Background
The spine is one of the most common sites for tumor metastasis.1 It is estimated that approximately 30% of all patients with cancer develop spinal metastasis at some point in their cancer course.2 Spinal metastatic disease can cause significant morbidity in patients, including pain, hypercalcemia, pathologic fractures, spinal instability, and compression of the spinal cord or cauda equina.3 Management of spinal metastases can be complex and may benefit from multimodal therapeutic strategies to achieve optimal outcomes.
Stereotactic spinal radiosurgery (SSRS) is an emerging technique that has been developed to deliver highly conformal ionizing radiation doses, designed to control gross disease while simultaneously minimizing dose to surrounding critical structures such as the spinal cord. Current data on SSRS suggest favorable local control rates of approximately 90% at 1 year, complete pain response of approximately 50%, and low rates of high-grade toxicity.4 However, as with all emerging technologies, understanding the potential complications of novel therapies such as SSRS is fundamental to safely implementing this technology throughout the wider oncology community. The focus of this case and review is the risk of vertebral fracture after SSRS.
Case presentation
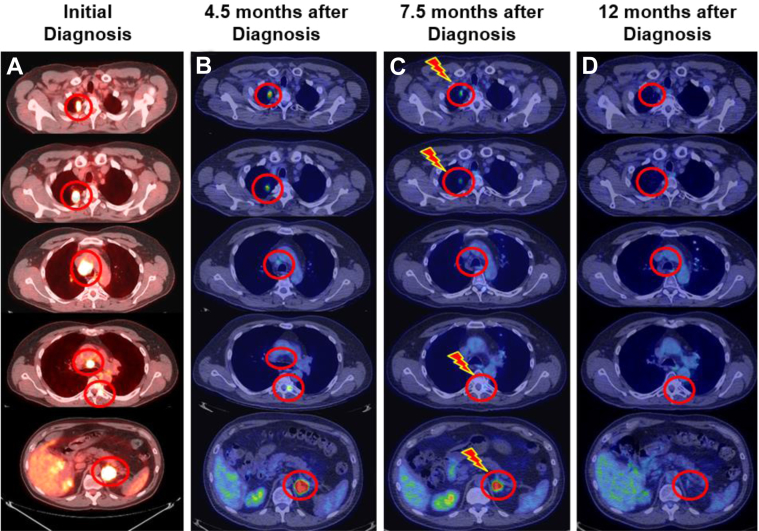
A 54-year old male patient presented with a progressively worsening cough. His medical history was notable for smoking half a pack of cigarettes daily for 35 years but was otherwise unremarkable. Further evaluation included an 18F-fluorodeoxyglucose positron emission/computed tomography (FDG-PET/CT) scan that revealed 2 right upper-lung nodules, 2 enlarged mediastinal lymph nodes, a left adrenal gland lesion, and a lesion involving the left posterolateral elements of the T6 vertebrae (Fig 1A). Endoscopically guided biopsy of a right upper-lung nodule and the left adrenal mass revealed concordant poorly differentiated adenocarcinoma of primary lung origin.
Figure 1.
Radiographic timeline of patient's 6 lesions. (A) Represents 18F-fluorodeoxyglucose (FDG) positron emission/computed tomography (PET/CT) axial slices of the patient at initial diagnosis. Identified FDG-avid lesions included 2 right upper-lung nodules, station 4R paratracheal and station 7 subcarinal lymph nodes, a left T6 vertebrae metastasis, and a left adrenal gland metastasis. (B) FDG-PET/CT reevaluation 4.5 months after initial diagnosis and 6 total cycles of chemotherapy showing a mixed response. (C) FDG-PET/CT reevaluation 7.5 months after initial diagnosis and 10 total cycles of chemotherapy. The patient completed radiation therapy to the 4 indicated sites 1 month after this scan was performed. (D) Complete radiographic response to all sites 12 months after initial diagnosis.
After multidisciplinary tumor board discussion, systemic therapy was recommended. The patient was treated with 6 cycles every 21 days of gemcitabine (1250 mg/m2/d on days 1 and 8), cisplatin (80 mg/m2/d on day 1), and bevacizumab (7.5 mg/kg/d on day 1). A reevaluation with FDG-PET/CT scan 4.5 months after the initial diagnosis showed a mixed response with decreased FDG uptake in the right upper-lung nodules and mediastinal lymph nodes, stable FDG uptake in the left adrenal gland, and increased FDG uptake in the left pedicle of T6 (Fig 1B). There was no evidence of new metastatic disease.
After further multidisciplinary discussion, continuation of systemic therapy was recommended. The patient was reinitiated on 1 additional cycle of gemcitabine, cisplatin, and bevacizumab (7 total cycles) and then switched to pemetrexed (500 mg/m2/d on day 1), carboplatin (area under the curve 6 on day 1), and bevacizumab (15 mg/kg/d on day 1) for 3 cycles (10 cycles total). Bevacizumab was omitted during the 10th total cycle in preparation for stereotactic radiation therapy.
A reevaluation with FDG-PET/CT scan 7.5 months after initial diagnosis showed further response in the right upper-lung nodules, T6 lesion, and left adrenal. There was complete response involving the mediastinal adenopathy per the Response Evaluation Criteria in Solid Tumors, version 1.1, and no evidence of new distant disease (Fig 1C). The patient had a total spinal instability neoplastic score (SINS) of 5.5 The SINS component scores included location (score 1), pain (score 1), bone lesion (score 2), radiographic spinal alignment (score 0), vertebral body collapse (score 0), and posterolateral involvement of spinal elements (score 1). At our institution, surgical consultation is recommended before SSRS for patients with SINS ≥7.
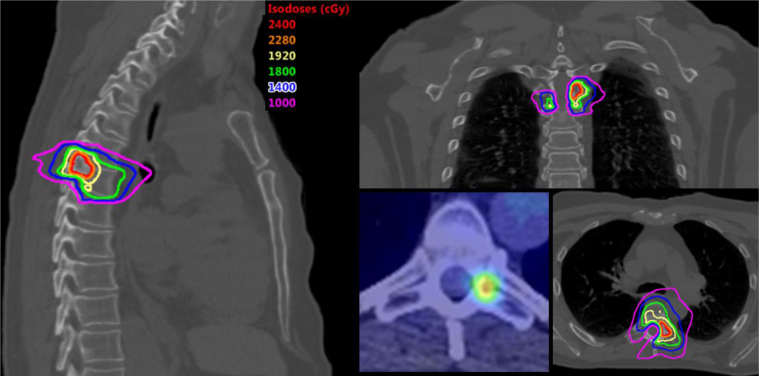
The patient proceeded with stereotactic body radiation therapy to the right upper-lung nodules (50 Gy in 5 fractions) and left adrenal metastasis (50 Gy in 5 fractions) along with SSRS to the T6 vertebral lesion (24 Gy in 1 fraction; Fig 2). Radiation therapy was completed 8.5 months after initial diagnosis. For the lung nodules, an internal gross tumor volume (GTV) was created, which represented gross disease on all respiratory phases. The left adrenal GTV was identified on the breath hold scan. The planning target volume (PTV) was created by expanding the internal GTV/GTV by 0.5 cm isometrically. For the spine lesion, 2 target volumes were delineated, including a high-dose 24 Gy volume, which included all T1 contrast-enhancing disease on magnetic resonance imaging, and a low-dose 18 Gy volume designed by treating approximately 1 cm of at-risk adjacent osseous elements, consistent with published guidelines.6 The spinal PTVs were created by expanding each respective target volume by 0.2 cm.
Figure 2.
Stereotactic spinal radiation surgery (SSRS) plan. Representative sagittal, coronal, and axial slices for the T6 vertebrae lesion plan. SSRS consisted of 24 Gy and 18 Gy in 1 fraction to the high-dose and low-dose clinical target volumes, respectively.
Treatment planning was done using the Eclipse Treatment Planning System (Varian, Palo Alto, CA) with a volumetric multiarc approach. Dose constraints were followed per the report of the American Association of Physicists in Medicine Task Group 101.7 Critical constraint objectives for the spinal lesion included cord maximum <14 Gy and D1.2cc[Gy] <7 Gy. Treatments were delivered on consecutive days. Image guidance was done with daily cone beam CT using a 3 degree-of-freedom robotic couch for lung and adrenal targets matched to PTV. An integrated ExacTrac system (Brainlab AG, Munich, Germany) was used for the T6 lesion with tolerance set at 1 mm and 0.5° matched to the T6 bony anatomy.
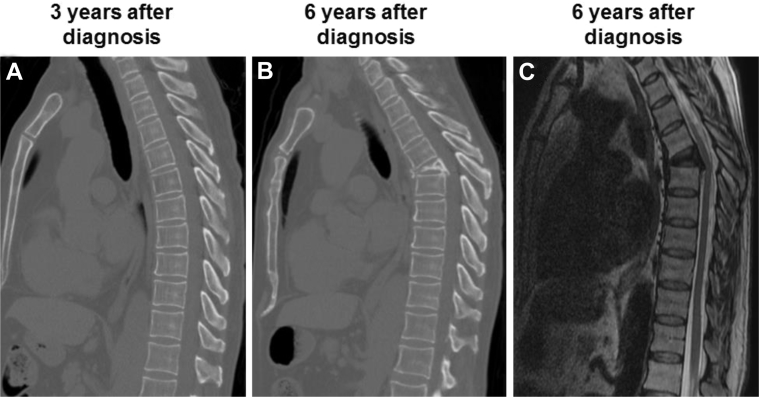
After completing radiation therapy, the patient completed 2 additional cycles of pemetrexed and carboplatin (12 total cycles), followed by reevaluation 12 months after the initial diagnosis; FDG-PET/CT scan showed no evidence of disease (Fig 1D). The patient remained disease-free more than 6 years after the initial diagnosis. However, after >5 years of freedom from disease after SSRS, the patient presented with sharp, stabbing pain at an area of kyphosis at the T6 level, associated with periscapular pain that radiated around to the anterior chest. Imaging revealed a compression fracture at T6 (Fig 3). Consequently, the patient underwent T5-T7 laminectomy, bilateral T6 nerve root rhizotomy, T5-6 and T6-7 discectomies, and T6 vertebrectomy along with posterior spinal fusion of T3-T9 and anterior spinal fusion of T5-T7. Pathology test results showed fragmented bone with degenerative changes and osteonecrosis with reactive fibrovascular proliferation. No malignancy was identified. The has since recovered from this operation and returned to his active lifestyle with expected spinal mobility limitations.
Figure 3.
Vertebral compression fracture at T6. (A) Sagittal computed tomography slices of patient's spine 3 years after diagnosis (2 years after stereotactic spinal radiation surgery [SSRS]), (B) which was the last imaging obtained before his T6 compression fracture 6 years after diagnosis (5 years after SSRS). (C) Sagittal T2-weighted magnetic resonance sequence at 6 years after diagnosis.
Ethics approval for this report was obtained by the ethics committee governed by the Mayo Clinic internal review board. Informed consent was obtained from the patient while under our care. Before the writing of this manuscript, patient consent was obtained to use his medical information for the purposes of research and advancing medicinal science, including publication of this report.
Discussion
SSRS has emerged as an excellent treatment option for patients with oligometastatic spinal disease, as outlined in the American College of Radiology appropriateness criteria.8 The decision to treat with SSRS should be made carefully by clinicians who are properly trained in this technologically advanced technique. Once appropriate selection of patients has occurred, recognition of treatment complications and every effort to mitigate such complications should be reviewed.
The present case highlights the complication of vertebral compression fracture post-SSRS. The patient had metastatic adenocarcinoma of the lung and after 10 cycles of chemotherapy was found to have no new evidence of distant metastasis despite 4 oligometastatic sites that demonstrated radiographic disease persistence. These 4 sites were treated with stereotactic radiation therapy, and the patient has had a sustained complete response. However, despite the excellent oncologic outcome, 5 years after SSRS, the patient developed a vertebral compression fracture that required extensive surgical intervention, stabilization, and fixation.
In a review of the current literature, 13 stereotactic radiosurgery studies have been identified that evaluated the risk of vertebral compression fracture.2, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 The rates of fracture are summarized with risk factors identified by univariate and multivariate analysis in Table 1. Of the 3860 treated vertebral bodies, 474 vertebral compression fractures (12.3%) were identified in this updated review. Risk factors of vertebral compression fracture include preexisting vertebral compression fracture, lytic-type lesion, spinal malalignment or deformity, a high SINS, higher radiation dose per fraction, baseline pain, age, and extent of involvement (Table 1).
Table 1.
Summarized risk factors for vertebral compression fracture after stereotactic spinal radiation surgery
| Study (Year; hospital) | Total patients, N | Total VBs | Dose/fx (median) | VB fractures, n (%) | Risk factors (UVA) | Risk factors (MVA) |
|---|---|---|---|---|---|---|
| Rose et al9 (2009; MSKCC) | 62 | 71 | 24/1 | 27 (39) | Not reported | CT appearance (lytic = 6.8x); lesion location (T10 and below = 4.6x); % VB involvement (>40%) |
| Boehling et al10 (2012; MDACC) | 93 | 123 | 18/1 27/3 30/5 |
25 (20) | Age >55 y; preexisting fracture; baseline pain; narcotic use before and after SBRT | Age >55 y; preexisting fracture; baseline pain |
| Cunha et al11 (2012; PMH) | 90 | 167 | 24/2 | 19 (11) | Spinal misalignment; lesion type (lytic); degree of preexisting VCF | Spinal misalignment; lesion type (lytic); dose per fraction (≥20 Gy); lung primary; hepatocellular primary |
| Sahgal et al12 (2013; PMH) | 252 | 410 | 24/1 | 57 (14) | Dose per fraction; preexisting VCF; lesion type (lytic); spinal deformity; spinal misalignment; paraspinal/epidural extension | Dose per fraction; preexisting VCF; lesion type (lytic); spinal deformity; spinal misalignment; paraspinal/ epidural extension |
| Sung et al13 (2014; KCCH) | 72 | 72 | 21/1∗ | 26 (36) | SINS; spinal deformity (<40% vs ≥40%); whole VB involvement (<40% vs ≥40%); VB osteolysis rate (<61% vs ≥61%) | VB osteolysis rate (<61% vs ≥61%) |
| Thibault et al14 (2014; Sunnybrook) | 37 | 71 | 24/2 | 10 (16) | Not reported | Single-fraction SBRT; preexisting VCF |
| Guckenburger et al15 (2014; Wurzburg) | 301 | 387 | 24/3 | 30 (8) | Not reported | Not reported |
| Germano et al16 (2016; Mt. Sinai) | 79 | 143 | 18/1 | 30 (21) | Colorectal primary; preexisting VCF; severe pain | Not reported |
| Jawad et al17 2016; Beaumont |
541 | 594 | 20/1 | 34 (5.7) | SBRT <36.8 d after diagnosis; no additional bone metastasis; no prior chemotherapy; preexisting VCF; tumor volume >37.3 cm3; EQD2 tumor >41.8 Gy; EQD2 spinal cord Dmax >46.1 Gy | Preexisting VCF; no additional bone metastasis; target volume >38.4 Gy |
| Lee et al18 (2016; MDACC) | 79 | 100 | 24-27/3 | 32 (41) | ESCC grade 1a and 1b; high SINS score (7-12) | High SINS score (7-12) |
| Thibault et al19 (2017; Sunnybrook) | 55 | 100 | 24/2 | 17 (17) | Dmax; D90; D80; D50 | Osteolytic percentage (≥11.6%); preexisting VCF; SBRT dose ≥20 Gy/fx |
| Boyce-Fappiano et al2 (2017; Henry Ford) | 448 | 1070 | 18/1 | 127 (12) | Preexisting VCF; hematologic primary; thoracic spine tumors; lesion type (lytic); female patients | Preexisting VCF; lesion type (lytic) |
| Virk et al20 (2017; MSKCC) | 323 | 552 | 24/1 | 40 (7.2) | Not reported | Not reported |
| Totals | 2432 | 3860 | 24/1 | 474 (12.3) |
Abbreviations: CT = computed tomography; Dmax = maximum dose; EQD2 = equivalent 2-Gy tumor dose; ESCC = epidural spinal cord compression classification; Fx = fraction; KCCH = Korean Cancer Center Hospital; MDACC = MD Anderson Cancer Center; MSKCC = Memorial Sloan Kettering Cancer Center; MVA = multivariate analysis; PMH. Princess Margaret Hospital; SBRT = stereotactic body radiation therapy; SINS = spinal instability neoplastic score; UVA = univariate analysis; VB = vertebral body; VCF = vertebral compression fracture.
Mean Dose/Fractionation.
Dose per fraction is an important consideration for both tumor control and risk of vertebral fracture. Three of the reviewed studies specifically identified dose as a risk factor on univariate and/or multivariate analysis.11, 12, 17 Sahgal et al and Cunha et al cautioned physicians of vertebral fracture risk when treating with single-fraction doses of ≥20 Gy.11, 12 A more detailed dosimetric analysis was performed by Jawad et al, who showed that an equivalent 2-Gy dose (EQD2) of ≥41.8 Gy and a prescription target volume dose of ≥38.4 Gy (EQD2) were predictive of vertebral compression fracture.17 These are important considerations when designing any high-quality SSRS treatment plan.
Unique features of this case include the delay in time to fracture along with the patient's durable disease control after aggressive metastasis-directed therapy. Previous reviews suggest that the time to fracture most commonly occurs at approximately 3 months post-SSRS.2, 21 The patient's delay in fracture of 5 years post-SSRS may be more related to small vessel changes and demineralization, as opposed to acute structural changes from tumor voids where fracture occurs relatively soon after SSRS. These differences highlight the potential issue of delayed fracture as systemic therapies (eg, targeted and immune-mediated therapies) improve and disease control becomes more durable.
Although previous reviews have suggested that time to fracture most commonly occurs approximately 3 months post-SSRS, delayed vertebral fracture risk has been demonstrated in some series. For example, Moussazadeh et al reported a median time to vertebral compression fracture of 25.7 months (range, 11.6-76.0).22 The authors suggested that their observed longer time to the development of a fracture may be accounted for by carefully screening patients for symptomatic and radiographic mechanical instability before SSRS. Any patient with a symptomatic fracture causing severe instability pain (visual analog score >6) was treated in the Moussazadeh et al series with prophylactic vertebroplasty with or without instrumented percutaneous stabilization. The presented patient did not have a visual analog pain score >6; in fact, he was largely asymptomatic at the time of SSRS. Furthermore, he was deemed to be at low risk for spinal instability using the SINS (SINS total score = 5). Nevertheless, he developed delayed vertebral fracture, which may suggest the need for effective mitigation strategies for post-SSRS vertebral compression fracture, even in lower-risk patients if followed long enough.
Ongoing studies are evaluating prophylactic cement augmentation (MDACC 2014-0561, ClinicalTrials.gov, number NCT02387905) to mitigate the risk of vertebral compression fracture for the highest-risk patients (eg, preexisting fracture, SINS of 7-12, and SSRS dose of 24 Gy). This study along with others will further inform our decision making to minimize the complication of vertebral fracture post-SSRS in the future. Minimizing late effects will be important as outcomes improve and patients are living longer but complications could arise.
Conclusions
This teaching case highlights the complication of vertebral compression fracture after SSRS. In review of 13 published SSRS studies, the average rate of vertebral compression fracture was 12.3%. This case emphasizes the continued need for effective mitigation strategies for post-SSRS complications such as delayed vertebral compression fracture.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Suva L.J., Washam C., Nicholas R.W., Griffin R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce-Fappiano D., Elibe E., Schultz L. Analysis of the factors contributing to vertebral compression fractures after spine stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2017;97:236–245. doi: 10.1016/j.ijrobp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 4.Husain Z.A., Sahgal A., De Salles A. Stereotactic body radiation therapy for de novo spinal metastases: Systematic review. J Neurosurg Spine. 2017;27:295–302. doi: 10.3171/2017.1.SPINE16684. [DOI] [PubMed] [Google Scholar]
- 5.Fourney D.R., Frangou E.M., Ryken T.C. Spinal instability neoplastic score: Aanalysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 6.Cox B.W., Spratt D.E., Lovelock M. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Radiation Oncology-Bone Metastatses, Lo S.S., Lutz S.T. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16P:9–19. doi: 10.1089/jpm.2012.0376. [DOI] [PubMed] [Google Scholar]
- 9.Rose P.S., Laufer I., Boland P.J. Risk of fracture after single fraction image guided intensity modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehling N.S., Grosshans D.R., Allen P.K. Vertebral compression fracture risk after stereotactic body radiation therapy for spinal metastases. J Neurosurg Spine. 2012;16:379–386. doi: 10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]
- 11.Cunha M.V., Al-Omair A., Atenafu E.G. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): Analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84:e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Sahgal A., Atenafu E.G., Chao S. Vertebral compression fracture after spine stereotactic body radiation therapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–3431. doi: 10.1200/JCO.2013.50.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung S.H., Chang U.K. Evaluation of risk factors for vertebral compression fracture after stereotactic radiosurgery in spinal tumor patients. Korean J Spine. 2014;11:103–108. doi: 10.14245/kjs.2014.11.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thibault I., Al-Omair A., Masucci G.L. Spine stereotactic body radiation therapy for renal cell cancer spinal metastases: Analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21:711–718. doi: 10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger M., Mantel F., Gerszten P.C. Safety and efficacy of stereotactic body radiation therapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiat Oncol. 2014;9:226. doi: 10.1186/s13014-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germano I.M., Carai A., Pawha P., Blacksburg S., Lo Y.C., Green S. Clinical outcome of vertebral compression fracture after single fraction spine radiosurgery for spinal metastases. Clin Exp Metastasis. 2016;33:143–149. doi: 10.1007/s10585-015-9764-8. [DOI] [PubMed] [Google Scholar]
- 17.Jawad M.S., Fahim D.K., Gerszten P.C. Vertebral compression fractures after stereotactic body radiation therapy: A large, multi-institutional, multinational evaluation. J Neurosurg Spine. 2016;24:928–936. doi: 10.3171/2015.10.SPINE141261. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.H., Tatsui C.E., Ghia A.J. Can the spinal instability neoplastic score before spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor?: A post hoc analysis of prospective phase II single-institution trials. J Neurooncol. 2016;126:509–517. doi: 10.1007/s11060-015-1990-z. [DOI] [PubMed] [Google Scholar]
- 19.Thibault I., Whyne C.M., Zhou S. Volume of lytic vertebral body metastatic disease quantified using computed tomography-based image segmentation predicts fracture risk after spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:75–81. doi: 10.1016/j.ijrobp.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Virk M.S., Han J.E., Reiner A.S. Frequency of symptomatic vertebral body compression fractures requiring intervention following single-fraction stereotactic radiosurgery for spinal metastases. Neurosurg Focus. 2017;42:E8. doi: 10.3171/2016.10.FOCUS16359. [DOI] [PubMed] [Google Scholar]
- 21.Faruqi S., Tseng C.L., Whyne C. Vertebral compression fracture after spine stereotactic body radiation therapy: A review of the pathophysiology and risk factors. Neurosurgery. 2018;83:314–322. doi: 10.1093/neuros/nyx493. [DOI] [PubMed] [Google Scholar]
- 22.Moussazadeh N., Lis E., Katsoulakis E. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2015;93:361–367. doi: 10.1016/j.ijrobp.2015.05.035. [DOI] [PubMed] [Google Scholar]