PRT versus XRT.
Methods and materials
Patients receiving curative-intent RT and concurrent chemotherapy for EC were identified. Lymphocyte nadir was defined as the lowest lymphocyte count during RT. G4L was defined as absolute lymphocyte count <200/mm3. Univariate and multivariable logistic regression analyses (MVA) were performed to assess patient and treatment factors associated with lymphopenia. A propensity-matched (PM) cohort was created using logistic regression, including baseline covariates.
Results
A total of 144 patients met the inclusion criteria. The median age was 66 years (range, 32-85 years). Of these patients, 79 received XRT (27% 3-dimensional chemo-RT and 73% intensity modulated RT) and 65 received PRT (100% pencil-beam scanning). Chemotherapy consisted of weekly carboplatin and paclitaxel (99%). There were no significant differences in baseline characteristics between the groups, except for age (median 4 years older in the PRT cohort). G4L was significantly higher in patients who received XRT versus those who received PRT (56% vs 22%; P < .01). On MVA, XRT (odds ratio [OR]: 5.13; 95% confidence interval [CI], 2.35-11.18; P < .001) and stage III/IV (OR: 4.54; 95% CI, 1.87-11.00; P < .001) were associated with G4L. PM resulted in 50 PRT and 50 XRT patients. In the PM cohort, G4L occurred in 60% of patients who received XRT versus 24% of patients who received PRT. On MVA, XRT (OR: 5.28; 95% CI, 2.14-12.99; P < .001) and stage III/IV (OR: 3.77; 95% CI, 1.26-11.30; P = .02) were associated with G4L.
Conclusions
XRT was associated with a significantly higher risk of G4L in comparison with PRT. Further work is needed to evaluate a potential association between RT modality and antitumor immunity as well as long-term outcomes.
Introduction
Worse clinical outcomes have been associated with treatment-induced lymphopenia for a number of malignancies, including esophageal cancer (EC).1, 2, 3, 4, 5, 6, 7 Lymphocytes are highly sensitive to ionizing radiation, with a 50% lethal dose of 1 to 2 Gy.8 Over a course of standard fractionated radiation therapy (RT), circulating lymphocytes are predicted to receive a mean dose of 2 Gy.9 Therefore, RT appears to be a significant mediator of lymphopenia, including in comparison with chemotherapy.2 For example, induction chemotherapy has been shown to have minimal impact on lymphocyte count, but precipitous declines are noted after the initiation of RT.2
RT modality and technique may affect the severity of treatment-induced lymphopenia. Through a reduction in moderate-to-low integral radiation dose, proton RT (PRT) may reduce the pool of lymphocytes exposed to lethal doses of radiation. A recent series from one institution investigating the relationship between RT modality and lymphopenia in EC showed a decrease in lymphopenia rates for patients treated with PRT compared with photon RT (XRT).3, 6 The purpose of this study was to validate the association of lower rates of grade 4 lymphopenia (G4L) in patients treated with PRT versus XRT.
Methods and materials
Patients
After institutional review board approval, medical records were reviewed for consecutive patients with EC who received RT and concurrent chemotherapy with curative intent between July 2015 and December 2017. Patients with histologies other than adenocarcinoma or squamous cell carcinoma or those who received a combination of PRT and XRT were excluded. A limited number of patients with stage IV disease on the basis of nonregional nodes (n = 3) or with oligometastasis (n = 2) undergoing definitive dosing were included.
Treatment
All patients received concurrent chemo-RT (CRT; generally 41.4-50.4 Gy) with 5 weekly cycles of carboplatin and paclitaxel, followed by surgical resection in operable patients. The clinical target volume typically included the primary esophageal tumor with a 3- to 4-cm longitudinal mucosal expansion and a 1-cm radial expansion, involved lymph nodes, and elective regional lymph nodes (supraclavicular for upper tumors and celiac for distal tumors). The choice of RT modality was at the discretion of the treating physician, most commonly dictated by insurance coverage. PRT was administered using the pencil-beam scanning technique with 2 posterior-oblique beams. XRT was 3-dimensional CRT (n = 21; 27%) and intensity modulated RT (IMRT; n = 58; 73%) using volumetric modulated arc therapy. Complete blood counts were done before initiation of CRT and most often weekly during treatment.
Statistical analysis
G4L was defined as absolute lymphocyte count <200/mm3 in accordance with the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0. Differences between the groups in baseline parameters were assessed using Wilcoxon, χ2, and Fisher exact tests where appropriate. Univariate and multivariable logistic regression analyses (UVA and MVA, respectively) were used to assess factors potentially associated with odds of lymphopenia. The clinical target volume (CTV) was considered codependent with stage and was therefore not included in the MVA. Tests were 2-sided with a P-value <.05 denoting statistical significance.
A propensity-matched (PM) analysis was performed to reduce the inherent biases of a retrospective analysis. We employed a 1:1 direct match using clinical, pathologic, and treatment parameters: age, sex, clinical stage, tumor histology, tumor location, surgical status, and CTV. Baseline covariates of age, sex, clinical stage, histology, median radiation dose, CTV, chemotherapy type, tumor location, and surgical status were considered when assessing G4L. A sensitivity analysis was performed excluding all patients who received 3-dimensional CRT, as was a χ2 comparison of 3-dimensional CRT with IMRT for rate of G4L.
Results
Entire cohort
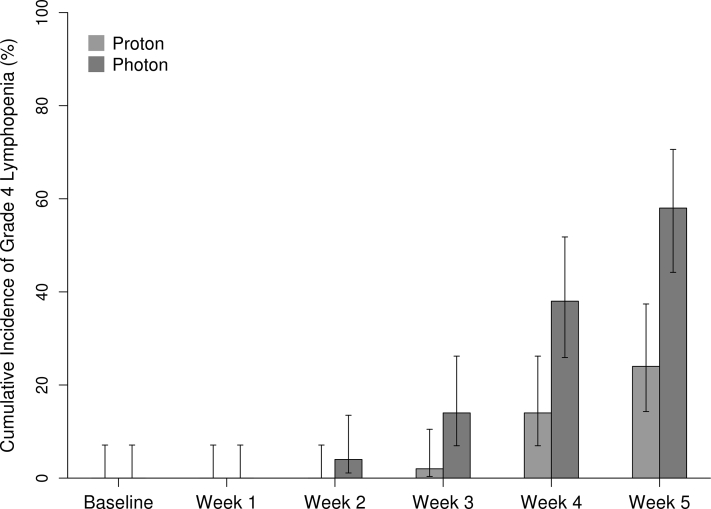
A total of 144 patients were included in the study. Patient characteristics are presented by RT modality in Table 1. The only statistically significant differences between the baseline characteristics of patients who received XRT and those who received PRT was age (median, 64 vs 68 years, respectively; P = .01). G4L occurred in 40% of patients overall (Fig 1).
Table 1.
Patient characteristics
| Overall cohort |
P-value | Propensity-matched cohort |
P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 144) | Proton (n = 65) | Photon (n = 79) | Total (N = 100) | Proton (n = 50) | Photon (n = 50) | |||
| Age at diagnosis | .011∗ | .541∗ | ||||||
| Median | 66.0 | 68.0 | 64.0 | 66.0 | 66.0 | 64.5 | ||
| Q1, Q3 | 59.0, 73.0 | 61.0, 76.0 | 57.0, 72.0 | 57.0, 72.5 | 57.0, 72.0 | 57.0, 73.0 | ||
| Range | (32.0-85.0) | (32.0-85.0) | (39.0-85.0) | (32.0-85.0) | (32.0-85.0) | (39.0-85.0) | ||
| Sex | .067† | .401† | ||||||
| Female | 22 (15.3%) | 6 (9.2%) | 16 (20.3%) | 15 (15.0%) | 6 (12.0%) | 9 (18.0%) | ||
| Male | 122 (84.7%) | 59 (90.8%) | 63 (79.7%) | 85 (85.0%) | 44 (88.0%) | 41 (82.0%) | ||
| Overall stage | .543‡ | .819‡ | ||||||
| I | 9 (6.3%) | 6 (9.2%) | 3 (3.8%) | 4 (4.0%) | 1 (2.0%) | 3 (6.0%) | ||
| IIA | 2 (1.4%) | 1 (1.5%) | 1 (1.3%) | 2 (2.0%) | 1 (2.0%) | 1 (2.0%) | ||
| IIB | 34 (23.6%) | 14 (21.5%) | 20 (25.3%) | 20 (20.0%) | 11 (22.0%) | 9 (18.0%) | ||
| III | 94 (65.3%) | 43 (66.2%) | 51 (64.6%) | 73 (73.0%) | 36 (72.0%) | 37 (74.0%) | ||
| IV | 5 (3.5%) | 1 (1.5%) | 4 (5.1%) | 1 (1.0%) | 1 (2.0%) | 0 (0.0%) | ||
| Surgery candidate | .362‡ | >.99† | ||||||
| No | 23 (16.0%) | 8 (12.3%) | 15 (19.0%) | 12 (12.0%) | 6 (12.0%) | 6 (12.0%) | ||
| Yes | 121 (84.0%) | 57 (87.7%) | 64 (81.0%) | 88 (88.0%) | 44 (88.0%) | 44 (88.0%) | ||
| Tumor location | .282† | .749† | ||||||
| Upper-middle | 18 (12.5%) | 6 (9.2%) | 12 (15.2%) | 11 (11.0%) | 6 (12.0%) | 5 (10.0%) | ||
| Lower | 126 (87.5%) | 59 (90.8%) | 67 (84.8%) | 89 (89.0%) | 44 (88.0%) | 45 (90.0%) | ||
| Tumor histology | .240† | .766† | ||||||
| Adenocarcinoma | 123 (85.4%) | 58 (89.2%) | 65 (82.3%) | 87 (87.0%) | 44 (88.0%) | 43 (86.0%) | ||
| Squamous cell | 21 (14.6%) | 7 (10.8%) | 14 (17.7%) | 13 (13.0%) | 6 (12.0%) | 7 (14.0%) | ||
| Grade | .051† | .034† | ||||||
| I/II | 60 (51.7%) | 30 (62.5%) | 30 (44.1%) | 41 (50.6%) | 24 (63.2%) | 17 (39.5%) | ||
| III/IV | 56 (48.3%) | 18 (37.5%) | 38 (55.9%) | 40 (49.4%) | 14 (36.8%) | 26 (60.5%) | ||
| Missing | 28 | 17 | 11 | 19 | 12 | 7 | ||
| Total radiation therapy dose (Gy) | .339∗ | .248∗ | ||||||
| Median | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | ||
| Q1, Q3 | 50.0, 50.0 | 50.0, 50.0 | 50.0, 50.4 | 50.0, 50.0 | 50.0, 50.0 | 45.0, 50.4 | ||
| Range | (41.4-56.3) | (41.4-50.4) | (41.4-56.3) | (41.4-56.3) | (41.4-50.4) | (41.4-56.3) | ||
| Clinical target volume (cc) | .238∗ | .637∗ | ||||||
| Mean (SD) | 49.0 (3.1) | 49.8 (1.1) | 48.2 (3.9) | 48.9 (3.0) | 49.8 (1.3) | 48.1 (4.0) | ||
| Median | 554.1 | 529.4 | 571.7 | 568.3 | 577.9 | 563.2 | ||
| Q1, Q3 | 408.2, 756.0 | 409.7, 708.5 | 405.0, 784.2 | 410.2, 757.3 | 456.2, 779.6 | 396.4, 737.2 | ||
| Range | (95.4-1395.3) | (95.4-1193.6) | (114.5-1395.3) | (95.4-1311.3) | (95.4-1193.6) | (114.5-1311.3) | ||
| Chemotherapy cycles | .249† | .476† | ||||||
| 3-4 | 33 (22.9%) | 12 (18.5%) | 21 (26.6%) | 23 (23.0%) | 10 (20.0%) | 13 (26.0%) | ||
| 5-6 | 111 (77.1%) | 53 (81.5%) | 58 (73.4%) | 77 (77.0%) | 40 (80.0%) | 37 (74.0%) | ||
Abbreviations: SD = standard deviation; Q = quarter.
Wilcoxon text.
χ2 test.
Fisher exact test.
Figure 1.
Cumulative incidence of grade 4 lymphopenia by week.
Univariate and multivariable models are presented in Table 2 for the entire cohort. On UVA, an increase in CTV per 100 cm3 (hazard ratio: 1.21; P < .01), stage III/IV (odds ratio [OR]: 3.92; P < .01), and XRT (OR: 4.58; P < .001) were associated with higher rates of G4L. CTV was considered codependent with stage, as noted earlier, and was not included in the MVA. The Pearson correlation coefficient between the variables of stage and CTV was 0.38. On MVA, XRT (OR: 5.13; 95% confidence interval [CI], 2.35-11.18; P < .001) and stage III/IV (OR: 4.54; 95% CI, 1.87-11.00; P < .001) were associated with G4L.
Table 2.
Univariate and multivariable logistic models for overall cohort (row percentages)
| Variable | Level | Lymphopenia | Univariate OR (95% CI) | Parsimonious multivariable OR (95% CI) |
|---|---|---|---|---|
| Age (per 10 y) | N/A | N/A | 0.90 (0.65-1.24) P = .51 |
|
| CTV (per 100 units) | N/A | N/A | 1.21 (1.05-1.40) P = .007 |
|
| Sex | M | 39.3% | 1.0 reference | |
| F | 45.5% | 1.28 (0.51-3.21) P = .59 |
||
| Stage group | I/II | 20.0% | 1.0 reference | 1.0 reference |
| III/IV | 49.5% | 3.92 (1.71-8.99) P = .001 |
4.54 (1.87-11.00) P < .001 |
|
| Surgery candidate | Yes | 40.5% | 1.0 reference | |
| No | 39.1% | 0.94 (0.38-2.35) P = .90 |
||
| Tumor location | Upper-middle | 44.4% | 1.0 reference | |
| Lower | 39.7% | 0.82 (0.30-2.23) P = .70 |
||
| Tumor histology | Squamous cell | 38.1% | 1.0 reference | |
| Adenocarcinoma | 40.7% | 1.11 (0.43-2.88) P = .83 |
||
| Grade | I/II | 40.0% | 1.0 reference | |
| III/IV | 44.6% | 1.21 (0.58-2.53) P = .61 |
||
| Radiation type | Proton | 21.5% | 1.0 reference | 1.0 reference |
| Photon | 55.7% | 4.58 (2.19-9.59) P < .001 |
5.13 (2.35-11.18) P < .001 |
|
| Total dose group | 5000+ | 38.5% | 1.0 reference | |
| <5000 | 50.0% | 1.60 (0.64-3.97) P = .32 |
||
| Concurrent chemotherapy cycles | 5-6 | 36.0% | 1.0 reference | |
| 3-4 | 54.5% | 2.13 (0.97-4.68) P = .06 |
Abbreviations: CI = confidence interval; CTV = clinical target volume; N/A = not applicable; OR = odds ratio.
In an exploratory MVA, both CTV and stage were included along with RT modality (XRT vs PRT). In this model, CTV was not significantly associated with G4L (OR: 1.12; P = .15), but stage III/IV (OR: 3.40; P = .01) and XRT (OR: 4.75; P < .001) remained significant.
PM group
The PM cohort included 50 XRT and 50 PRT patients (C-statistic logistic model: 0.690). Patient characteristics are presented by RT modality in Table 1. The PM XRT group had more grade III/IV tumors compared with the PRT group (61% vs 37%; P = .034), but no other differences between the groups were noted.
On UVA, stage III/IV and XRT were associated with high rates of G4L. An increase in CTV per 100 cm3 was not significantly associated with G4L and was not included in the parsimonious MVA. On MVA, stage III/IV (OR: 3.77; 95% CI, 1.26-11.30; P = .02) and XRT (OR: 5.28; 95% CI: 2.14-12.99; P < .001) were associated with G4L (Table 3). RT modality was most strongly associated with G4L, with 60% of XRT patients having G4L compared with 24% of PRT patients.
Table 3.
Univariate and multivariable logistic models for propensity-matched cohort (row percentages)
| Variable | Value | Rate of grade 4 lymphopenia | UVA OR (95% CI) | Parsimonious MVA OR (95% CI) |
|---|---|---|---|---|
| Age (per 10 y) | N/A | N/A | 1.11 (0.75-1.65) P = .59 |
|
| Sex | Male | 40.0% | 1.0 reference | |
| Female | 53.3% | 1.71 (0.57-5.17) P = .34 |
||
| Stage group | I/II | 23.1% | 1.0 reference | 1.0 reference |
| III/IV | 48.6% | 3.16 (1.14-8.76) P = .03 |
3.77 (1.26-11.30) P = .02 |
|
| Surgery candidate | Yes | 40.9% | 1.0 reference | |
| No | 50.0% | 1.44 (0.43-4.84) P = .55 |
||
| Tumor location | Upper-middle | 36.4% | 1.0 reference | |
| Lower | 42.7% | 1.30 (0.36-4.78) P = .69 |
||
| Tumor histology | Squamous cell | 30.8% | 1.0 reference | |
| Adenocarcinoma | 43.7% | 1.74 (0.50-6.10) P = .38 |
||
| Grade | I/II | 39.0% | 1.0 reference | |
| III/IV | 47.5% | 1.41 (0.58-3.42) P = .44 |
||
| Radiation type | Proton | 24.0% | 1.0 reference | 1.0 reference |
| Photon | 60.0% | 4.75 (2.01-11.24) P < .001 |
5.28 (2.14-12.99) P < .001 |
|
| Total radiation therapy dose (Gy; per 10 units) | N/A | N/A | 0.57 (0.15-2.09) P = .40 |
|
| Chemotherapy cycles | 5-6 | 39.0% | 1.0 reference | |
| 3-4 | 52.2% | 1.71 (0.67-4.36) P = .26 |
||
| CTV (Per 100 units) | N/A | N/A | 1.17 (0.99-1.39) P = .07 |
Abbreviations: CI = confidence interval; CTV = clinical target volume; MVA = multivariate analysis; N/A = not applicable; OR = odds ratio; UVA = univariate analysis.
Sensitivity analysis
The rate of G4L was not different for patients receiving 3DCRT versus IMRT, with 52% for patients who received 3-dimensional CRT and 57% for patients who received IMRT in the entire cohort (P = .72). The rate of G4L was 67% for 3-dimensional CRT and 57% for IMRT (P = .53) in the PM cohort. When removing all patients treated with 3-dimensional CRT from the overall and PM cohorts, the findings were unchanged, and RT modality remained significantly associated with G4L.
Discussion
In the present study, we showed an association of XRT and risk of G4L (55.7% of patients who received XRT compared with 21.5% of patients who received PRT for the entire cohort). XRT remained significant in a PM cohort on MVA (OR: 5.28; P < .001). Our results are similar to and validate those of Shiraishi et al, who showed an OR of 3.45 for G4L XRT compared with PRT.6 However, there are important differences between our cohort and that of Shiraishi et al. In our cohort, all patients received pencil-beam scanning PRT, few patients received induction chemotherapy, nearly all received concurrent carboplatin and paclitaxel chemotherapy, and we accounted for the number of cycles of chemotherapy.
The effect of RT on tumor-adapted immunity can be both stimulatory and suppressive.10 RT can facilitate the activation of lymphocytes, including populations of CD4+ and CD8+ T cells, but standard fractionation can lead to immunosuppression via substantial lymphocyte depletion.4, 11 Lymphopenia can persist after treatment, and RT appears to have an additional indirect effect on nonirradiated lymphocytes and nonirradiated hematopoietic stem cells.12 As novel methods to enhance the immune interaction with malignancies are developed, it is imperative that we examine the effect of existing treatments on the immune response and where possible optimize these treatments to favor a more robust response.
Our results emphasize important findings from previous investigations. First, lymphopenia has been associated with inferior outcomes, namely survival and incidence of distant metastases. Second, lymphopenia in the setting of immunotherapy has been shown to predict less favorable responses.13, 14 Our results are especially relevant in this context given that immunotherapy is under investigation in hundreds of trials and has shown promising results across various malignancies.15, 16, 17, 18, 19, 20 Limited prospective data are available in this regard for nonmetastatic EC, and recent results from a lung cancer trial provide an example of the potential relevance and need for further investigation of treatment-induced lymphopenia.
In the PACIFIC trial, Antonia et al demonstrated a substantial progression-free survival benefit for adjuvant durvalumab compared with placebo (16.8 vs 5.6 months) after definitive CRT for patients with locally advanced non-small cell lung cancer.16 The impact of RT-related lymphopenia on response rates to immunotherapy remains unclear, but we hypothesize that the preservation of lymphocyte counts may improve the efficacy of adjuvant immunotherapy.13
Here, we demonstrate that PRT compared with XRT may be one such way to reduce treatment-induced lymphopenia in patients with EC and potentially other disease sites. The exact mechanism by which proton therapy may reduce the rates of lymphopenia in EC remains to be determined, and dosimetric analyses, including an examination of the dose to organs at risk, are warranted. PRT may decrease rates of lymphopenia by reducing the dose to circulating lymphocytes and/or reducing dose to bone marrow. Studies in patients with glioma, in whom the majority of radiation exposure is to circulating lymphocytes, have identified intermediate dose volumes as significantly associated with severe treatment-induced lymphopenia.21 Dosimetric analysis of lung cancer revealed that the volume of the lung receiving ≥5 Gy was associated with the rate of lymphopenia.22 Therefore, the impact of PRT is most likely mediated by a reduction in a low-dose radiation bath and in the integral intermediate dose volumes in patients with EC.23
These results are limited by their retrospective nature and the potential for selection and other biases. We attempted to reduce these issues with the use of propensity matching. Another limitation of our series was the rate of G4L in our photon cohort compared with historic comparisons. For instance, 38% of patients in the Cancer and Leukemia Group B (CALGB) 9781 study experienced G4L compared with 56% in our analysis.24
Additionally, 41.40 Gy is standard of care per the ChemoRadiotherapy for Esophageal cancer followed by Surgery Study (CROSS) trial.25 The majority of patients treated in our series received approximately 50 Gy, and the impact of PRT relative to XRT on severe treatment-induced lymphopenia may be lower at reduced dose levels.
Conclusions
XRT was associated with a significantly higher risk of G4L compared with PRT. Dosimetric and prospective analyses are warranted to better understand the interaction between RT and G4L and assess the oncologic outcomes and potential impact on future therapies for EC.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary material for this article (https://doi.org/10.1016/j.adro.2018.09.004) can be found at advanceradonc.org.
Supplementary data
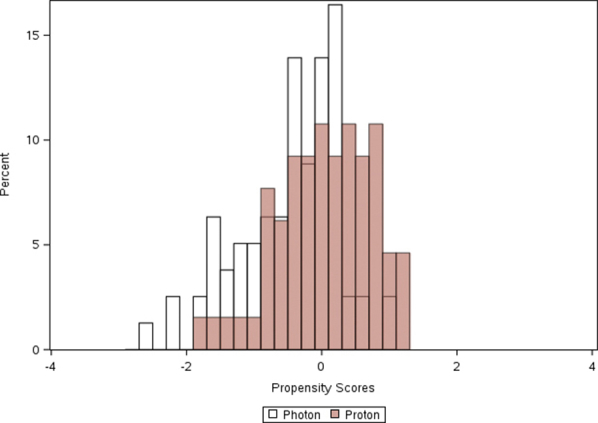
Supplementary Figure 1.
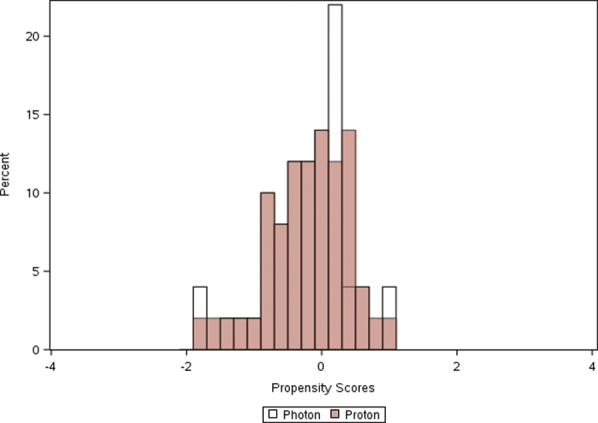
Supplementary Figure 2.
References
- 1.Campian J.L., Sarai G., Ye X., Marur S., Grossman S.A. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–1753. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campian J.L., Ye X., Brock M., Grossman S.A. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31:183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davuluri R., Jiang W., Fang P. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Grossman S.A., Ellsworth S., Campian J. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13:1225–1231. doi: 10.6004/jnccn.2015.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman S.A., Ye X., Lesser G. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraishi Y., Fang P., Xu C. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–160. doi: 10.1016/j.radonc.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu E.S., Oduyebo T., Cobb L.P. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140:76–82. doi: 10.1016/j.ygyno.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura N., Kusunoki Y., Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–227. [PubMed] [Google Scholar]
- 9.Yovino S., Kleinberg L., Grossman S.A., Narayanan M., Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiotto M., Fu Y.X., Weichselbaum R.R. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De La Maza L., Wu M., Wu L. In situ vaccination after accelerated hypofractionated radiation and surgery in a mesothelioma mouse model. Clin Cancer Res. 2017;23:5502–5513. doi: 10.1158/1078-0432.CCR-17-0438. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor V., Khudanyan A., de la Puente P. Stem cell transfusion restores immune function in radiation-induced lymphopenic C57BL/6 mice. Cancer Res. 2015;75:3442–3445. doi: 10.1158/0008-5472.CAN-15-1412. [DOI] [PubMed] [Google Scholar]
- 13.Diehl A., Yarchoan M., Hopkins A., Jaffee E., Grossman S.A. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8:114268–114280. doi: 10.18632/oncotarget.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku G.Y., Yuan J., Page D.B. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsaab H.O., Sau S., Alzhrani R. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont A.M., Chiarion-Sileni V., Grob J.J. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris R.L., Blumenschein G., Jr., Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J., Mandala M., Del Vecchio M. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 21.Huang J., DeWees T.A., Badiyan S.N. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92:1000–1007. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Tang C., Liao Z., Gomez D. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Welsh J., Gomez D., Palmer M.B. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81:1336–1342. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tepper J., Krasna M.J., Niedzwiecki D. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Hagen P., Hulshof M.C., van Lanschot J.J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]